Dmisteinbergite, CaAl2Si2O8, a Metastable Polymorph of Anorthite: Crystal-Structure and Raman Spectroscopic Study of the Holotype Specimen
Abstract
:1. Introduction
2. Materials and Methods
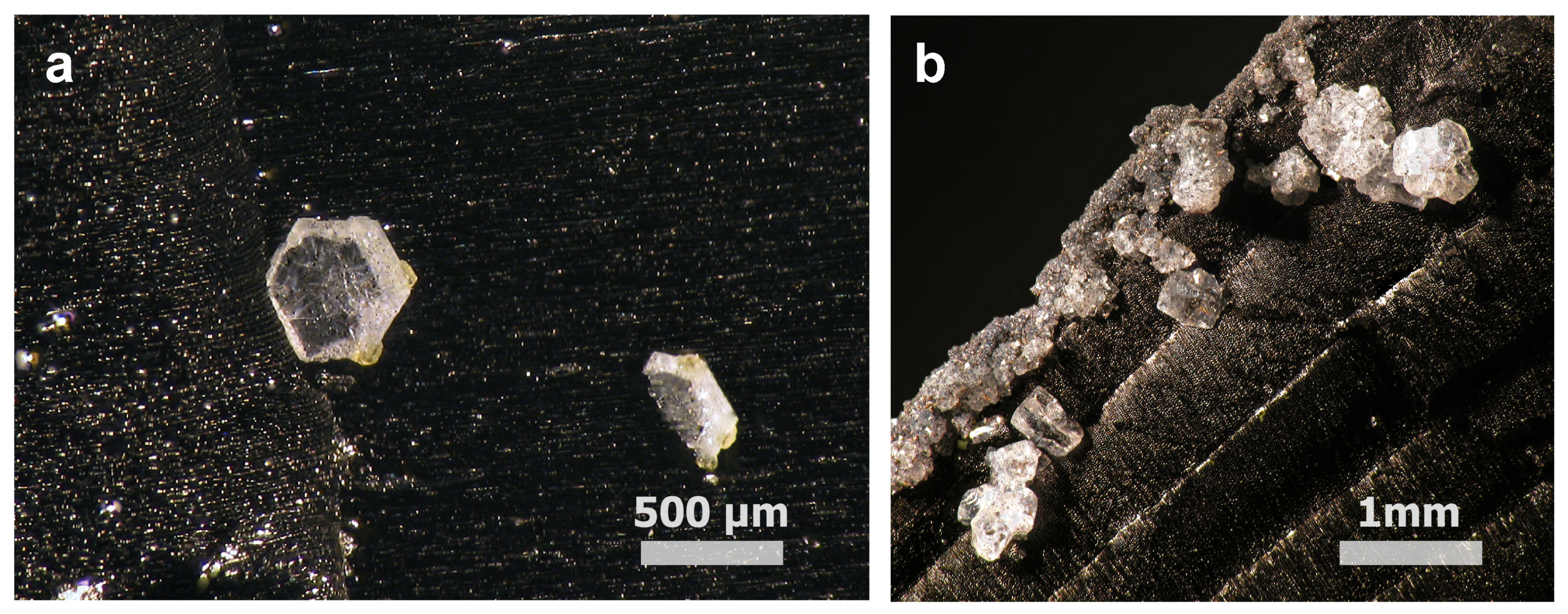
2.1. Materials
2.2. Single-Crystal X-ray Diffraction
2.3. Raman Spectroscopy
3. Results
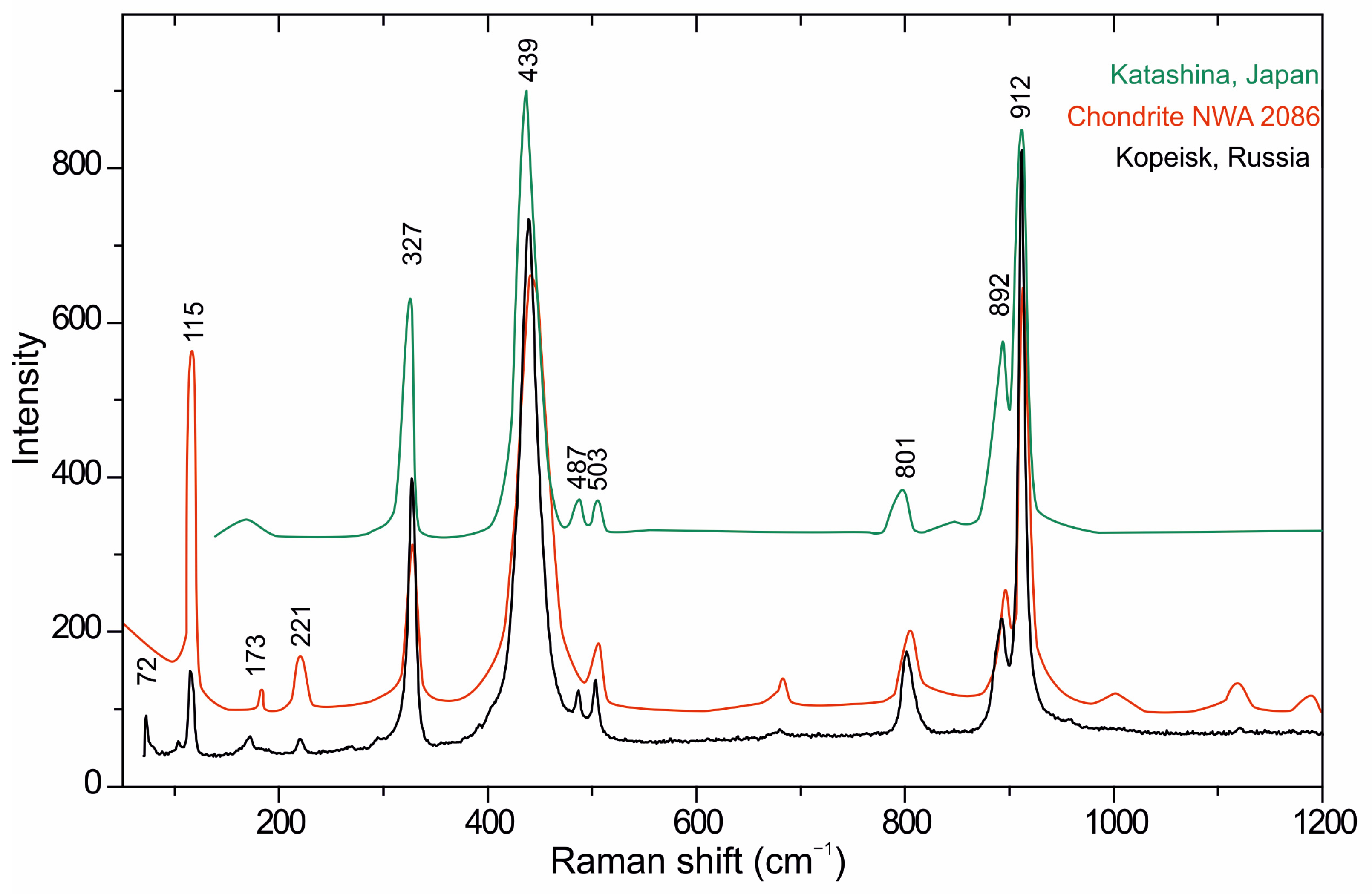
3.1. Raman Spectroscopy
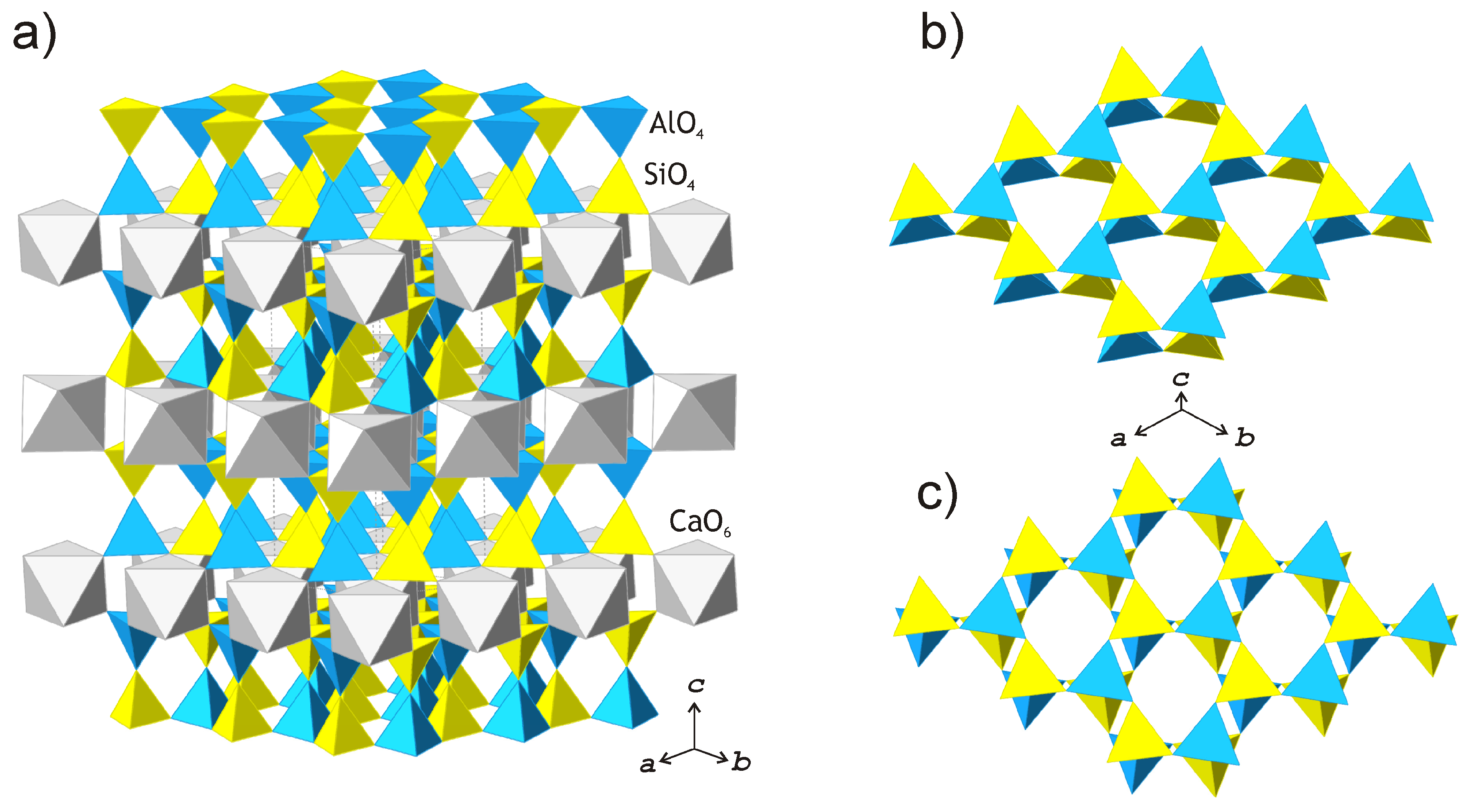
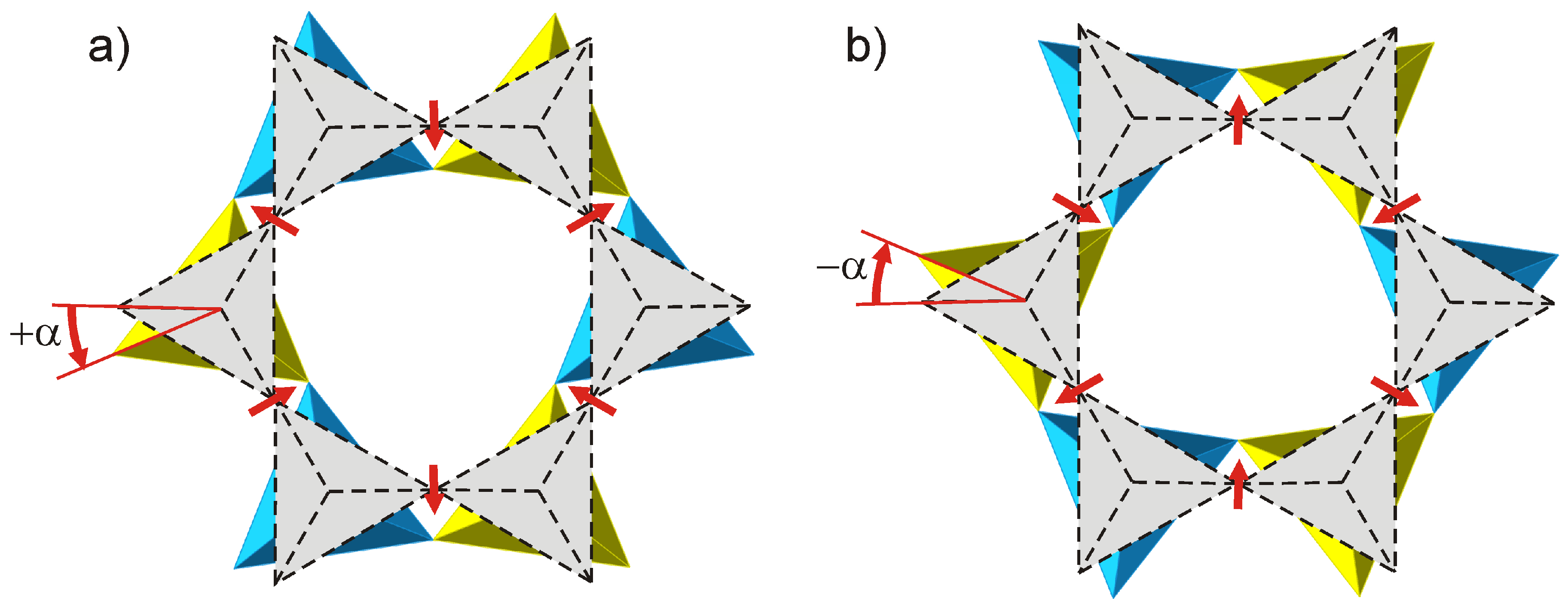
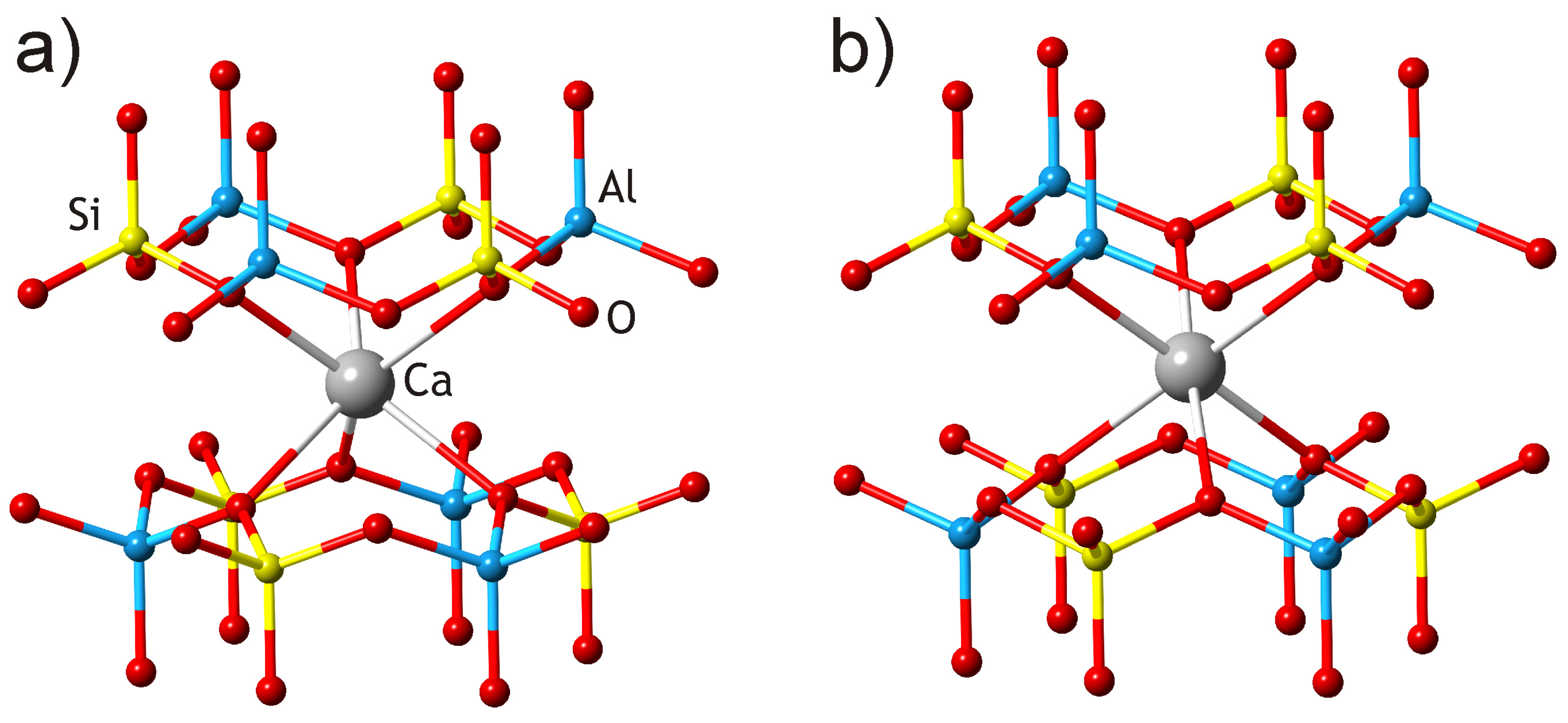
3.2. Crystal Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yaroshevsky, A.A.; Bulakh, A.G. The mineral composition of the Earth’s crust, mantle, meteorites, Moon, and planets. In Advanced Mineralogy; Marfunin, A.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; Volume 1, pp. 27–36. [Google Scholar]
- Ferrero, S.; Wunder, B.; Ziemann, M.A.; Wälle, M.; O’Brien, P.J. Carbonatitic and granitic melts produced under conditions of primary immiscibility during anatexis in the lower crust. Earth Planet. Sci. Lett. 2016, 454, 121–131. [Google Scholar] [CrossRef]
- Ferrero, S.; Ziemann, M.A.; Angel, R.; O’Brien, P.J.; Wunder, B. Kumdykolite, kokchetavite and cristobalite crystallized in nanogranites from felsic granulites, Orlica-Snieznik Dome (Bohemian Massif): Not an evidence for ultrahigh pressure conditions. Contrib. Mineral. Petrol. 2016, 171, 3. [Google Scholar] [CrossRef]
- Ferrero, S.; Angel, R. Micropetrology: Are inclusions in minerals grains of truth? J. Petrol. 2018, 9, 1671–1700. [Google Scholar]
- Carvalho, B.B.; Bartoli, O.; Ferri, F.; Cesare, B.; Ferrero, S.; Remusat, L.; Capizzi, L.S.; Poli, S. Anatexis and fluid regime of the deep continental crust: New clues from melt and fluid inclusions in metapelitic migmatites from Ivrea Zone (NW Italy). J. Metamorph. Geol. 2019, 37, 951–975. [Google Scholar] [CrossRef]
- Ma, C.; Krot, A.N.; Bizzarro, M. Discovery of dmisteinbergite (hexagonal CaAl2Si2O8) in the Allende meteorite: A new member of refractory silicates formed in the solar nebula. Am. Mineral. 2013, 98, 1368–1371. [Google Scholar] [CrossRef]
- Németh, P.; Lehner, S.W.; Petaev, M.I.; Buseck, P. Kumdykolite, a high-temperature feldspar from an enstatite chondrite. Am. Mineral. 2013, 98, 1070–1073. [Google Scholar] [CrossRef]
- Fintor, K.; Walter, H.; Nagy, S. Petrographic and micro-Raman analysis of chondrules and (Ca, Al)-rich inclusions of NWA 2086 CV3 type carbonaceous chondrite. Lunar Planet. Sci. 2013, 44, 1152. [Google Scholar]
- Fintor, K.; Park, C.; Nagy, S.; Pál-Molnár, E.; Krot, A.N. Hydrothermal origin of hexagonal CaAl2Si2O8 (dmisteinbergite) in a compact type A CAI from the Northwest Africa 2086 CV3 chondrite. Meteor. Planet. Sci. 2014, 49, 812–823. [Google Scholar] [CrossRef]
- Hwang, S.L.; Shen, P.; Chu, H.T.; Yui, T.F.; Liou, J.G.; Sobolev, N.V.; Zhang, R.Y.; Shatsky, V.S.; Zayachkovsky, A.A. Kokchetavite: A new polymorph of KAlSi3O8 from the Kokchetav UHP terrain. Contrib. Mineral. Petrol. 2004, 148, 380–389. [Google Scholar] [CrossRef]
- Hwang, S.L.; Shen, P.; Chu, H.T.; Yui, T.-F.; Liou, J.G.; Sobolev, N.V. Kumdykolite, an orthorhombic polymorph of albite, from the Kokchetav ultrahigh-pressure massif, Kazakhstan. Eur. J. Mineral. 2009, 21, 1325–1334. [Google Scholar] [CrossRef]
- Schertl, H.P.; Sobolev, N.V. The Kokchetav Massif, Kazakhstan: “Type locality” of diamond-bearing UHP metamorphic rocks. J. Asian Earth Sci. 2013, 63, 5–38. [Google Scholar] [CrossRef]
- Liou, J.G.; Tsujimori, T.; Yang, J.; Zhang, R.Y.; Ernst, W.G. Recycling of crustal materials through study of ultrahigh-pressure minerals in collisional orogens, ophiolites, and mantle xenoliths: A review. J. Asian Earth Sci. 2014, 96, 386–420. [Google Scholar] [CrossRef]
- Kotková, J.; Koda, R.; Machovi, V. Kumdykolite from the ultrahigh-pressure granulite of the Bohemian Massif. Am. Mineral. 2014, 99, 1798–1801. [Google Scholar] [CrossRef]
- Perraki, M.; Faryad, S.W. First finding of microdiamond, coesite and other UHP phases in felsic granulites in the Moldanubian Zone: Implications for deep subduction and a revised geodynamic model for Variscan Orogeny in the Bohemian Massif. Lithos 2014, 202, 157–166. [Google Scholar] [CrossRef]
- Di Pierro, S.; Gnos, E. Ca-Al-silicate inclusions in natural moissanite (SiC). Am. Mineral. 2016, 101, 71–81. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Galuskin, E.V.; Vapnik, Y.; Prusik, K.; Stasiak, M.; Dzierzanowski, P.; Murashko, M. Gurimite, Ba3(VO4)2 and hexacelsian, BaAl2Si2O8—Two new minerals from schorlomite-rich paralava of the Hatrurim Complex, Negev Desert, Israel. Mineral. Mag. 2017, 81, 1009–1019. [Google Scholar] [CrossRef]
- Nestola, F.; Mittempergher, S.; Toro, G.D.; Zorzi, F.; Pedron, D. Evidence of dmisteinbergite (hexagonal form of CaAl2Si2O8) in pseudotachylyte: A tool to constrain the thermal history of a seismic event. Am. Mineral. 2010, 95, 405–409. [Google Scholar] [CrossRef]
- Mittempergher, S.; Dallai, L.; Pennacchioni, G.; Renard, F.; Di Toro, G. Origin of hydrous fluids at seismogenic depth: Constraints from natural and experimental fault rocks. Earth Planet. Sci. Lett. 2014, 385, 97–109. [Google Scholar] [CrossRef]
- Dobson, D.P.; Thomas, R.W.; Mitchell, T.M. Diffusion profiles around quartz clasts as indicators of the thermal history of pseudotachylytes. Geochem. Geophys. Geosyst. 2018, 19, 4329–4341. [Google Scholar] [CrossRef]
- Goldsmith, J.R. A “simplexity principle” and its relation to “ease” of crystallization. J. Geol. 1953, 61, 439–451. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Cempírek, J.; Grew, E.S.; Kampf, A.R.; Ma, C.; Novák, M.; Gadas, P.; Škoda, R.; Vašinová-Galiová, M.; Pezzotta, F.; Groat, L.A.; et al. Vránaite, ideally Al16B4Si4O38, a new mineral related to boralsilite, Al16B6Si2O37, from the Manjaka pegmatite, Sahatany Valley, Madagascar. Am. Mineral. 2016, 101, 2108–2117. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Zhitova, E.S.; Spratt, J.; Zolotarev, A.A.; Krivovichev, S.V. Isolueshite, NaNbO3, from the Kovdor carbonatite, Kola peninsula, Russia: Composition, crystal structure and possible formation scenarios. N. Jb. Mineral. Abh. 2017, 194, 165–173. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Hydrogen bonding and structural complexity of the Cu3(AsO4)(OH)3 polymorphs (clinoclase, gilmarite): A theoretical study. J. Geosci. 2017, 62, 79–85. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Hawthorne, F.C.; Williams, P.A. Structural complexity and crystallization: The Ostwald sequence of phases in the Cu2(OH)3Cl system (botallackite–atacamite–clinoatacamite). Struct. Chem. 2017, 28, 153–159. [Google Scholar] [CrossRef]
- Plášil, J. Structural complexity of uranophane and uranophane-β: Implications for their formation and occurrence. Eur. J. Mineral. 2018, 30, 253–257. [Google Scholar] [CrossRef]
- Huskić, I.; Novendra, N.; Lim, D.-W.; Topić, F.; Titi, H.M.; Pekov, I.V.; Krivovichev, S.V.; Navrotsky, A.; Kitagawa, H.; Friščić, T. Functionality in metal-organic framework minerals: Proton conductivity, stability and potential for polymorphism. Chem. Sci. 2019, 10, 4923–4929. [Google Scholar] [CrossRef]
- Davis, G.L.; Tuttle, O.F. Two new crystalline phases of the anorthite composition, CaO-Al2O3-SiO2. Am. J. Sci. Bowen Volume. 1951, 1, 107–114. [Google Scholar]
- Takeuchi, Y.; Donnay, G. The crystal structure of hexagonal CaAl2Si2O8. Acta Crystallogr. 1959, 12, 465–470. [Google Scholar] [CrossRef]
- Takéuchi, Y.; Haga, N.; Ito, J. The crystal structure of monoclinic CaAl2Si2O8: A case of monoclinic structure closely simulating orthorhombic symmetry. Z. Kristallogr. 1973, 137, 380–398. [Google Scholar]
- Ito, J. High temperature solvent growth of anorthite on the join CaAl2Si2O8-SiO2. Contrib. Mineral. Petrol. 1976, 59, 187–194. [Google Scholar] [CrossRef]
- Abe, T.; Tsukamoto, K.; Sunagawa, I. Nucleation, growth and stability of CaAl2Si2O8 polymorphs. Phys. Chem. Miner. 1991, 17, 473–484. [Google Scholar] [CrossRef]
- Abe, T.; Sunagawa, I. Hexagonal CaAl2Si2O8 in a high temperature solution; metastable crystallization and transformation to anorthite. Mineral. J. 1995, 17, 257–281. [Google Scholar] [CrossRef]
- Daniel, I.; Gillet, P.; McMillan, P.F.; Richet, P. An in-situ high-temperature structural study of stable and metastable CaAl2Si2O8 polymorphs. Mineral. Mag. 1995, 59, 25–33. [Google Scholar] [CrossRef]
- Dimitrijević, R.; Dondur, V.; Kremenović, A. Thermally induced phase transformations of Ca-exchanged LTA and FAU zeolite frameworks: Rietveld refinement of the hexagonal CaAl2Si2O8 diphyllosilicate structure. Zeolites 1996, 16, 294–300. [Google Scholar] [CrossRef]
- Chesnokov, B.V.; Lotova, E.V.; Pavlyuchenko, V.S.; Nigmatulina, E.N.; Usova, L.V.; Bushmakin, A.R.; Nishanbaev, T.P. Svyatoslavite, CaAl2Si2O8 (orthorhombic), a new mineral. Zap. Vses. Mineral. Obshch. 1989, 118, 111–114. (In Russian) [Google Scholar]
- Krivovichev, S.V.; Shcherbakova, E.P.; Nishanbaev, T.P. The crystal structure of svyatoslavite and evolution of complexity during crystallization of a CaAl2Si2O8 melt: A structural automata description. Can. Mineral. 2012, 50, 585–592. [Google Scholar] [CrossRef]
- Chesnokov, B.V.; Lotova, E.V.; Nigmatulina, E.N.; Pavlyuchenko, V.S.; Bushmakin, A.F. Dmisteinbergite CaAl2Si2O8 (hexagonal)-A new mineral. Zap. Vses. Mineral. Obshch. 1990, 119, 43–45. (In Russian) [Google Scholar]
- Simakin, A.G.; Eremyashev, V.E.; Kucherinenko, Y.V. New data on dmisteinbergite. Zap. Ross. Mineral. Obshch. 2010, 139, 102–108. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Shcherbakova, E.P.; Nishanbaev, T.P. Minerals from Burned Dumps of Chelyabinsk Coal Basin; Institute of Mineralogy UrO RAS: Miass, Russia, 2008. (In Russian) [Google Scholar]
- Akatsuka, K.; Yasumori, A.; Maeda, K. Structure of crystalline CaAl2Si2O8 precipitated in a CaO-Al2O3-SiO2 glass-ceramic. Mater. Lett. 2019, 242, 163–165. [Google Scholar] [CrossRef]
- Bruker-AXS. APEX2, Version 2014.11-0; Bruker-AXS: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Goettingen: Goettingen, Germany, 2007. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: the RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Pilz, W. Raman spectra of silicates. Acta Phys. Hung. 1987, 61, 27–30. [Google Scholar]
- Brigatti, M.F.; Guggenheim, S. Mica crystal chemistry and the influence of pressure, temperature, and solid solution on atomistic models. Rev. Mineral. Geochem. 2002, 46, 1–97. [Google Scholar] [CrossRef]
- Ferraris, G.; Ivaldi, G. Structural features of micas. Rev. Mineral. Geochem. 2002, 46, 117–153. [Google Scholar] [CrossRef]
- Alietti, E.; Brigatti, M.F.; Poppi, L. Clintonite-1M: Crystal chemistry and its relationships to closely associated Al-rich phlogopite. Am. Mineral. 1997, 82, 936–945. [Google Scholar] [CrossRef]
- Freeman, J.J.; Wang, A.; Kuebler, K.E.; Jolliff, B.L.; Haskin, L.A. Characterization of natural feldspars by Raman spectroscopy for future planetary exploration. Can. Mineral. 2008, 46, 1477–1500. [Google Scholar] [CrossRef]
- Maeda, K.; Yasumori, A. Toughening of CaO–Al2O3–SiO2 glass by dmisteinbergite precipitation. Mater. Lett. 2016, 180, 231–234. [Google Scholar] [CrossRef]
- Maeda, K.; Yasumori, A. Nucleation and growth of hexagonal CaAl2Si2O8 crystals in CaO–Al2O3–SiO2 glass. Mater. Lett. 2017, 206, 241–244. [Google Scholar] [CrossRef]
- Maeda, K.; Iwasaki, K.; Urata, S.; Akatsuka, K.; Yasumori, A. 3D microstructure and crack pathways of toughened CaO–Al2O3–SiO2 glass by precipitation of hexagonal CaAl2Si2O8 crystal. J. Am. Ceram. Soc. 2019, 102, 5535–5544. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth. Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Izatulina, A.R.; Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Kuz’mina, M.A.; Leoni, M.; Frank-Kamenetskaya, O.V. Hydrated Calcium Oxalates: Crystal Structures, Thermal Stability and Phase Evolution. Cryst. Growth Des. 2018, 18, 5465–5478. [Google Scholar] [CrossRef]
- Izatulina, A.R.; Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Chukanov, N.V.; Panikorovskii, T.L. Thermal Behavior and Phase Transition of Uric Acid and Its Dihydrate Form, the Common Biominerals Uricite and Tinnunculite. Minerals 2019, 9, 373. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherland; London/Oxford, UK; New York, NY, USA; Paris, France; Tokyo, Japan, 2007. [Google Scholar]
- Smith, J.V.; Brown, W.L. Feldspar Minerals: 1. Crystal Structures, Physical, Chemical, and Microtextural Properties, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1988. [Google Scholar]
- Krivovichev, S.V. Structural and topological complexity of zeolites: An information-theoretic analysis. Micropor. Mesopor. Mater. 2013, 171, 223–229. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity and configurational entropy of crystals. Acta Crystallogr. 2016, 72, 274–276. [Google Scholar] [CrossRef]
| Crystal System | Trigonal |
|---|---|
| Space group | P312 |
| a, Å | 5.1123 (2) |
| c, Å | 14.7420 (7) |
| V, Å3 | 333.67 (3) |
| Z | 2 |
| Dcalc, g/cm3 | 2.769 |
| μ, mm−1 | 1.575 |
| Crystal dimensions, mm | 0.17 × 0.05 × 0.03 |
| F (000) | 276.0 |
| Radiation | MoKα (λ = 0.71073) |
| 2θ range, degree | 2.76–62.89 |
| Index ranges | −7 ≤ h ≤ 7, −7 ≤ k ≤ 7, −21 ≤ l ≤ 16 |
| Reflections collected | 2731 |
| Independent reflections | 762 [Rint = 0.0198, Rsigma = 0.0189] |
| Data/restraints/parameters | 762/0/48 |
| Goodness of Fit | 1.132 |
| Final R indices [I ≥ 2σ (I)] | R1 = 0.0451, wR2 = 0.1355 |
| Final R indices [all data] | R1 = 0.0478, wR2 = 0.1382 |
| Largest diff. peak/hole/eÅ−3 | 0.57/−1.08 |
| Atom | x | y | z | Ueq |
|---|---|---|---|---|
| Ca1 | 0 | 0 | 0 | 0.0099 (5) |
| Ca2 | 0 | 0 | ½ | 0.0154 (6) |
| T1 | 2/3 | 1/3 | 0.8622 (2) | 0.0057 (6) |
| T2 | 1/3 | 2/3 | 0.8592 (3) | 0.0114 (9) |
| T3 | 1/3 | 2/3 | 0.6358 (3) | 0.0152 (9) |
| T4 | 2/3 | 1/3 | 0.6400 (3) | 0.0066 (7) |
| O1 | 2/3 | 1/3 | 0.7536 (4) | 0.013 (2) |
| O2 | 1/3 | 2/3 | 0.7460 (5) | 0.021 (3) |
| O3 | 0.997 (1) | 0.3821 (9) | 0.9029 (2) | 0.0055 (6) |
| O4 * | 0.598 (2) | 0.617 (2) | 0.5964 (5) | 0.017 (2) |
| O4A ** | 0.394 (3) | 0.382 (3) | 0.5967 (8) | 0.019 (3) |
| Note: * s.o.f. = 0.60 (1), ** s.o.f. = 0.40 (1). | ||||
| T1–О1 | 1.600 (6) | T3–O2 | 1.625 (8) | T3–O2 | 1.625 (8) |
| T1–O3 | 1.688 (6) 3× | T3–O4 | 1.606 (9) 3× | T3–O4A | 1.732 (19) 3× |
| <T1–O> | 1.666 | <T3–O> | 1.611 | <T3–O> | 1.705 |
| T2–O2 | 1.669 (10) | T4–O1 | 1.675 (8) | T4–O1 | 1.675 (8) |
| T2–O3 | 1.728 (6) 3× | T4–O4 | 1.773 (10) 3× | T4–O4A | 1.66 (2) 3× |
| <T2–O> | 1.713 | <T4–O> | 1.748 | <T4–O> | 1.664 |
| Ca1–O3 | 2.429 (3) 6× | Ca2–O4 | 2.461 (7) 6× | Ca2–O4A | 2.444 (10) 6× |
| Atom | T1 | T2 | T3 | T4 | Ca1 | Ca2 | Total |
|---|---|---|---|---|---|---|---|
| O1 | 1.07 | 0.94 | 2.01 | ||||
| O2 | 0.94 | 1.00 | 1.94 | ||||
| O3 | 0.84↓ ×3 | 0.81↓ ×3 | 0.29↓ ×6 | 1.94 | |||
| O4 | 1.05↓ ×3 | 0.73↓ ×3 | 0.26↓ ×6 | 2.04 | |||
| O4A | [0.75↓ ×3] | [0.98↓ ×3] | [0.28↓ ×6] | [2.01] | |||
| Total | 3.58 | 3.38 | 4.15 [3.24] | 3.12 [3.84] | 1.73 | 1.57 [1.67] |
| Shift, cm−1 | Vibration | Type of Vibration |
|---|---|---|
| 912s, 892s, 801s | Si‒O, Al‒O | v1,3 |
| 487w, 503w | O‒Si‒O, O‒Al‒O | v4 |
| 327s, 439s | Si‒O, Al‒O | v2 |
| 72w, 115w, 173w, 221w | Ca‒O, lattice vibrations | Т, v1 |
| Polymorph | Topological Complexity of Tetrahedral Unit | Structural Complexity | ||||
|---|---|---|---|---|---|---|
| v | IG, bits/atom | IG,total, bits/cell | v | IG, bits/atom | IG,total, bits/cell | |
| anorthite | 24 | 2.752 | 66.039 | 104 | 5.700 | 592.846 |
| svyatoslavite | 12 | 1.585 | 19.020 | 26 | 3.700 | 96.211 |
| dmisteinbergite | 12 | 1.459 | 17.510 | 26 | 3.046 | 79.192 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolotarev, A.A.; Krivovichev, S.V.; Panikorovskii, T.L.; Gurzhiy, V.V.; Bocharov, V.N.; Rassomakhin, M.A. Dmisteinbergite, CaAl2Si2O8, a Metastable Polymorph of Anorthite: Crystal-Structure and Raman Spectroscopic Study of the Holotype Specimen. Minerals 2019, 9, 570. https://doi.org/10.3390/min9100570
Zolotarev AA, Krivovichev SV, Panikorovskii TL, Gurzhiy VV, Bocharov VN, Rassomakhin MA. Dmisteinbergite, CaAl2Si2O8, a Metastable Polymorph of Anorthite: Crystal-Structure and Raman Spectroscopic Study of the Holotype Specimen. Minerals. 2019; 9(10):570. https://doi.org/10.3390/min9100570
Chicago/Turabian StyleZolotarev, Andrey A., Sergey V. Krivovichev, Taras L. Panikorovskii, Vladislav V. Gurzhiy, Vladimir N. Bocharov, and Mikhail A. Rassomakhin. 2019. "Dmisteinbergite, CaAl2Si2O8, a Metastable Polymorph of Anorthite: Crystal-Structure and Raman Spectroscopic Study of the Holotype Specimen" Minerals 9, no. 10: 570. https://doi.org/10.3390/min9100570
APA StyleZolotarev, A. A., Krivovichev, S. V., Panikorovskii, T. L., Gurzhiy, V. V., Bocharov, V. N., & Rassomakhin, M. A. (2019). Dmisteinbergite, CaAl2Si2O8, a Metastable Polymorph of Anorthite: Crystal-Structure and Raman Spectroscopic Study of the Holotype Specimen. Minerals, 9(10), 570. https://doi.org/10.3390/min9100570








