Dritsite, Li2Al4(OH)12Cl2·3H2O, a New Gibbsite-Based Hydrotalcite Supergroup Mineral
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence, Appearance, Physical Properties, and Optical Data
2.2. Chemical Composition
2.3. Raman Spectroscopy
2.4. Single-Crystal X-ray Diffraction and Crystal Structure Determination
2.5. Powder X-ray Diffraction
3. Results
3.1. Chemical Composition
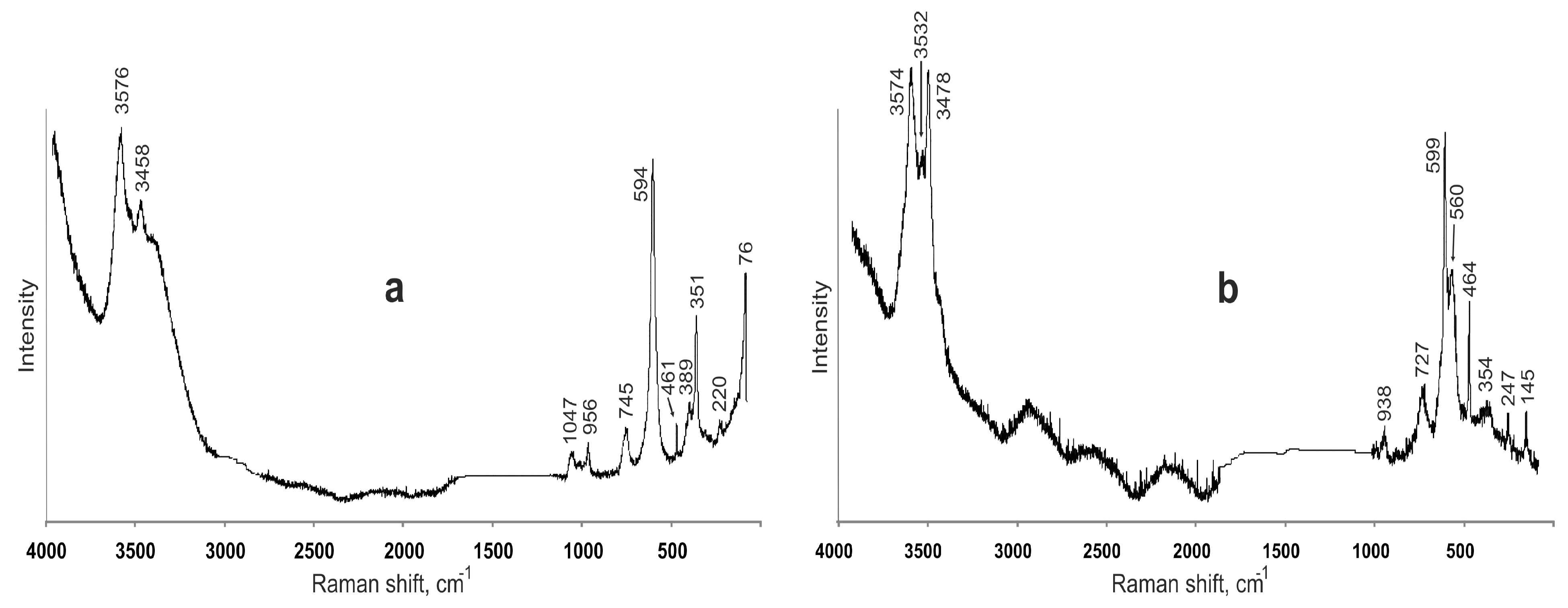
3.2. Raman Spectroscopy
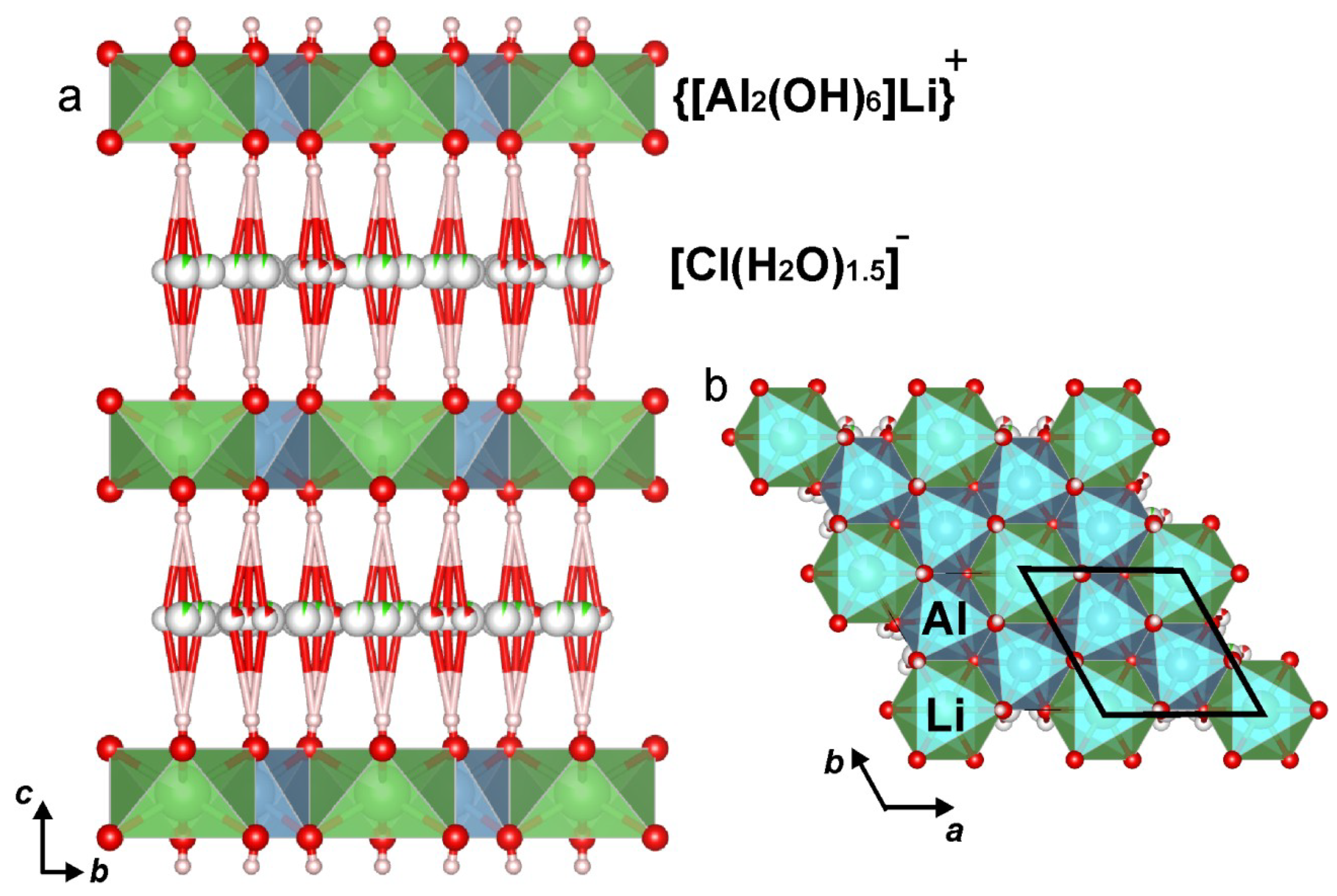
3.3. Single-Crystal X-ray Diffraction and Crystal Structure Determination
3.4. Powder X-ray Diffraction
4. Discussion
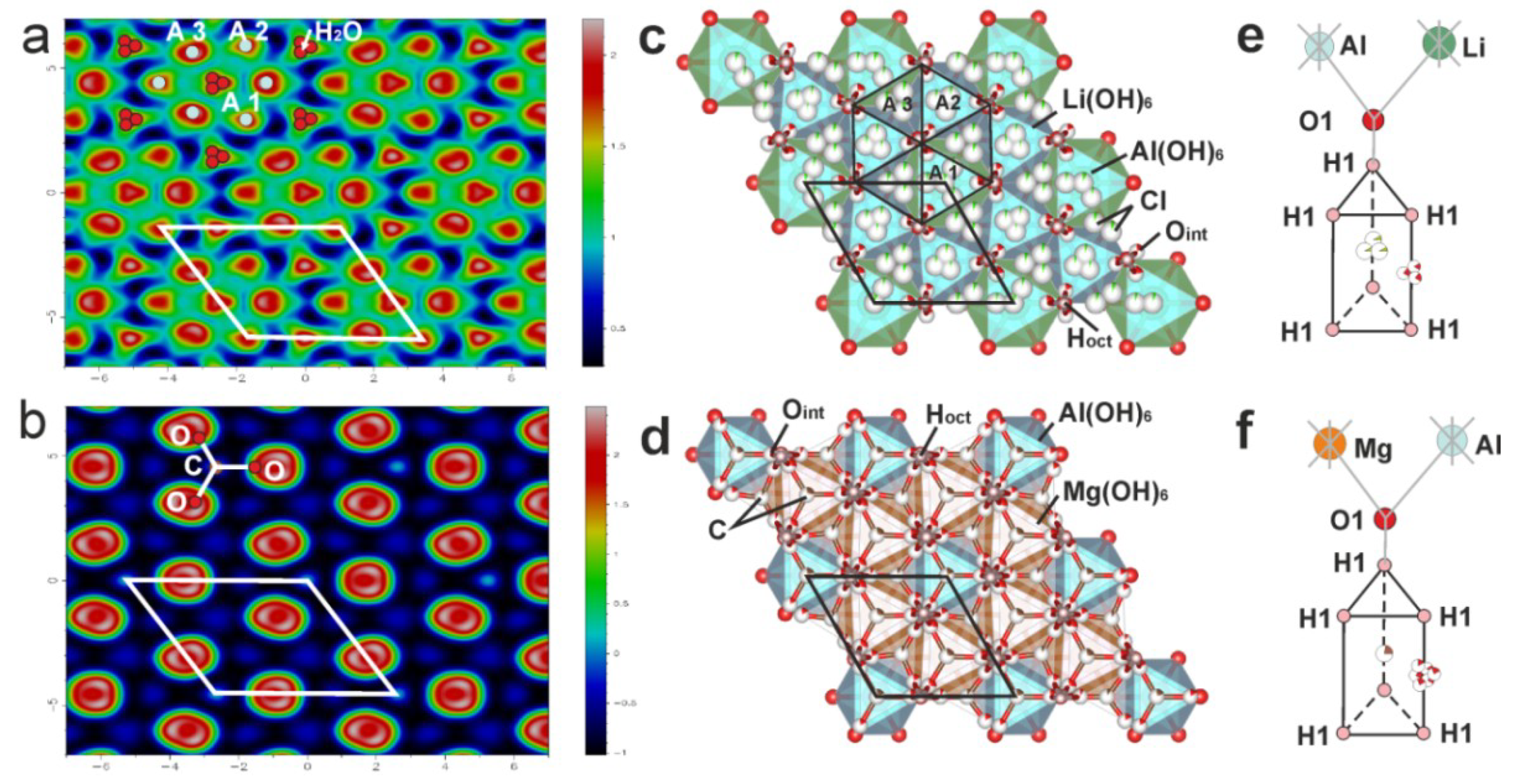
4.1. Crystal Structure of Dritsite-2H
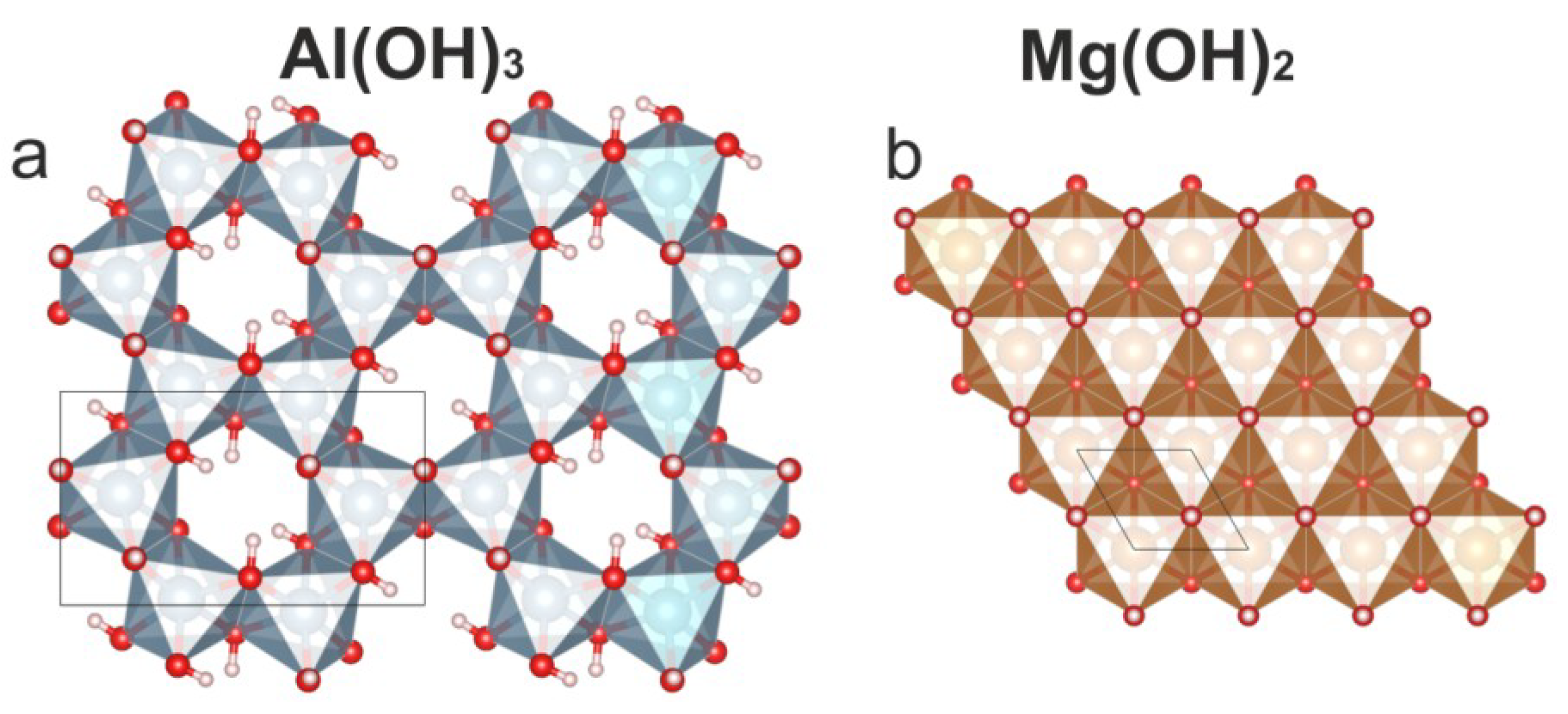
4.2. Comparison to Other Hydrotalcite Supergroup Minerals and Synthetic Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Science Publishers: New York, NY, USA, 2001. [Google Scholar]
- Evans, D.G.; Slade, R.C.T. Structural aspects of layered double hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin, Germany, 2006; Volume 119, pp. 1–87. [Google Scholar]
- Mills, S.J.; Christy, A.G.; Génin, J.-M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: natural layered double hydroxides. Miner. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef]
- Besserguenev, A.V.; Fogg, AM.; Francis, R.J.; Price, S.J.; O’Hare, D.; Isupov, V.P.; Tolochko, B.P. Synthesis and structure of the gibbsite intercalation compounds [LiAl2(OH)6]X {X = Cl, Br, NO3} and [LiAl2(OH)6]Cl·H2O using synchrotron X-ray and neutron powder diffraction. Chem. Mater. 1997, 9, 241–247. [Google Scholar] [CrossRef]
- Britto, S.; Kamath, P.V. Structure of Bayerite-Based Lithium–Aluminum Layered Double Hydroxides (LDHs): Observation of Monoclinic Symmetry. Inorg. Chem. 2009, 48, 11646–11654. [Google Scholar] [CrossRef] [PubMed]
- Britto, S.; Kamath, P.V. Polytypism in the lithium–aluminum layered double hydroxides: the [LiAl2(OH)6]+ layer as a structural synthon. Inorg. Chem. 2011, 50, 5619–5627. [Google Scholar] [CrossRef] [PubMed]
- Britto, S.; Thomas, G.S.; Kamath, P.V.; Kannan, S. Polymorphism and structural disorder in the carbonate containing layered double hydroxide of Li with Al. J. Phys. Chem. 2008, 112, 9510–9515. [Google Scholar] [CrossRef]
- Nagendran, S.; Kamath, P.V. Structure of the chloride- and bromide-intercalated Layered Double Hydroxides of Li and Al–interplay of coulombic and hydrogen-bonding interactions in the interlayer gallery. Eur. J. Inorg. Chem. 2013, 26, 4686–4693. [Google Scholar] [CrossRef]
- Niksch, A.; Pöllmannm, H. Synthesis and characterization of a [Li0+xMg2−2xAl1+x(OH)6][Cl·mH2O] Solid solution with X= 0–1 at different temperatures. Nat. Resour. J. 2017, 8, 445–459. [Google Scholar]
- Devyatkina, E.T.; Kotsupalo, N.P.; Tomilov, N.P.; Berger, A.S. On the lithium carbonate-hydroxyl-aluminate. Zhurnal Neorg. Khimii. 1983, 28, 1420–1425. (In Russian) [Google Scholar]
- Drewien, C.A.; Eatough, M.O.; Tallant, D.R.; Hills, C.R.; Buchheit, R.G. Lithium-aluminum-carbonate-hydroxide hydrate coatings on aluminum alloys: composition, structure, and processing bath chemistry. J. Mater. Res. 1996, 11, 1507–1513. [Google Scholar] [CrossRef]
- Hou, X.; Kirkpatrick, R.J. Thermal Evolution of the Cl-–LiAl2 Layered Double Hydroxide: A Multinuclear MAS NMR and XRD Perspective. Inorg. Chem. 2001, 40, 6397–6404. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Gao, Y.; Qiao, Y.; Zheng, Q.; Guo, Z.; Zhao, Y.; O'Hare, D.; Wang, Q. Synthesis of LiAl2-layered double hydroxides for CO2 capture over a wide temperature range. J. Mater. Chem. 2014, 2, 18454–18462. [Google Scholar] [CrossRef]
- Isupov, V.P. Intercalation compounds of aluminium hydroxide. J. Struct. Chem. 1999, 40, 672–685. [Google Scholar] [CrossRef]
- Isupov, V.P.; Chupakina, L.E.; Tarasov, K.A.; Shestakova, N.Yu. Synthesis of superfine carbonate form of Li–Al double hydroxide from sodium hydroaluminocarbonate. Chem. Sustain. Dev. 2007, 15, 63–69. [Google Scholar]
- Serna, C.J.; White, J.L.; Hem, S.L. Hydrolysis of aluminium-tri-(sec-butoxide) in ionic and nonionic media. Clay Clay Miner. 1977, 25, 384–391. [Google Scholar] [CrossRef]
- Serna, C.J.; Rendon, J.L.; Iglesias, J.E. Crystal-chemical study of layered [Al2Li(OH)6]+·nH2O. Clay Clay Miner. 1982, 30, 180–184. [Google Scholar] [CrossRef]
- Sissoko, I.; Iyagba, E.T.; Sahai, I.; Biloen, P. Anion intercalation and exchange in Al(OH)3− derived compounds. J. Solid State Chem. 1985, 60, 283–288. [Google Scholar] [CrossRef]
- Thiel, J.P.; Chiang, C.K.; Poeppelmeier, K.R. Structure of LiAl2(OH)7·H2O. Chem. Mater. 1993, 5, 297–304. [Google Scholar] [CrossRef]
- Génin, J.-M.R.; Mills, S.J.; Christy, A.G.; Guérin, O.; Herbillon, A.J.; Kuzmann, E.; Ona-Nguema, G.; Ruby, C.; Upadhyay, C. Mössbauerite, Fe3+6O4(OH)8[CO3]·3H2O, the fully oxidized ‘green rust’ mineral from Mont Saint-Michel Bay, France. Miner. Mag. 2014, 78, 447–465. [Google Scholar] [CrossRef]
- Karpenko, V.Yu.; Pautov, L.A.; Zhitova, E.S.; Agakhanov, A.A.; Krzhizhanovskaya, M.G.; Siidra, O.I.; Rassulov, V.A. Akopovaite, IMA 2018-095. CNMNC Newsletter No. 46, December 2018, page 1186. Eur. J. Miner. 2018, 30, 1181–1189. [Google Scholar]
- Drits, V.A.; Sokolova, T.N.; Sokolova, G.V.; Cherkashin, V.I. New members of hydrotalcite-manasseite group. Clay Clay Min. 1987, 35, 401–417. [Google Scholar] [CrossRef]
- Bookin, A.S.; Drits, V.A. Polytype diversity of the hydrotalcite-like minerals. I. Possible polytypes and their diffraction patterns. Clay Clay Min. 1993, 41, 551–557. [Google Scholar] [CrossRef]
- Saalfeld, H.; Wedde, M. Refinement of the crystal structure of gibbsite, Al(OH)3. Z. für Krist. 1974, 139, 129–135. [Google Scholar] [CrossRef]
- Zigan, F.; Rothbauer, R. Neutronenbeugungsmeeungen am Brucit. Neues JB Miner. Mon 1967, 1967, 137–143. [Google Scholar]
- Kudryashov, A.I. Verkhnekamskoe Salt Depos., 2nd ed.; Epsilon Plus: Moscow, Russia, 2013; pp. 1–368. (In Rusian) [Google Scholar]
- Chaikovskiy, I.I.; Chaikovskaya, E.V.; Korotchenkova, O.V.; Chirkova, E.P.; Utkina, T.A. Autogenic titanium and zirconium minerals at the Verkhnekamskoe salt deposit. Geochem. Int. 2019, 57, 184–196. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Voronova, M.L. Verkhnekamskoe Potash Salt Depos.; Nedra: Leningrad, Russian, 1975; pp. 1–219. [Google Scholar]
- Pekov, I.V.; Zubkova, N.V.; Chaikovskiy, I.I.; Chirkova, E.P.; Belakovskiy, D.I.; Yapaskurt, V.O.; Bychkova, Y.V.; Lykova, I.S.; Britvin, S.N.; Pushcharovsky, D.Y. Krasnoshteinite, IMA 2018-077. CNMNC Newsletter No. 46, December 2018, page 1182. Eur. J. Miner. 2018, 30, 1181–1189. [Google Scholar]
- Mandarino, J.A. The Gladstone–Dale compatibility of minerals and its use in selecting mineral species for further study. Can. Miner. 2007, 45, 1307–1324. [Google Scholar] [CrossRef]
- CrysAlisPro, version 1.171.37.35; Data Collection and Processing Software for Agilent X-ray Diffractometers; Agilent Technologies UK Ltd.: Oxford, UK, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Britvin, S.N.; Dolivo-Dobrovolsky, D.V.; Krzhizhanovskaya, M.G. Software for processing of X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zap. Ross. Miner. Obs. 2017, 146, 104–107, (In Russian with English abs.). [Google Scholar]
- Topas, version 4.2; General Profile and Structure Analysis Software for Powder Diffraction Data. Bruker-AXS: Karlsruhe, Germany, 2009.
- Brabers, V.A.M. Infrared spectra and ionic ordering of the lithium ferrite–aluminate and chromite systems. Spectrochim. Acta. A 1976, 32, 1709–1711. [Google Scholar] [CrossRef]
- Khosravi, I.; Yazdanbakhsh, M.; Eftekhar, M.; Haddadi, Z. Fabrication of nano delafossite LiCo0.5Fe0.5O2 as the new adsorbent in efficient removal of reactive blue from aqueous solutions. Mater. Res. Bull. 2013, 48, 2213–2219. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T. Comparison of Raman spectra in characterizing gibbsite, bayerite, diaspore and boehmite. J. Raman Spectrosc. 2001, 32, 745–750. [Google Scholar] [CrossRef]
- Mills, S.J.; Whitfield, P.S.; Wilson, S.A.; Woodhouse, J.N.; Dipple, G.M.; Raudsepp, M.; Francis, C.A. The crystal structure of stichtite, re-examination of barbertonite, and the nature of polytypism in MgCr hydrotalcites. Am. Miner. 2011, 96, 179–187. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Pekov, I.V.; Chukanov, N.V.; Yapaskurt, V.O.; Bocharov, V.N. Minerals of the stichtite-pyroaurite-iowaite-woodallite system from serpentinites of Terektinsky range, Altay Mountains, Russia. Russ. Geol. Geoph 2019, in press. [Google Scholar]
- Theiss, F.; López, A.; Frost, R.L.; Scholz, R. Spectroscopic characterisation of the LDH mineral quintinite Mg4Al2(OH)12CO3 × 3H2O. Spectrochim. Acta, A 2015, 150, 758–764. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Guinier, A.; Bokij, G.B.; Boll-Dornberger, K.; Cowley, J.M.; Durovic, S.; Jagodzinski, H.; Krishna, P.; DeWolff, P.M.; Zvyagin, B.B.; Cox, D.E.; et al. Nomenclature of polytype structures. Report of the International Union of Crystallography ad-hoc committee on the Nomenclature of discordered, modulated and polytype structures. Acta Cryst. 1984, A40, 399–404. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Zhitova, E.S.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal chemistry of natural layered double hydroxides. 1. Quintinite-2H-3c from the Kovdor alkaline massif, Kola peninsula, Russia. Miner. Mag. 2010, 74, 821–832. [Google Scholar]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Zhitova, E.S.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal chemistry of natural layered double hydroxides. 2. Quintinite-1M: First evidence of a monoclinic polytype in M2+–M3+ layered double hydroxides. Miner. Mag. 2010, 74, 833–840. [Google Scholar]
- Zhitova, E.S.; Yakovenchuk, V.N.; Krivovichev, S.V.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal chemistry of natural layered double hydroxides. 3. The crystal structure of Mg, Al-disordered quintinite-2H. Miner. Mag. 2010, 74, 841–848. [Google Scholar]
- Zhitova, E.S.; Ivanyuk, G.Y.; Krivovichev, S.V.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Mikhailova, Y.A. Crystal Chemistry of Pyroaurite from the Kovdor Pluton, Kola Peninsula, Russia, and the Långban Fe–Mn deposit, Värmland, Sweden. Geol Ore Depos. 2017, 59, 652–661. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Krivovichev, S.V.; Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Mikhailova, J.A. Crystal chemistry of natural layered double hydroxides. 4. Crystal structures and evolution of structural complexity of quintinite polytypes from the Kovdor alkaline massif, Kola peninsula, Russia. Miner. Mag. 2018, 82, 329–346. [Google Scholar]
- Zhitova, E.S.; Krivovichev, S.V.; Pekov, I.V.; Greenwell, H.C. Crystal chemistry of natural layered double hydroxides. 5. Single-crystal structure refinement of hydrotalcite, [Mg6Al2(OH)16](CO3)(H2O)4. Miner. Mag. 2019, 83, 269–280. [Google Scholar]
- Mills, S.J.; Whitfield, P.S.; Kampf, A.R.; Wilson, S.A.; Dipple, G.M.; Raudsepp, M.; Favreau, G. Contribution to the crystallography of hydrotalcites: The crystal structures of woodallite and takovite. J. Geosci. 2012, 58, 273–279. [Google Scholar] [CrossRef]
- Lozano, R.P.; Rossi, C.; La Iglesia, A.; Matesanz, E. Zaccagnaite-3R, a new Zn–Al hydrotalcite polytype from El Soplao cave (Cantabria, Spain). Am. Miner. 2012, 97, 513–523. [Google Scholar] [CrossRef]
- Hou, X.; Kalinichev, A.G.; Kirkpatrick, R.J. Interlayer structure and dynamics of Cl–LiAl2-Layered Double Hydroxides: 35Cl NMR observation and molecular dynamic simulation. Chem. Mater. 2002, 14, 2078–2085. [Google Scholar] [CrossRef]
- Wachowiak, J.; Pieczk, A. Motukoreaite from the Kłodawa Salt Dome, Central Poland. Miner. Mag. 2016, 80, 277–289. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Krivovichev, S.V.; Pekov, I.V.; Yapaskurt, V.O. Crystal Chemistry of Chlormagaluminite, Mg4Al2(OH)12Cl2(H2O)2, a Natural Layered Double Hydroxide. Minerals 2019, 9, 221. [Google Scholar] [CrossRef]
- Jensen, N.D.; Duong, N.T.; Bolanz, R.; Nishiyama, Y.; Rasmussen, C.A.; Gottlicher, J.; Steininger, R.; Prevot, V.; Nielsen, U.G. Synthesis and structural characterization of a pure ZnAl4(OH)12(SO4)·2.6H2O Layered Double Hydroxide. Inorg. Chem 2019, in press. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Cooper, M.A. The crystal structure of chalcoalumite: mechanisms of Jahn-Teller-driven distortion in [6]Cu2+-containing oxysalts. Miner. Mag. 2013, 77, 2901–2912. [Google Scholar] [CrossRef]
- Uvarova, Yu. A.; Sokolova, E.; Hawthorne, F.C.; Karpenko, V.V.; Agakhanov, A.A.; Pautov, L.A. The crystal chemistry of the “nickelalumite”-group minerals. Can. Miner. 2005, 43, 1511–1519. [Google Scholar] [CrossRef]
- Karpenko, V.V.; Agakhanov, A.A.; Pautov, L.A.; Dikaya, T.V.; Bekenova, G.K. New occurrence of nickelalumite on Kara-Chagyr, South Kirgizia. New Data Miner. 2004, 39, 32–39. [Google Scholar]
- Agakhanov, A.A.; Karpenko, V.Y.; Pautov, L.A.; Uvarova, Y.A.; Sokolova, E.; Hawthorne, F.C.; Bekenova, G.K. Kyrgyzstanite, ZnAl4(SO4)(OH)12(H2O)3—A new mineral from the Kara-Tangi, Kyrgyzstan. New Data Miner. 2005, 40, 23–28. [Google Scholar]
- Williams, S.; Khin, B.S. Chalcoalumite from Bisbee, Arizona. Miner. Rec. 1971, 2, 126–127. [Google Scholar]
- Larsen, E.S.; Vassar, H.E. Chalcoalumite, a new mineral from Bisbee, Arizona. Am. Miner. 1925, 10, 79–83. [Google Scholar]
- Pertlik, F.; Dunn, P.J. Crystal structure of alvanite, (Zn,Ni)Al4(VO3)2(OH)12·2H2O, the first example of an unbranched zweier-single chain vanadate in nature. Neues JB Miner. Mon. 1990, 9, 385–392. [Google Scholar]
- Karpenko, V.V.; Pautov, L.A.; Sokolova, E.; Hawthorne, F.C.; Agakhanov, A.A.; Dikaya, T.V.; Bekenova, G.K. Ankinovichite, nickel analogue of alvanite, a new mineral from Kurunsak (Kazakhstan) and Kara-Chagyr (Kirgizia). Zap. RMO 2004, 133, 59–70. [Google Scholar]
- Martini, J.E.J. Mbobomkulite, hydrombobomkulite, and nickelalumite, new minerals from Mbobo Mkulu cave, eastern Transvaal. Ann. Geol. Surv. S. Afr. 1980, 14, 1–10. [Google Scholar]

| Constituent | Mean | Range | Standart Deviation |
|---|---|---|---|
| Li2O | 6.6 | ||
| Al2O3 | 45.42 | 45.20–45.67 | 0.23 |
| SiO2 | 0.11 | 0.05–0.25 | 0.08 |
| SO3 | 0.21 | 0.00–0.47 | 0.18 |
| Cl | 14.33 | 14.14–14.46 | 0.13 |
| H2Ocalc. * | 34.86 | ||
| –O=Cl | –3.24 | ||
| Total | 98.29 |
| Dritsite | Tentative Assignment | |
|---|---|---|
| a * | b * | |
| 3576, 3520 sh, 3458 | 3574, 3532, 3478 | O–H stretching of OH groups forming hydrogen bonds with Cl− (above 3500 cm−1) and O (below 3500 cm−1) |
| 3373 sh, 3288 sh | 3416 | O–H stretching of H2O groups |
| 1047 | - | Combination mode |
| 956 | 938 | Al···O–H and Li···O–H bending vibrations involving OH groups forming strong hydrogen bonds with O atoms |
| 745 | 727 | Al···O–H and/or Li···O–H bending vibrations involving OH groups forming weak hydrogen bonds (with Cl−(?)) |
| 594 | 599, 560 | Al···O stretching |
| 461 | 464 | Li···O, Al···O stretching |
| 389, 351, 303, 220 | 354, 247, 145 | Lattice modes |
| Crystal Data | |
|---|---|
| Crystal system | Hexagonal |
| Space group | P63/mcm |
| Unit-cell dimensions a, c (Å) | 5.0960(3),15.3578(13) |
| Unit-cell volume (Å3) | 345.4(5) |
| Structural formula, Z | Li2Al4(OH)12Cl2(H2O)2.6, Z = 1 |
| Absorption coefficient (mm−1) | 0.762 |
| Data Collection | |
| Diffractometer | Xcalibur S CCD |
| Temperature (K) | 293 |
| Radiation, wavelength (Å) | MoKα, 0.71073 |
| θ range for data collection (°) | 2.65-32.71 |
| h, k, l ranges | –7→7, –7→7, –22→23 |
| Axis, frame width (°), time per frame (s) | ω, 1, 30 |
| Reflections collected | 6039 |
| Unique reflections (Rint) | 257 (0.1035) |
| Unique reflections F > 2σ(F) | 250 |
| Data completeness to θmax (%) | 98.8 |
| Structure Refinement | |
| Refinement method | Full-matrix least-squares on F2 |
| Weighting coefficients, a, b | 0.199700, 1.606800 |
| Data/restrains/parameters | 257/1/43 |
| R1 [F > 4σ(F)], wR2 [F > 4σ(F)] | 0.0885, 0.2606 |
| R1 all, wR2 all | 0.0910, 0.2648 |
| Goodness-of-fit on F2 | 1.053 |
| Largest diff. peak and hole (ēÅ−3) | 0.82, –0.67 |
| Atom | W.P. | x | y | z | Ueq | s.o.f | s.s. ref (ē) | Assigned Site Populations |
|---|---|---|---|---|---|---|---|---|
| Octahedral (gibbsite-based) layer | ||||||||
| Al | 4d | 1/3 | 2/3 | 0 | 0.0128(3) | 1 * | 48 | Al4.0 |
| Li | 2b | 0 | 0 | 0 | 0.034(6) | 1 * | 6 | Li2.0 |
| O1 | 12k | 0 | 0.3653(4) | 0.0635(1) | 0.0143(9) | 1 * | 96 | (OH)12.0 |
| H1 | 12k | 0 | 0.368(12) | 0.125(2) | 0.015(17) | 1 * | 12 | |
| Interlayer components | ||||||||
| O2 | 6g | 0 | 0.416(14) | ¼ | 0.04(2) | 0.12(4) | 5.8 | (H2O)2.6 |
| O3 | 12j | −0.220(10) | 0.687(9) | ¼ | 0.042(13) | 0.16(3) | 15.4 | |
| Cl11 | 6g | 0 | 0.11(3) | ¼ | 0.04(3) | 0.04(3) | 4.1 | Cl2 |
| Cl12 | 2a | 0 | 0 | ¼ | 0.02(5) | 0.04(6) | 1.4 | |
| Cl22 | 4c | −1/3 | 1/3 | ¼ | 0.02(4) | 0.03(3) | 2.0 | |
| Cl23 | 12j | −0.311(9) | 0.456(17) | ¼ | 0.038(14) | 0.046(16) | 9.4 | |
| Cl31 | 6g | 0 | 0.68(3) | ¼ | 0.00(5) | 0.01(3) | 1.0 | |
| Cl32 | 6g | 0 | 0.77(3) | ¼ | 0.05(3) | 0.06(3) | 6.1 | |
| Cl33 | 12j | −0.094(13) | 0.566(14) | ¼ | 0.034(12) | 0.045(13) | 9.2 | |
| Octahedral (Gibbsite-Like) Layer | |||||||
|---|---|---|---|---|---|---|---|
| Al–O1 × 6 | 1.8934(19) | Li–O1 × 6 | 2.101(3) | ||||
| Hydrogen Bonding Scheme | |||||||
| D–H | d(D–H) | d(H···A) | <DHA | d(D···A) | A | ||
| O1–H1 | 0.94(4) | 2.34(9) | 144(7) | 3.15(5) | Cl11 | ||
| O1–H1 | 0.94(4) | 2.92(9) | 130(7) | 3.60(5) | Cl11 | ||
| O1–H1 | 0.94(4) | 2.69(6) | 134(6) | 3.417(3) | Cl12 | ||
| O1–H1 | 0.94(4) | 2.51(4) | 140(3) | 3.293(3) | Cl22 | ||
| O1–H1 | 0.94(4) | 2.66(6) | 136(4) | 3.41(3) | Cl23 | ||
| O1–H1 | 0.94(4) | 2.21(6) | 151(4) | 3.06(3) | Cl23 | ||
| O1–H1 | 0.94(4) | 2.82(6) | 133(4) | 3.53(3) | Cl23 | ||
| O1–H1 | 0.94(4) | 2.51(11) | 141(7) | 3.30(7) | Cl31 | ||
| O1–H1 | 0.94(4) | 2.60(11) | 137(7) | 3.36(7) | Cl31 | ||
| O1–H1 | 0.94(4) | 2.83(12) | 134(6) | 3.55(8) | Cl32 | ||
| O1–H1 | 0.94(4) | 2.53(12) | 139(6) | 3.29(8) | Cl32 | ||
| O1–H1 | 0.94(4) | 2.33(6) | 146(6) | 3.159(19) | Cl33 | ||
| O1–H1 | 0.94(4) | 2.50(6) | 140(6) | 3.274(19) | Cl33 | ||
| O1–H1 | 0.94(4) | 2.99(6) | 130(6) | 3.660(19) | Cl33 | ||
| O1–H1 | 0.94(4) | 1.94(4) | 173(8) | 2.877(7) | O2 | ||
| O1–H1 | 0.94(4) | 2.03(7) | 160(6) | 2.94(2) | O3 | ||
| Cl—H Distances | |||||||
| Interlayer prism A1 | Interlayer prism A2 | Interlayer prism A3 | |||||
| Cl12–H1 × 6 | 2.69(4) | Cl22–H1 × 6 | 2.51(3) | Cl31–H1 × 2 | 2.51(5) | Cl33–H1× 2 | 2.33(4) |
| <Cl12–H1> | 2.69 | <Cl22–H1> | 2.51 | Cl31–H1 × 4 | 2.60(3) | Cl33–H1× 2 | 2.50(3) |
| Cl11–H1 × 4 | 2.92(5) | Cl21–H1 × 2 | 2.67(4) | <Cl31–H1> | 2.57 | Cl33–H1× 2 | 2.99(3) |
| Cl11–H1 × 2 | 2.34(4) | Cl21–H1 × 2 | 2.20(3) | Cl32–H1 × 4 | 2.53(3) | <Cl33–H1> | 2.61 |
| <Cl11–H1> | 2.73 | Cl21–H1 × 2 | 2.82(3) | Cl32–H1 × 2 | 2.83(5) | ||
| <Cl21–H1> | 2.56 | <Cl32–H1> | 2.63 | ||||
| Dritsite-2H (This Study) | Synthetic Analogue of Dritsite-2H* [4] | |||||||
|---|---|---|---|---|---|---|---|---|
| Imeas | dmeas (Å) | Icalc | dcalc (Å) | h | k | L | Imeas | dmeas (Å) |
| 100 | 7.68 | 100 | 7.68 | 0 | 0 | 2 | 100 | 7.65 |
| 61 | 4.422 | 15 | 4.417 | 0 | 1 | 0 | 11 | 4.413 |
| 99 | 3.832 | 34 | 3.840 | 0 | 0 | 4 | 60 | 3.823 |
| 20 | 3.829 | 0 | 1 | 2 | ||||
| 4 | 2.899 | 3 | 2.898 | 0 | 1 | 4 | 4 | 2.890 |
| 30 | 2.561 | 5 | 2.560 | 0 | 0 | 6 | 5 | 2.549 |
| 8 | 2.516 | 8 | 2.515 | 1 | 1 | 1 | 10 | 2.513 |
| 2 | 2.421 | 4 | 2.420 | 1 | 1 | 2 | 4 | 2.417 |
| 25 | 2.283 | 23 | 2.283 | 1 | 1 | 3 | 25 | 2.279 |
| 3 | 2.216 | 1 | 2.215 | 0 | 1 | 6 | 2 | 2.207 |
| 14 | 2.124 | 12 | 2.124 | 1 | 1 | 4 | 14 | 2.120 |
| >1 | 2.122 | 0 | 2 | 2 | ||||
| 19 | 1.963 | 10 | 1.962 | 1 | 1 | 5 | 13 | 1.958 |
| 7 | 1.920 | >1 | 1.920 | 0 | 0 | 8 | 2 | 1.911 |
| 1.914 | 2 | 1.914 | 0 | 2 | 4 | |||
| 20 | 1.807 | 14 | 1.807 | 1 | 1 | 6 | 16 | 1.802 |
| 7 | 1.672 | 2 | 1.672 | 0 | 2 | 6 | 2 | 1.668 |
| 2 | 1.632 | 1 | 1.631 | 2 | 1 | 2 | 1 | 1.630 |
| 1 | 1.587 | 1 | 1.587 | 2 | 1 | 3 | <1 | 1.585 |
| 13 | 1.534 | 12 | 1.534 | 1 | 1 | 8 | 12 | 1.529 |
| 19 | 1.472 | 8 | 1.472 | 0 | 3 | 0 | 9 | 1.471 |
| 26 | 1.445 | 10 | 1.446 | 0 | 3 | 2 | 12 | 1.445 |
| Cl-LDHs | CO3-LDHs | ||||
|---|---|---|---|---|---|
| Phase | Dritsite | Synthetic analogue of dritsite | Chlormag-aluminite | Quintinite * | |
| Symbol | LiAl2-Cl | Mg2Al-Cl | Mg2Al-CO3 | ||
| Polytype | 2H | 2H | 2H | 2T | 2H |
| Crystal chemical formula | [Al4Li2(OH)12] | [Mg4Al2(OH)12] | [Mg4Al2(OH)12] | ||
| [Cl2(H2O)3] | [Cl2(H2O)3] | [(CO3)(H2O)3] | |||
| Crystal system | Hexagonal | Trigonal | Hexagonal | ||
| Space group | P63/mcm** | P-3c1 | P63/mmc | ||
| a, Å | 5.0960(3) | 5.0963(3) | 5.268(3) | 5.2720(6) | 3.0455(10) |
| c, Å | 15.3578(13) | 15.2919(9) | 15.297(8) | 15.113(3) | 15.125(7) |
| β, ° | 90 | 90 | 90 | 90 | 90 |
| V, Å3 | 345.4(5) | 344.0 | 367.6(4) | 363.8 | 121.5 |
| The strongest lines in the PXRD pattern: d, Å (I, %) | 7.68 (100) | 7.65 (100) | 7.72 (100) | 7.56 (100) | 7.57 (100) |
| 4.422 (61) | 3.823 (60) | 3.856 (38) | 3.778 (58) | 3.785 (57) | |
| 3.832 (99) | 2.279 (25) | 2.350 (38) | 2.600 (14) | 2.600 (11) | |
| 2.561 (30) | 2.210 (14) | 2.179 (34) | 2.519 (10) | 2.524 (10) | |
| 2.283 (25) | 1.958 (13) | 1.843 (29) | 2.489 (9) | 2.492 (8) | |
| 1.445 (26) | 1.802 (16) | 1.558 (16) | 1.987 (7) | 1.824 (6) | |
| Reference | This study | [4] | [53] | [47] | [47] |
| Title | M2+ | A | n | Space Group | a, Å | b, Å | c, Å | β, º | Reference |
|---|---|---|---|---|---|---|---|---|---|
| “Nickelalumite” | Ni | SO4 | 3 | P21/n | 10.2567 | 8.8815 | 17.0989 | 95.548 | [56,57] |
| Kyrgyzstanite | Zn | SO4 | 3 | P21/n | 10.246 | 8.873 | 17.220 | 96.41 | [58] |
| Chalcoalumite | Cu | SO4 | 3 | P21/n | 10.228 | 8.929 | 17.098 | 95.800 | [55,59,60] |
| Alvanite | Zn | VO3 | 2 | P21/n | 17.808 | 5.132 | 8.881 | 92.11 | [61] |
| Ankinovichite | Ni | VO3 | 2 | P21/n | 17.8098 | 5.1228 | 8.8665 | 92.141 | [62] |
| Mbobomkulite | Ni | NO3 | 3 | unknown | 10.171 | 8.865 | 17.145 | 95.37 | [63] |
| Hydro-Mbobomkulite | Ni | NO3 | 12 | unknown | 10.145 | 17.155 | 20.870 | 90.55 | [63] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhitova, E.S.; Pekov, I.V.; Chaikovskiy, I.I.; Chirkova, E.P.; Yapaskurt, V.O.; Bychkova, Y.V.; Belakovskiy, D.I.; Chukanov, N.V.; Zubkova, N.V.; Krivovichev, S.V.; et al. Dritsite, Li2Al4(OH)12Cl2·3H2O, a New Gibbsite-Based Hydrotalcite Supergroup Mineral. Minerals 2019, 9, 492. https://doi.org/10.3390/min9080492
Zhitova ES, Pekov IV, Chaikovskiy II, Chirkova EP, Yapaskurt VO, Bychkova YV, Belakovskiy DI, Chukanov NV, Zubkova NV, Krivovichev SV, et al. Dritsite, Li2Al4(OH)12Cl2·3H2O, a New Gibbsite-Based Hydrotalcite Supergroup Mineral. Minerals. 2019; 9(8):492. https://doi.org/10.3390/min9080492
Chicago/Turabian StyleZhitova, Elena S., Igor V. Pekov, Ilya I. Chaikovskiy, Elena P. Chirkova, Vasiliy O. Yapaskurt, Yana V. Bychkova, Dmitry I. Belakovskiy, Nikita V. Chukanov, Natalia V. Zubkova, Sergey V. Krivovichev, and et al. 2019. "Dritsite, Li2Al4(OH)12Cl2·3H2O, a New Gibbsite-Based Hydrotalcite Supergroup Mineral" Minerals 9, no. 8: 492. https://doi.org/10.3390/min9080492
APA StyleZhitova, E. S., Pekov, I. V., Chaikovskiy, I. I., Chirkova, E. P., Yapaskurt, V. O., Bychkova, Y. V., Belakovskiy, D. I., Chukanov, N. V., Zubkova, N. V., Krivovichev, S. V., & Bocharov, V. N. (2019). Dritsite, Li2Al4(OH)12Cl2·3H2O, a New Gibbsite-Based Hydrotalcite Supergroup Mineral. Minerals, 9(8), 492. https://doi.org/10.3390/min9080492









