Geochemical Investigations of Fe-Si-Mn Oxyhydroxides Deposits in Wocan Hydrothermal Field on the Slow-Spreading Carlsberg Ridge, Indian Ocean: Constraints on Their Types and Origin
Abstract
1. Introduction
2. Sampling Stations and Study Area
3. Materials and Methods
4. Results
4.1. Bulk Sediment Mineralogy
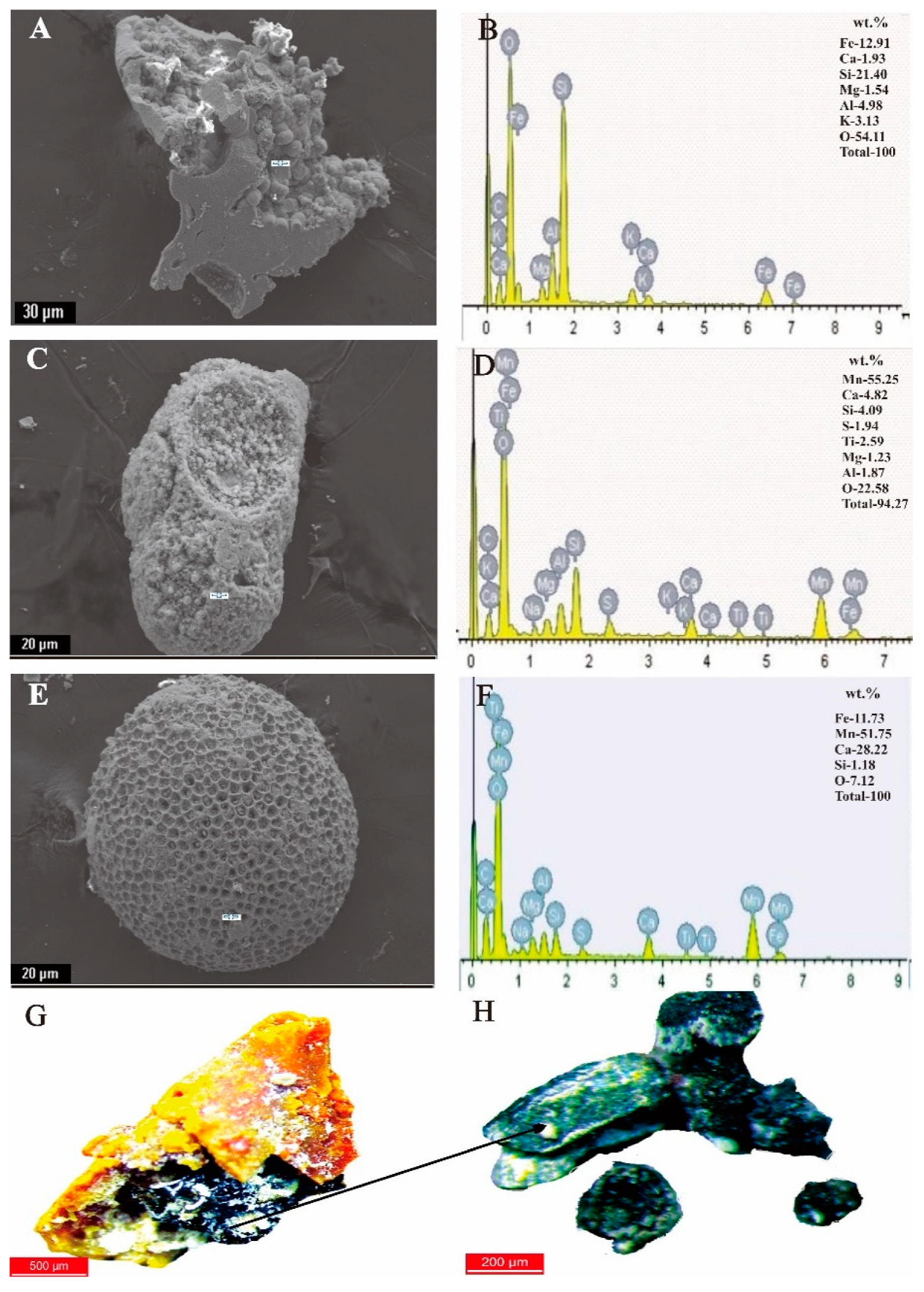
4.2. Morphology and Relative Abundance of Fe-Oxyhydroxide Separates
4.3. Mineral Chemistry of Fe-Oxyhydroxide Grains
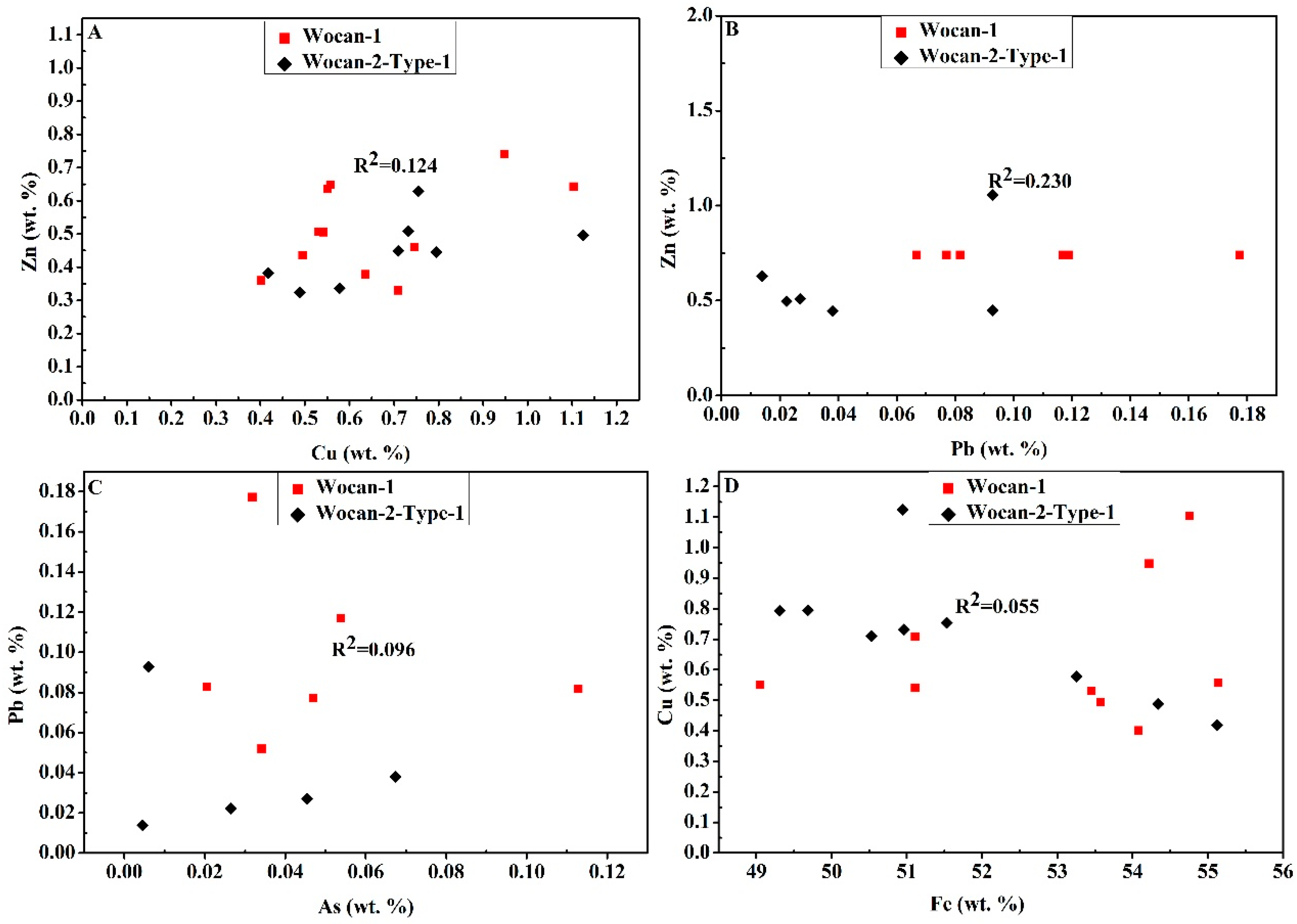
5. Discussion
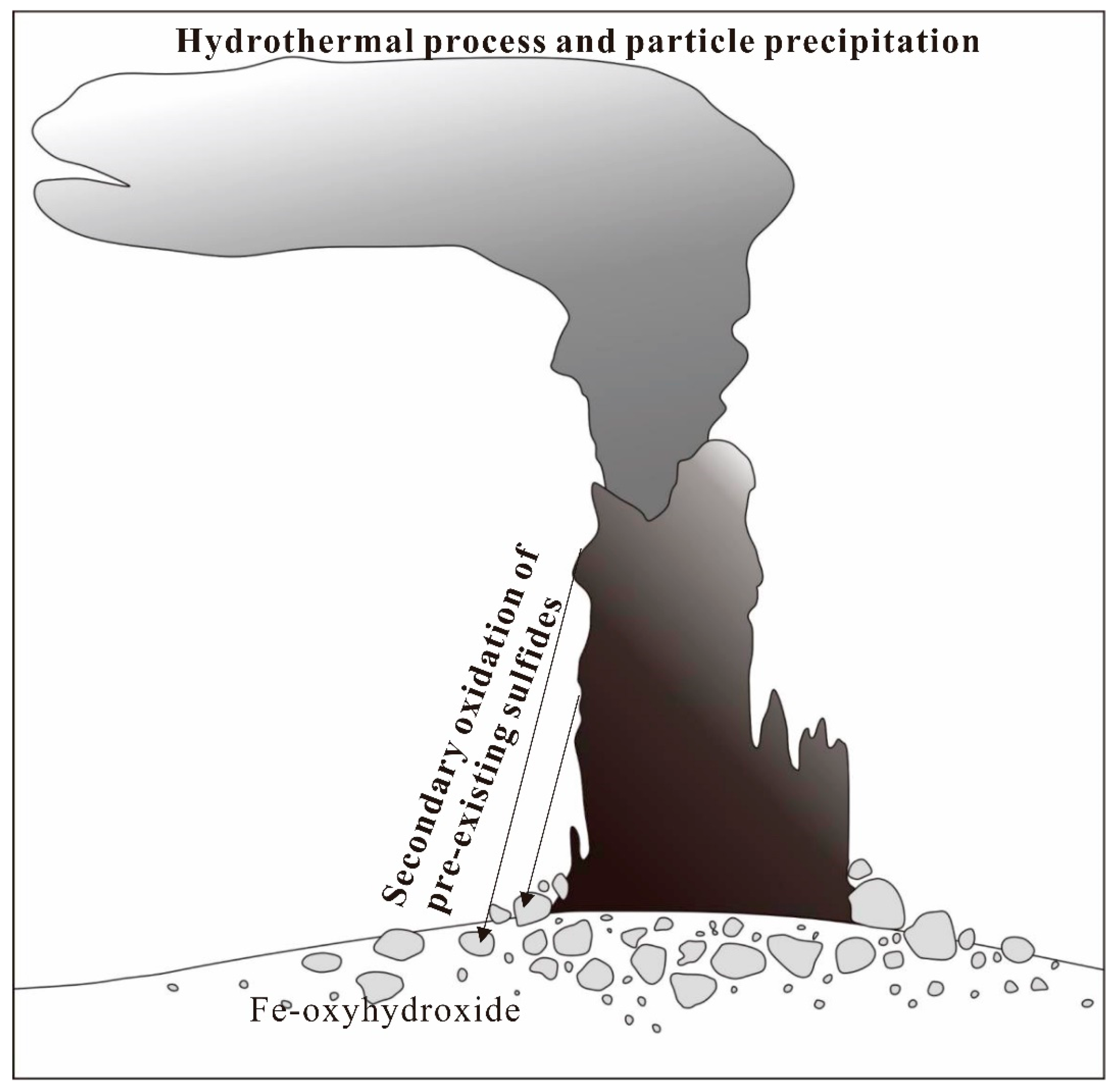
5.1. Origin and Formation Process of Wocan-1 Fe-Oxyhydroxides
5.2. Origin and Formation Process of Wocan-2 Fe-Oxyhydroxides
5.3. Origin and Formation Process of Oxyhydroxide Separates of the Ridge Flanks
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hekinian, R.; Hoffert, M.; Larqué, P.; Cheminée, J.L.; Stoffers, P.; Bideau, D. Hydrothermal Fe and Si oxyhydroxide deposits from South Pacific intraplate volcanoes and East Pacific Rise axial and off-axial regions. Econ. Geol. 1993, 88, 2099–2121. [Google Scholar] [CrossRef]
- Hrischeva, E.; Scott, S.D. Geochemistry and morphology of metalliferous sediments and oxyhydroxides from the Endeavour segment, Juan de Fuca Ridge. Geochim. Cosmochim. Acta 2007, 71, 3476–3497. [Google Scholar] [CrossRef]
- Dekov, V.M.; Petersen, S.; Garbe-Schönberg, C.D.; Kamenov, G.D.; Perner, M.; Kuzmann, E.; Schmidt, M. Fe-Si-oxyhydroxide deposits at a slow-spreading centre with thickened oceanic crust: The Lilliput hydrothermal field (9°33′S, Mid-Atlantic Ridge). Chem. Geol. 2010, 278, 186–200. [Google Scholar] [CrossRef]
- Fouquet, Y.; von Stackelberg, U.; Charlou, J.L.; Donval, J.L.; Erzinger, J.; Foucher, J.P.; Herzig, P.; Muhe, R.; Soakai, S.; Wiedicke, M. Metallogenesis in back-arc environments: The Lau Basin example. Econ. Geol. 1993, 88, 2154–2181. [Google Scholar] [CrossRef]
- Lizasa, K.; Kawasaki, K.; Maeda, K.; Matsumoto, T.; Saito, N.; Hirai, K. Hydrothermal sulfide-bearing Fe-Si oxyhydroxide deposits from the Coriolis Troughs, Vanuatu backarc, southwestern Pacific. Mar. Geol. 1998, 145, 1–21. [Google Scholar]
- Dekov, V.; Savelli, C. Hydrothermal activity in the SE Tyrrhenian Sea: An overview of 30 years of research. Mar. Geol. 2004, 204, 161–185. [Google Scholar] [CrossRef]
- Kato, S.; Kobayashi, C.; Kakegawa, T.; Yamagishi, A. Microbial communities in iron-silica-rich microbial mats at deep-sea hydrothermal fields of the Southern Mariana Trough. Environ. Microbiol. 2009, 11, 2094–2111. [Google Scholar] [CrossRef]
- Alt, J.C.; Lonsdale, P.; Haymon, R.; Muehlenbachs, K. Hydrothermal sulfide and oxide deposits on seamounts near 21°N, East Pacific Rise. Geol. Soc. Am. Bull. 1987, 98, 157–168. [Google Scholar] [CrossRef]
- Boyd, T.D.; Scott, S.D. Two-XRD-line ferrihydrite and Fe-Si-Mn oxyhydroxide mineralization from Franklin Seamount, western Woodlark Basin, Papua New Guinea. Can. Miner. 1999, 37, 973–990. [Google Scholar]
- Dekov, V.M.; Kamenov, G.D.; Savelli, C.; Stummeyer, J.; Thirye, M.; Shanks, W.C.; Willingham, A.L.; Boycheva, T.B.; Rochette, P.; Kuzmann, E.; et al. Metalliferous sediments from Eolo Seamount (Tyrrhenian Sea): Hydrothermal deposition and re-deposition in a zone of oxygen depletion. Chem. Geol. 2009, 264, 347–363. [Google Scholar] [CrossRef]
- Hein, J.R.; Schulz, M.S.; Dunham, R.E.; Stern, R.J.; Bloomer, S.H. Diffuse flow hydrothermal manganese mineralization along the active Mariana and southern Izu-Bonin arc system, western Pacific. J. Geophys. Res. 2008, 113, Bo8S14. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, H.; Yang, Q.; Sun, Z.; Bao, S.; Yao, H. Hydrothermal Fe-Si-Mn oxide deposits from the Central and South Valu Fa Ridge, Lau Basin. Appl. Geochem. 2011, 26, 1192–1204. [Google Scholar] [CrossRef]
- Mills, R.A.; Elderfield, H. Rare Earth Element geochemistry of hydrothermal deposits from the active TAG Mound 26°N Mid-Atlantic Ridge. Geochem. Cosmochim. Acta 1995, 59, 3511–3524. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, X.; Zhang, G.; Yin, X.; Chen, D.; Wang, X. Formation of Fe-oxyhydroxides from the East Pacific Rise near latitude 13°N: Evidence from mineralogical and geochemical data. Sci. China Ser. D Earth Sci. 2008, 51, 206–215. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, S.; Wang, X.; Ouyang, H.; Yin, X.; Li, Z. Mineralogical and micromorphological characteristics of Si-Fe-Mn oxyhydroxides from the PACMANUS hydrothermal field, Eastern Manus Basin. Sci. China Earth Sci. 2012, 55, 2039–2048. [Google Scholar] [CrossRef]
- Hekinian, R. Hydrothermal Activity and Metalliferous Deposits, Sea Floor Exploration: Scientific Adventures Diving into the Abyss; Springer International Publishing: Cham, Switzerland, 2014; pp. 145–164. [Google Scholar]
- Marchig, V.; Gundlach, H.; Moller, P.; Schley, F. Some geochemical indicators for discrimination between diagenetic and hydrothermal metalliferous sediments. Mar. Geol. 1982, 50, 241–256. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Huang, W.; Dong, H.; Little, C.T.S.; Li, J. Generation of hydrothermal Fe-Si oxyhydroxide deposit on the Southwest Indian Ridge and its implication for the origin of ancient banded iron formations. J. Geophys. Res. Biogeosci. 2015, 120, 187–203. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Petersen, S.; Frische, M.; Qiu, Z.; Li, H.; Li, H.; Wu, Z.; Cui, R. Mineralogy and trace element geochemistry of sulfide minerals from the Wocan Hydrothermal Field on the slow-spreading Carlsberg Ridge, Indian Ocean. Ore Geol. Rev. 2017, 84, 1–19. [Google Scholar] [CrossRef]
- Murton, B.J.; Rona, P.A. Carlsberg ridge and mid-Atlantic ridge: Comparison of slow spreading centre analogues. Deep Sea Res. 2015, 121, 71–84. [Google Scholar] [CrossRef]
- Dias, Á.S.; Mills, R.A.; Taylor, R.N.; Ferreira, P.; Barriga, F.J.A.S. Geochemistry of a sediment push-core from the Lucky Strike hydrothermal field, Mid-Atlantic Ridge. Chem Geol. 2008, 247, 339–351. [Google Scholar] [CrossRef]
- Mohapatra, B.K.; Jena, S.; Singh, P.P. Microstructure-compositional variation in iron oxy-hydroxide minerals formed with manganese mineralization, Eastern Ghats Supergroup, Orissa. J. Geol. Soc. India 2011, 77, 450–458. [Google Scholar] [CrossRef]
- Mange, M.A.; Maurer, H.F.W. Methods, Heavy Minerals in Colour; Springer: Dordrecht, The Netherlands, 1992; pp. 11–25. [Google Scholar]
- Ramdohr, P. Goethite from Hind low, Derbyshire. Bull. Geol. Surv. G. B. 1980, 52, 51–54. Available online: http://handbookofmineralogy.org/pdfs/manganosite (accessed on 28 November 2018).
- Criddle, A.J.; Stanley, C.J. (Eds.) Quantitative Data File for Ore Minerals, 3rd ed.; Chapman & Hall: London, UK, 1955; Volume 351, Available online: http://handbookofmineralogy.org/pdfs/Goethite (accessed on 28 November 2018).
- Popoola, S.O.; Han, X.; Wang, Y.; Qiu, Z.; Cai, Y. Mineralogical and geochemical signature of metalliferous sediments in Wocan-1 and Wocan-2 hydrothermal sites, on the Carlsberg Ridge, Indian Ocean. Mineral 2018. submitted. [Google Scholar]
- Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Slattery, A.; Verdugo-Ihl, M.R.; Courtney-Davies, L.; Gao, W. Advances and Opportunities in Ore Mineralogy. Minerals 2017, 7, 233. [Google Scholar] [CrossRef]
- Reich, M.; Large, R.; Deditius, A.P. New advances in trace element geochemistry of ore minerals and accessory phases. Ore Geol. Rev. 2017, 81, 1215–1217. [Google Scholar] [CrossRef]
- Batanova, V.G.; Sobolev, A.V.; Magnin, V. Trace element analysis by EPMA in geosciences: Detection limit, precision and accuracy. IOP Conf. Ser. Mater. Sci. Eng. 2018, 304, 012001. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, H.; Glasby, G.P.; Sun, Z.; Yang, Q.; Yin, X.; Li, J. Mineralogical characterization and formation of Fe-Si oxyhydroxide deposits from modern seafloor hydrothermal vents. Am. Mineral. 2013, 85–97. [Google Scholar] [CrossRef]
- Benites, M.; Millo, C.; Hein, J.; Nath, B.N.; Murton, B.; Galante, D.; Jovane, L. Integrated Geochemical and Morphological Data Provide Insights into the Genesis of Ferromanganese Nodules. Minerals 2018, 8, 488. [Google Scholar] [CrossRef]
- Yeo, I.A.; Dobson, K.; Josso, P.; Pearce, R.B.; Howarth, S.A.; Lusty, P.A.J.; Le Bas, T.P.; Murton, B.J. Assessment of the Mineral Resource Potential of Atlantic Ferromanganese Crusts Based on Their Growth History, Microstructure, and Texture. Minerals 2018, 8, 327. [Google Scholar] [CrossRef]
- Marino, E.; González, F.J.; Lunar, R.; Reyes, J.; Medialdea, T.; Castillo-Carrión, M.; Bellido, E.; Somoza, L. High-Resolution Analysis of Critical Minerals and Elements in Fe-Mn Crusts from the Canary Island Seamount Province (Atlantic Ocean). Minerals 2018, 8, 285. [Google Scholar] [CrossRef]
- Halbach, P. Processes controlling the heavy metal distribution in Pacific ferromanganese nodules and crusts. Geologische Rundschau 1986, 75, 235–247. [Google Scholar] [CrossRef]
- Varentsov, I.M.; Drits, V.A.; Gorshkov, A.I.; Sivtsov, A.V.; Sakharov, B.A. Mn-Fe oxyhydroxide crusts from Krylov Seamount (Eastern Atlantic): Mineralogy, geochemistry and genesis. Mar. Geol. 1991, 96, 53–70. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Z.; Qi, H.; Chen, S.; Yin, X.; Yang, B. Fe-Si-Mn-oxyhydroxide encrustations on basalts at East Pacific Rise near 13°N: An SEM-EDS study. J. Ocean Univ. China 2014, 13, 917–925. [Google Scholar] [CrossRef]
- Boström, K. The origin and fate of ferromanganoan active ridge sediments. Stock. Contrib. Geol. 1973, 27, 149–243. [Google Scholar]
- Bonatti, E. Metal deposits in the oceanic lithosphere. In The Sea; Emiliani, C., Ed.; John Willey and Sons: New York, NY, USA, 1981; Volume 7, pp. 639–686. [Google Scholar]
- Dias, Á.S.; Barriga, F.J.A.S. Mineralogy and geochemistry of hydrothermal sediments from the serpentinite-hosted Saldanha hydrothermal field (36°34′N; 33°26′W) at MAR. Mar. Geol. 2006, 225, 157–175. [Google Scholar] [CrossRef]
- Münch, U.; Blum, N.; Halbach, P. Mineralogical and geochemical features of sulfide chimneys from the MESO zone, Central Indian Ridge. Chem. Geol. 1999, 155, 29–44. [Google Scholar] [CrossRef]
- Moorby, S.A.; Cronan, D.S.; Glasby, G.P. Geochemistry of hydrothermal Mn oxide deposits from the SW Pacific island arc. Geochim. Cosmochim. Acta 1984, 48, 433–441. [Google Scholar] [CrossRef]
- Kennedy, C.B.; Martinez, R.E.; Scott, S.D.; Ferris, F.G. Surface chemistry and reactivity of bacteriogenic iron oxides from Axial Volcano, Juan de Fuca Ridge, north-east Pacific Ocean. Geobiology 2003, 1, 59–69. [Google Scholar] [CrossRef]
- Tsikos, H.; Matthews, A.; Erel, Y.; Moore, J.M. Iron isotopes constrain biogeochemical redox cycling of iron and manganese in a Palaeoproterozoic stratified basin. Earth Planet. Sci. Lett. 2010, 298, 125–134. [Google Scholar] [CrossRef]
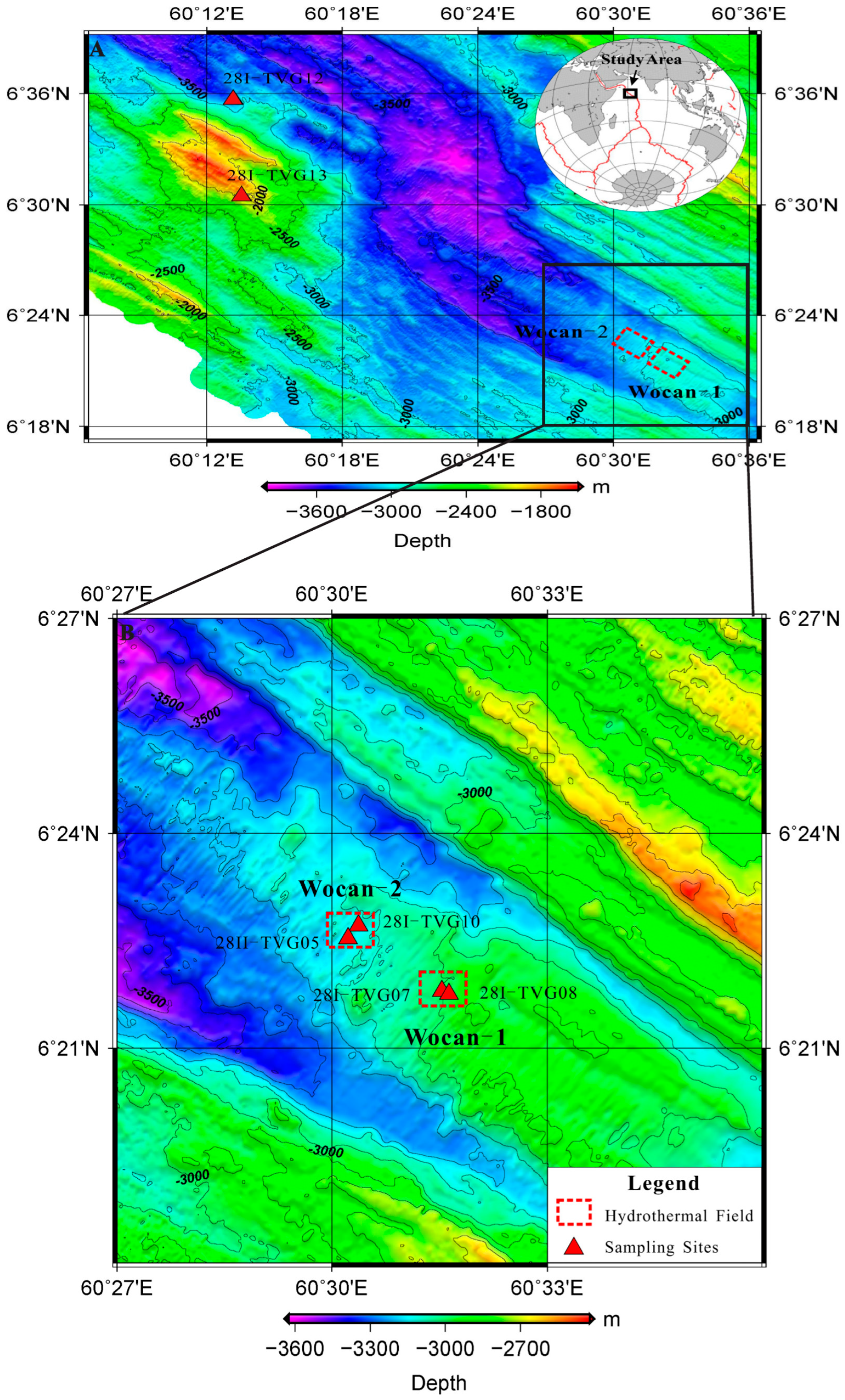
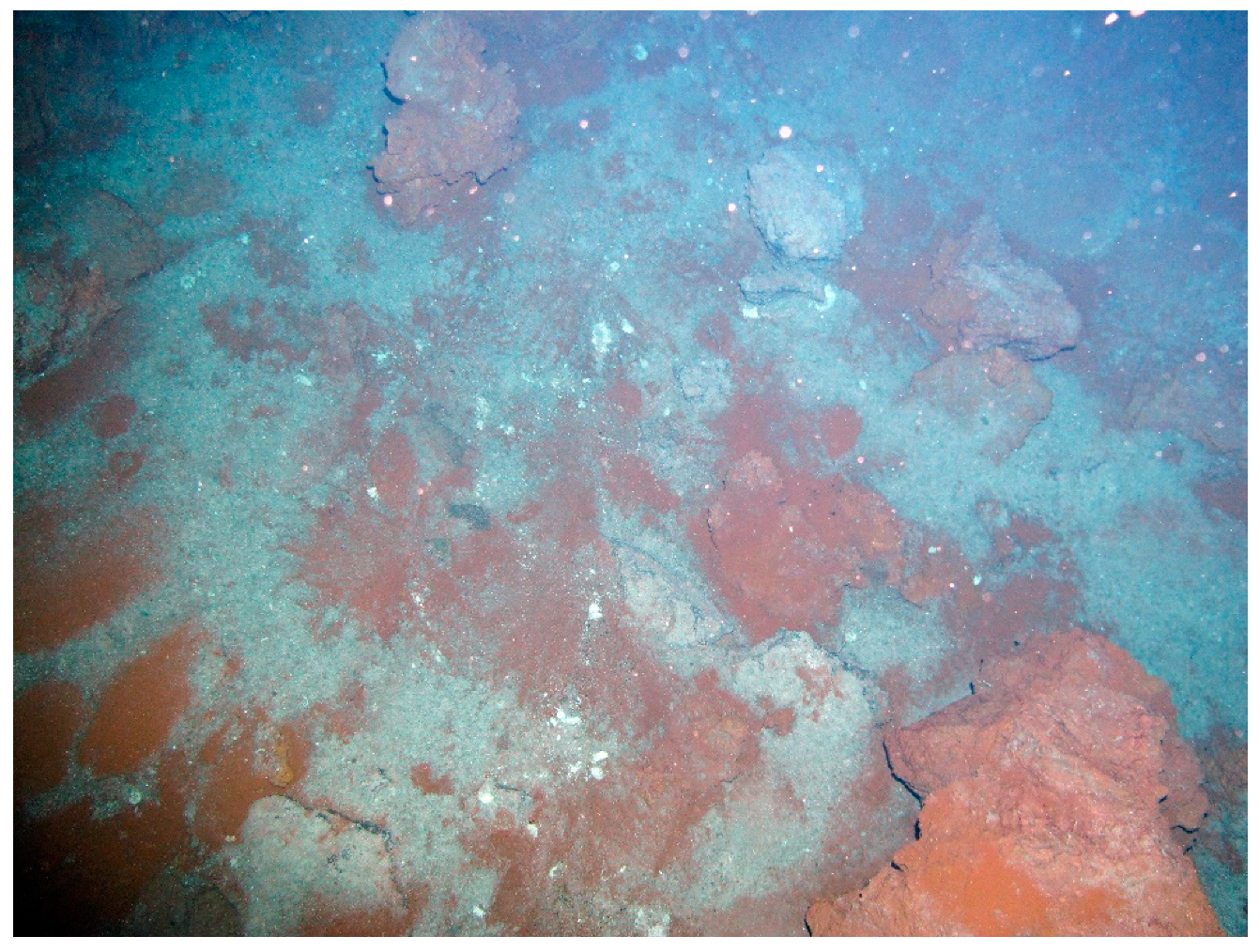
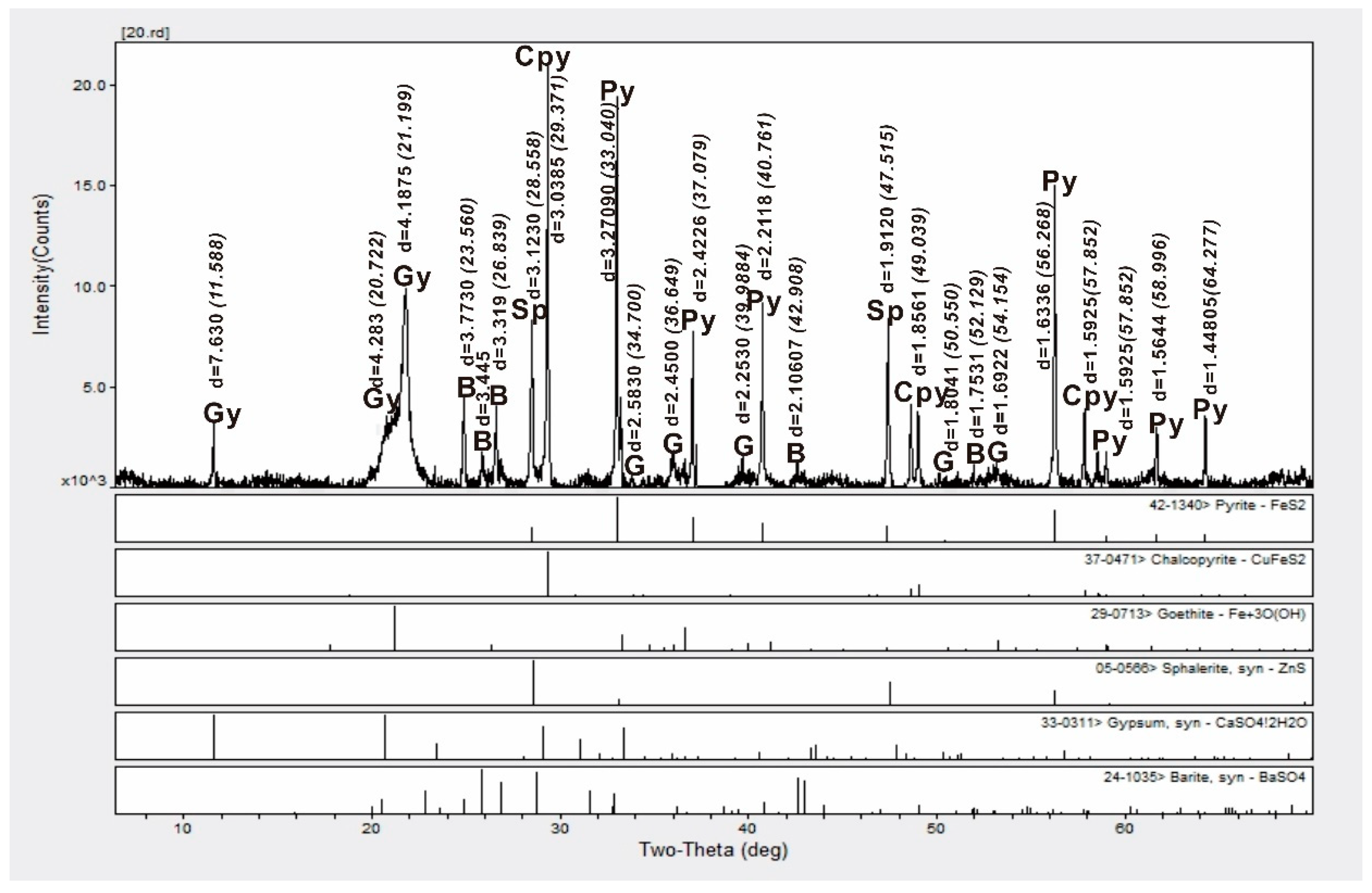
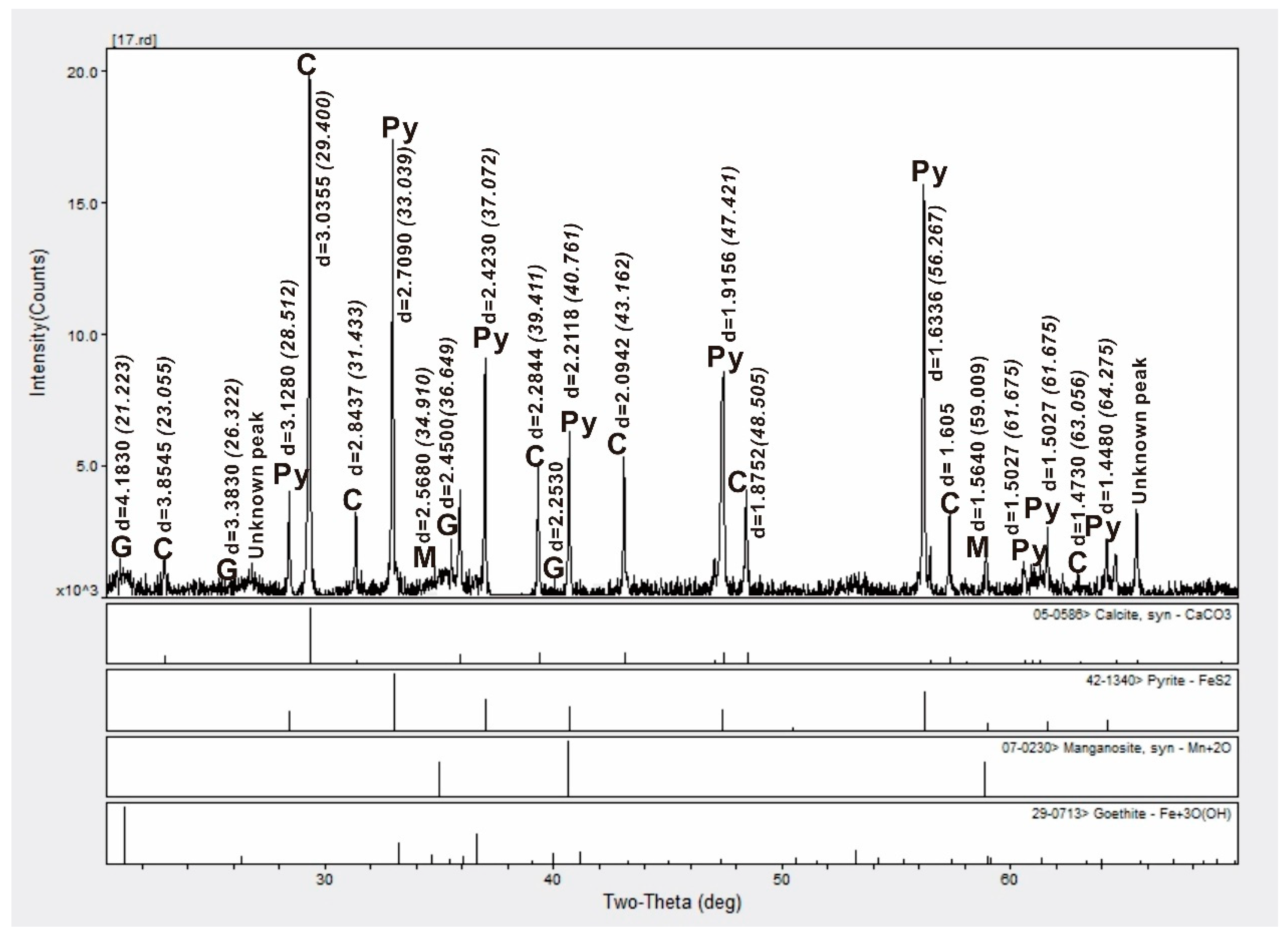

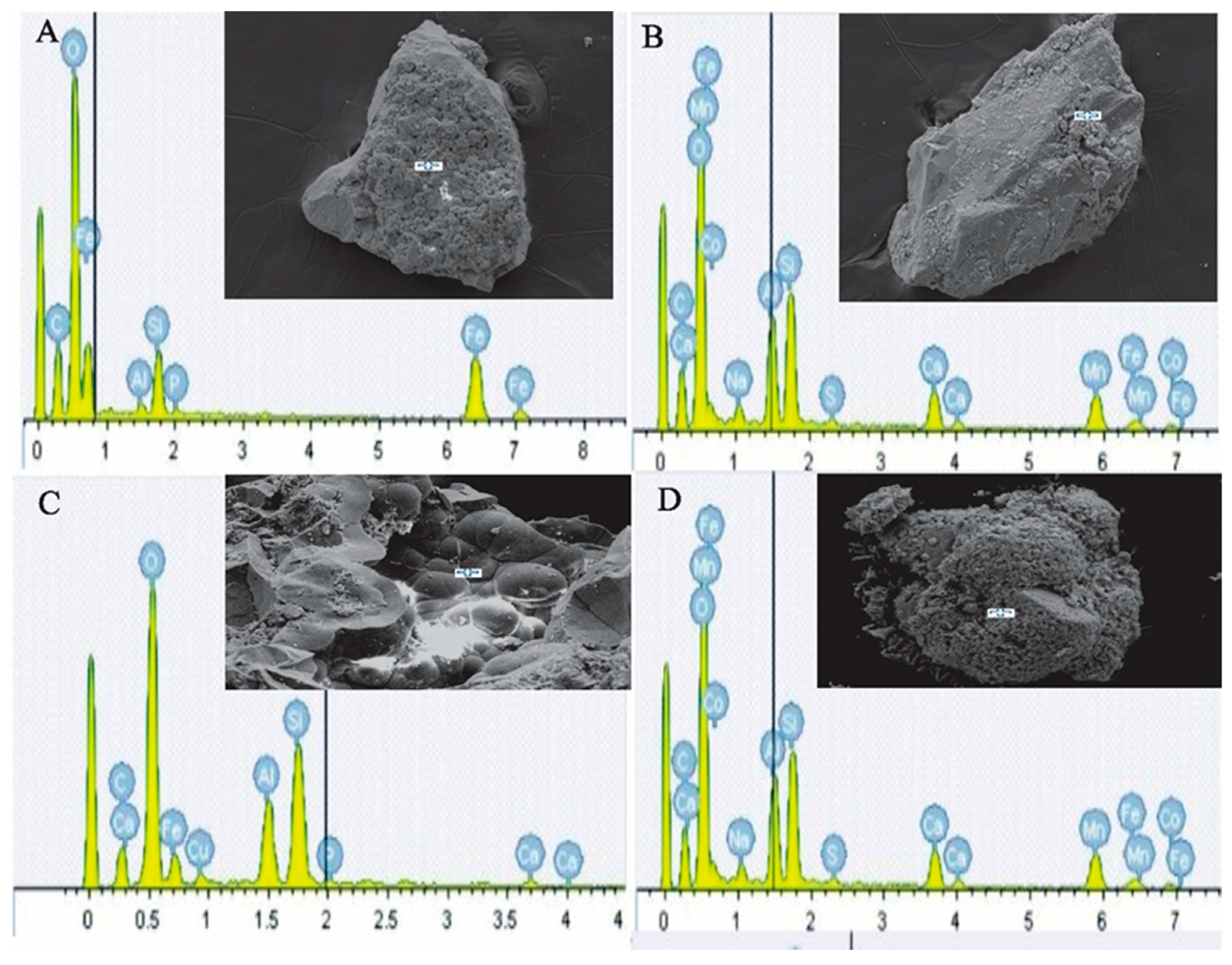

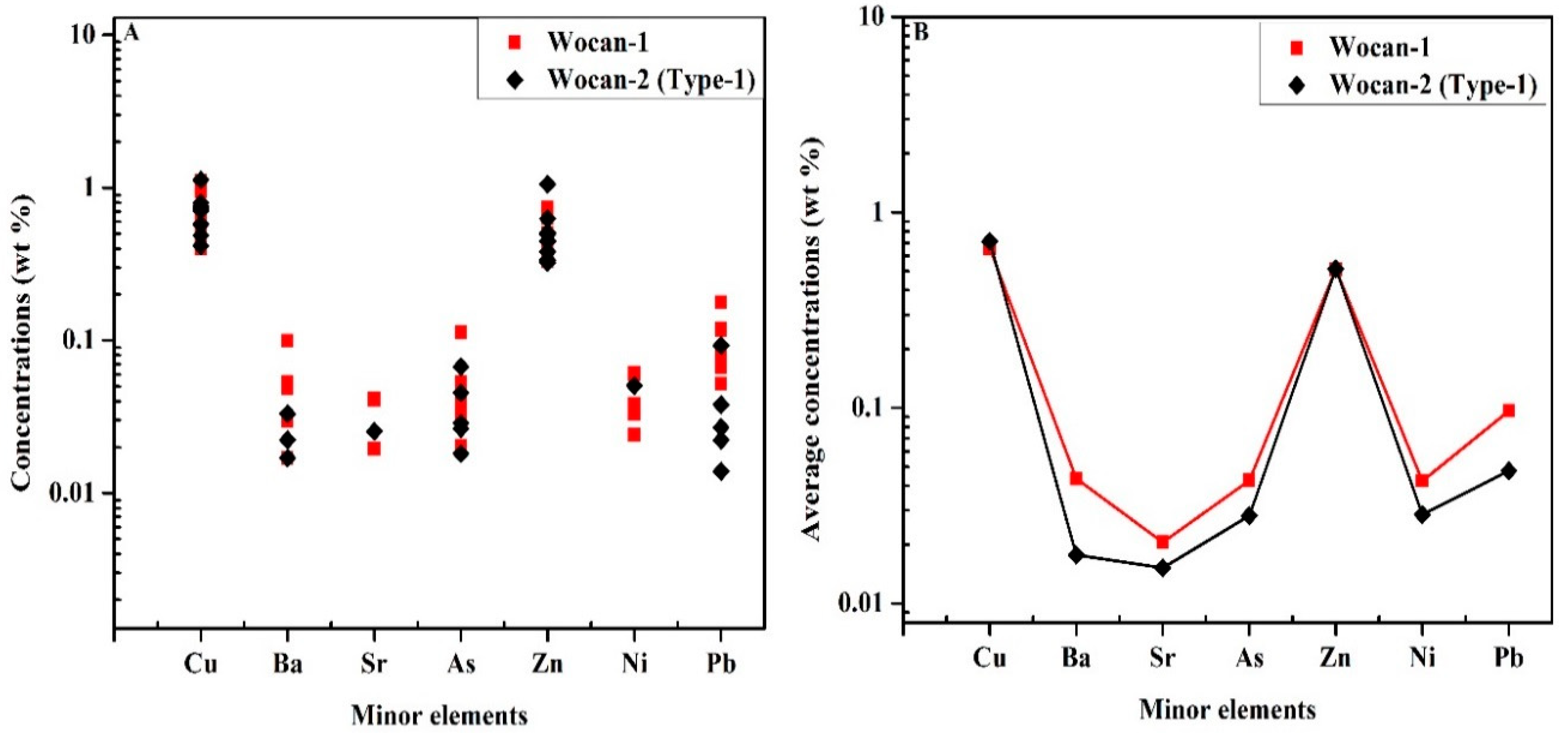
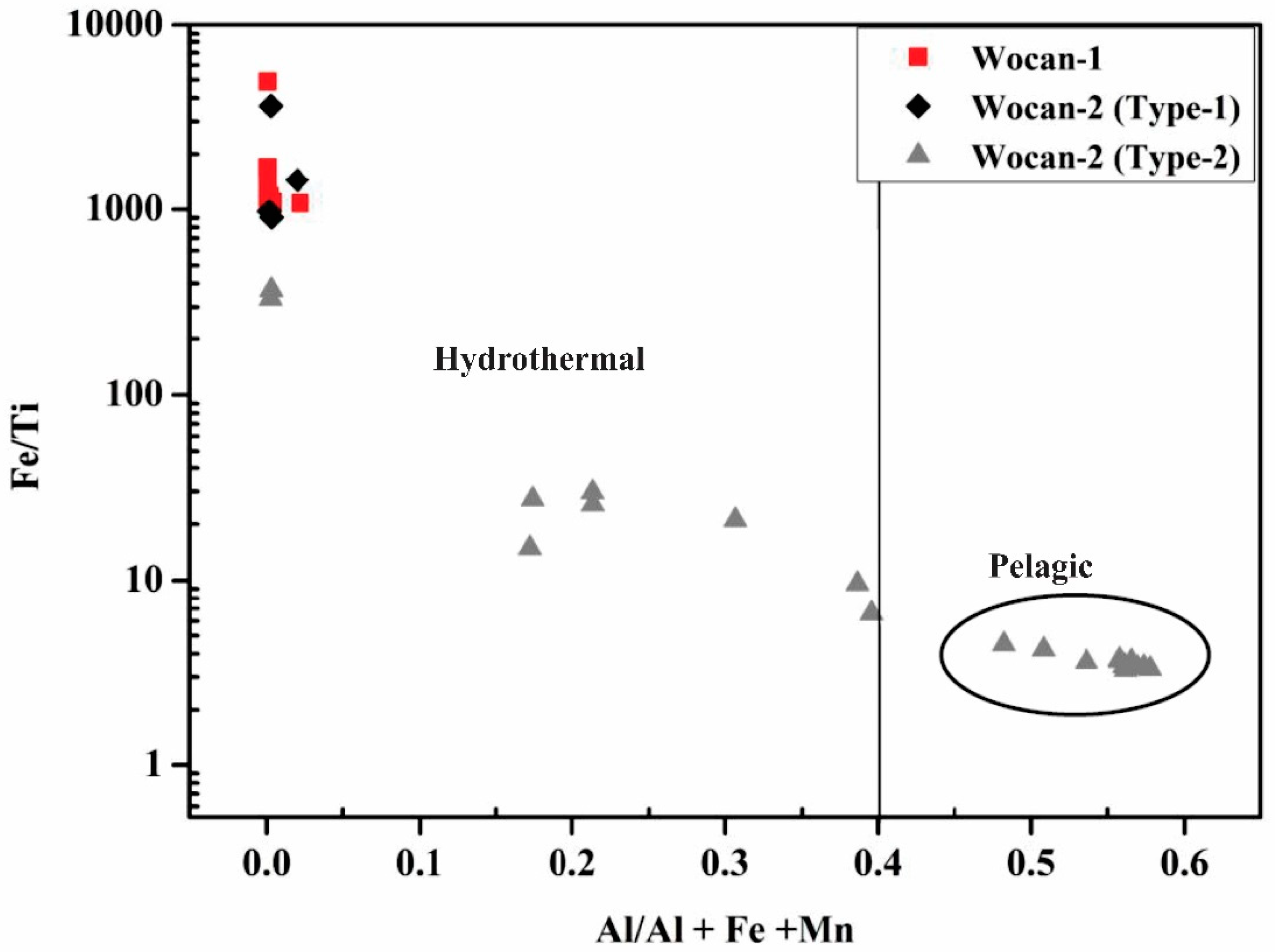
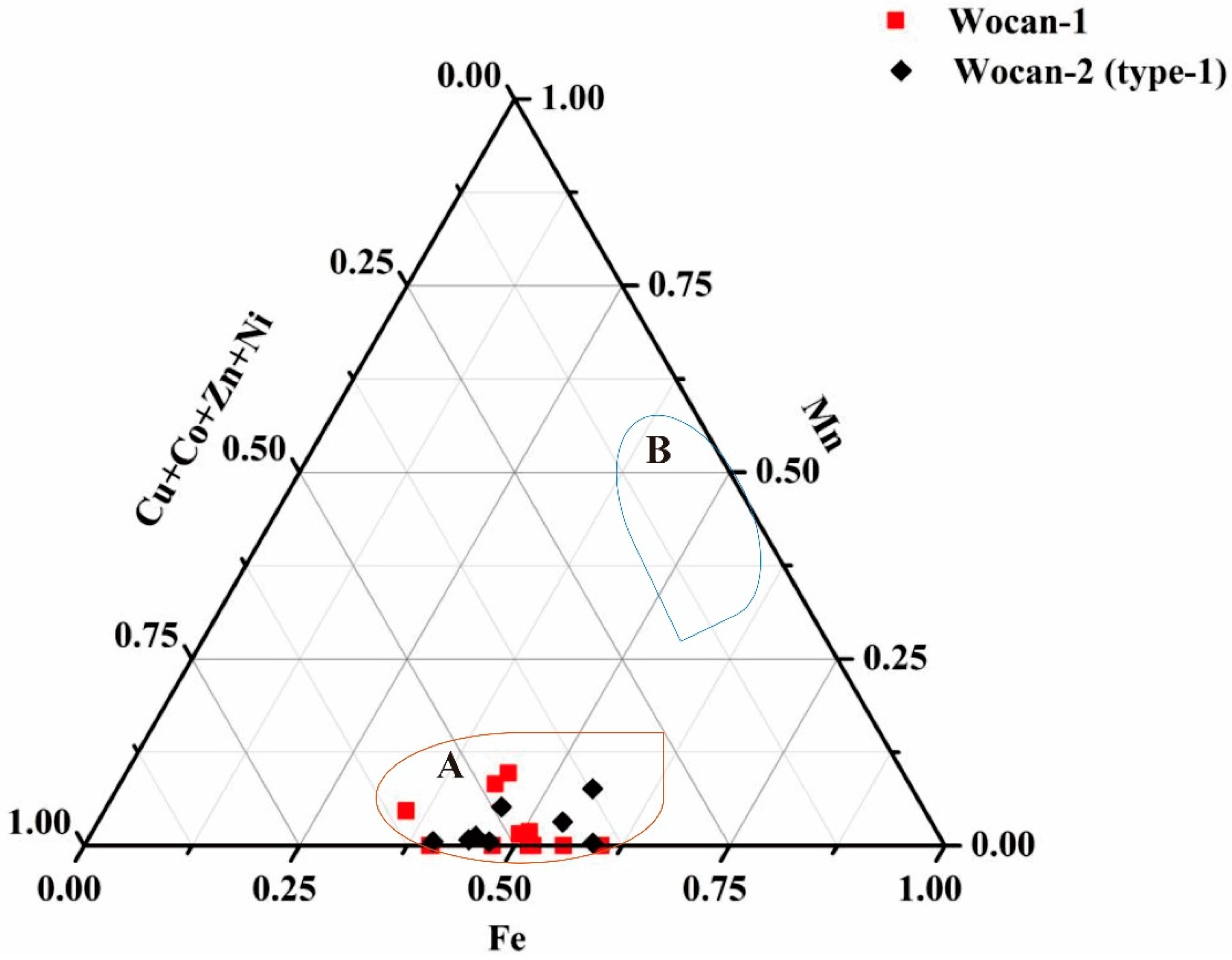
| Site | Station | Longitude (E) | Latitude (N) | Depth (m) |
|---|---|---|---|---|
| Wocan-1 | 28I-TVG07 | 60°31.534′ | 6°21.796′ | 2989 |
| 28I-TVG08 | 60°31.635′ | 6°21.756′ | 2973 | |
| Wocan-2 | 28I-TVG10 | 60°30.372′ | 6°21.866′ | 3104 |
| 28II-TVG05 | 60°30.226′ | 6°22.534′ | 3105 | |
| Ridge Flank | 28I-TVG13 | 60°13.190′ | 6°35.675′ | 3254 |
| 28I-TVG12 | 60°13.550′ | 6°30.462′ | 2009 |
| Grain Counts | 28I-TVG07 | 28I-TVG08 | 28I-TVG10 | 28II-TVG05 | 28I-TVG13 | 28I-TVG12 |
|---|---|---|---|---|---|---|
| Fe-oxyhydroxide grains | 132 | 139 | 176 | 119 | 29 | 13 |
| Non-Fe-oxyhydroxide grains | 122 | 118 | 87 | 132 | 219 | 233 |
| Total grain counts | 254 | 257 | 263 | 251 | 248 | 246 |
| % Fe-oxyhydroxide | 51.97 | 54.09 | 66.92 | 47.41 | 11.69 | 5.28 |
| Element | SiO2 | SO3 | FeO | MnO | TiO2 | Al2O3 | Al/(Al + Fe + Mn) | Fe/Mn | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 6.85 | 0.04 | 63.10 | Bdl | 0.02 | 0.04 | 0.0004 | 659.19 | |||
| Max | 16.31 | 0.26 | 70.93 | 0.11 | 0.08 | 2.30 | 0.02 | 4,642.62 | |||
| Av | 11.82 | 0.10 | 67.47 | 0.06 | 0.06 | 0.31 | 0.003 | 2,486.05 | |||
| STD-EV | ±3.06 | ±0.08 | ±2.76 | ±0.05 | ±0.02 | ±0.67 | ±0.01 | ±66.06 | |||
| n = 11 | CuO | CoO | NiO | BaO | SrO | As2O5 | ZnO | PbO | Total | H2O+ (calc) | Cu + Co + Zn + Ni |
| Min | 0.50 | Bdl | 0.03 | 0.02 | Bdl | Bdl | 0.41 | 0.06 | 71.11 | 28.89 | 0.79 |
| Max | 1.38 | Bdl | 0.08 | 0.11 | 0.05 | 0.15 | 0.92 | 0.19 | 93.35 | 6.65 | 1.78 |
| Av | 0.82 | Bdl | 0.05 | 0.05 | 0.02 | 0.06 | 0.64 | 0.10 | 81.72 | 18.28 | 1.19 |
| STDEV | ±0.26 | - | ±0.02 | ±0.04 | ±0.02 | ±0.04 | ±0.17 | ±0.04 | ±7.37 | - | ±0.31 |
| Element | SiO2 | SO3 | FeO | MnO | TiO2 | Al2O3 | Al/(Al + Fe + Mn) | Fe/Mn | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 8.40 | 0.08 | 63.44 | Bdl | 0.03 | Bdl | 0.0002 | 1062 | |||
| Max | 15.19 | 0.65 | 70.91 | 0.07 | 0.09 | 1.96 | 0.02 | 35,080 | |||
| Av | 12.63 | 0.24 | 66.57 | 0.03 | 0.07 | 0.35 | 0.0036 | 9,557 | |||
| STD-EV | ±2.07 | ±0.19 | ±2.63 | ±0.03 | ±0.03 | ±0.61 | ±0.006 | ±25.59 | |||
| n = 9 | CuO | CoO | NiO | BaO | SrO | As2O5 | ZnO | PbO | Total | H2O+ (calc) | Cu + Co + Zn + Ni |
| Min | 0.52 | Bdl | Bdl | Bdl | Bdl | Bdl | 0.40 | 0.02 | 77.14 | 22.86 | 0.80 |
| Max | 1.41 | Bdl | 0.06 | 0.04 | 0.03 | 0.09 | 1.31 | 0.10 | 84.64 | 15.36 | 1.85 |
| Av | 0.89 | Bdl | 0.04 | 0.02 | 0.02 | 0.04 | 0.64 | 0.05 | 81.54 | 18.46 | 1.23 |
| STD-EV | ±0.26 | ±0.04 | ±0.01 | ±0.01 | ±0.03 | ±0.28 | ±0.04 | ±2.59 | - | ±0.36 |
| Element | SiO2 | SO3 | FeO | MnO | TiO2 | Al2O3 | Al/(Al + Fe + Mn) | Fe/Mn | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 34.78 | Bdl | 8.60 | 0.07 | 0.09 | 0.07 | 0.003 | 25.81 | |||
| Max | 49.96 | Bdl | 26.61 | 0.35 | 5.46 | 19.08 | 0.577 | 37.66 | |||
| Av | 46.97 | - | 14.28 | 0.18 | 3.09 | 11.36 | 0.404 | 90.38 | |||
| STD-EV | ±4.24 | - | ±4.30 | ±0.07 | ±2.13 | ±7.32 | ±0.20 | ±13.94 | |||
| n = 21 | CuO | CoO | NiO | BaO | SrO | AS2O5 | ZnO | PbO | Total | H2O+ (calc) | Cu + Co + Zn + Ni |
| Min | Bdl | Bdl | Bdl | Bdl | Bdl | Bdl | Bdl | Bdl | 61.55 | 38.45 | Bdl |
| Max | Bdl | Bdl | Bdl | Bdl | Bdl | Bdl | Bdl | Bdl | 87.91 | 12.09 | Bdl |
| Av | - | - | - | - | - | - | - | - | 75.87 | 24.13 | - |
| STD-EV | - | - | - | - | - | - | - | - | ±10.49 | - | - |
| Element | SiO2 | SO3 | FeO | MnO | TiO2 | Al2O3 | Fe/Mn | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | 81.09 | Bdl | 0.02 | 4.04 | 0.03 | 0.03 | 0.004 | |||
| Max | 93.79 | Bdl | 0.09 | 5.11 | 0.08 | 0.58 | 0.022 | |||
| Av | 89.08 | - | 0.05 | 4.75 | 0.05 | 0.43 | 0.011 | |||
| STD-EV | ±6.42 | - | ±0.04 | ±0.54 | 0.03 | ±0.28 | ±0.01 | |||
| n = 3 | CuO | CoO | NiO | BaO | SrO | AS2O5 | ZnO | PbO | Total | Cu + Co + Zn + Ni |
| Min | Bdl | Bdl | Bdl | Bdl | Bdl | Bdl | Bdl | Bdl | 86.83 | Bdl |
| Max | Bdl | Bdl | Bdl | 0.05 | Bdl | Bdl | Bdl | Bdl | 98.03 | Bdl |
| Av | - | - | - | - | - | - | - | - | 94.28 | - |
| STD-EV | - | - | - | - | - | - | - | - | ±5.70 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olatunde Popoola, S.; Han, X.; Wang, Y.; Qiu, Z.; Ye, Y. Geochemical Investigations of Fe-Si-Mn Oxyhydroxides Deposits in Wocan Hydrothermal Field on the Slow-Spreading Carlsberg Ridge, Indian Ocean: Constraints on Their Types and Origin. Minerals 2019, 9, 19. https://doi.org/10.3390/min9010019
Olatunde Popoola S, Han X, Wang Y, Qiu Z, Ye Y. Geochemical Investigations of Fe-Si-Mn Oxyhydroxides Deposits in Wocan Hydrothermal Field on the Slow-Spreading Carlsberg Ridge, Indian Ocean: Constraints on Their Types and Origin. Minerals. 2019; 9(1):19. https://doi.org/10.3390/min9010019
Chicago/Turabian StyleOlatunde Popoola, Samuel, Xiqiu Han, Yejian Wang, Zhongyan Qiu, and Ying Ye. 2019. "Geochemical Investigations of Fe-Si-Mn Oxyhydroxides Deposits in Wocan Hydrothermal Field on the Slow-Spreading Carlsberg Ridge, Indian Ocean: Constraints on Their Types and Origin" Minerals 9, no. 1: 19. https://doi.org/10.3390/min9010019
APA StyleOlatunde Popoola, S., Han, X., Wang, Y., Qiu, Z., & Ye, Y. (2019). Geochemical Investigations of Fe-Si-Mn Oxyhydroxides Deposits in Wocan Hydrothermal Field on the Slow-Spreading Carlsberg Ridge, Indian Ocean: Constraints on Their Types and Origin. Minerals, 9(1), 19. https://doi.org/10.3390/min9010019






