1. Introduction
Serpentinization and carbonation of ultramafic rocks is a ubiquitous process within the oceanic lithosphere, which results in intense rock alteration and widespread redistribution of chemical elements. Over the last few years, ultramafic rocks alteration reactions have been the subject of growing interests among scientists, firstly because they significantly influence rock rheology [
1] and volatile recycling [
2], but also because they raise realistic societal and environmental expectations, e.g., in the search for long-term carbon dioxide sequestration strategies [
3,
4] or for a source of renewable H
2 [
5].
Serpentinization is a hydration reaction during which olivine ((Mg,Fe)SiO
4) is transformed into serpentine ((Mg,Fe)
3Si
2O
5(OH)
3), along with a limited number of additional phases. The composition of aqueous fluids, and in particular the presence of carbon dioxide (CO
2) among the volatile phases, plays an important role in the determination of mineral species in the reaction products. In the absence of carbon dioxide, serpentine is typically accompanied by brucite ((Mg,Fe)(OH)
2). In contrast, when carbon dioxide is present, a carbonation reaction occurs leading to the formation of magnesite (MgCO
3), dolomite (CaMg(CO
3)
2), or calcite (CaCO
3). In addition to hydration and carbonation, serpentinization involves redox reactions during which divalent iron is oxidized and incorporated into serpentine and an oxide phase, typically magnetite (Fe
3O
4) or hematite (Fe
2O
3). The alteration of olivine through serpentinization is accompanied by a significant increase in the rock volume, with differences of up to 50% between the molar volumes of the starting minerals and the reaction products [
6,
7].
Serpentinization is directly dependent on the ability of aqueous fluids to percolate towards the reaction fronts. Therefore, the evolution of rock total porosity over time is one of the key features of serpentinization reactions. This is a nontrivial issue, especially in a context where the increase in volume associated with phase transformations tends to form veins, obstructing circulation pathways, which may in turn impede fluid flow and inhibit the reaction [
8,
9]. However, even if serpentinization is in theory self-limiting in essence, the large bodies of fully serpentinized rocks outcropping in various geological settings around the world [
9,
10] indicate that situations of pervasive and sustained fluid flow may persist for long periods of time. For that to happen, pre-existing fluid circulation pathways have to remain open, and/or new ones have to be created in the course of the reaction [
10]. In the former case, significant mass transfer is required between dissolution and precipitation sites [
7,
11,
12], while the latter is achieved through rock fracturing [
9,
10] or the build-up of interconnected porosity [
10,
13]. Even if those features are well established and corroborated by various field observations e.g., [
14,
15] and in laboratory experiments e.g., [
16,
17], the mechanisms at play and the conditions under which they occur are still not well constrained. For instance, several processes have been utilized to account for the pervasive rock fracturation observed during serpentinization. These include mechanisms not directly related to the reaction itself, such as thermal cracking of olivine upon cooling [
18] and fracturing resulting from local tectonic stresses [
19]. More recently, an intrinsic mechanism of reaction-induced cracking, possibly triggered by the major volume increase associated with phase transformations, has been put forward [
8,
20]. While there are recent convincing experimental evidence for the occurrence of intercrystalline reaction-induced cracking [
13,
21], equivalent data supporting the existence of intracrystalline reaction-induced cracking is still lacking. In addition, other critical issues such as the role of carbon dioxide in dissolution-precipitation mechanisms, or the mutual evolution and interdependence of fracturation and porosity networks are important topics that remain to be investigated further.
Most of the recent experimental studies dealing with flow-through [
20,
22], and static [
23] alteration of natural peridotites were faced with the challenge of slow reaction rates. To overcome this issue, many of the experimental studies were performed under static confined pressure using either fine grain (<<100 μm) starting materials [
17,
24,
25,
26,
27], or sintered olivine grains [
13,
26] or thermally cracked samples [
16]. While the large surface area associated with smaller grain sizes may enhance reaction kinetics, it may also hinder key processes typically occurring in coarser-grained natural rocks. For instance, the occurrence of intracrystalline reaction-induced cracking and the development of interconnected porosity may be hampered or biased in fine-grained materials by the wealth of readily available interfaces. To avoid these effects and to allow for a more realistic comparison with the textures observed in natural serpentinites, here we have decided to use larger olivine grains (>1 mm) as starting materials. The experiments have been carried out for 3 to 12 months at a temperature of 200 °C and vapor saturated pressure. Alkaline aqueous solutions have been used as reactive fluids in a setup simulating no fluid flow and static conditions. The present study reports the first experimental evidence for intracrystalline reaction-induced cracking in olivine during serpentinization, and clearly demonstrates the critical role played by carbon dioxide in the degree of coupling between dissolution and precipitation reactions. We also report new findings on the creation and evolution of porosity over time, in particular on the ability of the system to develop porosity in the absence of fracturation.
2. Materials and Methods
2.1. Sample Preparation and Experimental Conditions
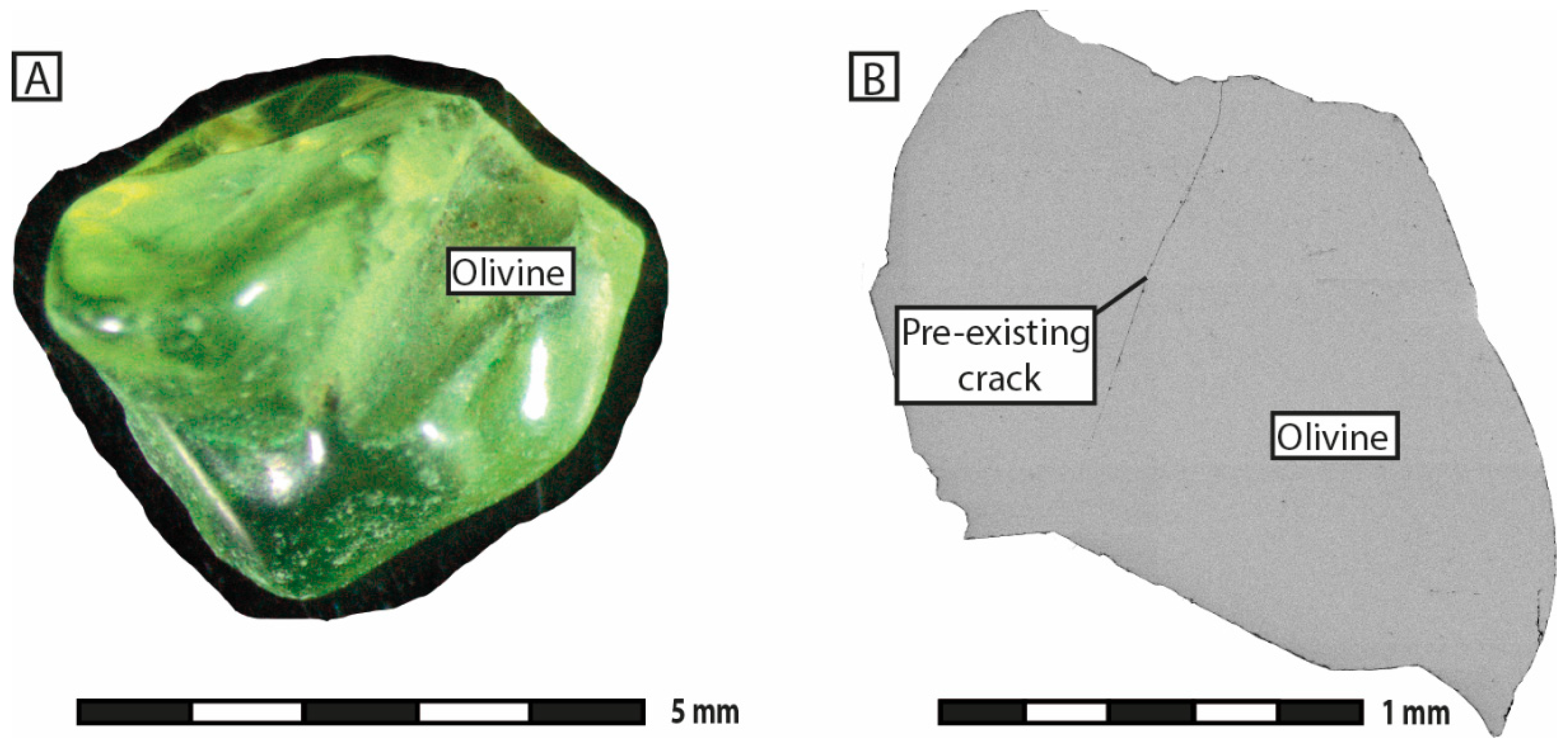
Serpentinization experiments were conducted at the ISTerre laboratory of the University of Grenoble Alpes (Grenoble, France). The starting material consisted of fresh natural olivine grains of unknown origin, 1 to 5 mm in size (
Figure 1). This choice was motivated firstly because this size range is observed in many peridotite rocks, and secondly because these olivine grains had a chemical composition similar to the well-documented San Carlos olivine (see
Supplementary Materials S1). The grains contained a few mineral inclusions of clinopyroxene and orthopyroxene and showed very little porosity or fracturation. Sample preparation involved washing in concentrated 3 M HCl solution for 1 to 2 min to remove any surface contaminants, followed by rinsing in deionized water. No thermally induced cracking of the grains was applied.
The selected olivine grains were divided into two groups, each being reacted with a different aqueous solution, namely (i) a highly alkaline 1 M NaOH solution to induce hydration of the grains, and (ii) a mildly alkaline NaHCO
3 solution to induce carbonation of the grains. These solutions were selected as reactants because of their ability to generate a wide spectrum of alteration features similar to those observed in nature during serpentinization reactions, and additionally because they significantly enhanced reaction kinetics [
14,
15], thus keeping the experiments within realistic time frames. This choice was also well in line with previous observations reporting the enhancing effect of alkaline fluids on the rate of serpentinization reactions [
24], and the well-established increase in fluid alkalinity over time [
28].
At the beginning of the experiments, four to ten olivine grains (~200 mg) along with 2 mL of solution were put in a static-batch Teflon capsule fitted into a closed steel autoclave (see
Supplementary Materials S2). The choice of such a high solution-to-mineral ratio (~10) was justified to avoid major changes in the composition of the solution as the reaction proceeded. Experimental runs were conducted for durations of 3, 6 and 12 months at a temperature of 200 °C (
Table 1), which is considered a relevant temperature value for both hydration and carbonation of ultramafic rocks [
29,
30]. At the end of the experiments, the autoclaves were quenched in cold water to stop the reaction and freeze the reaction products. The samples were then rinsed and oven-dried at 60 °C for 24 h.
In addition, a seventh experiment was run on similar olivine grains using high-purity deionized water as a reactive solution (See
Supplementary Materials S2). This experiment was carried out for a duration of 12 months at a temperature of 200 °C. The observed features served as references to attest to the validity of the serpentinization experiments performed under alkaline conditions.
2.2. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
Reacted olivine grains and slurry residues were imaged at the Institute of Earth Sciences of the University of Lausanne (Lausanne, Switzerland) using a Tescan Mira II LMU Schottky field emission-scanning electron microscope (FE-SEM) operated at an acceleration voltage of 20 kV and a probe current of ~0.5 nA. The goal here was to identify mineral phases and textures, with a particular emphasis on the reaction products, porosity and fracturation. Backscattered electron (BSE) images were taken on finely-polished epoxy mounts coated with a 15 nm carbon layer, while a few olivine grains were hand-picked on aluminum stubs and gold-coated for secondary electron (SE) imaging.
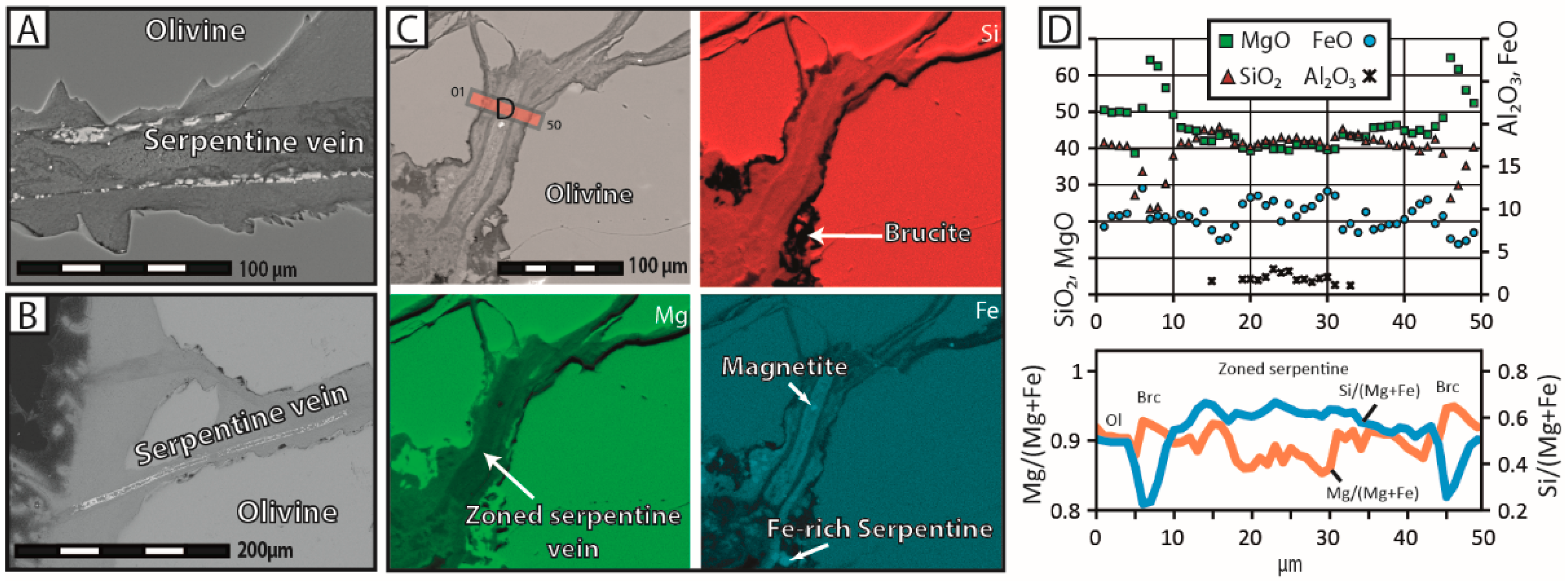
Energy dispersive spectroscopy (EDS) analyses were carried out at the same facility to determine the chemical composition of the reaction products. This data was complemented by elemental maps on specific user-defined areas, in order to visualize the spatial distribution of key chemical elements, such as Si, Fe and Mg.
2.3. Raman Spectroscopy
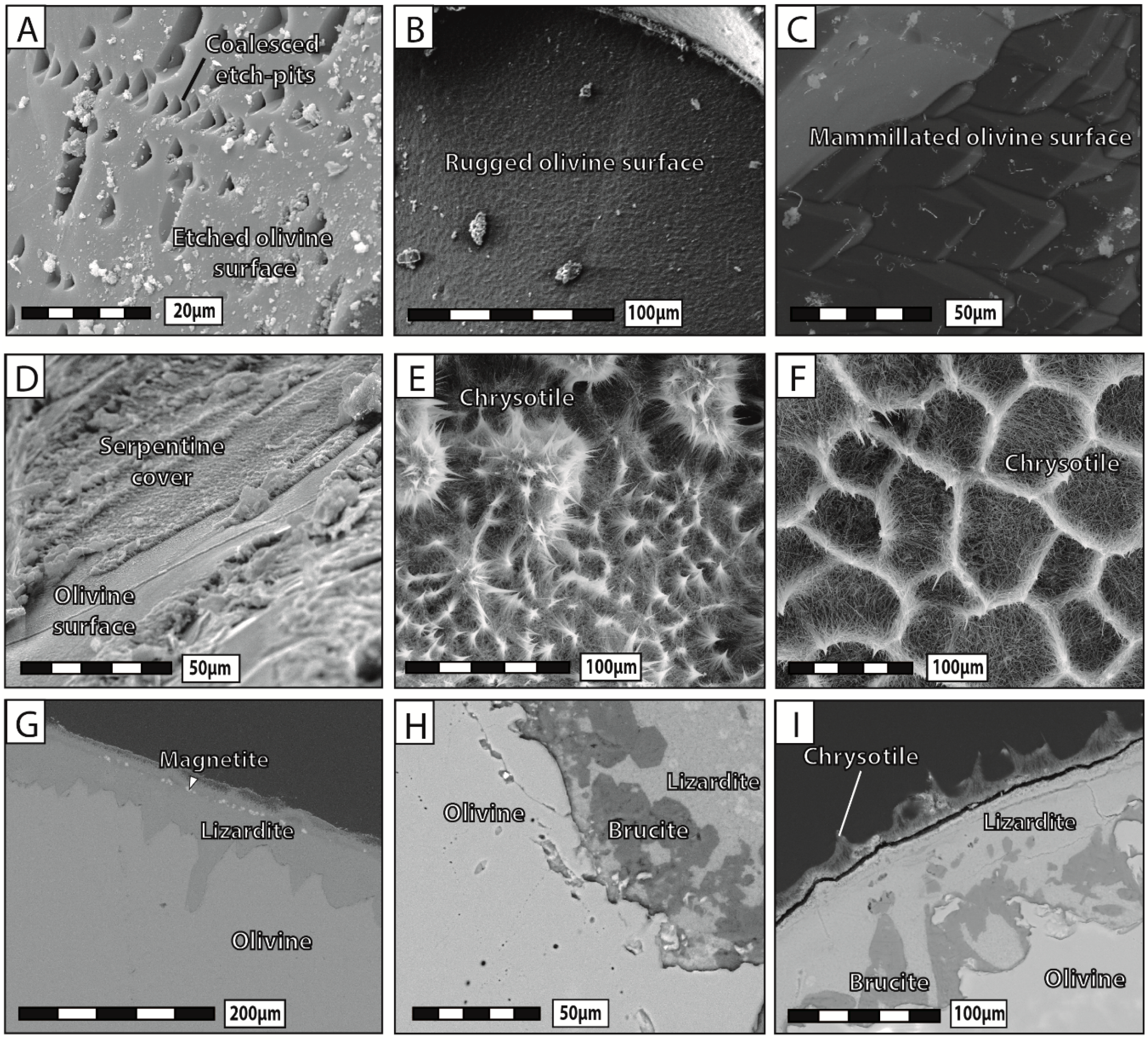
In-situ Raman spectroscopy was used to differentiate the serpentine polymorphs chrysotile and lizardite, as they are characterized by distinct Raman peaks on the OH stretching range, with a single band at 3697 cm−1, and two bands at 3682 cm−1 and 3704 cm−1, respectively. Raman spectra of the reaction products were collected using a Horiba LabRam HR800 (Jobin-Yvon Technologies) apparatus generating an argon laser beam, 532 nm in wavelength. Measurements were performed at the Institute of Earth Sciences of the University of Lausanne (Lausanne, Switzerland). The instrument was equipped with an Olympus™ BX30 open microscope used for fine focusing of the laser beam on the sample surface (probe size <1 μm in diameter). The reflected Raman signal was sampled for 60 s in two cycles, and analyzed using a 600 lines/mm grating.
2.4. X-ray Synchrotron Microtomography
The 3D morphologies of olivine grains were investigated using X-ray microtomography images acquired at the beamline ID19 of the European Synchrotron Radiation Facility in Grenoble (France). The main advantages of synchrotron microtomography over standard desktop micro-computed tomography alternatives include better spatial resolution and limited beam hardening artefacts due to the use of highly-coherent monochromatic X-ray radiation. The instrumentation was operated at an energy of 35 keV and the setting was adjusted to image either X-ray absorption or phase contrast. Radiographs were taken over 360° at 0.18° intervals, all having a 16-bit grayscale depth and 2.2 µm pixel size. Cross-sectional tomographic slices and 3D volume renderings were then reconstructed and post-processed using the commercial AvizoFire™ software 9.4.
4. Discussion
4.1. Coupled vs. Decoupled Dissolution-Precipitation
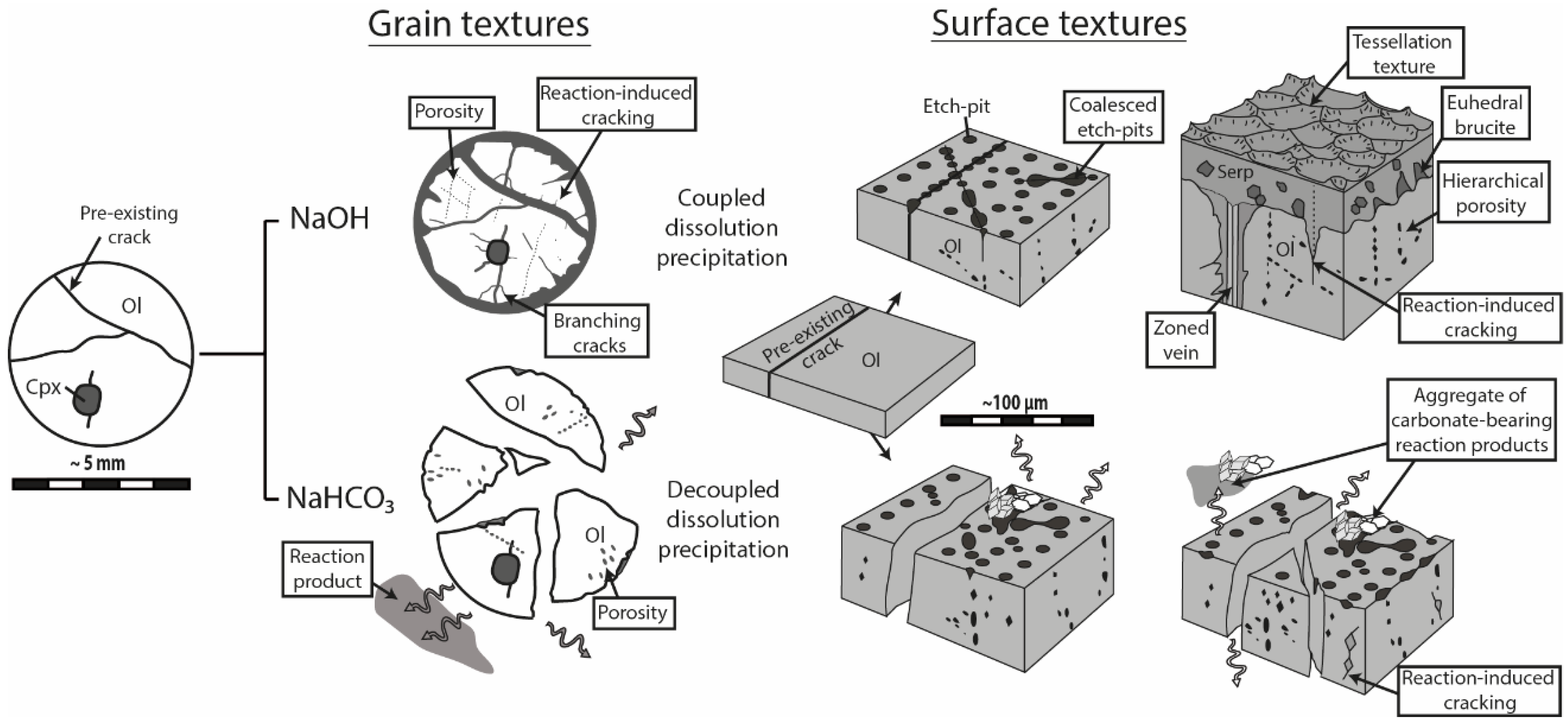
Serpentinization experiments carried out in alkaline NaOH and NaHCO
3 solutions revealed strikingly different macro- and microscopic textures (
Figure 10). During reaction in NaOH solution, olivine grains remained whole—as previously reported for smaller grain fraction [
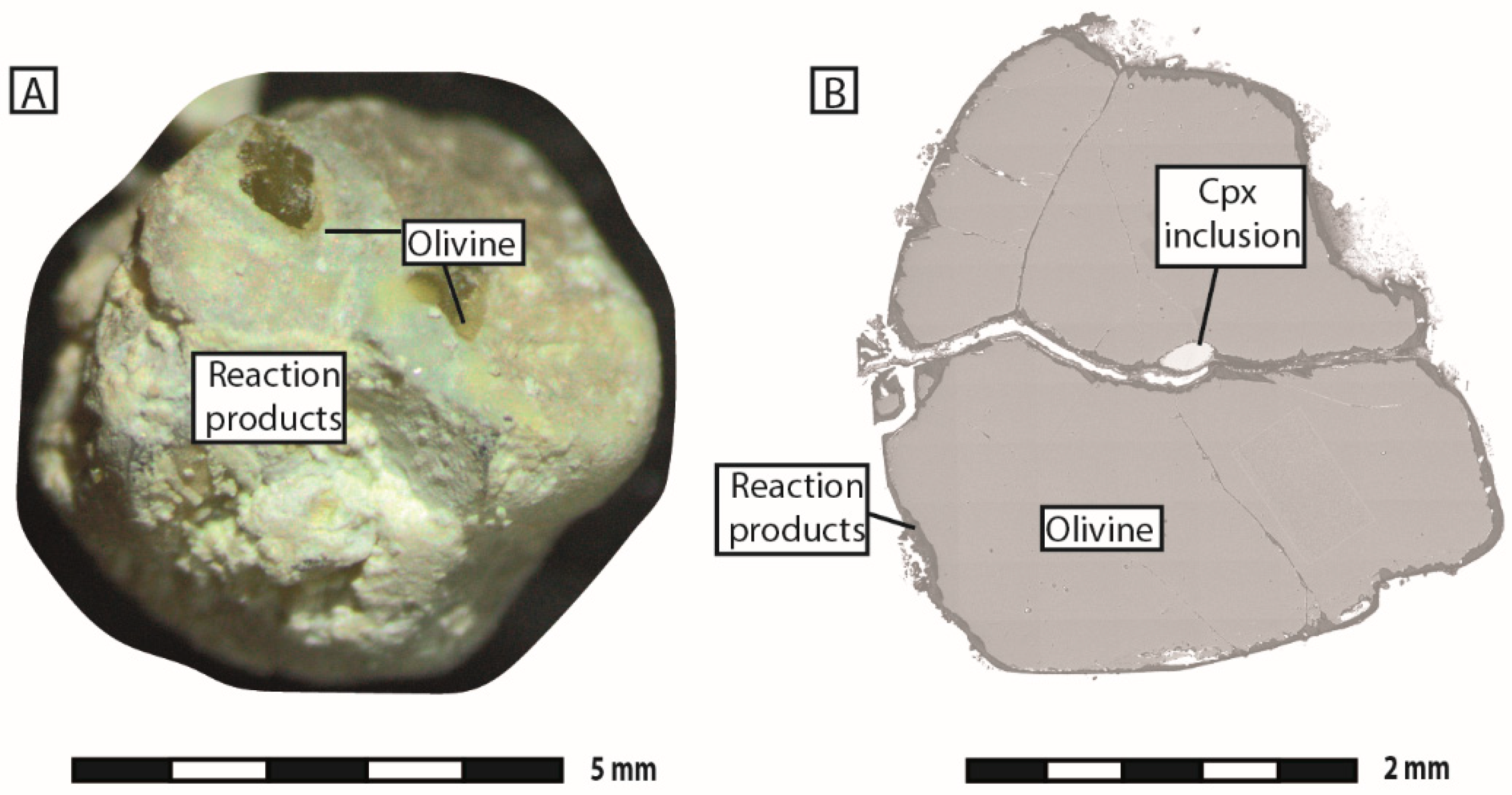
24]—and the reaction products precipitated in-situ, either as infilling material within pre-existing and new cracks or as a coherent cover onto the olivine surface. The extent of grain fracturation increased significantly, as the reaction proceeded mainly through intracrystalline reaction-induced cracking, and a hierarchical porosity network formed due to the progressive growth and coalescence of pores along microcracks. In contrast, olivine grains reacted in NaHCO
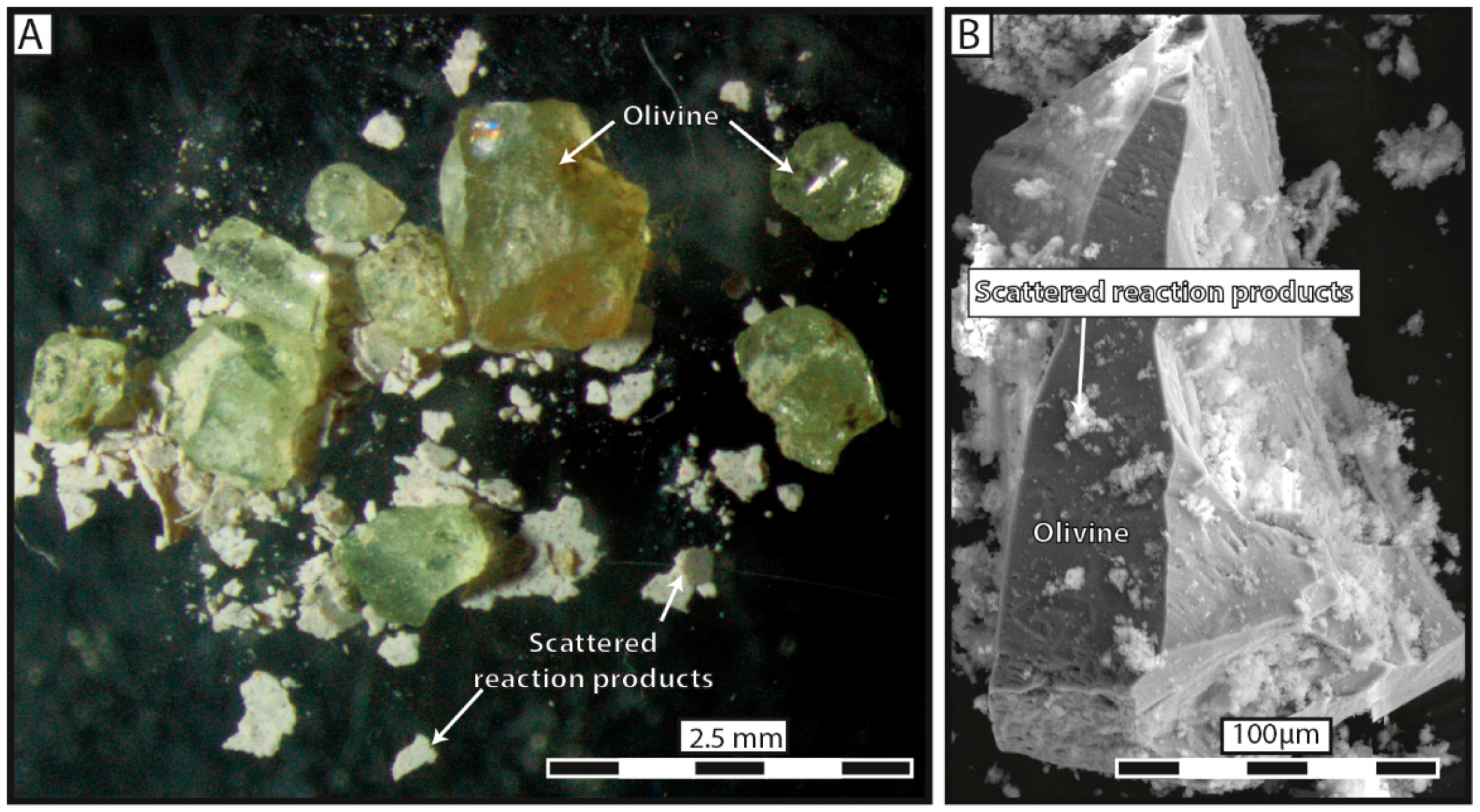
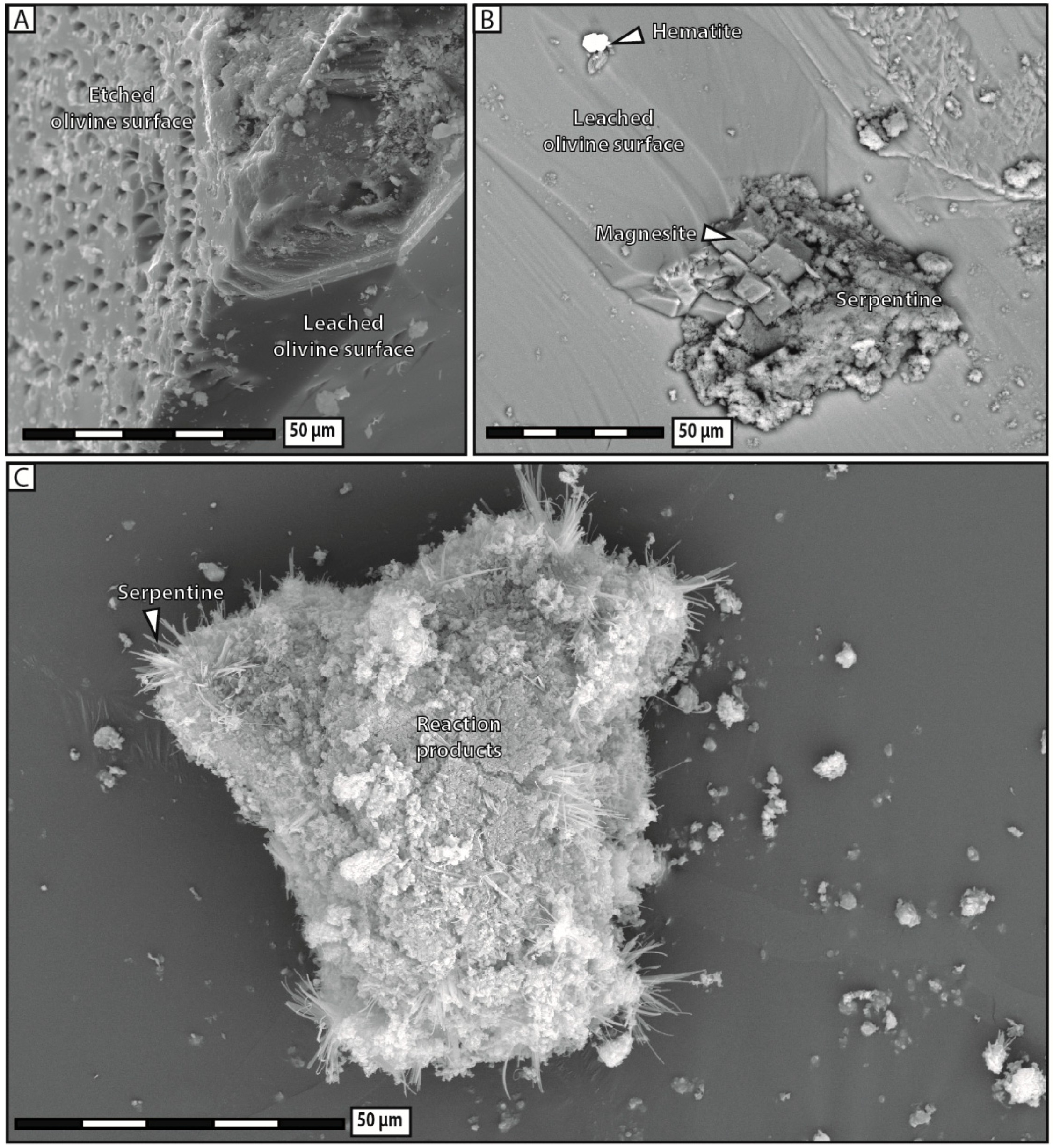
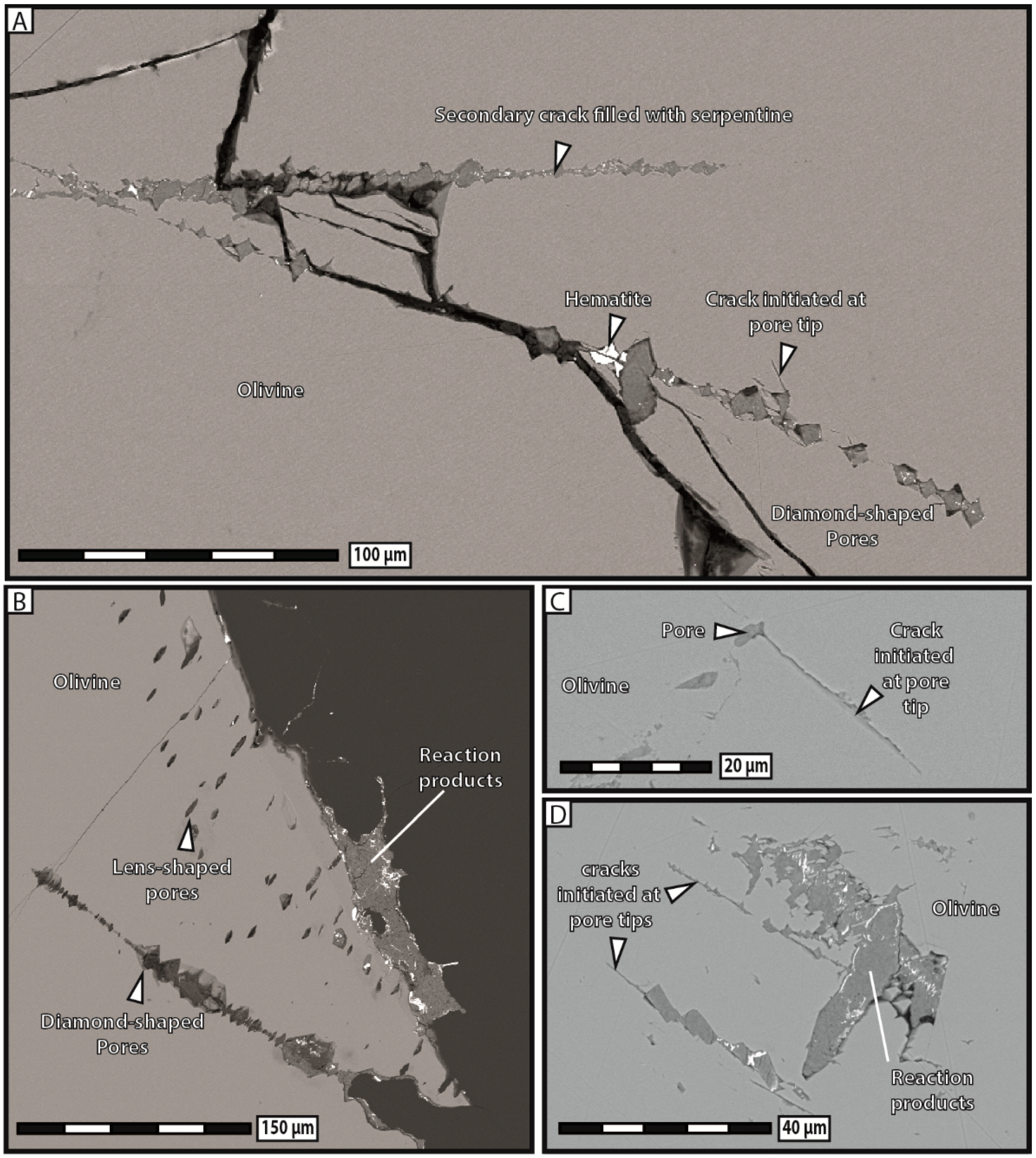
3 solution systematically broke apart into several fragments and most of the reaction products precipitated in aggregates widely scattered away from dissolution sites. Substantial porosity developed across olivine grains and no reaction-induced cracking occurred, except at the tips of the very few diamond-shaped pores filled with reaction products.
Our experimental data indicate a very different degree of coupling between dissolution and precipitation processes, depending on whether they have occurred in carbon dioxide-free (NaOH) or carbon dioxide-rich (NaHCO
3) solutions. In the former, dissolution of olivine and precipitation of the reaction products are intimately correlated, as they occur concomitantly and with minimum mass transfer, while in the latter, precipitation mainly takes place away from dissolution sites, thus indicating clear spatial decoupling between both processes. The reason for such a distinct reaction mechanism is still unclear, but it is surely not a consequence of the difference in alkalinity between the two reactive solutions. The reference experiment run in deionized water (pH
start = 5.8) displays no decoupling between dissolution and precipitation (see
Supplementary Materials S2). It has therefore closer similarities with the experiments carried out in highly alkaline NaOH solution (pH
start = 13.5), than with those conducted in mildly alkaline NaHCO
3 solution (pH
start = 8.7). Consequently, while the respective pH values can explain to some extent the different reaction rates in two types of experiments, they cannot account for the discrepant dissolution–precipitation behavior observed here.
A possible explanation for the distinct degree of coupling between dissolution and precipitation could be the disequilibrium between the respective rates of precipitation and fluid flow [
13]. In a situation in which precipitation is sluggish and slow, as expected for carbonate minerals [
35], fluid flow and transport of chemical species may overtake crystal growth. The high chemical potential between the crack interfaces and the precipitation sites would sustain the constant removal of the reaction products, and shift precipitation far away from dissolution sites. In contrast, a high precipitation and growth rate of the reaction products, such as that of brucite and serpentine during hydration experiments, triggers veining and partial clogging of the circulation pathways. Consequently, fluid flow and mass transfer capabilities may drop down drastically, which further favors in-situ precipitation. This interpretation is well in line with the recent experimental work of Malvoisin and Brunet (2014) on sintered olivine aggregates [
28], who reported that the nucleation and growth of brucite directly onto the olivine surface is kinetically favored by the system, as it minimizes surface energy. We therefore consider that the rapid growth of brucite and serpentine has a local inhibitory effect on fluid flow, leaving the reaction products with no other option but to precipitate in situ. In contrast, the sluggishness of carbonate precipitation can keep pre-existing fluid circulation pathways open over long periods, sustaining efficient mass transport, and therefore, delocalized precipitation.
The clear-cut results reported here have far-reaching consequences on the way the massive increase in volume accompanying serpentinization reactions is accommodated. A high degree of spatial coupling between dissolution and precipitation implies that the volumetric changes have to be fitted in-situ, e.g., through reaction-induced cracking, otherwise the reaction will stop. In contrast, when both mechanisms are spatially decoupled, the increase in volume is compensated for through long-range transportation of the reaction products. The fact that carbonation tends to prioritize mass transfer is not anecdotal, as the reaction is mostly self-driven and likely to persist over time. This positive loop, in which the constant removal of the reaction products enhances rock permeability, and therefore fluid flow and mass transfer, suggests that the presence of carbon dioxide in the reactive fluids facilitates the widespread dispersal of the reaction products and the redistribution of chemical elements within the oceanic lithosphere. This process leads to sharp mineralogical and chemical dissociation between the parent rock and the carbonated reaction products, as illustrated by the textures of natural ophicarbonates [
11,
36,
37,
38]. The pervasive break-up of the olivine grains observed after the carbonation experiments can be interpreted as a low-pressure analogue for the intensely brecciated textures commonly reported for natural ophicarbonates found at the top of serpentinite units [
39,
40,
41,
42], even where no significant tectonic activity is invoked. In both cases, fragmentation can be easily explained by the lack of cohesion commonly provided by veins, in which serpentine acts as a glue, welding olivine fragments together.
4.2. Reaction-Induced Cracking
Our experimental data have provided ample evidence for the formation of new intracrystalline cracks during serpentinization. These results are in good agreement with those reported in natural serpentinites by other authors [
9,
21], who proposed that the nucleation and propagation of new cracks is driven by the crystallization stress accumulated at the tips of dissolution notches during progressive serpentinization [
43,
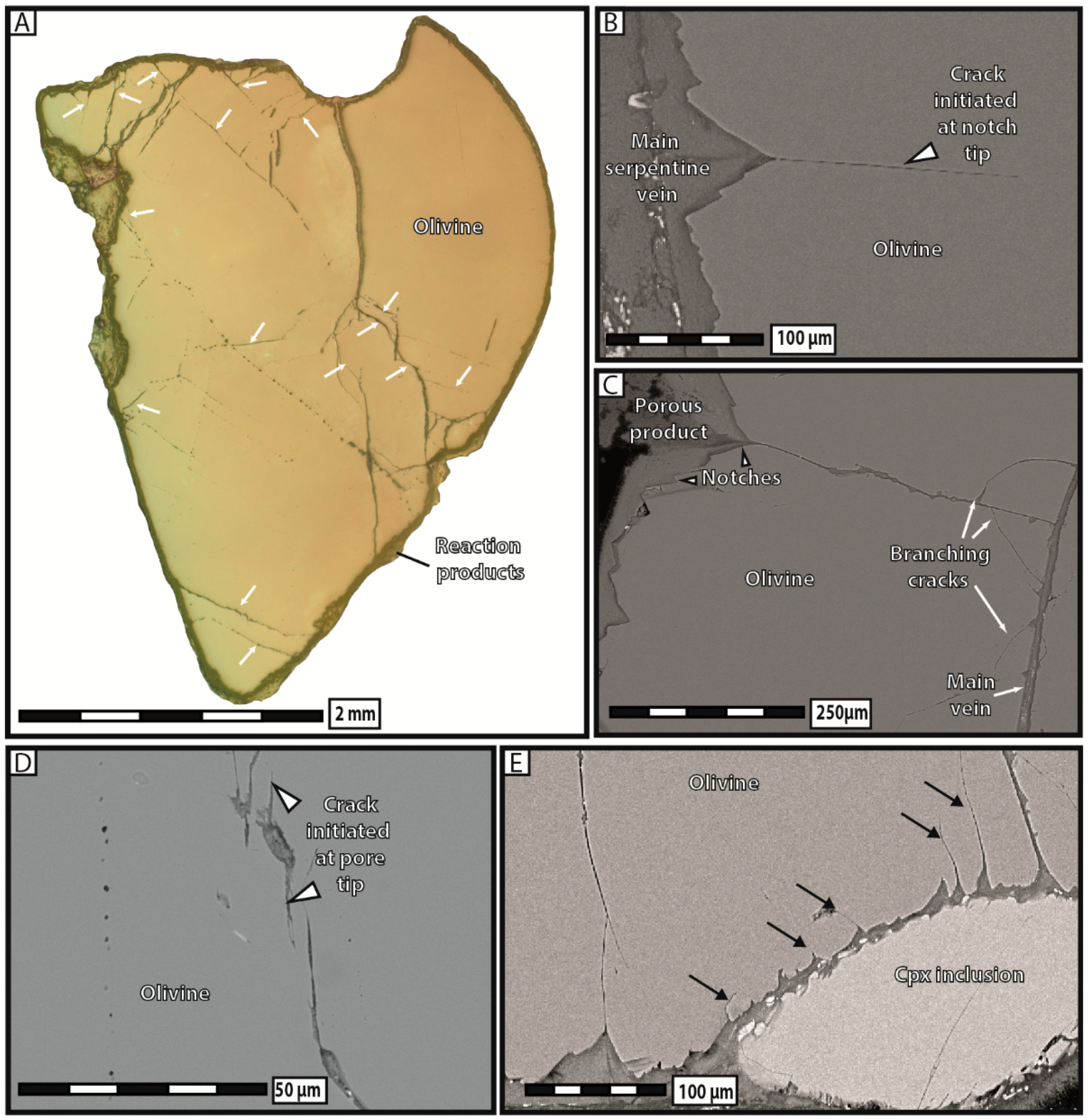
44]. We therefore support the idea that the large increase in volume associated with phase transformation does not necessarily impede the extent of serpentinization reactions, but rather triggers the formation of a hierarchical fracturation network allowing for serpentinization to continue during hydration. The fact that reaction-induced cracking is abundant in hydration experiments, i.e., reactions in which no long-range mass transfer and no spatial decoupling between dissolution and precipitation have occurred, indicates that, at least in serpentinite systems, in-situ precipitation of the reaction products is a prerequisite to raise stress at notch tips and trigger mineral/rock fracturation. This point is further evidenced in our carbonation reactions, in which only the diamond-shaped pores filled with reaction products have generated new cracks. Consequently, our experimental work unequivocally argues for a cause-and-effect relationship linking in-situ phase transformation and intracrystalline reaction-induced cracking whatever the reaction (hydration or carbonation). The striking similarities between the reaction-induced cracks observed experimentally here and in natural ophiolites give further credence to this hypothesis.
There is little doubt that if the reaction products had massively precipitated in situ during our carbonation experiments, substantially more reaction-induced cracking, similar to that observed after hydration experiments, would have taken place in that case too. Indeed, carbonation in no way constitutes an intrinsic obstacle to reaction-induced cracking, as long as dissolution and precipitation mechanisms are spatially coupled and dominant over mass transfer. This finding leads us to stress that a high degree of spatial coupling between dissolution and precipitation constitutes an essential condition for reaction-induced cracking, much more than the type of reaction itself, at least when the confining pressure is very low. However, carbonation reactions show a weaker tendency for in situ precipitation, and consequently also, reaction-induced cracking rather, than hydration reactions. Alternatively, carbonation reactions could also give rise to delayed reaction-induced cracking occurring in the nearby rocks where precipitation takes place, but our experiments on single grains did not allow for such observations.
It is worth noting that, in our experiments, cracking has been locally enhanced by the occurrence of less-reactive mineral inclusions of pyroxene or spinel. This process is common and has been widely reported in the literature for natural peridotites [
32,
45]. Pyroxene and spinel are highly resistant to alteration and act as hard spots around which stress accumulates and radial cracks nucleate. This effect may be further amplified by the high serpentinization efficiency attributed to a greater silica activity at the olivine-pyroxene boundary [
46]. We therefore conclude that natural, inclusion-rich olivine grains and peridotites may be more prone to intracrystalline reaction-induced cracking than monomineralic rocks or manufactured samples of dunite sintered from synthetic olivine powder.
4.3. Porosity, an Alternative and Complementary Way for Reaction Propagation
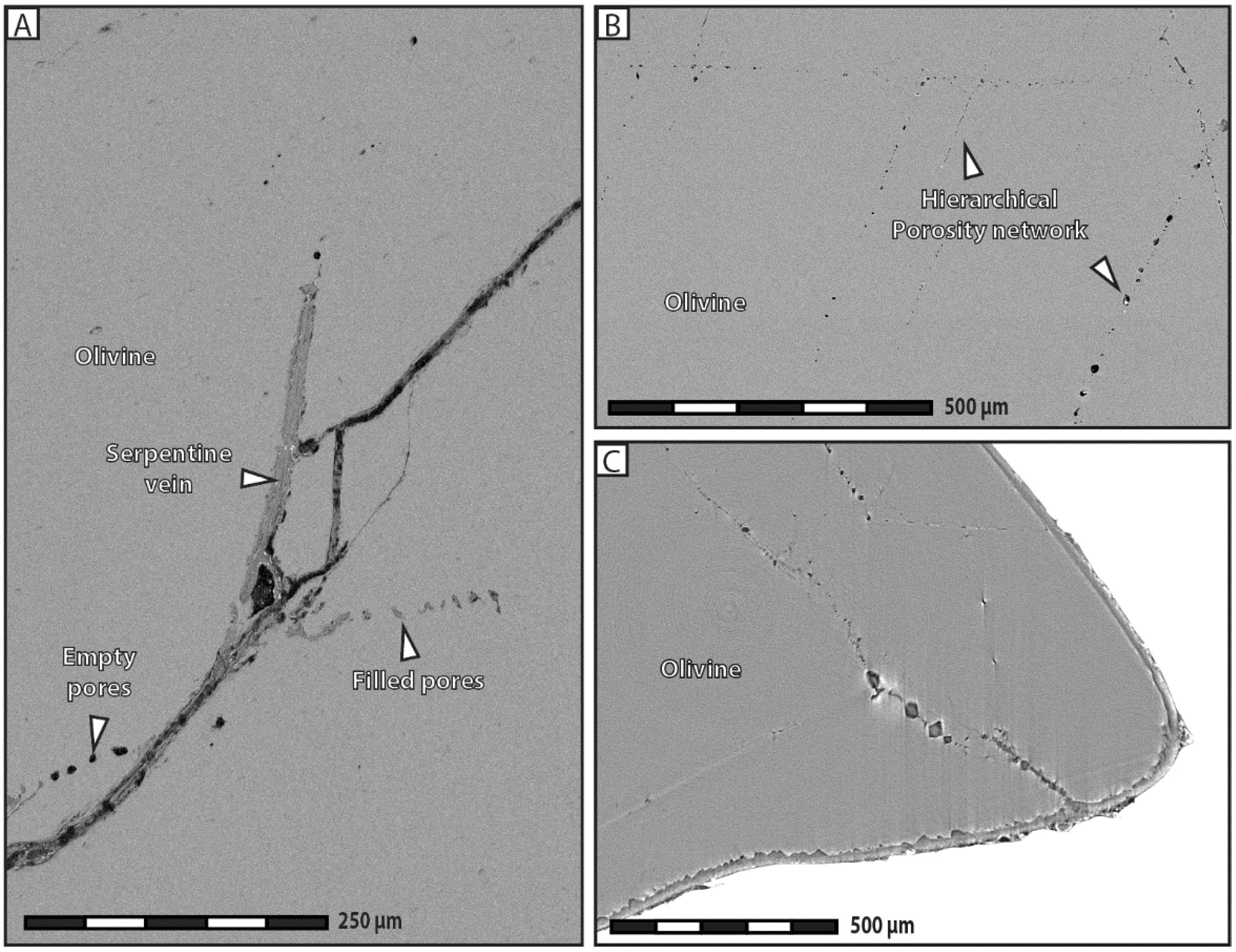
Our experimental data indicate that the development of porosity is an essential aspect of serpentinization reactions, whatever the composition of the reactive fluid. As a general trend, our results show that the apparent pore abundance is positively correlated with fracturation, in the sense that highly fractured grains contain more pores than less fractured ones in hydration experiments. Moreover, we have proposed several lines of evidence to demonstrate that porosity and fracturation networks tend to mutually self-propagate in the course of the reaction. The observation that pores preferably line up along microcracks indicates that fracturation actively participates in spreading porosity towards the interior of olivine grains. The fact that juvenile cracks obviously nucleate at the tips of diamond-shaped pores shows that the reverse is also true, i.e., that pores play an equally important role in the propagation of the fracture network. This self-reinforcing loop, in which more cracks generate more pores, and vice versa, is noteworthy, as it guarantees efficient progression of the reaction front through the olivine grains, and therefore the survival of serpentinization reactions over time in a context of massive volume increase and limited mass transfer.
Besides highlighting the strong mutual correlation between porosity and fracturation, during both hydration and carbonation, our experimental results suggest that some form of porosity may develop in absence of cracks too. The growth of isolated pores in the outer fringes of olivine grains during carbonation experiments indicates that fracturation is not an absolute requirement for pore formation. According to this view, porosity is thought to propagate spontaneously, e.g., in response to high chemical gradients between dissolution and precipitation sites. Alternatively, those pores could also be 2D cross-sectional views of dissolution channels, similar to those recently reported by Xing et al. [
21]. Whatever the interpretation, the occurrence of porosity features disconnected from cracks—in good agreement with previous studies [
47,
48]—is not irrelevant, as it suggests that serpentinization could, to some extent, take place with little or no fracturation at all. This result stresses the multivalent nature of serpentinization, which can follow alternative reaction paths to keep progressing over time.
Finally, the coexistence of pores filled with reaction products immediately adjacent to empty pores suggests that serpentinization continuously creates and eliminates porosity, in accordance with the physicochemical conditions at a specific time and place. It must be noted that both filled and empty pores can positively influence the course of serpentinization reactions. While empty pores facilitate fluid flow and mass transfer, pores filled with reaction products participate in the spreading of the fracturation network through reaction-induced cracking. This observation was already noted in other studies [
49,
50].
5. Implications and Conclusions
In the present study, hydration and carbonation experiments were carried out on coarse olivine grains in order to better understand the processes at play during serpentinization reactions. The striking parallels that can be drawn between the textural features observed here and in natural outcrops are excellent indicators of the relevance of the experimental set-up and starting materials. The tendency of hydrated olivine grains to accommodate the reaction products in situ while remaining whole is characterized in hydrated peridotites by the widespread occurrence of mesh texturing and veining. In contrast, the delocalized precipitation of the reaction products and the pervasive break-up of olivine grains after carbonation reactions is in good agreement with the bimodal mineral distribution and the highly brecciated and often chaotic nature of many ophicarbonates. We therefore concluded that the fluid composition, and in particular the carbon dioxide content of the volatile phase, not only determines the mineral species occurring in the reaction products, but also drastically controls the textures of serpentinized rocks and the degree of redistribution of the chemical elements in the oceanic lithosphere.
Our study has provided the first experimental evidence for a cause-and-effect relationship between in situ precipitation and intracrystalline reaction-induced cracking in olivine especially during hydration experiments. We have shown that this process occurs regardless of the type of reactive fluid, commencing as soon as precipitation of the reaction products occurs in situ and exerts a sufficient differential stress at the tips of dissolution notches or pores. This finding is particularly timely, as two very recent experimental serpentinization studies have questioned the very existence of such a feature at the expense of intercrystalline reaction-induced cracking [
13,
21]. Based on our own experimental results and observations, however, we strongly advocate that intracrystalline reaction-induced cracking does occur in olivine, and that it is an essential component of rock fracturation during serpentinization reactions.
Finally, our study has highlighted the extreme versatility and multi-faceted nature of serpentinization. Processes that could at first be misidentified as detrimental, such as massive volume increase during phase transformations and ensuing pore clogging, are in reality not major obstacles for the continuation of serpentinization reactions, due to the ability of the system to adapt through a series of positive loops, making it resilient and enduring over time. Among the most striking features are the paired and concomitant development of fracturation and porosity networks, and the possibility to extend (or reduce) porosity even in absence of fracturation. The many strings serpentinization has to its bow undoubtedly explains at least part of its considerable success over time and the widespread occurrence of fully serpentinized rocks in very different geological contexts all over the world.