Crystal Chemistry of Birefringent Uvarovite Solid Solutions
Abstract
1. Introduction
2. Experimental Methods
2.1. Sample Description
2.2. Electron-Probe Microanalysis (EPMA)
2.3. Synchrotron High-Resolution Powder X-Ray Diffraction (HRPXRD)
2.4. Rietveld Structure Refinement
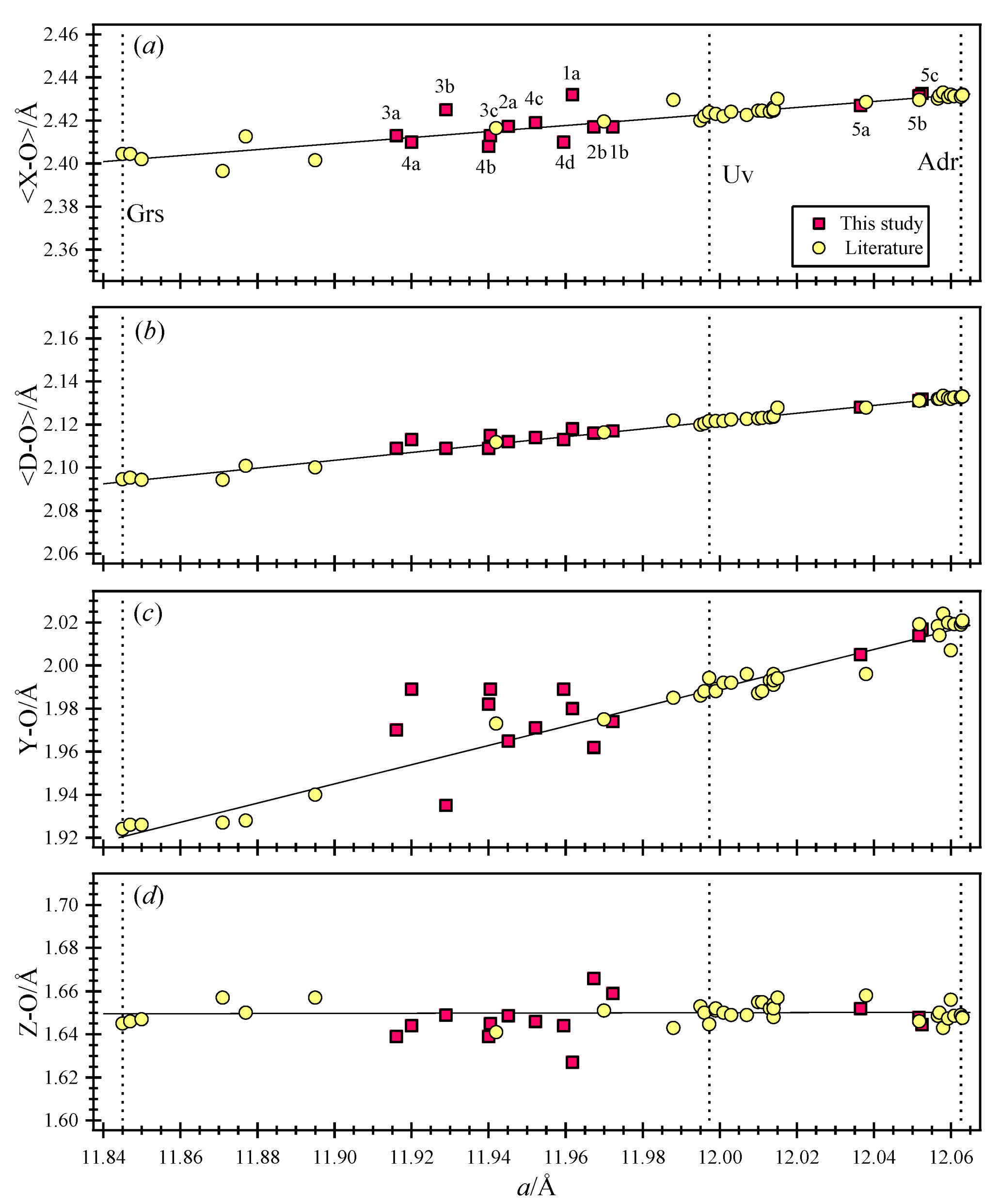
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akizuki, M. Origin of optical variations in grossular-andradite garnet. Am. Mineral. 1984, 66, 403–409. [Google Scholar]
- Ivanova, T.I.; Shtukenberg, A.G.; Punin, Y.O.; Frank-Kamenetskaya, O.V.; Sokolov, P.B. On the complex zonality in grandite garnets and implications. Mineral. Mag. 1998, 62, 857–868. [Google Scholar] [CrossRef]
- Jamtveit, B. Oscillatory zonation patterns in hydrothermal grossular-andradite garnet: Nonlinear dynamics in regions of immiscibility. Am. Mineral. 1991, 76, 1319–1327. [Google Scholar]
- Pollok, K.; Jamtveit, B.; Putnis, A. Analytical transmission electron microscopy of oscillatory zoned grandite garnets. Contrib. Mineral. Petrol. 2001, 141, 358–366. [Google Scholar] [CrossRef]
- Antao, S.M.; Zaman, M.; Gontijo, V.L.; Camargo, E.S.; Marr, R.A. Optical anisotropy, zoning, and coexistence of two cubic phases in andradites from Quebec and New York. Contrib. Mineral. Petrol. 2015, 169, 10. [Google Scholar] [CrossRef]
- Ague, J.J.; Axler, J.A. Interface coupled dissolution-reprecipitation in garnet from subducted granulites and ultrahigh-pressure rocks revealed by phosphorous, sodium, and titanium zonation. Am. Mineral. 2016, 101, 1696–1699. [Google Scholar] [CrossRef]
- Antao, S.M. Three cubic phases intergrown in a birefringent andradite-grossular garnet and their implications. Phys. Chem. Miner. 2013, 40, 705–716. [Google Scholar] [CrossRef]
- Brewster, D. On the optical figures produced by the disintegrated surfaces of crystals. Philos. Mag. Ser. 4 1853, 6, 16–30. [Google Scholar] [CrossRef]
- Mallard, E. Anomalies optiques. Ann. Mines. Mem. VII Ser. 1876, 10, 60. [Google Scholar]
- Allen, F.M.; Buseck, P.R. XRD, FTIR, and TEM studies of optically anisotropic grossular garnets. Am. Mineral. 1988, 73, 568–584. [Google Scholar]
- Brown, D.; Mason, R.A. An occurrence of sectored birefringence in almandine from the Gangon terrane, Labrador. Can. Mineral. 1994, 32, 105–110. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-forming minerals: Volume 1A Orthosilicates; Longman Group Limited: New York, NY, USA, 1982. [Google Scholar]
- Rossman, G.R.; Aines, R.D. The hydrous components in garnets: Grossular-hydrogrossular. Am. Mineral. 1991, 76, 1153–1164. [Google Scholar]
- Frank-Kamenetskaya, O.V.; Rozhdestvenskaya, L.V.; Shtukenberg, A.G.; Bannova, I.I.; Skalkina, Y.A. Dissymmetrization of crystal structures of grossular-andradite garnets Ca3(Al, Fe)2(SiO4)3. Struct. Chem. 2007, 18, 493–503. [Google Scholar] [CrossRef]
- Takéuchi, Y.; Haga, N.; Umizu, S.; Sato, G. The derivative structure of silicate garnets in grandite. Z. Krist. 1982, 158, 53–99. [Google Scholar] [CrossRef]
- Antao, S.M.; Klincker, A.M. Origin of birefringence in andradite from Arizona, Madagascar, and Iran. Phys. Chem. Miner. 2013, 40, 575–586. [Google Scholar] [CrossRef]
- Andrut, M.; Wildner, M. The crystal chemistry of birefringent natural uvarovites: Part I. Optical investigations and UV-VIS-IR absorption spectroscopy. Am. Mineral. 2001, 86, 1219–1230. [Google Scholar] [CrossRef]
- Andrut, M.; Wildner, M. The crystal chemistry of birefringent natural uvarovites. Part III. Application of the superposition model of crystal fields with a characterization of synthetic cubic uvarovite. Phys. Chem. Miner. 2002, 29, 595–608. [Google Scholar] [CrossRef]
- Wildner, M.; Andrut, M. The crystal chemistry of birefringent natural uvarovites: Part II. Single-crystal X-ray structures. Am. Mineral. 2001, 86, 1231–1251. [Google Scholar] [CrossRef]
- Baur, W.H.; Fischer, R.X. On the significance of small deviations from higher symmetry. Mineral. Mag. 2003, 67, 793–797. [Google Scholar] [CrossRef]
- Baur, W.H.; Tillmanns, E. How to avoid unnecessarily low symmetry in crystal structure determination. Acta Crystallogr. 1986, B42, 95–111. [Google Scholar] [CrossRef]
- Bank, H. Über grossular und hydrogrossular. Z. Dtsch. Gemmol. Ges. 1982, 31, 93–96. [Google Scholar]
- Hirai, H.; Nakazawa, H. Visualizing low symmetry of a grandite garnet on precession photographs. Am. Mineral. 1986, 71, 1210–1213. [Google Scholar]
- Hirai, H.; Nakazawa, H. Grandite garnet from Nevada: Confirmation of origin of iridescence by electron microscopy and interpretation of a moiré-like texture. Am. Mineral. 1986, 71, 123–126. [Google Scholar]
- Koritnig, S.; Rösch, H.; Schneider, A.; Seifert, F. Der Titan-zirkon-granat aus den Kalksilikatfels-Einschlüssen des Gabbro im Radautal, Harz, Bundesrepublik Deutschland. Tsch. Mineral. Petrogr. Mitt. 1978, 25, 305–313. [Google Scholar] [CrossRef]
- Manning, P.G.; Owens, D.R. Electron microprobe, X-ray diffraction, and spectral studies of South African and British Columbian “jades”. Can. Mineral. 1977, 15, 512–517. [Google Scholar]
- Zabinski, W. “Hydrogarnets”, Polska Akademia Nauk Oddzial Krakowie, Komisja Nauk Mineralogicznych, Prace Mineralogiczne. Geol. Warszawa 1966, 3, 1–69. [Google Scholar]
- Antao, S.M. Is near-endmember birefringent grossular non-cubic? New evidence from synchrotron diffraction. Can. Mineral. 2013, 51, 771–784. [Google Scholar] [CrossRef]
- Schingaro, E.; Lacalamita, M.; Mesto, E.; Ventruti, G.; Pedrazzi, G.; Ottolini, L.; Scordari, F. Crystal chemistry and light elements analysis of Ti-rich garnets. Am. Mineral. 2016, 101, 371–384. [Google Scholar] [CrossRef]
- Armbruster, T. Structure refinement of hydrous andradite, Ca3Fe1.54Mn0.02Al0.26(SiO4)1.65(O4H4)1.35, from the Wessels mine, Kalahari manganese field, South Africa. Eur. J. Mineral. 1995, 7, 1221–1225. [Google Scholar] [CrossRef]
- Novak, G.A.; Gibbs, G.V. The crystal chemistry of the silicate garnets. Am. Mineral. 1971, 56, 1769–1780. [Google Scholar]
- Ungaretti, L.; Leona, M.; Merli, M.; Oberti, R. Non-ideal solid-solution in garnet: Crystal-structure evidence and modelling. Eur. J. Mineral. 1995, 7, 1299–1312. [Google Scholar] [CrossRef]
- Antao, S.M.; Cruickshank, L.A. Two cubic phases in kimzeyite garnet from the type locality Magnet Cove, Arkansas. Acta Crystallogr. 2016, B72, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Hålenius, U. Nomenclature of the garnet supergroup. Am. Mineral. 2013, 98, 785–811. [Google Scholar] [CrossRef]
- Antao, S.M. Schorlomite and morimotoite: what’s in a name? Powder Diffr. 2014, 29, 346–351. [Google Scholar] [CrossRef]
- Antao, S.M.; Cruickshank, L.A. Crystal structure refinements of tetragonal (OH,F)-rich spessartine and henritermierite garnets. Acta Crystallogr. 2018, B74, 104–114. [Google Scholar] [CrossRef]
- Antao, S.M.; Mohib, S.; Zaman, M.; Marr, R.A. Ti-rich andradites: Chemistry, structure, multi-phases, optical anisotropy, and oscillatory zoning. Can. Mineral. 2015, 53, 133–158. [Google Scholar] [CrossRef]
- Antao, S.M.; Suarez Nieto, N.S.; Cruickshank, L.A.; Gwanmesia, G.D. Crystal structure refinements of pyrope-majorite solid solutions between Prp100Mj0 and Prp17Mj83. Adv. X-Ray Anal. 2015, 59, 192–211. [Google Scholar]
- Antao, S.M.; Zaman, M.; Suarez Nieto, N.S.; Gontijo, V.L.; Marr, R.A. Structural variations in pyrope-almandine solid solutions. Adv. X-Ray Anal. 2014, 58, 90–107. [Google Scholar]
- Locock, A.J. An excel spreadsheet to recast analyses of garnet into end-member components, and a synopsis of the crystal chemistry of natural silicate garnets. Comput. Geosci. 2008, 34, 1769–1780. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Wang, J.; Lee, P.L.; Toby, B.H. State-of-the-art high-resolution powder X-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite. Can. Mineral. 2008, 46, 1501–1509. [Google Scholar] [CrossRef]
- Lee, P.L.; Shu, D.; Ramanathan, M.; Preissner, C.; Wang, J.; Beno, M.A.; Von Dreele, R.B.; Ribaud, L.; Kurtz, C.; Antao, S.M.; et al. A twelve-analyzer detector system for high-resolution powder diffraction. J. Synchrotron Radiat. 2008, 15, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Toby, B.H.; Lee, P.L.; Ribaud, L.; Antao, S.M.; Kurtz, C.; Ramanathan, M.; Von Dreele, R.B.; Beno, M.A. A dedicated powder diffraction beamline at the advanced photon source: Commissioning and early operational results. Rev. Sci. Instrum. 2008, 79, 085105. [Google Scholar] [CrossRef] [PubMed]
- Antao, S.M.; Duane, M.J.; Hassan, I. DTA, TG, and XRD studies of sturmanite and ettringite. Can. Mineral. 2002, 40, 1403–1409. [Google Scholar] [CrossRef]
- Ehm, L.; Antao, S.M.; Chen, J.H.; Locke, D.R.; Michel, F.M.; Martin, C.D.; Yu, T.; Parise, J.B.; Lee, P.L.; Chupas, P.J.; et al. Studies of local and intermediate range structure in crystalline and amorphous materials at high pressure using high-energy X-rays. Powder Diffr. 2007, 22, 108–112. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. Thermal analyses of sodalite, tugtupite, danalite, and helvite. Can. Mineral. 2002, 40, 163–172. [Google Scholar] [CrossRef]
- Skinner, L.B.; Benmore, C.J.; Antao, S.M.; Soignard, E.; Amin, S.A.; Bychkov, E.; Rissi, E.; Parise, J.B.; Yarger, J.L. Structural changes in vitreous GeSe4 under pressure. J. Phys. Chem. C 2011, 116, 2212–2217. [Google Scholar] [CrossRef]
- Hassan, I.; Antao, S.M.; Parise, J.B. Haüyne: Phase transition and high-temperature structures obtained from synchrotron radiation and Rietveld refinements. Mineral. Mag. 2004, 68, 499–513. [Google Scholar] [CrossRef]
- Antao, S.M. Structural trends for celestite (SrSO4), anglesite (PbSO4), and barite (BaSO4): Confirmation of expected variations within the SO4 groups. Am. Mineral. 2012, 97, 661–665. [Google Scholar] [CrossRef]
- Ehm, L.; Michel, F.M.; Antao, S.M.; Martin, C.D.; Lee, P.L.; Shastri, S.D.; Chupas, P.J.; Parise, J.B. Structural changes in nanocrystalline mackinawaite (FeS) at high presure. J. Appl. Crystallogr. 2009, 42, 15–21. [Google Scholar] [CrossRef]
- Parise, J.B.; Antao, S.M.; Michel, F.M.; Martin, C.D.; Chupas, P.J.; Shastri, S.; Lee, P.L. Quantitative high-pressure pair distribution function analysis. J. Synchrotron Radiat. 2005, 12, 554–559. [Google Scholar] [CrossRef]
- Antao, S.M. Crystal structure of morimotoite from Ice River, Canada. Powder Diffr. 2014, 29, 325–330. [Google Scholar] [CrossRef]
- Antao, S.M. Crystal-structure analysis of four mineral samples of anhydrite, CaSO4, using synchrotron high-resolution powder X-ray diffraction data. Powder Diffr. 2011, 26, 326–330. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Crichton, W.A.; Parise, J.B. Effects of high pressure and temperature on cation ordering in magnesioferrite, MgFe2O4, using in situ synchrotron X-ray powder diffraction up to 1430 K and 6 GPa. Am. Mineral. 2005, 90, 1500–1505. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. Gaudefroyite, Ca8Mn3+6[(BO3)6(CO3)2O6]: High-temperature crystal structure. Can. Mineral. 2008, 46, 183–193. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Parise, J.B. The structure of danalite at high temperature obtained from synchrotron radiation and Rietveld refinements. Can. Mineral. 2003, 41, 1413–1422. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report; LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000.
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Cagliotti, G.; Paoletti, A.; Ricci, F.P. Choice of collimators for a crystal spectrometer for neutron diffraction. Nucl. Instrum. 1958, 3, 223–228. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Debye-Scherrer synchrotron X-ray data from alumina. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Kitamura, K.; Komatsu, H. Optical anisotropy associated with growth striation of yttrium garnet, Y3(Al,Fe)5O12. Krist. und Technik. 1978, 13, 811–816. [Google Scholar] [CrossRef]
- Antao, S.M. Crystal chemistry of birefringent hydrogrossular. Phys. Chem. Miner. 2015, 42, 455–474. [Google Scholar] [CrossRef]
- Basso, R.; Cimmino, F.; Messiga, B. Crystal chemical and petrological study of hydrogarnets from a Fe-gabbro metarodingite (Gruppo Di Voltri, Western Liguria, Italy). Neues Jahrb. Fur Mineral. Abh. 1984, 150, 247–258. [Google Scholar]
- Basso, R.; Cimmino, F.; Messiga, B. Crystal chemistry of hydrogarnets from three different microstructural sites of a basaltic metarodingite from the Voltri Massif (Western Liguria, Italy). Neues Jahrb. Fur Mineral. Abh. 1984, 148, 246–258. [Google Scholar]
- Basso, R.; Dellagiusta, A.; Zefiro, L. A crystal chemical study of a Ti-containing hydrogarnet. Neues Jahrb. Fur Mineral. Mon. 1981, 5, 230–236. [Google Scholar]
- Basso, R.; Dellagiusta, A.; Zefiro, L. Crystal-structure refinement of plazolite—A highly hydrated hatural hydrogrossular. Neues Jahrb. Fur Mineral. Mon. 1983, 6, 251–258. [Google Scholar]
- Adamo, I.; Gatta, G.D.; Rotitoti, N.; Diella, V.; Pavese, A. Green andradite stones: Gemological and mineralogical characterisation. Eur. J. Mineral. 2010, 23, 91–100. [Google Scholar] [CrossRef]
- Agrosì, G.; Schingaro, E.; Pedrazzi, G.; Scandale, E.; Scordari, R. A crystal chemical insight into sector zoning of a titanian andradite (“melanite”) crystal. Eur. J. Mineral. 2002, 14, 785–794. [Google Scholar] [CrossRef]
- Armbruster, T.; Birrer, J.; Libowitzky, E.; Beran, A. Crystal chemistry of Ti-bearing andradites. Eur. J. Mineral. 1998, 10, 907–921. [Google Scholar] [CrossRef]
- Armbruster, T.; Geiger, C.A. Andradite crystal chemistry, dynamic X-site disorder and structural strain in silicate garnets. Eur. J. Mineral. 1993, 5, 59–71. [Google Scholar] [CrossRef]
- Armbruster, T.; Geiger, C.A.; Lager, G.A. Single crystal X-ray structure study of synthetic pyrope almandine garnets at 100 and 293 K. Am. Mineral. 1992, 77, 518–527. [Google Scholar]
- Chakhmouradian, A.R.; McCammon, C.A. Schorlomite: A discussion of the crystal chemistry, formula, and inter-species boundaries. Phys. Chem. Miner. 2005, 32, 277–289. [Google Scholar] [CrossRef]
- Ferro, O.; Galli, E.; Papp, G.; Quartieri, S.; Szakall, S.; Vezzalini, G. A new occurrence of katoite and re-examination of the hydrogrossular group. Eur. J. Mineral. 2003, 15, 419–426. [Google Scholar] [CrossRef]
- Geiger, C.A.; Armbruster, T. Mn3Al2Si3O12 spessartine and Ca3Al2Si3O12 grossular garnet: Structural dynamic and thermodynamic properties. Am. Mineral. 1997, 82, 740–747. [Google Scholar] [CrossRef]
- Geiger, C.A.; Armbruster, T.; Lager, G.A.; Jiang, K.; Lottermoser, W.; Amthauer, G. A combined temperature dependent 57Fe mössbauer and single crystal X-ray diffraction study of synthetic almandine: Evidence for the Gol’danskii-Karyagin effect. Phys. Chem. Miner. 1992, 19, 121–126. [Google Scholar] [CrossRef]
- Gramaccioli, C.M.; Pilati, T.; Demartin, F. Atomic displacement parameters for spessartine Mn3Al2Si3O12 and their lattice-dynamical interpretation. Acta Crystallogr. 2002, B58, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Lager, G.A.; Armbruster, T.; Faber, J. Neutron and X-ray-diffraction study of hydrogarnet Ca3Al2(O4H4)3. Am. Mineral. 1987, 72, 756–765. [Google Scholar]
- Lager, G.A.; Armbruster, T.; Rotella, F.J.; Rossman, G.R. OH substitution in garnets: X-ray and neutron diffraction, infrared, and geometric-modeling studies. Am. Mineral. 1989, 74, 840–851. [Google Scholar]
- Lager, G.A.; Rossman, G.R.; Rotella, F.J.; Schultz, A.J. Neutron-diffraction structure of a low-water grossular at 20 K. Am. Mineral. 1987, 72, 766–768. [Google Scholar]
- Munno, R.; Rossi, G.; Tadini, C. Crystal chemistry of kimzeyite from Stromboli, Aeolian Islands, Italy. Am. Mineral. 1980, 65, 188–191. [Google Scholar]
- Novak, G.A.; Meyer, H.O.A. Refinement of the crystal structure of a chrome pyrope garnet: An inclusion in natural diamond. Am. Mineral. 1970, 55, 2124–2127. [Google Scholar]
- Peterson, R.C.; Locock, A.J.; Luth, R.W. Positional disorder of oxygen in garnet: The crystal-structure refinement of schorlomite. Can. Mineral. 1995, 33, 627–631. [Google Scholar]
- Sacerdoti, M.; Passaglia, E. The crystal structure of katoite and implications within the hydrogrossular group of minerals. Bull. Minéral. 1985, 108, 1–8. [Google Scholar] [CrossRef]
- Schingaro, E.; Scordari, F.; Capitanio, F.; Parodi, G.; Smith, D.C.; Mottana, A. Crystal chemistry of kimzeyite from Anguillara, Mts. Sabatini, Italy. Eur. J. Mineral. 2001, 13, 749–759. [Google Scholar] [CrossRef]
- Schingaro, E.; Scordari, F.; Pedrazzi, G.; Malitesta, C. Ti and Fe speciation by X-ray photoelectron spectroscopy (XPS) and mössbauer spectroscopy for a full crystal chemical characterisation of Ti-garnets from Colli Albani (Italy). Annali Di Chimica 2004, 94, 185–196. [Google Scholar] [CrossRef]
- Scordari, F.; Schingaro, E.; Pedrazzi, G. Crystal chemistry of melanites from Mt. Vulture (Southern Italy). Eur. J. Mineral. 1999, 11, 855–869. [Google Scholar] [CrossRef]
- Smyth, J.R.; Madel, R.E.; McCormick, T.C.; Munoz, J.L.; Rossman, G.R. Crystal-structure refinement of a F-bearing spessartine garnet. Am. Mineral. 1990, 75, 314–318. [Google Scholar]
- Weber, H.P.; Virgo, D.; Huggins, F.E. A neutron-diffraction and 57Fe Mössbauer study of a synthetic Ti-rich garnet. Carnegie Inst. Wash. Year Book 1975, 74, 575–579. [Google Scholar]
- Baikie, T.; Schreyer, M.K.; Wong, C.L.; Pramana, S.S.; Klooster, W.T.; Ferraris, C.; McIntyre, G.J.; White, T.J. A multi-domain gem-grade Brazilian apatite. Am. Mineral. 2012, 97, 1574–1581. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. A two-phase intergrowth of genthelvite from Mont Saint-Hilaire, Quebec. Can. Mineral. 2010, 48, 1217–1223. [Google Scholar] [CrossRef]
- Antao, S.M.; Nicholls, J.W. Crystal chemistry of three volcanic K-rich nepheline samples from Oldoinyo Lengai, Tanzania and Mount Nyiragongo, Eastern Congo, Africa. Front. Earth Sci. 2018, 6, 155. [Google Scholar] [CrossRef]
- Akizuki, M. Growth structure and crystal symmetry of grossular garnets from the Jeffrey mine, Asbestos, Quebec, Canada. Am. Mineral. 1989, 74, 859–864. [Google Scholar]
- Antao, S.M.; Dhaliwal, I. Growth Oscillatory Zoning in Erythrite, Ideally Co3(AsO4)2·8H2O: Structural Variations in Vivianite-Group Minerals. Minerals 2017, 7, 136. [Google Scholar] [CrossRef]
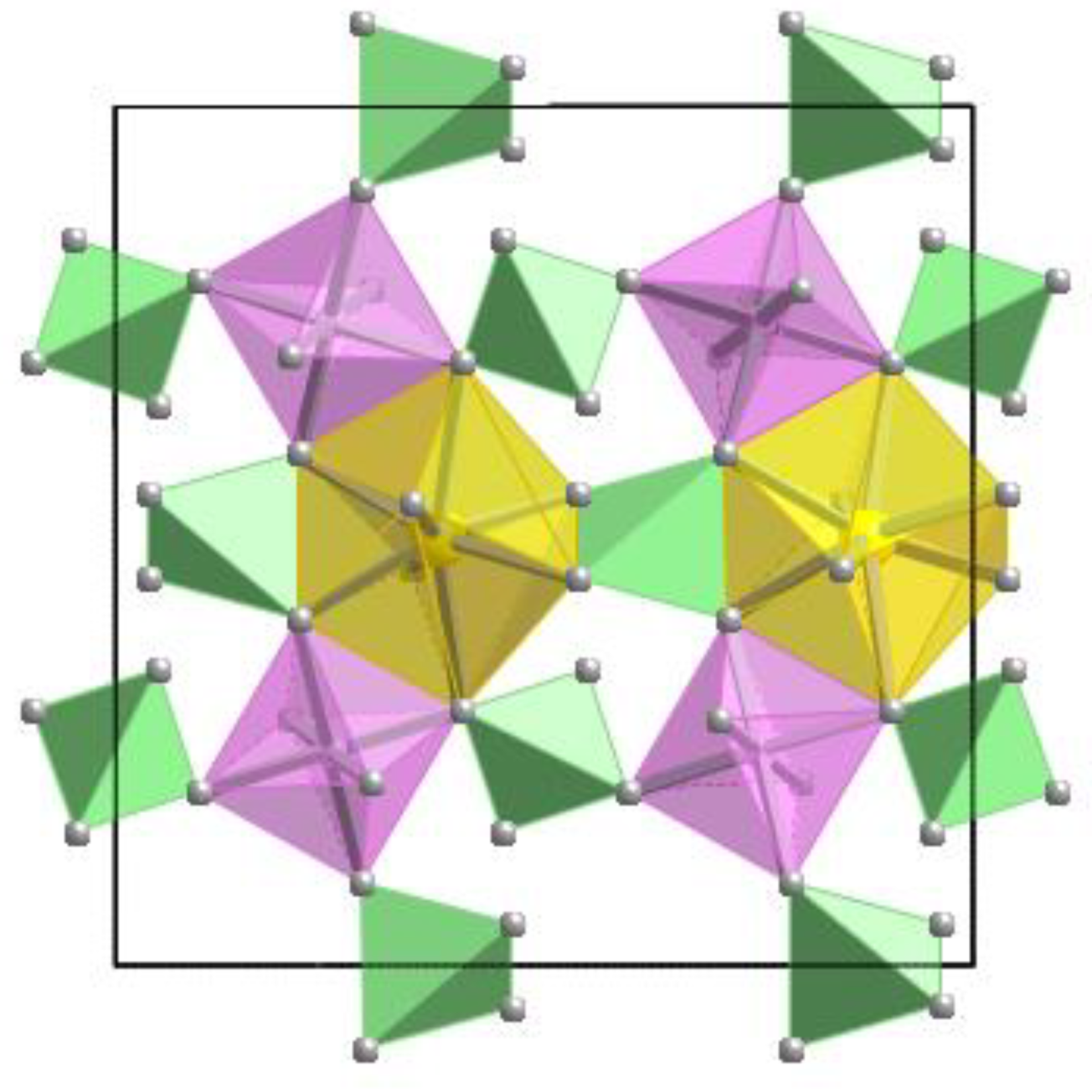
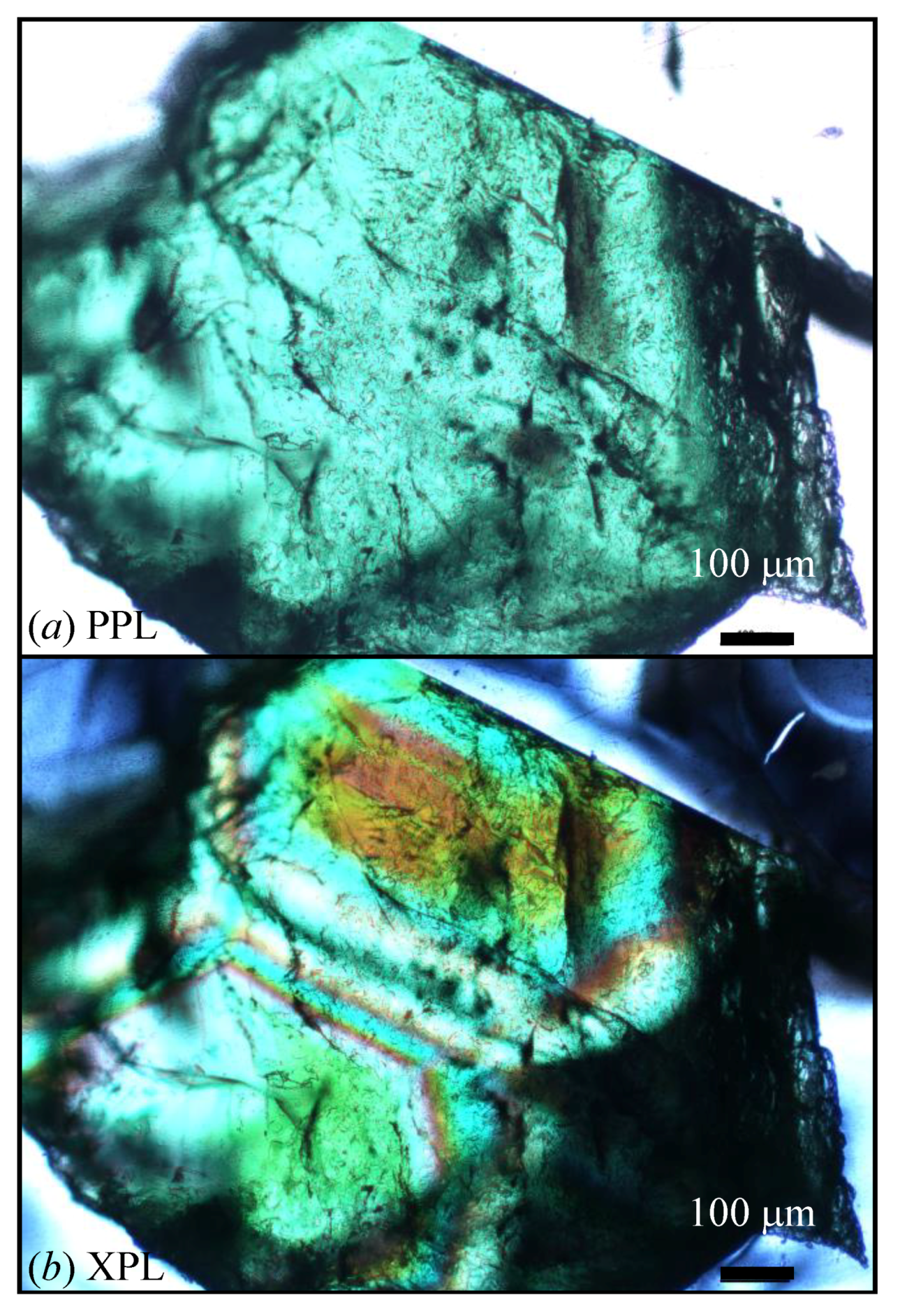
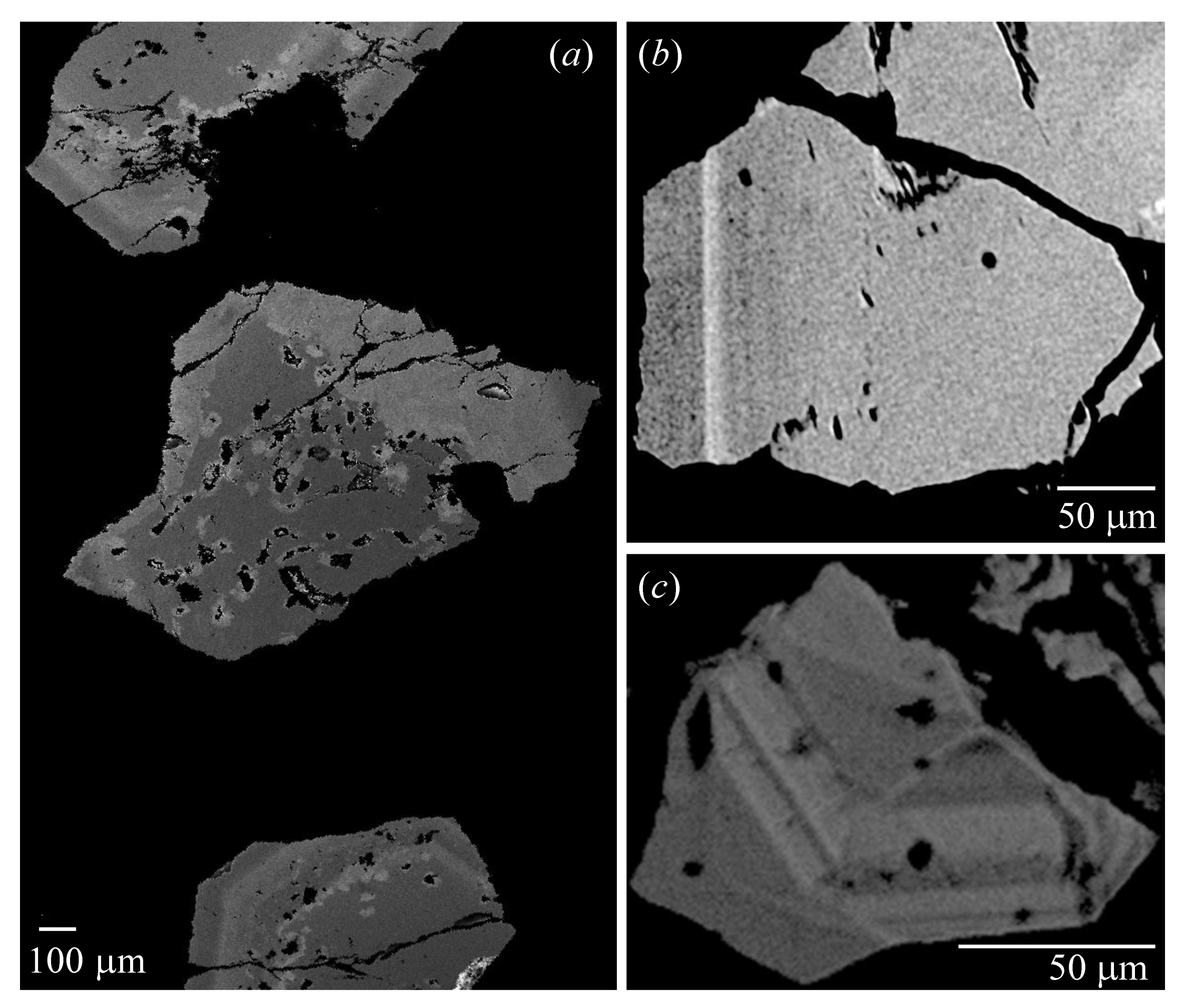
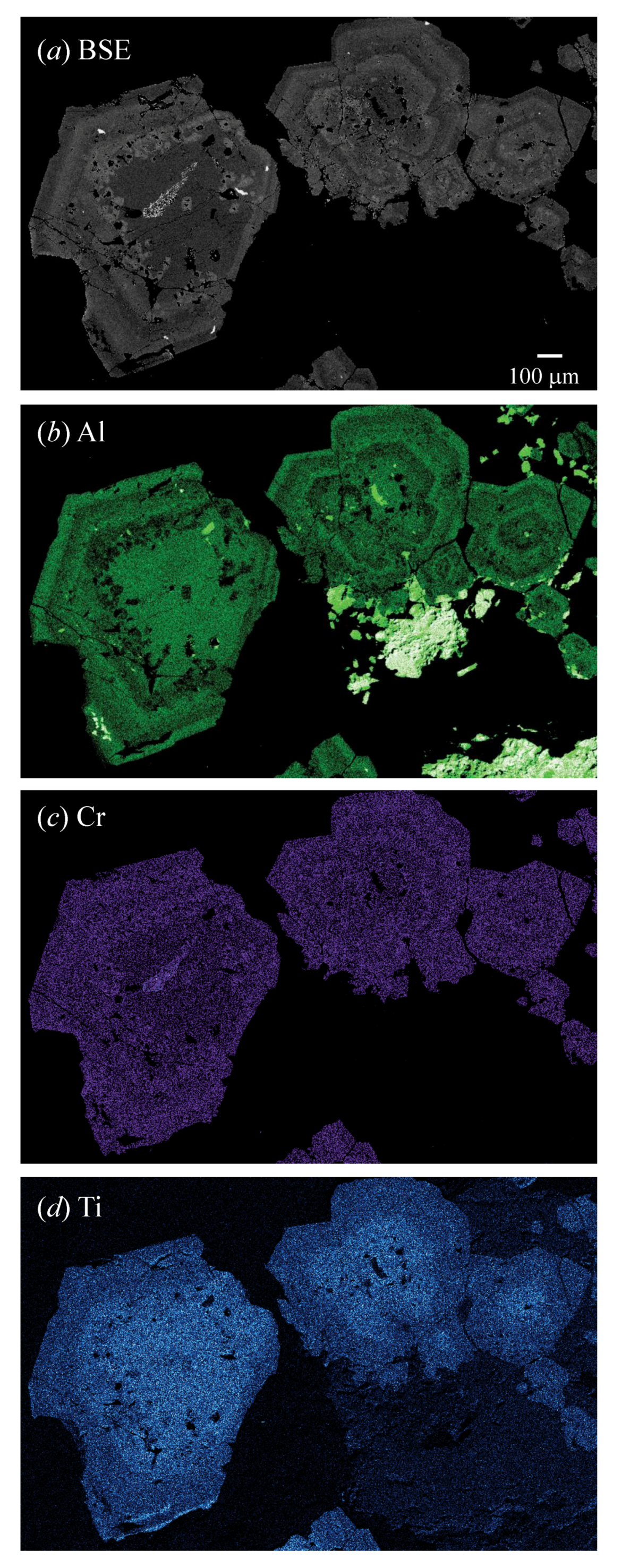
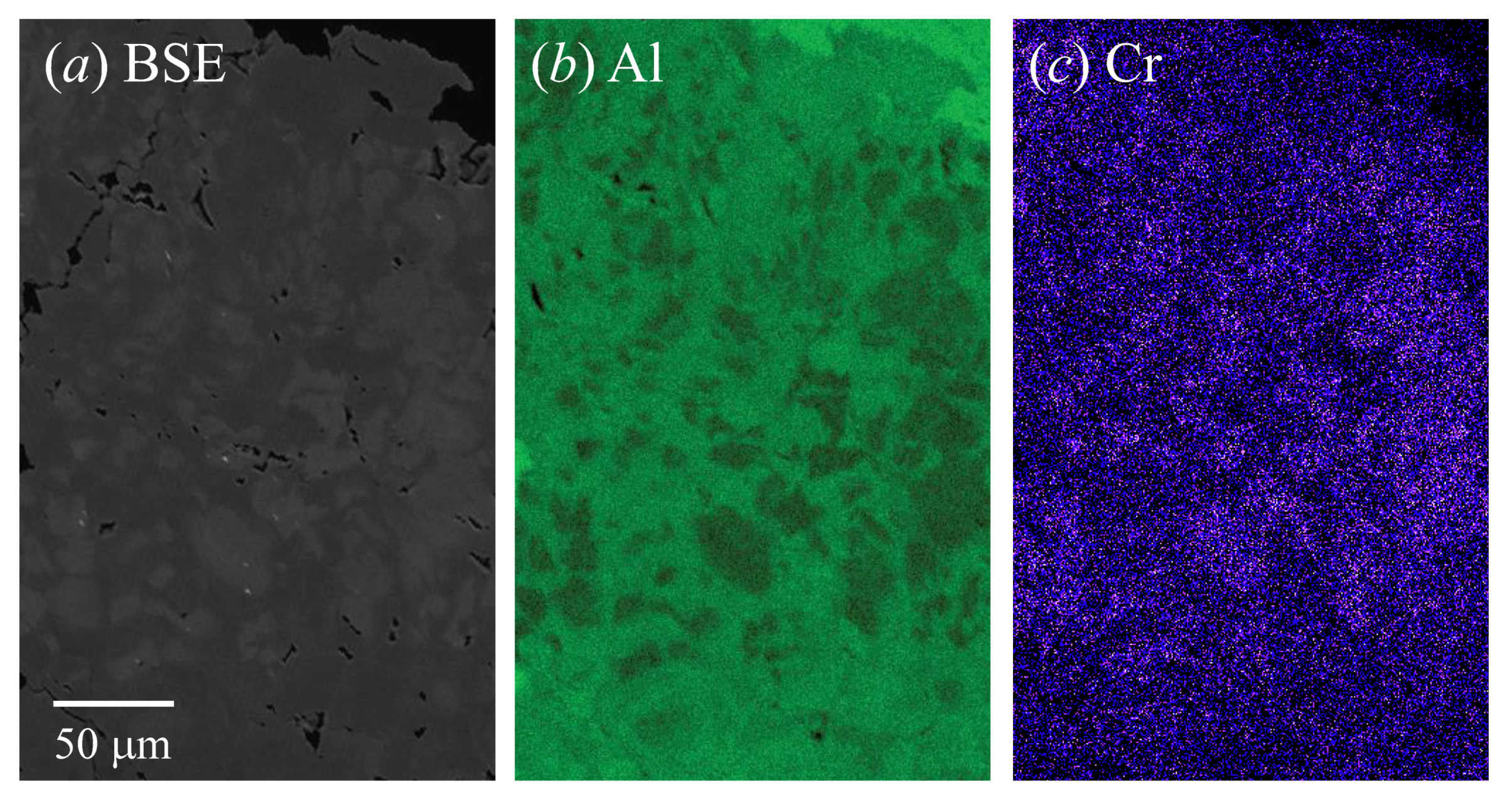
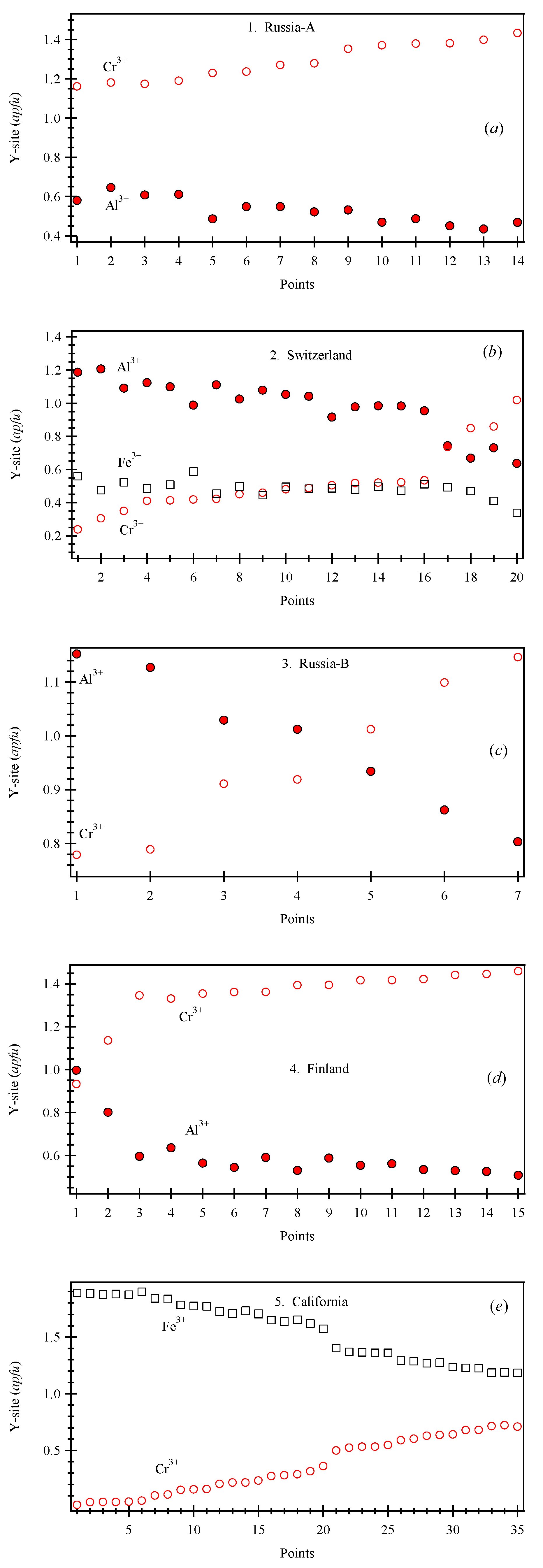
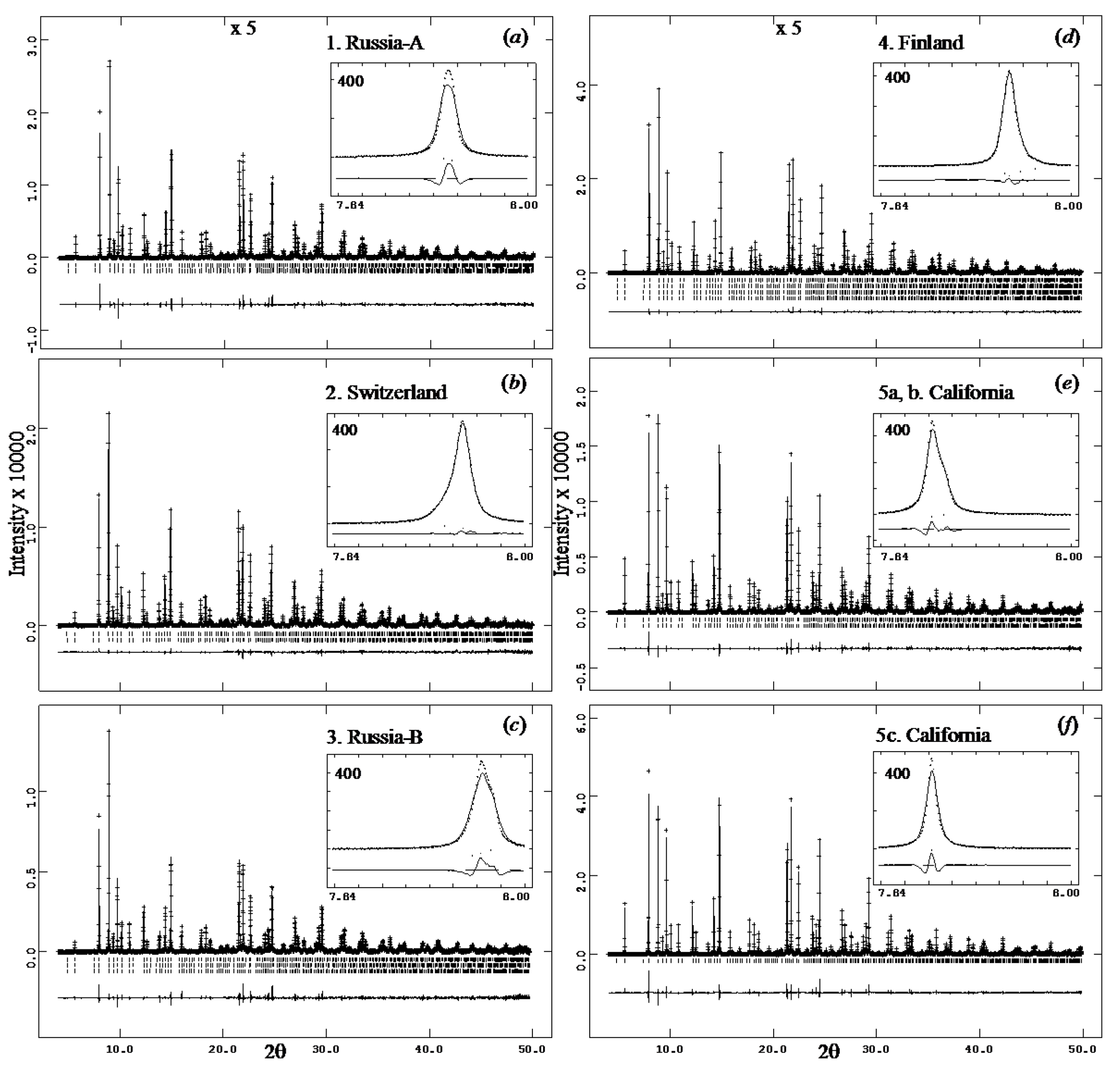
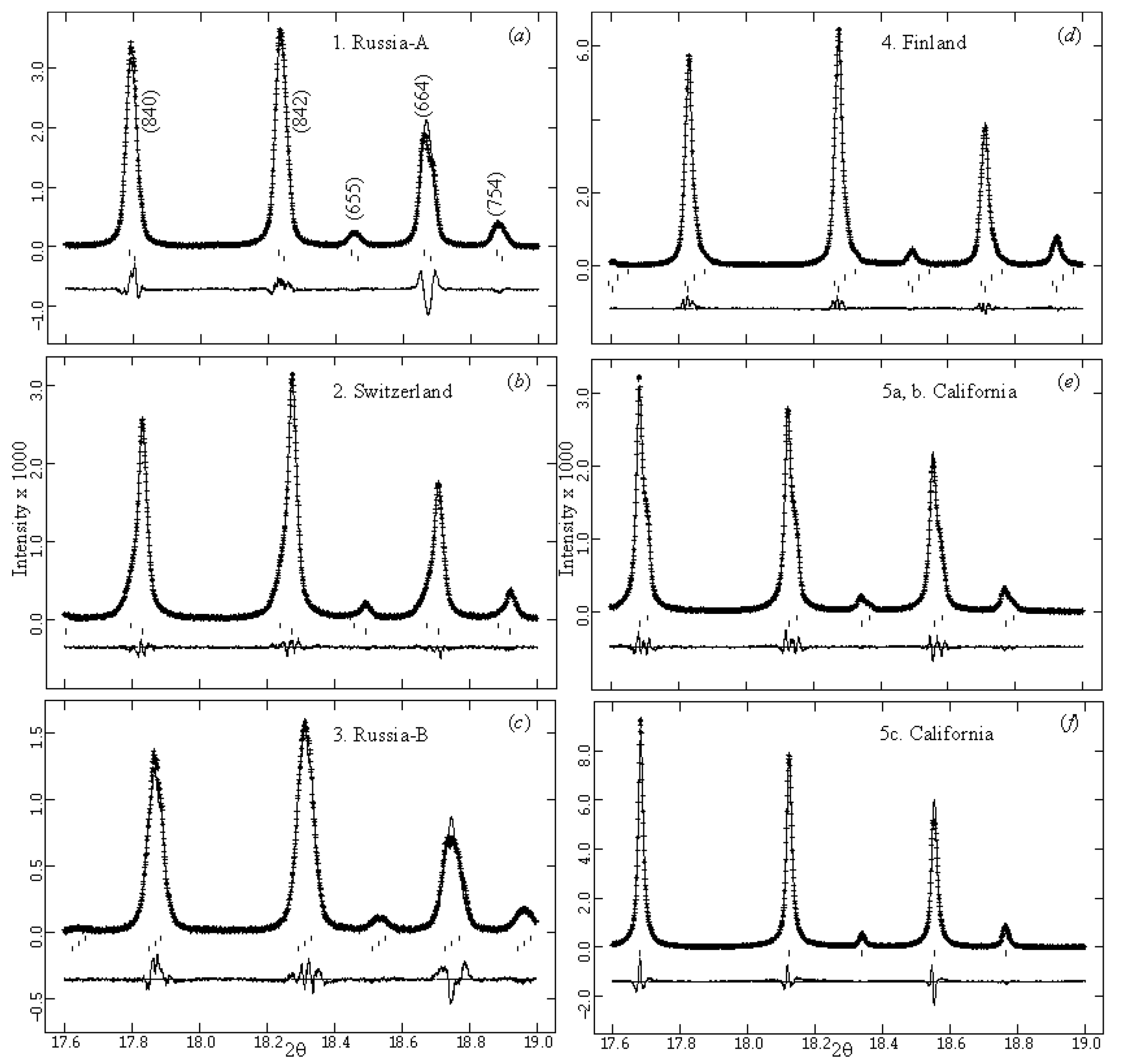
| Sample # | Locality |
|---|---|
| 1. Russia-A | Sarany, near Perm, Russia; Royal Ontario Museum (ROM #M51847) sample. |
| 2. Switzerland | Zermatt area, Switzerland (ROM #M33537). |
| 3. Russia-B | Sarany, Urals, Russia. |
| 4. Finland | Outokumpo, Finland. |
| 5a, 5b. California | Jacksonville, Toulumne Co., California, USA. These crystals are dark green. |
| 5c. California | Jacksonville, Toulumne Co., California, USA. The crystals are light green, isotropic, and chemically homogeneous. All crystals from sample 5 are from the same hand specimen. |
| Oxide (wt. %) | ϕ 1a | ϕ 1b | ϕ 2a | ϕ 2b | ϕ 3a | ϕ 3b | ϕ 3c | ϕ 4a | ϕ 4b | ϕ 4c | ϕ 4d | ϕ 5a | ϕ 5b, c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | 33.54 | 33.66 | 34.89 | 33.78 | 35.71 | 35.23 | 34.75 | 33.55 | 33.42 | 32.94 | 33.94 | 33.52 | 32.60 |
| MnO | 0.07 | 0.05 | 0.51 | 0.31 | 0.05 | 0.08 | 0.06 | 0.69 | 0.55 | 0.47 | 0.52 | 0.03 | 0.10 |
| MgO | 0.06 | 0.03 | 0.20 | 0.11 | 0.00 | 0.00 | 0.00 | 0.10 | 0.10 | 0.08 | 0.10 | 0.02 | 0.05 |
| Cr2O3 | 17.95 | 21.85 | 3.82 | 15.73 | 12.58 | 14.65 | 18.01 | 14.41 | 17.45 | 20.30 | 22.73 | 0.28 | 10.46 |
| Al2O3 | 6.59 | 4.79 | 12.79 | 6.59 | 12.48 | 10.83 | 8.47 | 10.33 | 8.26 | 6.03 | 5.31 | 0.89 | 0.79 |
| Fe2O3 | 0.83 | 0.63 | 9.46 | 5.47 | 0.97 | 0.94 | 0.66 | 0.52 | 0.51 | 0.47 | 0.44 | 30.06 | 18.38 |
| TiO2 | 1.92 | 0.93 | 0.27 | 0.13 | 0.20 | 0.20 | 0.18 | 0.50 | 0.33 | 0.14 | 0.12 | 0.11 | 0.21 |
| ZrO2 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SiO2 | 36.07 | 36.15 | 38.12 | 36.62 | 38.30 | 37.81 | 37.28 | 36.65 | 36.43 | 35.77 | 36.95 | 35.97 | 35.02 |
| ∑ | 97.06 | 98.09 | 100.07 | 98.75 | 100.28 | 99.74 | 99.40 | 96.81 | 97.20 | 96.44 | 100.11 | 100.88 | 97.70 |
| Cations for 12 O atoms (apfu) | |||||||||||||
| Ca2+ | 2.989 | 2.993 | 2.942 | 2.965 | 2.997 | 2.995 | 2.996 | 2.943 | 2.949 | 2.960 | 2.952 | 2.996 | 2.993 |
| Mn2+ | 0.005 | 0.004 | 0.034 | 0.022 | 0.003 | 0.005 | 0.004 | 0.048 | 0.039 | 0.033 | 0.036 | 0.002 | 0.007 |
| Mg2+ | 0.007 | 0.003 | 0.024 | 0.013 | 0.000 | 0.000 | 0.000 | 0.012 | 0.012 | 0.010 | 0.012 | 0.002 | 0.006 |
| ∑X | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.002 | 3.000 | 3.004 | 2.999 | 3.000 | 3.006 |
| Cr3+ | 1.180 | 1.434 | 0.238 | 1.019 | 0.779 | 0.919 | 1.146 | 0.933 | 1.136 | 1.346 | 1.459 | 0.019 | 0.709 |
| Al3+ | 0.646 | 0.468 | 1.186 | 0.636 | 1.152 | 1.012 | 0.803 | 0.997 | 0.801 | 0.596 | 0.507 | 0.087 | 0.079 |
| Fe3+ | 0.052 | 0.040 | 0.560 | 0.337 | 0.057 | 0.056 | 0.040 | 0.032 | 0.032 | 0.030 | 0.027 | 1.887 | 1.185 |
| Ti4+ | 0.120 | 0.058 | 0.016 | 0.008 | 0.012 | 0.012 | 0.011 | 0.031 | 0.021 | 0.009 | 0.008 | 0.007 | 0.014 |
| Zr4+ | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| V3+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.005 | 0.010 | 0.016 | 0.000 | 0.000 | 0.007 |
| ∑Y | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 1.998 | 2.000 | 1.996 | 2.001 | 2.000 | 1.994 |
| Si4+ = Z | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 |
| End-member mole % | |||||||||||||
| Uvarovite (Uv) | 59 | 72 | 12 | 51 | 39 | 46 | 57 | 47 | 57 | 67 | 73 | 1 | 35 |
| Grossular (Grs) | 32 | 23 | 57 | 31 | 57 | 50 | 40 | 48 | 38 | 28 | 24 | 4 | 4 |
| Andradite (Adr) | 3 | 2 | 28 | 17 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 94 | 59 |
| F(000) | 143 | 145 | 138 | 144 | 137 | 139 | 141 | 139 | 141 | 144 | 145 | 153 | 151 |
| 1. Russia-A (ROM #M51847) | 2. Switzerland (ROM #M33537) | 3. Russia-B | |||||
| ϕ 1a | ϕ 1b | ϕ 2a | ϕ 2b | ϕ 3a | ϕ 3b | ϕ 3c | |
| wt. % | 41.6(5) | 58.4(3) | 85.2(1) | 14.8(2) | 29.2(5) | 34.8(9) | 36.0(4) |
| a (Å) | 11.9617(1) | 11.97226(8) | 11.94509(2) | 11.96731(9) | 11.91603(9) | 11.92890(6) | 11.9405(1) |
| aΔa (Å) | --- | 0.0106b-a | --- | 0.0222b-a | --- | 0.0129b-a | 0.0116c-b |
| bLY | 4.85 | 8.68 | 11.50 | 17.31 | 16.30 | 13.53 | 16.27 |
| Reduced χ2 | 2.310 | 1.163 | 1.437 | ||||
| cR (F2) | 0.0497 | 0.0601 | 0.0868 | ||||
| Nobs | 1361 | 1390 | 2017 | ||||
| λ (Å) | 0.41390(2) | 0.41390(2) | 0.41424(2) | ||||
| Data points | 47992 | 47992 | 47996 | ||||
| 4. Finland | 5a, b. California | 5c. California | |||||
| ϕ 4a | ϕ 4b | ϕ 4c | ϕ 4d | ϕ 5a | ϕ 5b | ϕ 5c | |
| wt. % | 1.2(6) | 13.0(3) | 61.7(3) | 24.0(4) | 25.1(2) | 74.9(1) | 100.0 |
| a (Å) | 11.9200(1) | 11.9400(1) | 11.95213(3) | 11.95942(5) | 12.03656(3) | 12.05168(2) | 12.05247(1) |
| aΔa (Å) | --- | 0.0200b-a | 0.0121c-b | 0.0073d-c | --- | 0.0151b-a | 0.0008c-b |
| bLY | 7.27 | 12.04 | 8.53 | 6.95 | 10.65 | 10.91 | 8.82 |
| Reduced χ2 | 1.558 | 1.180 | 3.098 | ||||
| cR (F2) | 0.0425 | 0.0385 | 686 | ||||
| Nobs | 2656 | 1366 | 0.41424(2) | ||||
| λ (Å) | 0.41424(2) | 0.41424(2) | 47996 | ||||
| Data points | 47996 | 47996 | 686 | ||||
| 1. Russia-A | 2. Switzerland | 3. Russia-B | 4. Finland | 5a, b. California | 5c. California | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ϕ 1a | ϕ 1b | ϕ 2a | ϕ 2b | ϕ 3a | ϕ 3b | ϕ 3c | ϕ 4a† | ϕ 4b | ϕ 4c | ϕ 4d | ϕ 5a | ϕ 5b | ϕ 5c | ||
| Ca(X) | U | 0.44(1) | 0.44(1) | 0.474(8) | 0.474(8) | 0.47(1) | 0.47(1) | 0.47(1) | 0.453(7) | 0.453(7) | 0.453(7) | 0.453(7) | 0.463(9) | 0.463(9) | 0.440(9) |
| Y | U | 0.206(6)Cr | 0.206(6)Cr | 0.331(6)Al | 0.331(6)Al | 0.183(8)Cr | 0.183(8)Cr | 0.183(8)Cr | 0.184(4)Cr | 0.184(4)Cr | 0.184(4)Cr | 0.184(4)Cr | 0.274(4)Fe | 0.274(4)Fe | 0.275(5)Fe |
| Si(Z) | U | 0.27(1) | 0.27(1) | 0.29(1) | 0.29(1) | 0.23(2) | 0.23(2) | 0.23(2) | 0.30(1) | 0.30(1) | 0.30(1) | 0.30(1) | 0.32(1) | 0.32(1) | 0.36(1) |
| O | x | 0.0399(1) | 0.0389(1) | 0.03853(5) | 0.0384(2) | 0.0398(1) | 0.0369(2) | 0.0412(2) | 0.0379(2) | 0.0379(2) | 0.03875(8) | 0.0383(2) | 0.0390(1) | 0.03937(6) | 0.03959(5) |
| y | 0.0454(1) | 0.0484(1) | 0.04652(4) | 0.0479(2) | 0.0479(1) | 0.0423(2) | 0.0509(2) | 0.0464(2) | 0.0464(2) | 0.04664(8) | 0.0478(1) | 0.0483(1) | 0.04839(5) | 0.04838(4) | |
| z | 0.6541(1) | 0.6528(1) | 0.65300(5) | 0.6520(2) | 0.6532(2) | 0.6522(2) | 0.6532(2) | 0.6548(2) | 0.6548(2) | 0.65332(8) | 0.6546(2) | 0.6546(1) | 0.65502(6) | 0.65523(4) | |
| U | 0.82(2) | 0.82(2) | 0.87(1) | 0.87(1) | 0.80(2) | 0.80(2) | 0.80(2) | 0.80(1) | 0.80(1) | 0.80(1) | 0.80(1) | 0.85(1) | 0.85(1) | 0.88(2) | |
| Ca(X) | sof | 0.926(3) | 0.962(3) | 0.953(2) | 0.954(5) | 0.907(4) | 1.011(5) | 0.897(4) | 0.80(2) | 0.919(6) | 0.966(2) | 0.900(4) | 0.944(4) | 0.943(2) | 0.944(2) |
| Y | sof | 0.773(3)Cr | 0.825(2)Cr | 1.397(2)Al | 1.595(8)Al | 0.661(3)Cr | 0.722(4)Cr | 0.708(3)Cr | 0.63(2)Cr | 0.731(4)Cr | 0.805(2)Cr | 0.818(3)Cr | 0.881(3)Fe | 0.909(1)Fe | 0.905(1)Fe |
| Si(Z) | sof | 0.930(4) | 0.908(3) | 0.924(2) | 0.932(5) | 0.944(4) | 0.888(5) | 0.943(5) | 0.89(2) | 0.907(6) | 0.930(2) | 0.932(4) | 0.946(4) | 0.936(2) | 0.937(2) |
| Ca(X) | EPMA | 0.999 | 1.000 | 1.000 | 1.000 | 1.000 | 1.001 | 1.000 | 1.003 | 1.002 | 1.003 | 1.001 | 1.000 | 1.002 | 1.002 |
| Y | EPMA | 0.850Cr | 0.892Cr | 1.386Al | 1.603Al | 0.738Cr | 0.770Cr | 0.817Cr | 0.770Cr | 0.817Cr | 0.862Cr | 0.885Cr | 0.977Fe | 0.948Fe | 0.948Fe |
| Si(Z) | EPMA | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| X | bΔ(sof) | −0.073 | −0.038 | −0.047 | −0.046 | −0.093 | 0.010 | −0.103 | −0.203 | −0.083 | −0.037 | −0.101 | −0.056 | −0.059 | −0.059 |
| Y | Δ(sof) | −0.077 | −0.067 | 0.011 | −0.008 | −0.077 | −0.048 | −0.109 | −0.140 | −0.086 | −0.057 | −0.067 | −0.096 | −0.039 | −0.039 |
| Z | Δ(sof) | −0.070 | −0.092 | −0.076 | −0.068 | −0.056 | −0.112 | −0.057 | −0.110 | −0.093 | −0.070 | −0.068 | −0.054 | −0.064 | −0.064 |
| X | cΔe | −1.5 | −0.8 | −0.9 | −0.9 | −1.9 | 0.2 | −2.1 | −4.1 | −1.7 | −0.7 | −2.0 | −1.1 | −1.2 | −1.2 |
| Y | Δe | −1.8 | −1.6 | 0.1 | −0.1 | −1.8 | −1.1 | −2.6 | −3.4 | −2.1 | −1.4 | −1.6 | −2.5 | −1.0 | −1.0 |
| Z | Δe | −1.0 | −1.3 | −1.1 | −1.0 | −0.8 | −1.6 | −0.8 | −1.5 | −1.3 | −1.0 | −1.0 | −0.8 | −0.9 | −0.9 |
| 1a − 1b | 2a − 2b | 5a − 5b | 5b − 5c | ||||||||||||
| Ca(X) | −0.036 | −0.001 | 0.001 | −0.001 | |||||||||||
| Y | −0.052 | −0.198 | −0.028 | 0.004 | |||||||||||
| Si(Z) | 0.022 | −0.008 | 0.010 | −0.001 | |||||||||||
| 1. Russia-A | 2. Switzerland | 3. Russia-B | ||||||||
| ϕ 1a | ϕ1b | 1a − 1b | ϕ2a | ϕ2b | 2a − 2b | ϕ3a | ϕ3b | ϕ3c | ||
| Z–O | ×4 | 1.627(2) | 1.659(1) | −0.032 | 1.6487(6) | 1.666(3) | −0.017 | 1.639(2) | 1.649(2) | 1.645(2) |
| Y–O | ×6 | 1.980(2) | 1.974(1) | 0.006 | 1.9649(6) | 1.962(2) | 0.003 | 1.970(2) | 1.935(2) | 1.989(2) |
| X–O | ×4 | 2.345(2) | 2.353(1) | −0.008 | 2.3382(5) | 2.351(2) | −0.013 | 2.349(2) | 2.312(2) | 2.375(2) |
| X′–O | ×4 | 2.518(2) | 2.481(1) | 0.037 | 2.4963(5) | 2.483(2) | 0.013 | 2.477(2) | 2.538(2) | 2.451(2) |
| <X–O> | [8] | 2.432 | 2.417 | 0.015 | 2.4173 | 2.417 | 0.000 | 2.413 | 2.425 | 2.413 |
| a<D–O> | [4] | 2.118 | 2.117 | 0.001 | 2.1120 | 2.116 | −0.004 | 2.109 | 2.109 | 2.115 |
| ∠Y–O–Z | ×1 | 135.7(1) | 133.96(7) | 1.74 | 134.84(3) | 134.2(1) | 0.64 | 134.5(1) | 136.8(1) | 133.1(1) |
| 4. Finland | 5a, b. California | 5c. California | ||||||||
| ϕ4a | ϕ4b | ϕ4c | ϕ4d | ϕ5a | ϕ5b | 5a − 5b | ϕ5c | 5b − 5c | ||
| Z–O | ×4 | 1.644(2) | 1.639(3) | 1.646(1) | 1.644(2) | 1.652(2) | 1.6478(7) | 0.004 | 1.6445(6) | 0.003 |
| Y–O | ×6 | 1.989(2) | 1.982(3) | 1.971(1) | 1.989(2) | 2.005(2) | 2.0138(7) | −0.009 | 2.0169(6) | −0.003 |
| X–O | ×4 | 2.332(2) | 2.320(3) | 2.340(1) | 2.332(2) | 2.356(2) | 2.3610(7) | −0.005 | 2.3621(5) | −0.001 |
| X′–O | ×4 | 2.487(2) | 2.496(3) | 2.497(1) | 2.487(2) | 2.498(2) | 2.5019(7) | −0.004 | 2.5031(5) | −0.001 |
| <X–O> | [8] | 2.410 | 2.408 | 2.419 | 2.410 | 2.427 | 2.4315 | −0.005 | 2.4326 | −0.001 |
| <D–O> | [4] | 2.113 | 2.109 | 2.114 | 2.113 | 2.128 | 2.1311 | −0.003 | 2.1317 | −0.001 |
| ∠Y–O–Z | ×1 | 133.6(1) | 134.2(2) | 134.78(5) | 133.6(1) | 133.6(1) | 133.59(4) | 0.01 | 133.62(3) | −0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antao, S.M.; Salvador, J.J. Crystal Chemistry of Birefringent Uvarovite Solid Solutions. Minerals 2019, 9, 395. https://doi.org/10.3390/min9070395
Antao SM, Salvador JJ. Crystal Chemistry of Birefringent Uvarovite Solid Solutions. Minerals. 2019; 9(7):395. https://doi.org/10.3390/min9070395
Chicago/Turabian StyleAntao, Sytle M., and Jeffrey J. Salvador. 2019. "Crystal Chemistry of Birefringent Uvarovite Solid Solutions" Minerals 9, no. 7: 395. https://doi.org/10.3390/min9070395
APA StyleAntao, S. M., & Salvador, J. J. (2019). Crystal Chemistry of Birefringent Uvarovite Solid Solutions. Minerals, 9(7), 395. https://doi.org/10.3390/min9070395





