Infra Red Spectroscopy of the Regulated Asbestos Amphiboles
Abstract
1. Introduction
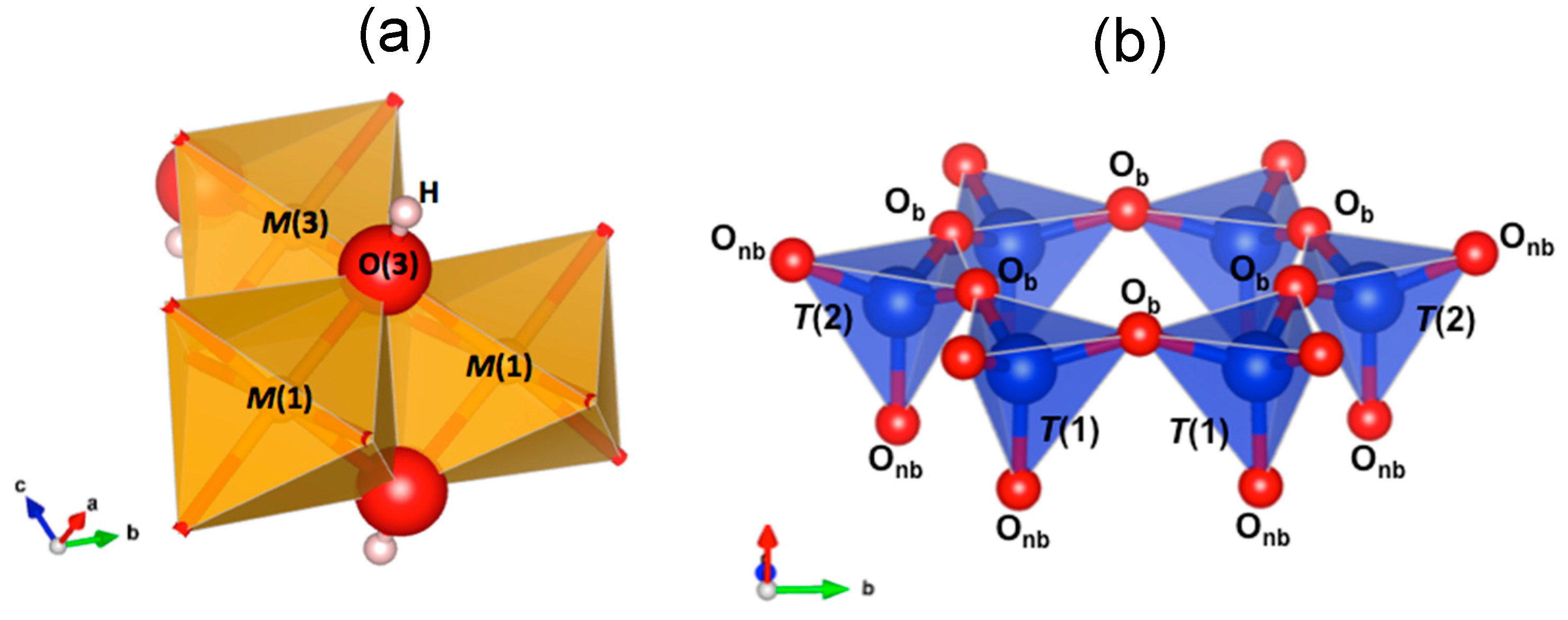
2. Vibrational Spectroscopy Applied to the Crystal Chemistry of Fibrous Amphiboles
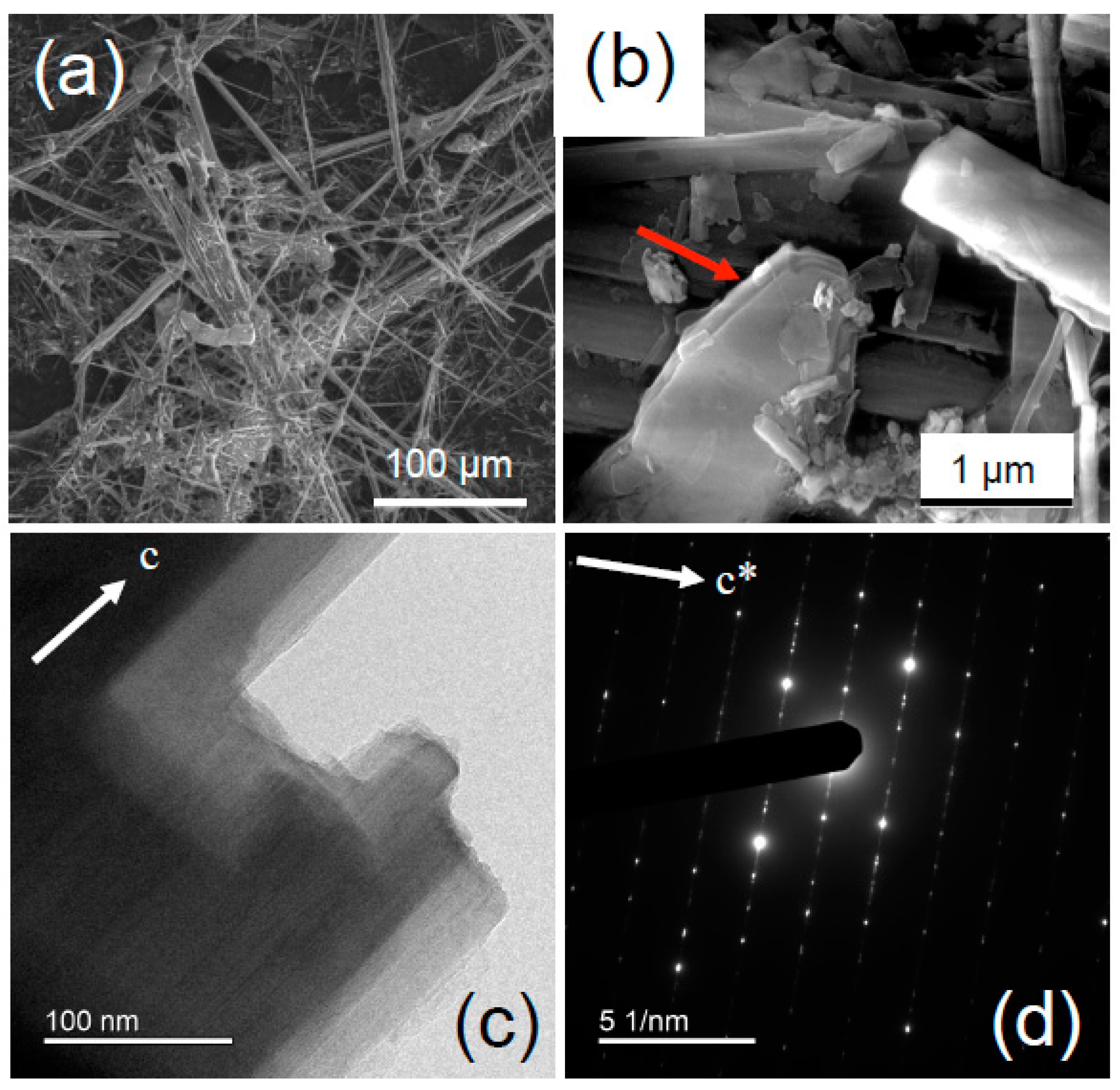
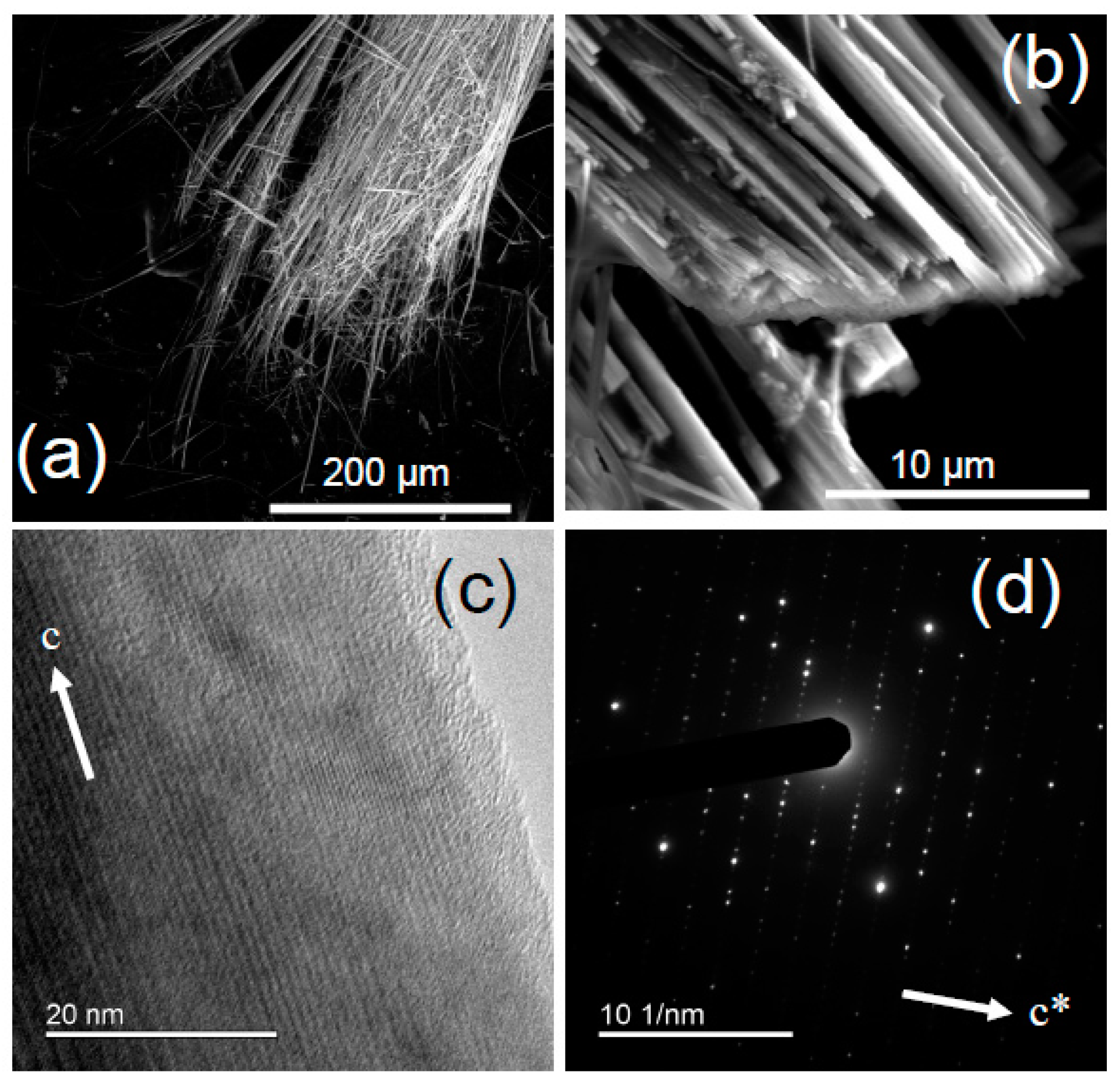
3. Materials and Methods
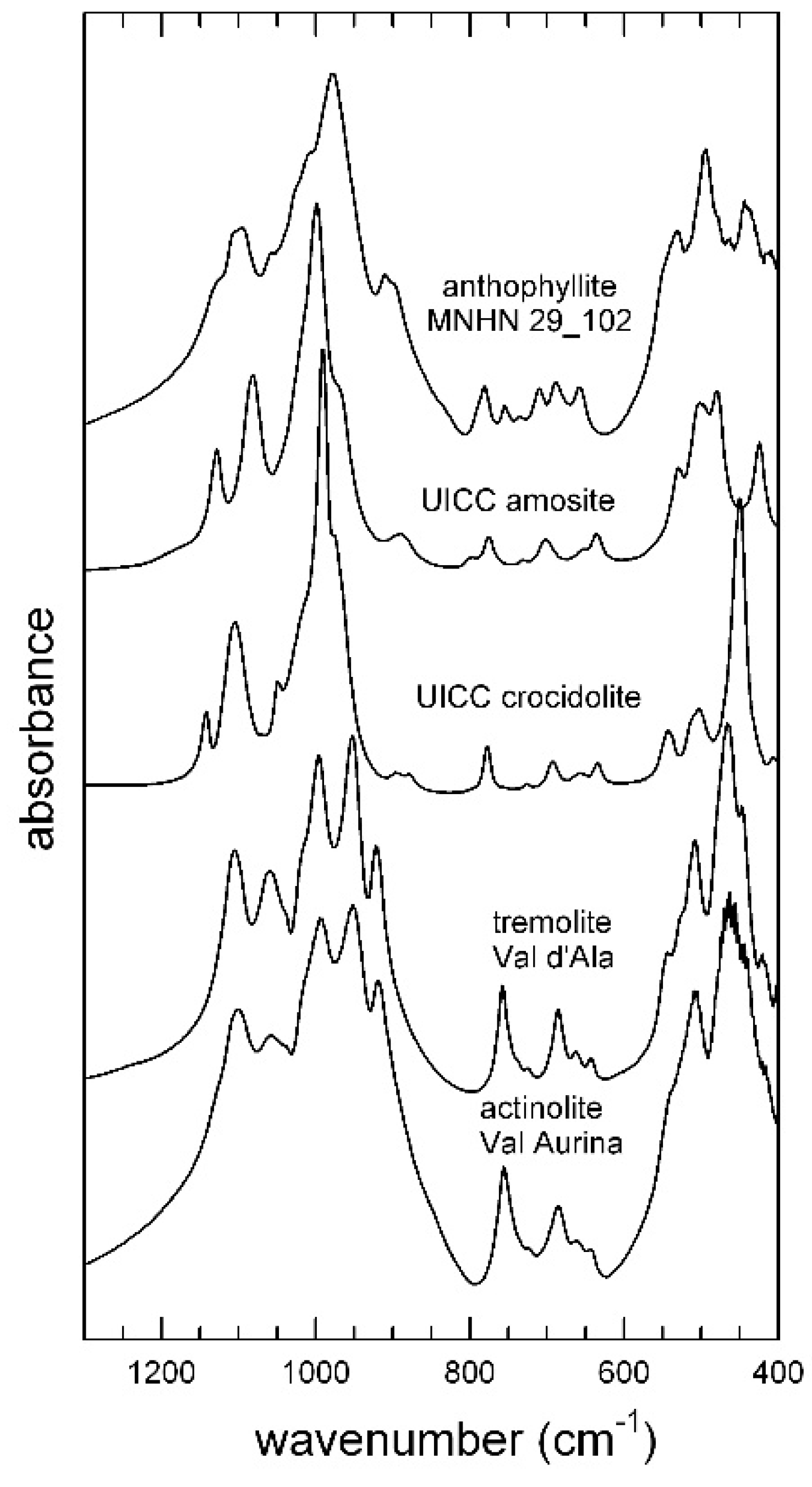
4. Results and Discussion
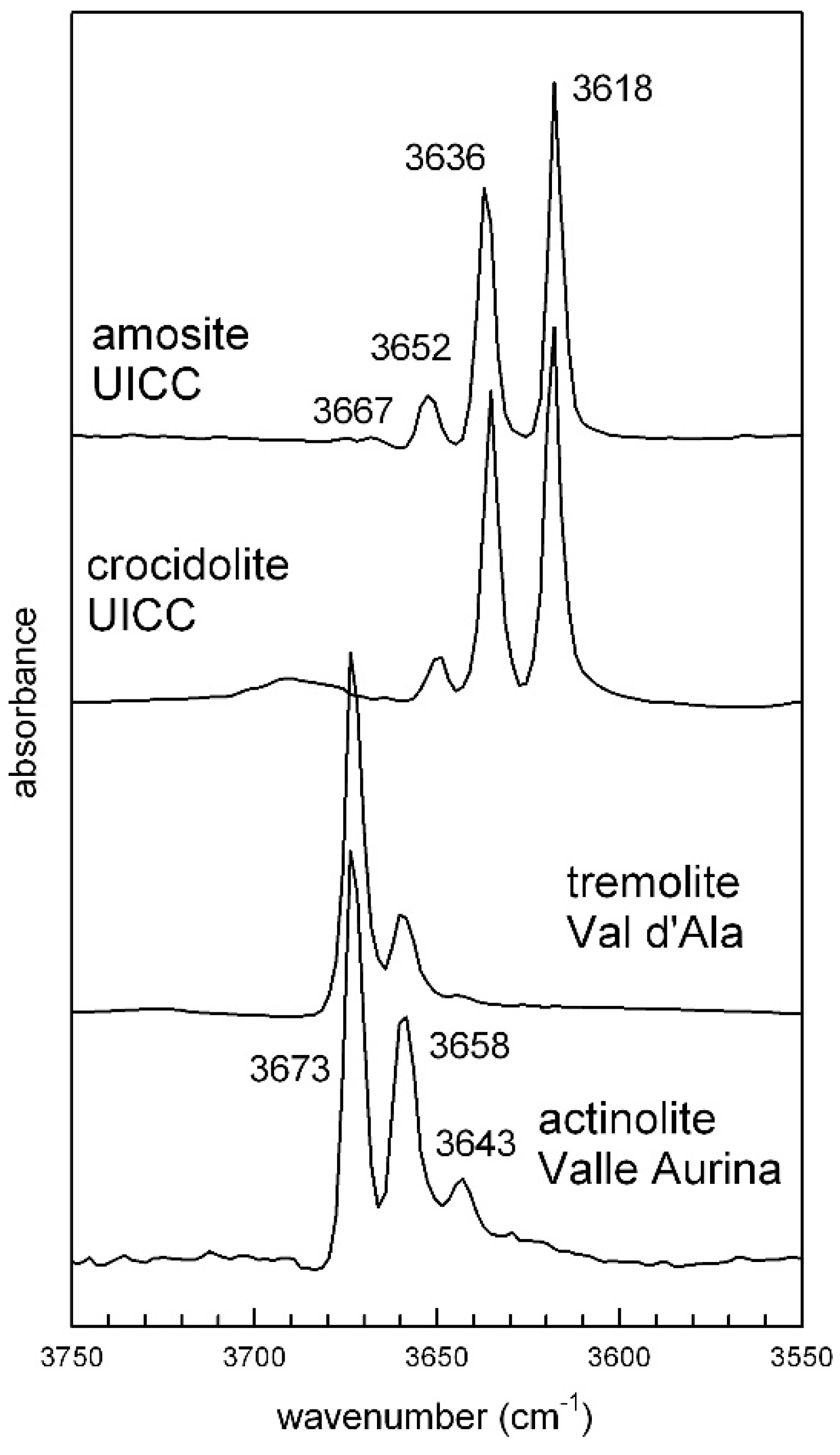
4.1. OH-Stretching Spectra and Crystal Chemistry
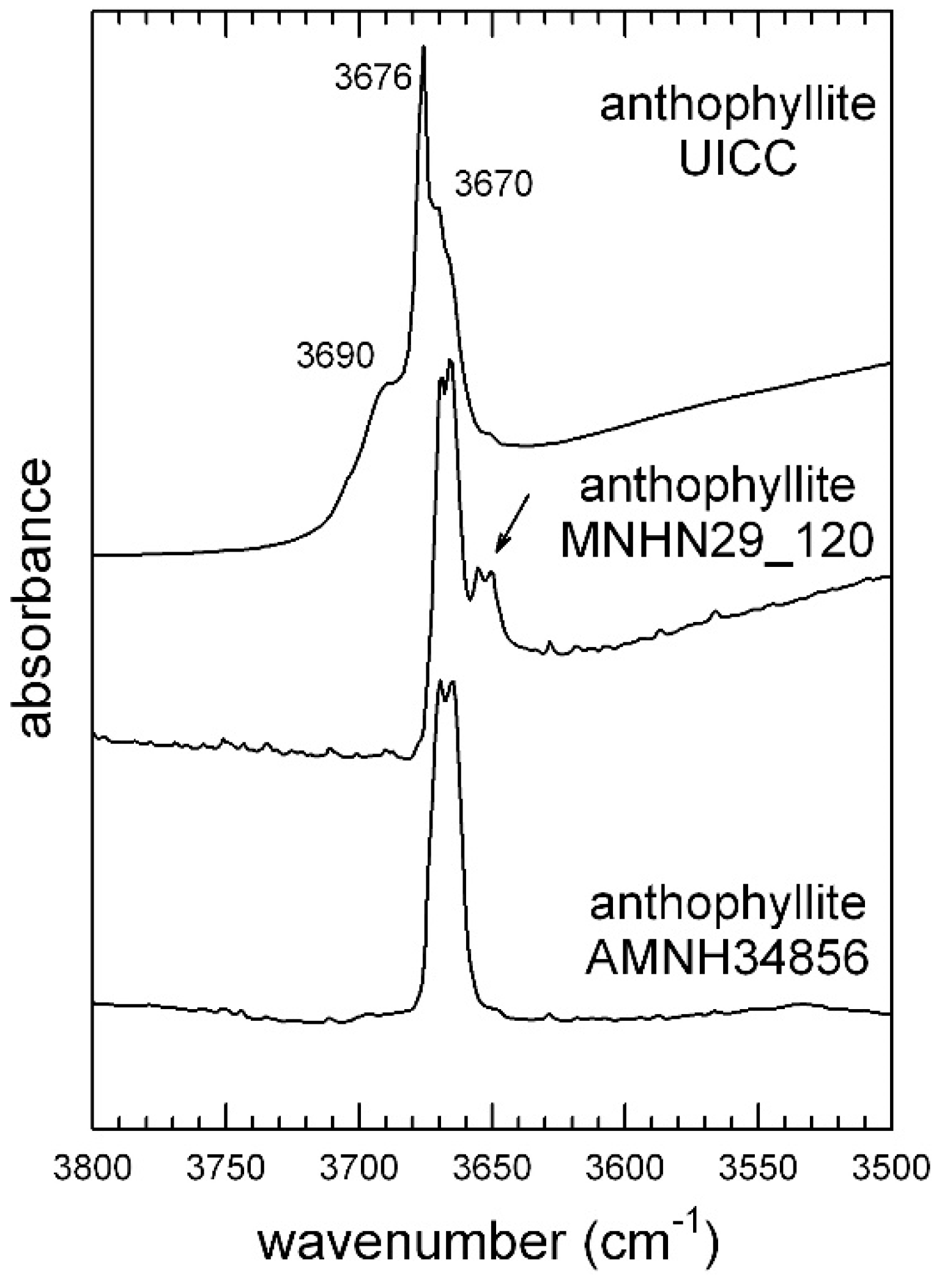
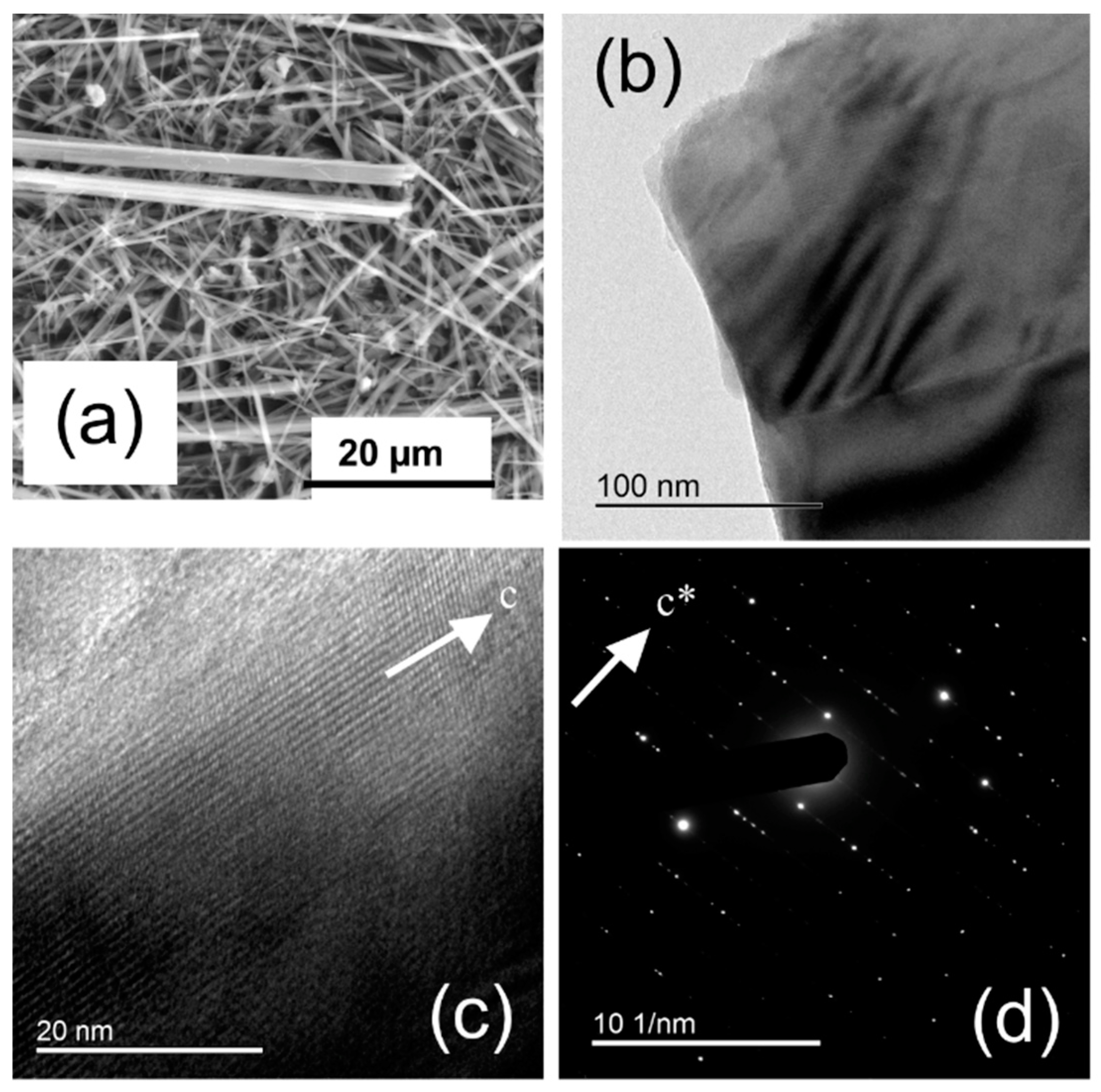
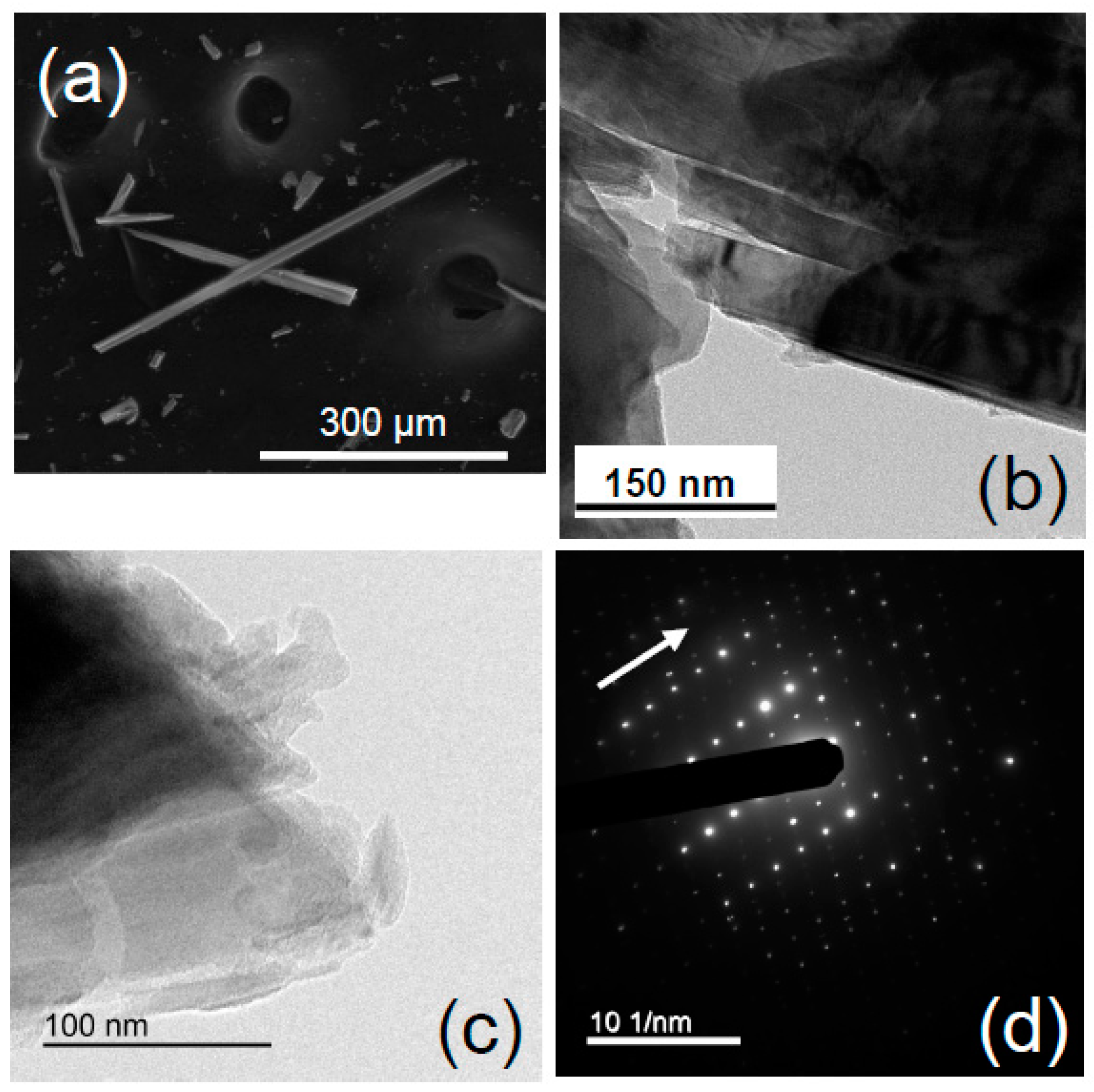
4.1.1. UICC Anthophyllite Asbestos
4.1.2. UICC Amosite (Grunerite Asbestos)
4.1.3. UICC Crocidolite (Riebeckite Asbestos)
4.1.4. Tremolite Asbestos from Val d’Ala
4.1.5. Actinolite Asbestos from Valle Aurina
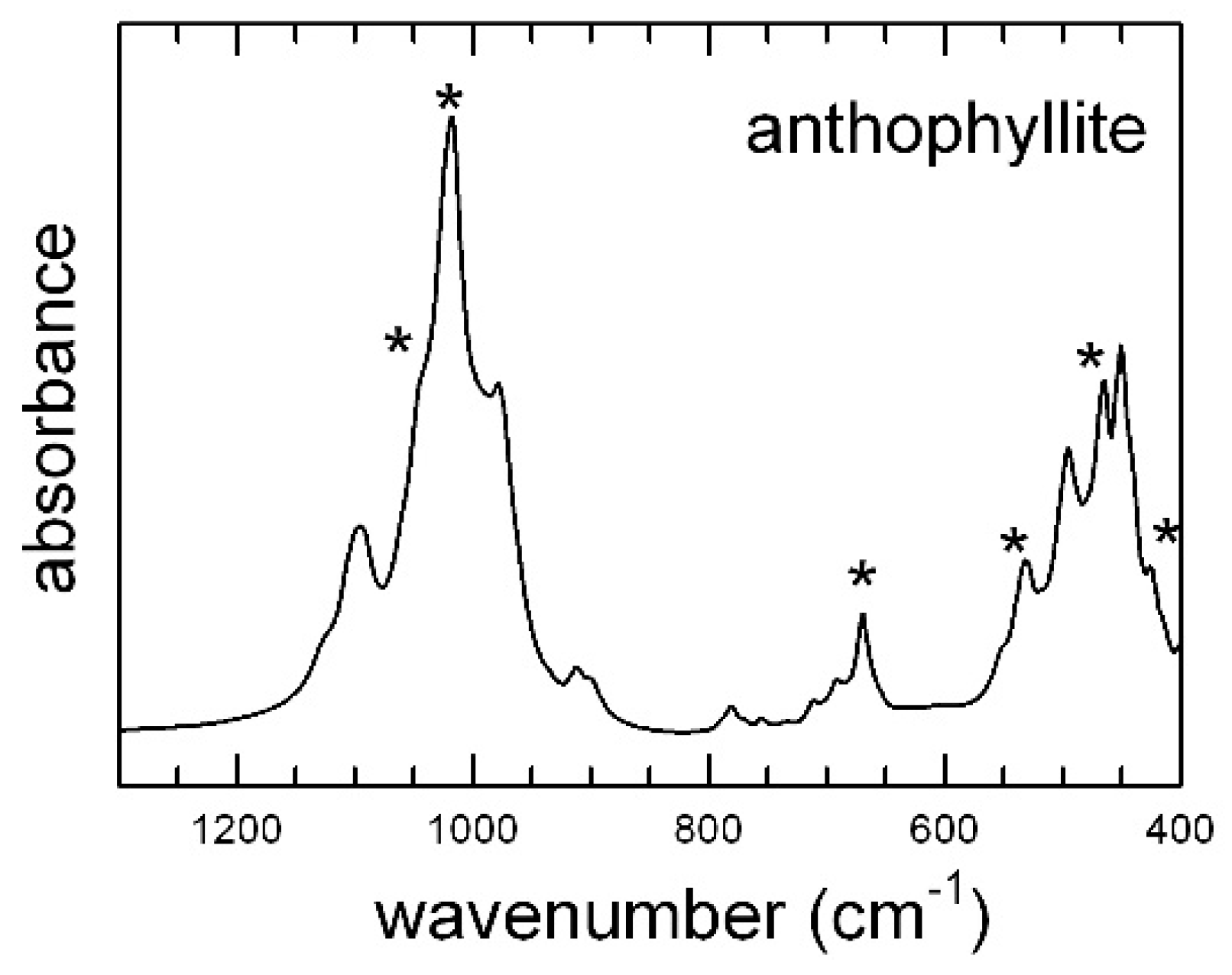
4.2. FTIR Spectra in the Low-Wavenumber Region
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, C.S. Discussion of the mineralogy of industrial talcs: U.S. Bureau of Mines Information Circular. In Proceedings of the Symposium on Talc, Washington, DC, USA, 8 May 1973; Volume 8639, pp. 22–42. [Google Scholar]
- Mossman, B.T.; Mignon, J.; Corn, M.; Seaton, A.; Gee, J.B.L. Asbestos: Scientific developments and implications for public policy. Science 1990, 247, 294–301. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Occupational Safety and Health (NIOSH). Asbestos Fibres and Other Elongate Mineral Particles: State of the Science and Roadmap for Research, revised ed.; Center for Disease Control and Prevention, Department of Health and Human Services: Washington, DC, USA, 2011; p. 174.
- Carbone, M.; Ly, B.H.; Dodson, R.F.; Pagano, I.; Morris, P.T.; Dogan, U.A.; Azdar, A.F.Z.; Pass, H.I.; Yang, H. Malignant mesothelioma: Facts myths, and hypotheses. J. Cell. Physiol. 2013, 227, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Oury, T.D.; Sporn, T.A.; Roggli, V.L. Pathology of Asbestos-Associated Diseases, 3rd ed.; Springer: Heidelberg, Germany, 2014; p. 358. [Google Scholar]
- Wiewiora, A. Crystallochemical classifications of phyllosilicates based on the unified system of projection of chemical composition: III. The serpentine-kaolin group. Clay Miner. 1990, 25, 93–98. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Double-Chain Silicates, 2nd ed.; The Geological Society: London, UK, 1997; 764p. [Google Scholar]
- Iezzi, G.; Della Ventura, G.; Bellatreccia, F.; Lomastro, S.; Bandli, B.R.; Gunter, M.E. Site occupancy in richterite-winchite from Libby, Montana USA, by FTIR spectroscopy. Mineral. Mag. 2007, 71, 93–104. [Google Scholar] [CrossRef]
- Gunter, M.E.; Belluso, E.; Mottana, A. Amphiboles: Environmental and health concerns. Rev. Mineral. Geochem. 2017, 67, 453–516. [Google Scholar] [CrossRef]
- Bozhilov, K.N. Structures and microstructures of non-classical pyriboles. EMU Notes Mineral. 2013, 14, 109–152. [Google Scholar]
- Plumlee, G.S.; Morman, S.A.; Ziegler, T.L. The toxicological geochemistry of Earth materials: An overview of processes and the interdisciplinary methods used to understand them. Rev. Mineral. Geochem. 2006, 64, 5–57. [Google Scholar] [CrossRef]
- Fubini, B.; Fenoglio, I. Toxic potential of mineral dusts. Elements 2007, 3, 407–414. [Google Scholar] [CrossRef]
- Roggli, V.L. The so-called short-fibre controversy. Literature review and critical analysis. Arch. Pathol. Lab. Med. 2015, 139, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Bursi Gandolfi, N.; Gualtieri, A.F.; Pollastri, S.; Ribaldi, E.; Belpoggi, F. Assessment of asbestos body formation by high resolution FEG-SEM after exposure of Sprague-Dawley rats to chrysotile, crocidolite, or erionite. J. Hazard. Mater. 2016, 306, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Belluso, E.; Cavallo, A.; Halterman, D. Crystal habit of mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; Volume 18, pp. 65–110. [Google Scholar]
- Yao, S.; Iezzi, G.; Della Ventura, G.; Bellatreccia, F.; Petibois, C.; Marcelli, A.; Nazzari, M.; Lazzarin, F.; Di Gioacchino, M.; Petrarca, C. Mineralogy and textures of riebeckitic asbestos (crocidolite): The role of single versus agglomerated fibres in toxicological experiments. J. Hazard. Mater. 2017, 340, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, F.C. Spectroscopic methods in Mineralogy and Geology. Rev. Mineral. 1988, 18, 698. [Google Scholar]
- Beran, A.; Libowitzky, E. Spectroscopic methods in Mineralogy. EMU Notes Mineral. 2004, 6, 661. [Google Scholar]
- Henderson, G.S.; Neuville, D.R.; Downs, R.T. Spectroscopic methods. Rev. Mineral. Geochem. 2014, 78, 800. [Google Scholar]
- Rinaudo, C.; Belluso, E.; Gastaldi, D. Assessment of the use of Raman spectroscopy for the determination of amphibole asbestos. Mineral. Mag. 2004, 68, 455–465. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Della Ventura, G. Short-range order in amphiboles. Rev. Mineral. Geochem. 2017, 67, 173–222. [Google Scholar] [CrossRef]
- Gottschalk, M.; Andrut, M.; Melzer, S. The determination of cummingtonite content of synthetic tremolite. Eur. J. Mineral. 1999, 11, 967–982. [Google Scholar] [CrossRef]
- Yao, S.; Chen, H.H.; Harte, E.; Della Ventura, G.; Petibois, C. The role of asbestos morphology on their cellular toxicity: An in vitro 3D Raman/Raley imaging study. Anal. Bioanal. Chem. 2013, 405, 8701–8707. [Google Scholar] [CrossRef] [PubMed]
- Della Ventura, G.; Marcelli, A.; Bellatreccia, F. SR-FTIR microscopy and FTIR imaging in the Earth Sciences. Rev. Mineral. Geochem. 2014, 78, 447–479. [Google Scholar] [CrossRef]
- Leissner, L.; Schlüter, J.; Horn, I.; Mihailova, B. Exploring the potential of Raman spectroscopy for crystallochemical analyses of complex hydrous silicates: I. Amphiboles. Am. Mineral. 2015, 100, 2682–2694. [Google Scholar] [CrossRef]
- Della Ventura, G. The analysis of asbestos minerals using vibrational spectroscopies (FTIR, Raman): Crystal Chemistry, identification and environmental applications. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; Volume 18, pp. 135–169. [Google Scholar]
- Lewis, I.R.; Chaffin, N.C.; Gunter, M.E.; Griffiths, P.R. Vibrational spectroscopic studies of asbestos and comparison of suitability for remote analysis. Spectroch. Acta A 1996, 52, 315–328. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Oberti, R. Amphiboles: Crystal Chemistry. Rev. Mineral. Geochem. 2007, 67, 1–54. [Google Scholar] [CrossRef]
- Wang, A.; Dhamelincourt, P.; Turrell, G. Raman and infrared spectroscopic investigation of the cation distributions in amphiboles. J. Mol. Struct. 1988, 175, 183–188. [Google Scholar] [CrossRef]
- Sbroscia, M.; Della Ventura, G.; Iezzi, G.; Sodo, A. Quantifying the A-site occupancy in amphiboles: A Raman study in the OH-stretching region. Eur. J. Mineral. 2018, in press. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Grundy, H.D. The crystal chemistry of the amphiboles. IV. X-ray and neutron refinements of the crystal structure of tremolite. Can. Mineral. 1976, 14, 334–345. [Google Scholar]
- Hawthorne, F.C.; Della Ventura, G.; Oberti, R.; Robert, J.-L.; Iezzi, G. Short-range order in minerals: Amphiboles. Can. Mineral. 2005, 43, 1895–1920. [Google Scholar] [CrossRef]
- Della Ventura, G.; Mottana, A.; Caprilli, E.; Bellatreccia, F.; De Benedetti, A. Asbestiform tremolite within the late pyroclastic deposits of the Alban Hills volcano (Latium, Italy): FTIR spectroscopy and crystal chemistry. Rendiconti Accademia Lincei 2014, 25, 229–236. [Google Scholar]
- Pollastri, S. Crystal Chemistry and Physical-Chemical Characterization of Mineral Fibres Aimed at Understanding Their Toxicity Potential. Unpublished. Ph.D. Thesis, University of Modena and Reggio Emilia, Modena, Italy, March 2016. [Google Scholar]
- Pollastri, S.; Perchiazzi, N.; Gigli, L.; Ferretti, P.; Cavallo, A.; Gandolf, N.B.; Pollok, K.; Gualtieri, A.F. The crystal structure of mineral fibres. 2. Amosite and fibrous anthophyllite. Period. Mineral. 2017, 86, 55–63. [Google Scholar]
- Welch, M.D.; Cámara, F.; Oberti, R. Thermoelasticity and high-T behaviour of anthophyllite. Phys. Chem. Miner. 2011, 38, 321–334. [Google Scholar] [CrossRef]
- Pollastri, S.; Gualtieri, A.F.; Gualtieri, M.L.; Hanuskova, M.; Cavallo, A.; Gaudino, G. The zeta potential of mineral fibres. J. Hazard. Mater. 2014, 276, 469–679. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; D’Acapito, F.; Trapananti, A.; Colantoni, I.; Andreozzi, G.B.; Gualtieri, A.F. The chemical environment of iron in mineral fibres. A combined X-ray absorption and Mössbauer spectroscopic study. J. Hazard. Mater. 2015, 298, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Della Ventura, G.; Mihailova, B.; Susta, U.; Guidi, M.C.; Marcelli, A.; Schlüter, J.; Oberti, R. The dynamics of Fe oxidation in riebeckite: A model for amphiboles. Am. Mineral. 2018, 103, 1103–1111. [Google Scholar] [CrossRef]
- Pacella, A.; Andreozzi, G.B.; Ballirano, P.; Gianfagna, A. Crystal chemical and structural characterization of fibrous tremolite from Ala di Stura (Lanzo Valley, Italy). Period. Mineral. 2008, 77, 51–62. [Google Scholar]
- Pollastri, S.; Gigli, L.; Ferretti, P.; Andreozzi, G.B.; Gandolfi, N.B.; Pollok, K.; Gualtieri, A.F. The crystal structure of mineral fibres. 3. Actinolite asbestos. Period. Mineral. 2017, 86, 89–98. [Google Scholar]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Ross, M. The geological occurrences and health hazards of amphibole and serpentine asbestos. Rev. Mineral. 1981, 9, 279–323. [Google Scholar]
- Fantauzzi, M.; Pacella, A.; Atzei, D.; Gianfagna, A.; Andreozzi, G.B.; Rossi, A. Combined use of X-ray photoelectron and Mössbauer spectroscopic techniques in the analytical characterization of iron oxidation state in amphibole asbestos. Anal. Bioanal. Chem. 2010, 396, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K. Identification of infrared OH librational bands of talc-willemseite solid-solutions and Al(VI)-free amphiboles through deuteration. Miner. J. 1990, 15, 93–104. [Google Scholar] [CrossRef]
- Ishida, K. Cation disordering in heat-treated anthophyllites through oxidation and dehydrogenation. Phys. Chem. Miner. 1998, 25, 160–167. [Google Scholar] [CrossRef]
- Ishida, K.; Hawthorne, F.C. Fine structure of infrared OH-stretching bands of holmquistite and anthophyllite. Phys. Chem. Miner. 2003, 30, 330–336. [Google Scholar] [CrossRef]
- Hirschmann, M.; Evans, B.W.; Yang, H. Composition and temperature dependence of Fe-Mg ordering in cummingtonite-grunerite as determined by X-ray diffraction. Am. Mineral. 1994, 79, 862–877. [Google Scholar]
- Yang, H.; Hirschmann, M.M. Crystal structure of P21/m ferromagnesian amphibole and the role of cation ordering and composition in the P21/m-C2/m transition in cummingtonite. Am. Mineral. 1995, 80, 916–922. [Google Scholar] [CrossRef]
- Boffa Ballaran, T.; Carpenter, M.A.; Domeneghetti, M.C. Phase transition and mixing behaviour of the cummingtonite-grunerite solid solution. Phys. Chem. Miner. 2001, 28, 87–101. [Google Scholar]
- Iezzi, G.; Della Ventura, G.; Cámara, F.; Pedrazzi, G.; Robert, J.L. BNa-BLi solid-solution in A-site-vacant amphiboles: Synthesis and cation ordering along the ferri-clinoferroholmquistite-riebeckite join. Am. Mineral. 2003, 88, 955–961. [Google Scholar] [CrossRef]
- Iezzi, G.; Della Ventura, G.; Tribaudino, M. Synthetic P21/m amphiboles in the system Li2O-Na2O-MgO-SiO2-H2O (LNMSH). Am. Mineral. 2006, 91, 425–429. [Google Scholar] [CrossRef]
- Della Ventura, G.; Bellatreccia, F.; Iezzi, G.; Oberti, R.; Camara, F. The P21/m-C2/m phase transition in synthetic amphiboles in the system Li2O-Na2O-MgO-SiO2-H2O: A high-T FTIR study. Eur. J. Mineral. 2008, 20, 1019–1026. [Google Scholar] [CrossRef]
- Ross, M.; Papike, J.J.; Weiblen, P.W. Exsolution in clinoamphiboles. Science 1968, 159, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Prewitt, C.T.; Papike, J.J.; Ross, M. Cummingtonite: A reversible, nonquenchable transition from P21/m to C2/m symmetry. Earth Planet. Sci. Lett. 1970, 8, 448–450. [Google Scholar] [CrossRef]
- Dreyer, C.J.B.; Robinson, H.A. The Occurrence and Exploitation of Amphibole Asbestos in South Africa; Preprint No. 78-H-64; Society of Mining Engineers; AIME: Salt Iake City, UT, USA, 1978; pp. 1–21. [Google Scholar]
- Iezzi, G.; Della Ventura, G.; Hawthorne, F.C.; Pedrazzi, G.; Robert, J.-L.; Novembre, D. The (Mg,Fe2+) substitution in ferri-clinoholmquistite, □Li2(Mg,Fe2+)3Fe3+2Si8O22(OH)2. Eur. J. Mineral. 2005, 17, 733–740. [Google Scholar] [CrossRef]
- Della Ventura, G.; Iezzi, G.; Redhammer, G.J.; Hawthorne, F.C.; Scaillet, B.; Novembre, D. Synthesis and crystal-chemistry of amphiboles along the magnesioriebeckite—Magnesio-arfvedsonite series as a function of fO2. Am. Mineral. 2005, 90, 1375–1383. [Google Scholar] [CrossRef]
- Strens, G.J. Infrared study of cation ordering and clustering in some (Fe,Mg) amphibole solid solutions. Chem. Commun. 1966, 519b–520. [Google Scholar] [CrossRef]
- Burns, R.G.; Strens, R.G.J. Infrared study of the hydroxyl bands in clinoamphiboles. Science 1966, 153, 890–892. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, F.C.; Della Ventura, G.; Robert, J.-L. Short-range order and long-range order in amphiboles: A model for the interpretation of infrared spectra in the principal OH-stretching region. Geochim. Cosmochim. Acta 1996, 5, 49–54. [Google Scholar]
- Della Ventura, G.; Robert, J.-L.; Hawthorne, F.C. Infrared spectroscopy of synthetic (Ni,Mg,Co)-potassium-richterite. Geochim. Cosmochim. Acta 1996, 5, 55–63. [Google Scholar]
- Della Ventura, G.; Robert, J.L.; Raudsepp, M.; Hawthorne, F.C.; Welch, M.D. Site occupancies in synthetic monoclinic amphiboles: Rietveld structure refinement and infrared spectroscopy of (nickel, magnesium, cobalt)-richterite. Am. Mineral. 1997, 82, 291–301. [Google Scholar] [CrossRef]
- Della Ventura, G.; Redhammer, G.J.; Robert, J.L.; Sergent, J.; Iezzi, G. Crystal-chemistry of synthetic amphiboles along the richterite-ferro-richterite join: A combined spectroscopic (FTIR, Mössbauer), XRPD, and microchemical study. Can. Mineral. 2016, 54, 97–114. [Google Scholar] [CrossRef]
- Iezzi, G.; Cámara, F.; Della Ventura, G.; Oberti, R.; Pedrazzi, G.; Robert, J.-L. Synthesis, crystal structure and crystal-chemistry of ferri-clinoholmquistite, □Li2Mg3Fe3+2Si8O22(OH)2. Phys. Chem. Mineral. 2004, 31, 375–385. [Google Scholar] [CrossRef]
- Vignaroli, G.; Rossetti, F.; Belardi, G.; Billi, A. Linking rock fabric to fibrous mineralisation: A basic tool for the asbestos hazard. Nat. Hazards Earth Syst. Sci. 2011, 11, 1267–2011. [Google Scholar] [CrossRef]
- Vignaroli, G.; Ballirano, P.; Belardi, G.; Rossetti, F. Asbestos fibre identification vs. evaluation of asbestos hazard in ophiolitic rock mélanges, a case study from the Ligurian Alps (Italy). Environ. Earth Sci. 2014. [Google Scholar] [CrossRef]
- Lazarev, A.N. Vibrational Spectra and Structure of Silicates; Consultants Bureau (Plenum Publishing Company Ltd.): New York, NY, USA; London, UK, 1972; 302p. [Google Scholar]
- Andrut, M.; Gottschalk, M.; Melzer, S.; Najorka, J. Lattice vibrational modes in synthetic tremolite-Sr-tremolite and tremolite-richterite solid solutions. Phys. Chem. Miner. 2000, 27, 301–309. [Google Scholar] [CrossRef]
- Hofmeister, A.M.; Bowey, J.E. Quantitative infrared spectra of hydrosilicates and related minerals. Mon. Not. R. Astron. Soc. 2006, 367, 577–591. [Google Scholar] [CrossRef]
- Ishida, K. Infrared spectra of alkali amphiboles of glaucophane–riebeckite series and their relation to chemical composition. Mineral. J. 1990, 15, 147–161. [Google Scholar] [CrossRef]
- Ishida, K.; Jenkins, D.M.; Hawthorne, F.C. Mid-IR bands of synthetic calcic amphiboles of tremolite-pargasite series and of natural calcic amphiboles. Am. Mineral. 2008, 93, 1112–1118. [Google Scholar] [CrossRef]
- Bard, D.; Yarwood, J.; Tylee, B. Asbestos fibre identification by Raman microspectroscopy. J. Raman Spectrosc. 1997, 28, 803–809. [Google Scholar] [CrossRef]
- Blaha, J.J.; Rosasco, G.J. Raman microprobe spectra of individual microcrystals and fibers of talc, tremolite, and related silicate minerals. Anal. Chem. 1978, 50, 892–896. [Google Scholar] [CrossRef]
- Rinaudo, C.; Gastaldi, D.; Belluso, E.; Capella, S. Application of Raman Spectroscopy on asbestos fibre identification. Neues Jahrbuch für Mineralogie-Abhandlungen 2005, 182, 31–36. [Google Scholar] [CrossRef]
- Rinaudo, C.; Cairo, S.; Gastaldi, D.; Gianfagna, A.; Mazziotti Tagliani, S.; Tosi, G.; Conti, C. Characterization of fluoro-edenite by µ-Raman and µ-FTIR spectroscopy. Mineral. Mag. 2006, 70, 291–298. [Google Scholar] [CrossRef]
- Rinaudo, C.; Allegrina, M.; Fornero, E.; Musa, M.; Croce, A.; Belli, D. Micro-Raman spectroscopy and VP-SEM/EDS applied to the identification of mineral particles and fibres in histological sections. J. Raman Spec. 2009, 41, 27–32. [Google Scholar] [CrossRef]
- Rinaudo, C.; Croce, A.; Musa, M.; Fornero, E.; Allegrina, M.; Trivero, P.; Bellis, D.; Sferch, D.; Toffalorio, F.; Veronesi, G.; et al. Study of inorganic particles, fibers, and asbestos bodies by variable pressure scanning electron microscopy with annexed energy dispersive spectroscopy and micro-raman spectroscopy in thin sections of lung and pleural plaque. Appl. Spectrosc. 2010, 64, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Campopiano, A.; Olori, A.; Cannizzaro, A.; Iannò, A.; Capone, P.P. Quantification of Tremolite in Friable Material Coming from Calabrian Ophiolitic Deposits by Infrared Spectroscopy. J. Spectrosc. 2015. [Google Scholar] [CrossRef]
- Della Ventura, G.; Robert, J.L.; Beny, J.M. Tetrahedrally coordinated Ti4+ in synthetic Ti-rich potassic richterite: Evidence from XRD, FTIR, and Raman studies. Am. Mineral. 1991, 76, 1134–1140. [Google Scholar]
- Apopei, A.I.; Buzgar, N. The Raman study of amphiboles. Analele Stiintifice de Univ. AI Cuza din Iasi. Sect. 2 Geol. 2010, 56, 57–83. [Google Scholar]
- Oberti, R.; Boiocchi, M.; Zema, M.; Hawthorne, F.C.; Redhammer, G.J.; Susta, U.; Della Ventura, G. The high-temperature behaviour of riebeckite: Expansivity, deprotonation, selective Fe oxidation and a novel cation disordering scheme for amphiboles. Eur. J. Mineral. 2018, in press. [Google Scholar] [CrossRef]
| Amphibole Species | Provenance | Reference |
|---|---|---|
| Anthophyllite | UICC standard Anthophyllite from Paakkila, NB #4173-111-5 | [34,35] |
| Anthophyllite | AMNH34856, Talcville, New York State (USA) | [36] |
| Anthophyllite | MNHN 29_102 | Della Ventura, unpublished |
| Amosite | UICC standard Amosite South African NB #4173-111-4 | [34,35] |
| Crocidolite | UICC standard Crocidolite South African NB #4173-111-3 | [34,37,38] |
| Crocidolite | Madagascar | [39] |
| Tremolite | Val d’Ala, Turin (Italy) | [34,40] |
| Actinolite | Valle Aurina (Bolzano, Italy) | [41] |
| Sample | a (Å) | b (Å) | c (Å) | β (°) | Space Group | Ref. |
|---|---|---|---|---|---|---|
| Anthophyllite UICC | 18.5770(8) | 18.0353(22) | 5.27285(9) | 90 | Pnma | [35] |
| Amosite UICC | 9.5484(2) | 18.3395(4) | 5.3346(1) | 101.825(2) | C2/m | [35] |
| Crocidolite UICC | 9.73238(7) | 18.0414(1) | 5.32794(4) | 103.515(1) | C2/m | [34] |
| Tremolite Ala | 9.8424(1) | 18.0712(2) | 5.28354(7) | 104.680(1) | C2/m | [40] |
| Actinolite Aurina | 9.84896(4) | 18.07748(8) | 5.28860(2) | 104.817(2) | C2/m | [41] |
| Sample | Anthophyllite UICC | Amosite UICC | Crocidolite UICC | Tremolite | Actinolite |
|---|---|---|---|---|---|
| reference | [35] | [35] | [34] | [40] | [41] |
| SiO2 | 57.3(4) | 49.8(2) | 51.7(3) | 57.8(1) | 54.83(5) |
| TiO2 | 0.02(2) | 0.04(2) | 0.03(3) | 0.02(2) | 0.04(9) |
| Al2O3 | 0.11(5) | 0.04(2) | 0.12(4) | 0.11(7) | 2.70(6) |
| FeO | 9.9(7) | 37.8(4) | 17.3(9) * | 2.4(4) * | 6.76(5) |
| Fe2O3 | 0.0 | 3.3(4) | 18.7(9) * | 0.3(4) * | 1.01(5) |
| Cr2O3 | 0.04(3) | 0.01(1) | 0.01(1) | 0.02(2) | 0.08(9) |
| NiO | 0.04(4) | 0.02(4) | 0.01(2) | 0.08(5) | 0.18(2) |
| MnO | 0.4(1) | 0.42(6) | 0.04(2) | 0.15(5) | 0.40(7) |
| MgO | 28.5(2) | 6.23(9) | 2.3(4) | 22.8(2) | 18.21(3) |
| CaO | 0.27(7) | 0.09(1) | 0.22(7) | 12.9(1) | 12.77(6) |
| Na2O | 0.02(1) | 0.03(2) | 6.7(2) | 0.23(6) | 0.48(6) |
| K2O | 0.02(1) | 0.02(1) | 0.05(4) | 0.05(2) | 0.13(3) |
| H2O | 2.30 | 1.94 | 2.18 | 2.02 | 2.47 |
| Total | 98.92 | 99.74 | 99.36 | 98.88 | 100.06 |
| Anthophyllite 29_102 | Amosite UICC | Crocidolite UICC | Tremolite | Actinolite | Assignment |
|---|---|---|---|---|---|
| 1126 | 1128 | 1142 | 1105 | 1102 | Region 1 Si–O–Si, O–Si–O and Si–O stretching modes |
| 1106 | 1082 | 1105 | 1059 | 1058 | |
| 1095 | 1020 | 1048 | 1038 | 1038 | |
| 1058 | 997 | 1017 | 1015 | 1012 | |
| 1026 | 969 | 990 | 995 | 993 | |
| 1008 | 888 | 976 | 953 | 952 | |
| 978 | 896 | 921 | 919 | ||
| 910 | 878 | ||||
| 898 | |||||
| 781 | 797 | 778 | 757 | 756 | Region 2 chain deformations and OH libration modes |
| 755 | 775 | 726 | 738 | 736 | |
| 736 | 729 | 693 | 724 | 724 | |
| 709 | 700 | 655 | 685 | 684 | |
| 688 | 654 | 634 | 661 | 661 | |
| 657 | 635 | 642 | 642 | ||
| 530 | 528 | 543 | 543 | 540 | Region 3 M–O modes |
| 495 | 499 | 506 | 526 | 508 | |
| 480 | 479 | 450 | 508 | 463 | |
| 463 | 424 | 465 | 446 | ||
| 440 | 445 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Ventura, G.; Vigliaturo, R.; Gieré, R.; Pollastri, S.; Gualtieri, A.F.; Iezzi, G. Infra Red Spectroscopy of the Regulated Asbestos Amphiboles. Minerals 2018, 8, 413. https://doi.org/10.3390/min8090413
Della Ventura G, Vigliaturo R, Gieré R, Pollastri S, Gualtieri AF, Iezzi G. Infra Red Spectroscopy of the Regulated Asbestos Amphiboles. Minerals. 2018; 8(9):413. https://doi.org/10.3390/min8090413
Chicago/Turabian StyleDella Ventura, Giancarlo, Ruggero Vigliaturo, Reto Gieré, Simone Pollastri, Alessandro F. Gualtieri, and Gianluca Iezzi. 2018. "Infra Red Spectroscopy of the Regulated Asbestos Amphiboles" Minerals 8, no. 9: 413. https://doi.org/10.3390/min8090413
APA StyleDella Ventura, G., Vigliaturo, R., Gieré, R., Pollastri, S., Gualtieri, A. F., & Iezzi, G. (2018). Infra Red Spectroscopy of the Regulated Asbestos Amphiboles. Minerals, 8(9), 413. https://doi.org/10.3390/min8090413







