Sulfide Formation as a Result of Sulfate Subduction into Silicate Mantle (Experimental Modeling under High P,T-Parameters)
Abstract
1. Introduction
2. Materials and Methods
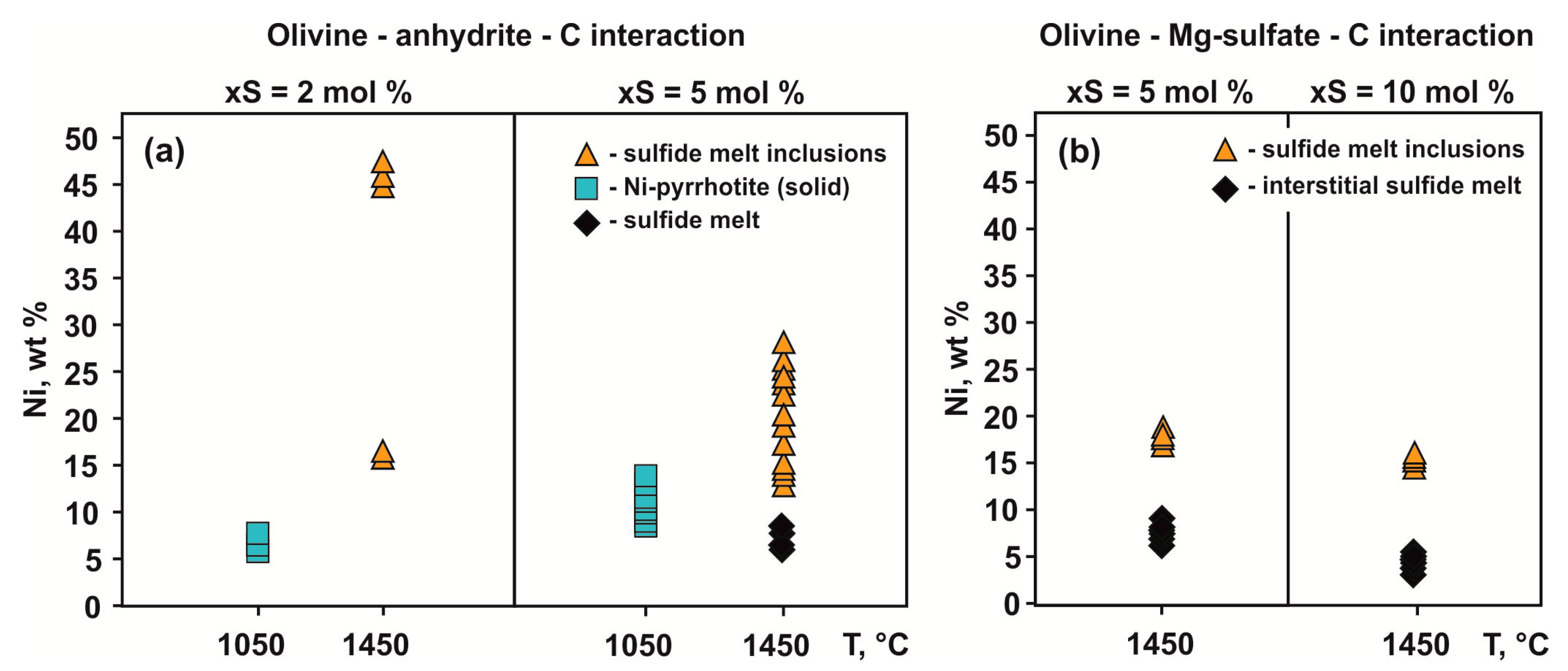
3. Results
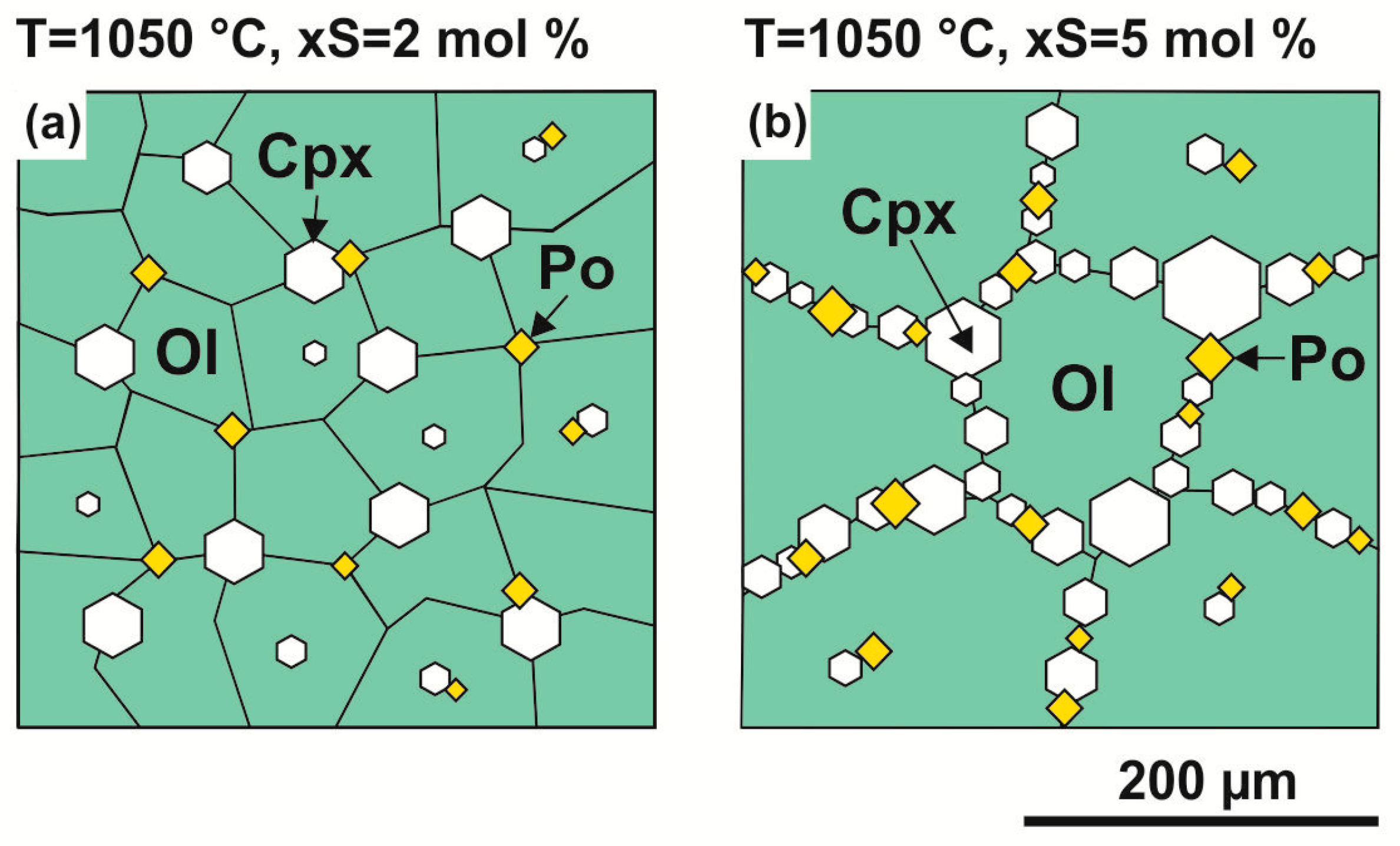
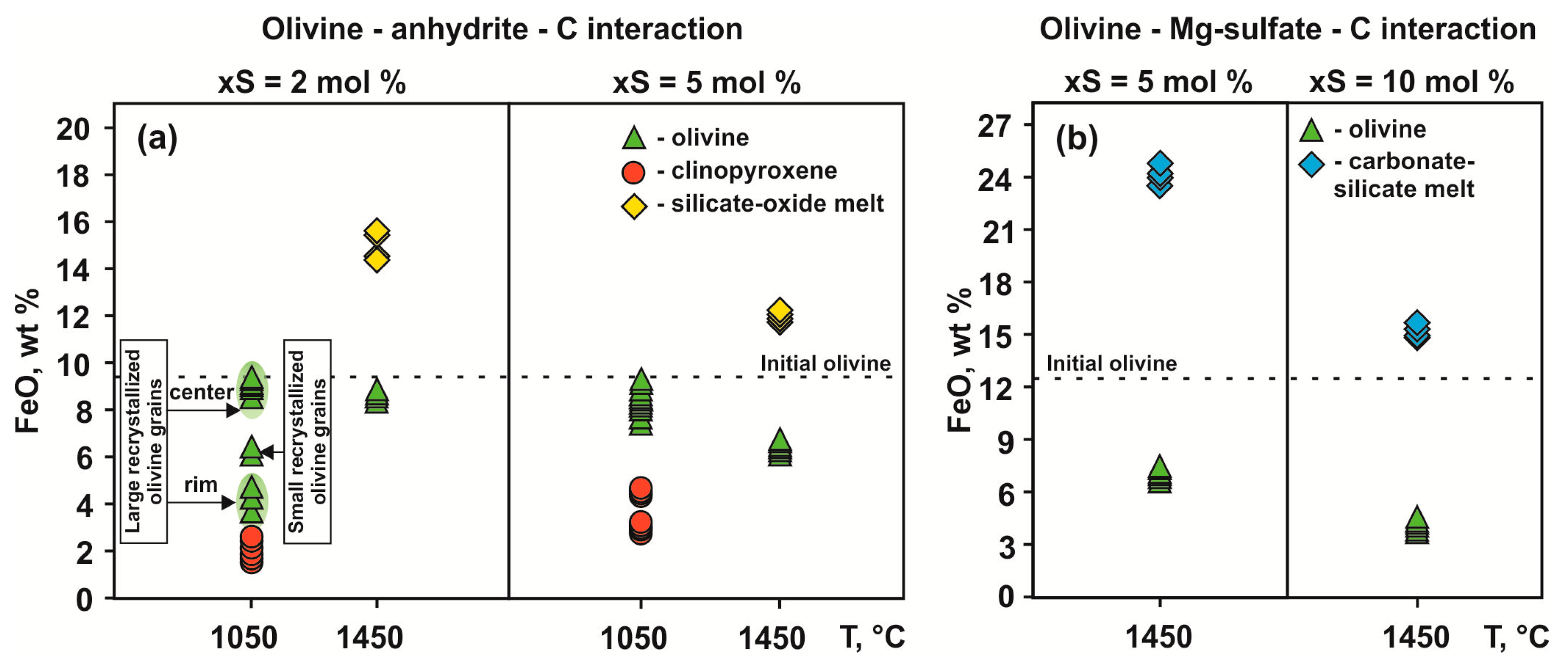
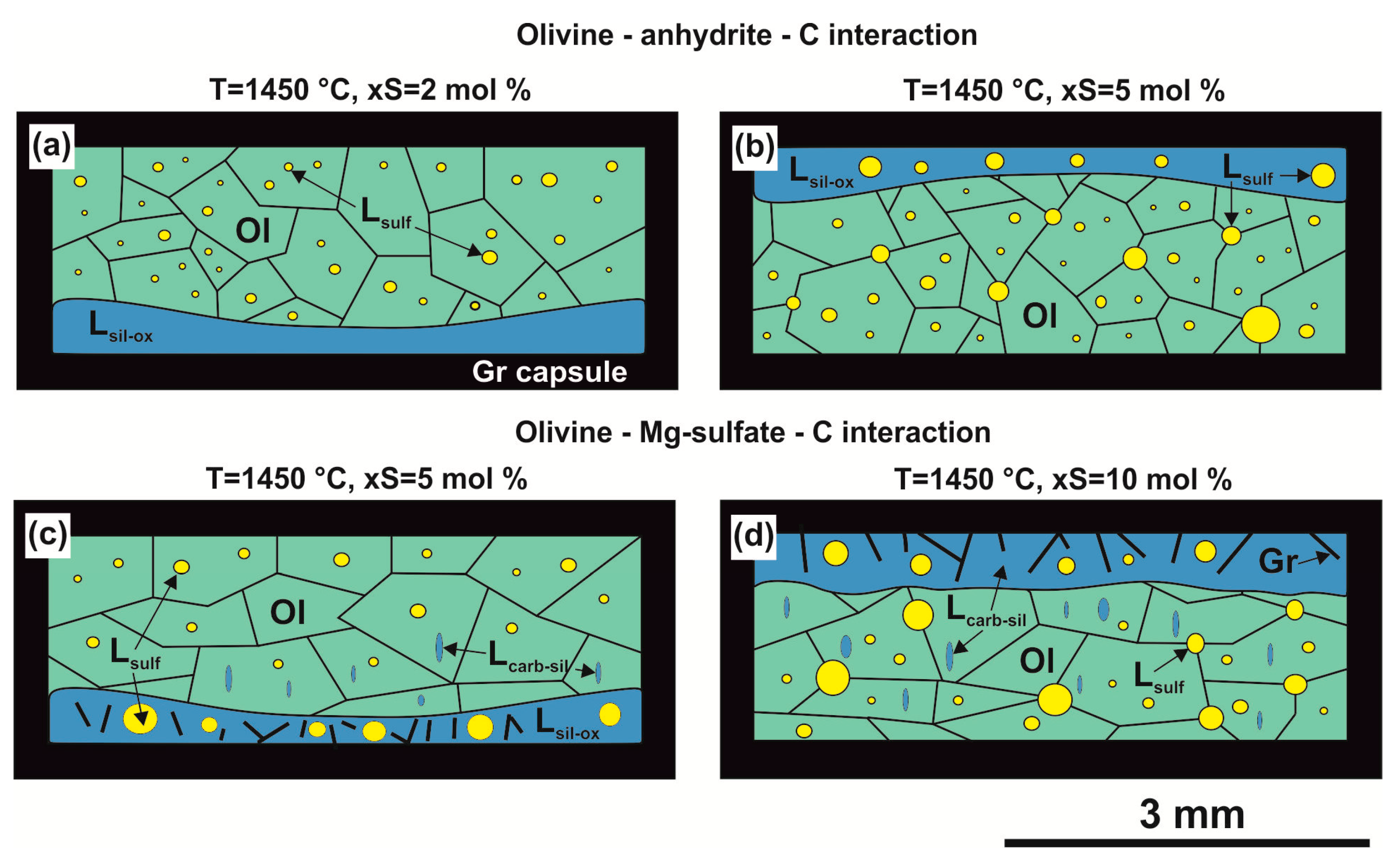
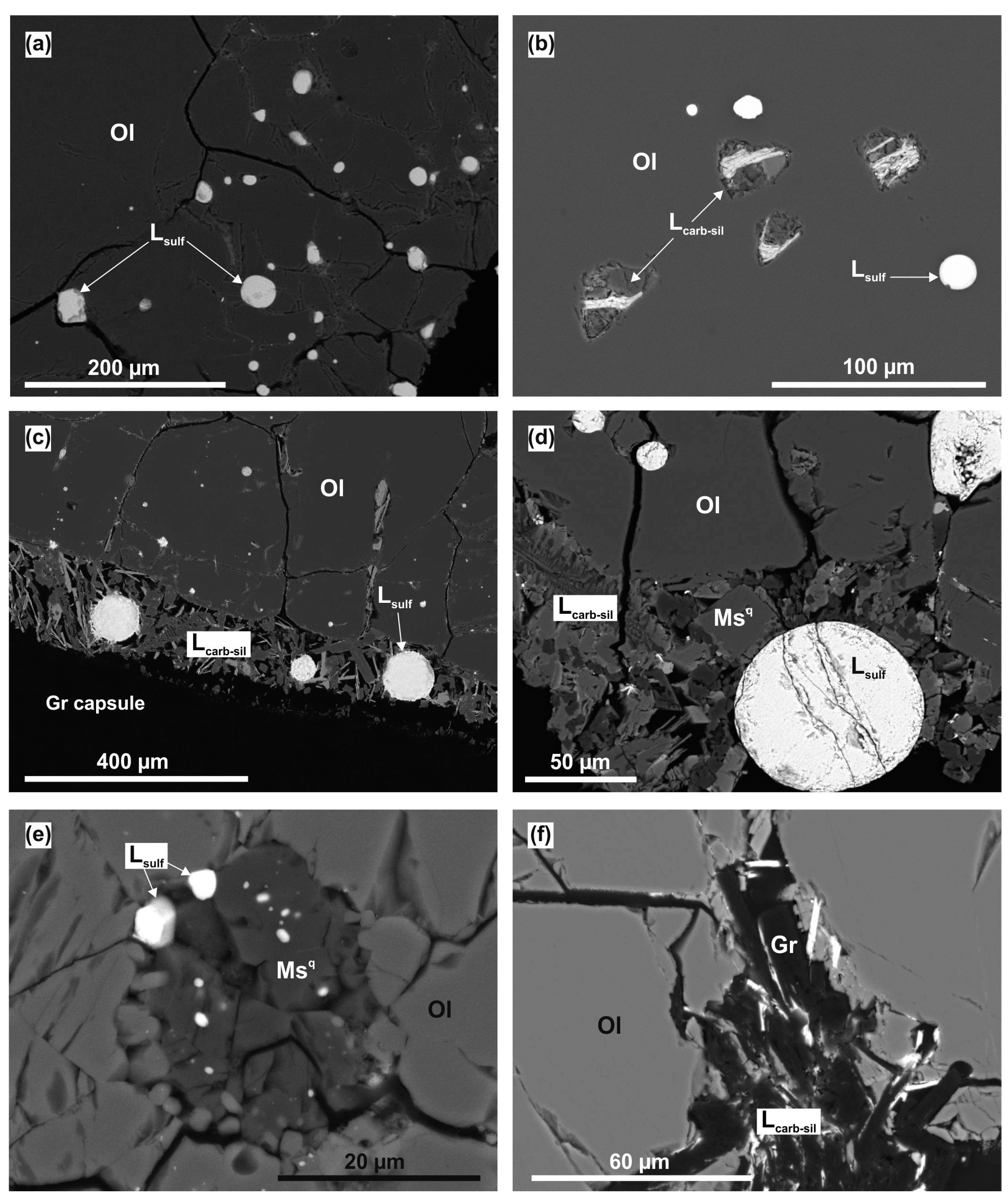
3.1. Interaction in the Olivine–Anhydrite–C System
3.2. Interaction in the Olivine—Mg-sulfate—C System
4. Discussion
4.1. Reconstruction of Sulfate-Olivine Interaction Processes
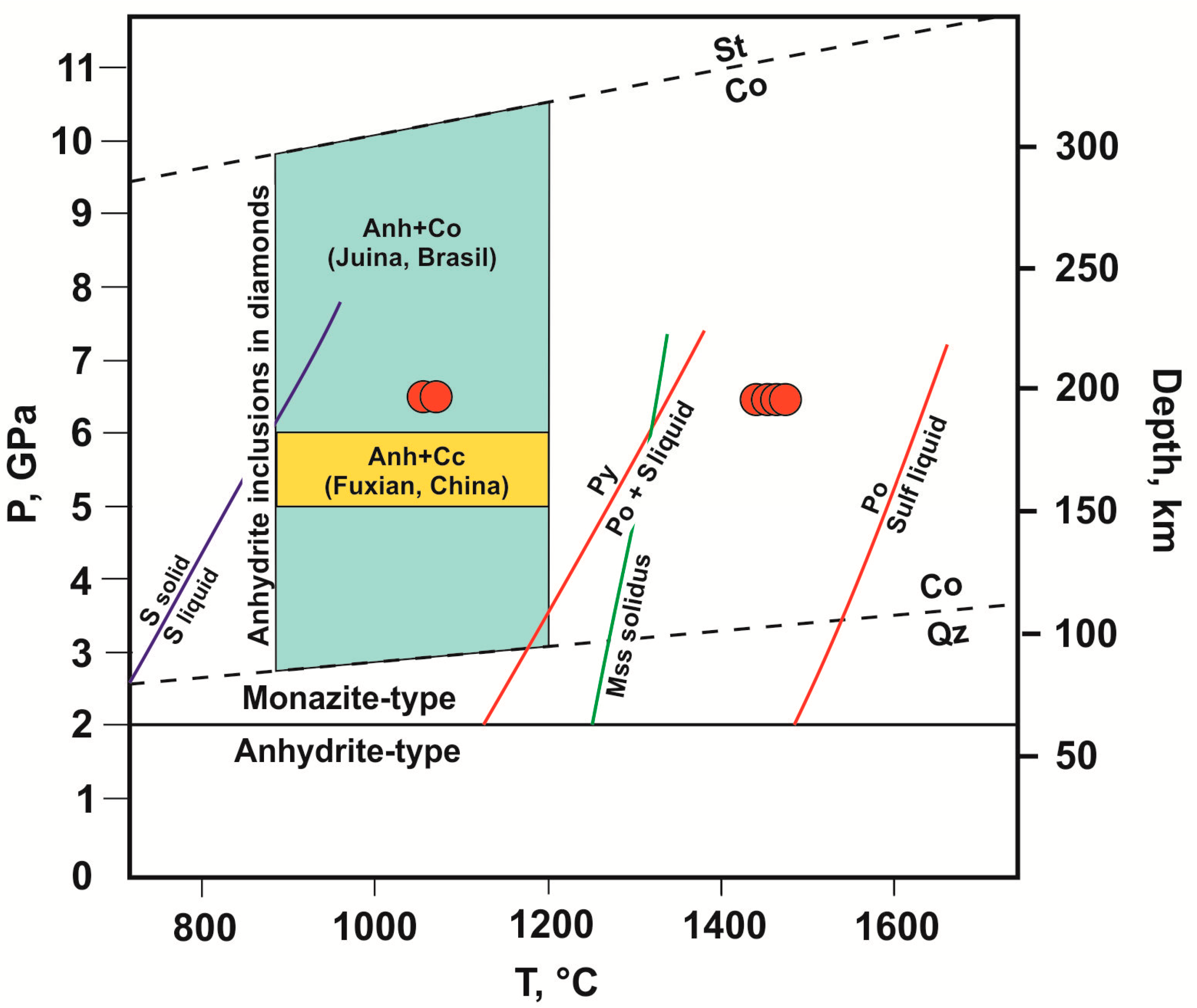
4.1.1. S-Rich Fluid Formation Processes in Olivine–Anhydrite–C System
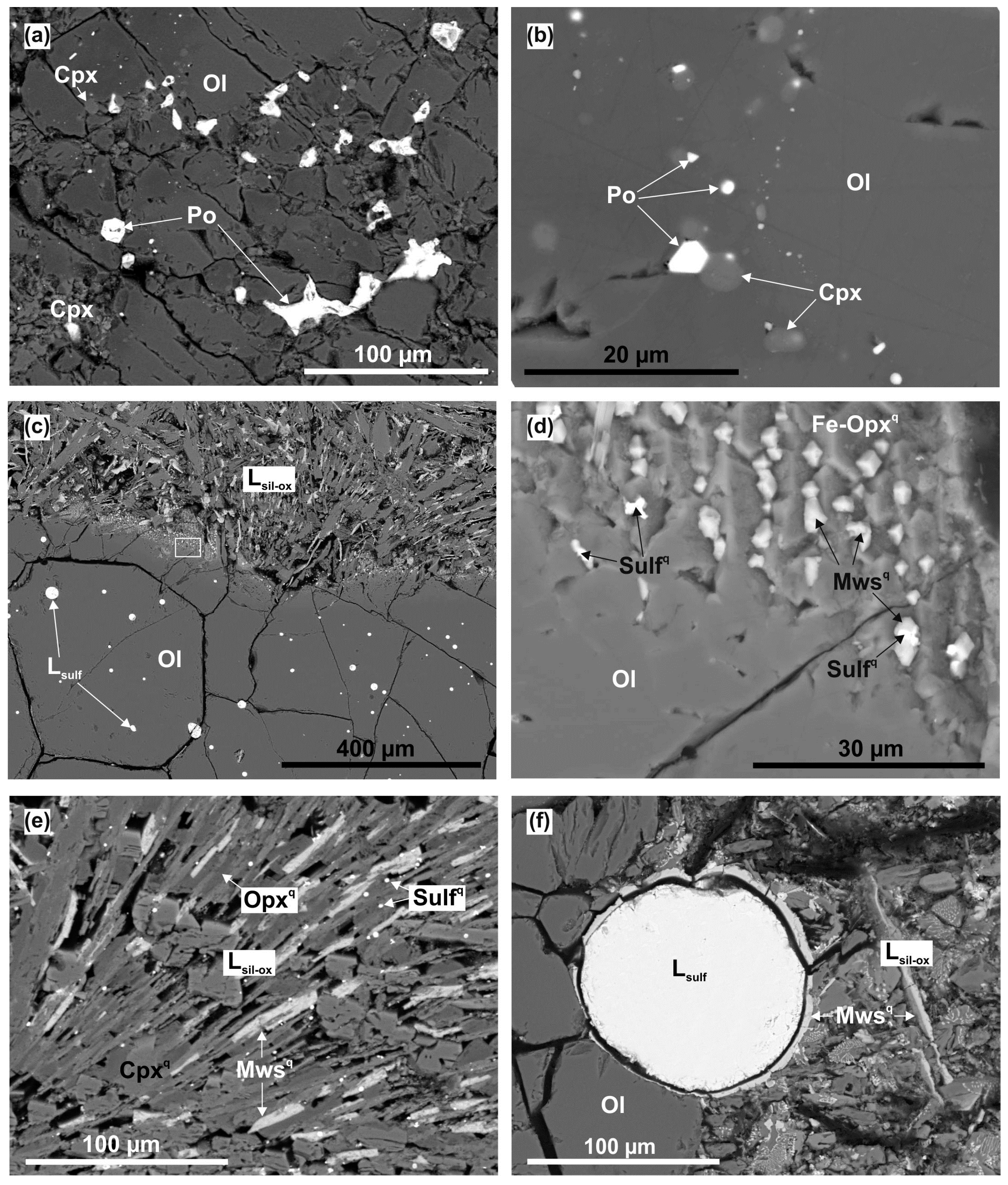
4.1.2. Fe,Ni-Sulfide Formation via Olivine Interaction with S-rich Fluid in the Olivine–Anhydrite–C System
4.1.3. The Formation of Silicate-Oxide and Sulfide Melts in the Olivine–Anhydrite–C System
4.1.4. The Formation of Carbonate-Silicate and Sulfide Melts in the Olivine–Mg-Sulfate–C System
4.2. Scenarios of Ca,Mg-Sulfates Interaction with Olivine Under High P,T-Conditions: Implications to Mantle Sulfide, Silicate and Carbonate Formation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alard, O.; Lorand, J.-P.; Reisberg, L.; Bodinier, J.-L.; Dautria, J.-M.; O’Reilly, S. Volatile-rich metasomatism in Montferrier xenoliths (Southern France): Implications for the abundances of chalcophile and highly siderophile elements in the subcontinental mantle. J. Petrol. 2011, 52, 2009–2045. [Google Scholar] [CrossRef]
- Delpech, G.; Lorand, J.P.; Grégoire, M.; Cottin, J.-Y.; O’Reilly, S.Y. In-situ geochemistry of sulfides in highly metasomatized mantle xenoliths from Kerguelen, southern Indian Ocean. Lithos 2012, 154, 296–314. [Google Scholar] [CrossRef]
- Evans, K.A.; Powell, R. The effect of subduction on the sulphur, carbon and redox budget of lithospheric mantle. J. Metamorph. Geol. 2015, 33, 649–670. [Google Scholar] [CrossRef]
- Zajacz, Z. The effect of melt composition on the partitioning of oxidized sulfur between silicate melts and magmatic volatiles. Geochim. Cosmochim. Acta 2015, 158, 223–244. [Google Scholar] [CrossRef]
- Kitayama, Y.; Thomassot, E.; Galy, A.; Golovin, A.; Korsakov, A.; d’Eyrames, E.; Assayag, N.; Bouden, N.; Ionov, D. Co-magmatic sulfides and sulfates in the Udachnaya-East pipe (Siberia): A record of the redox state and isotopic composition of sulfur in kimberlites and their mantle sources. Chem. Geol. 2017, 455, 315–330. [Google Scholar] [CrossRef]
- Alt, J.C.; Shanks, W.C.; Jackson, M.C. Cycling of sulfur in subduction zones: The geochemistry of sulfur in the Mariana-Island Arc and back-arc trough. Earth Planet. Sci. Lett. 1993, 119, 477–494. [Google Scholar] [CrossRef]
- Evans, K.A. The redox budget of subduction zones. Earth Sci. Rev. 2012, 113, 11–32. [Google Scholar] [CrossRef]
- Jégo, S.; Dasgupta, R. The fate of sulfur during fluid-present melting of subducting basaltic crust at variable oxygen fugacity. J. Petrol. 2014, 55, 1019–1050. [Google Scholar] [CrossRef]
- Tomkins, A.; Evans, K.A. Separate zones of sulfate and sulfide release from subducted mafic oceanic crust. Earth Planet. Sci. Lett. 2015, 428, 73–83. [Google Scholar] [CrossRef]
- Wirth, R.; Kaminsky, F.; Matsyuk, S.; Schreiber, A. Unusual micro- and nano-inclusions in diamonds from the Juina Area, Brazil. Earth Planet. Sci. Lett. 2009, 286, 292–303. [Google Scholar] [CrossRef]
- Leung, I.S. Silicon carbide cluster entrapped in a diamond from Fuxian, China. Am. Mineral. 1990, 65, 1110–1119. [Google Scholar]
- Evans, K.A.; Tomkins, A.G.; Cliff, J.; Fiorentini, M.L. Insights into subduction zone sulfur recycling from isotopic analysis of eclogite-hosted sulfides. Chem. Geol. 2014, 365, 1–19. [Google Scholar] [CrossRef]
- Stephens, D.R. The hydrostatic compression of eight rocks. J. Geophys. Res. 1968, 69, 2967–2978. [Google Scholar] [CrossRef]
- Borg, I.Y.; Smith, D.K. A high pressure polymorph of CaSO4. Contrib. Mineral. Petrol. 1975, 50, 127–133. [Google Scholar] [CrossRef]
- Langenhorst, F.; Deutsch, A.; Homeman, U.; Ivano, B.A.; Lounejeva, E. On the shock ehaviour of anhydrite: Experimental results and natural observations. In Proceedings of the 34th Annual Lunar and Planetary Science Conference, League City, TX, USA, 17–21 March 2003. [Google Scholar]
- Bradbury, S.E.; Williams, Q. X-ray diffraction and infrared spectroscopy of monazite-structured CaSO4 at high pressures: Implications for shocked anhydrite. J. Phys. Chem. Solids 2009, 70, 134–141. [Google Scholar] [CrossRef]
- Fujii, T.; Ohfuji, H.; Inoue, T. Phase relation of CaSO4 at high pressure and temperature up to 90 GPa and 2300 K. Phys. Chem. Miner. 2016, 43, 353–361. [Google Scholar] [CrossRef]
- Jégo, S.; Dasgupta, R. Fluid-present melting of sulfide-bearing ocean-crust: Experimental constraints on the transport of sulfur from subducting slab to mantle wedge. Geochim. Cosmoсhim. Acta 2013, 110, 106–134. [Google Scholar] [CrossRef]
- Haggerty, S.E. Diamond genesis in a multiply-constrained model. Nature 1986, 320, 34–38. [Google Scholar] [CrossRef]
- Sato, K.; Katsura, T. Sulfur: A new solvent-catalyst for diamond synthesis under high-pressure and high-temperature conditions. J. Cryst. Growth 2001, 223, 189–194. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Borzdov, Y.M.; Kupriyanov, I.N.; Gusev, V.A.; Khokhryakov, A.F.; Sokol, A.G. High pressure synthesis and characterization of diamond from sulfur-carbon system. Diam. Relat. Mater. 2001, 10, 2145–2152. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Kupriyanov, I.N.; Borzdov, Y.M.; Sokol, A.G.; Khokhryakov, A.F. Diamond Crystallization from a sulfur−carbon system at HPHT Conditions. Cryst. Growth Des. 2009, 9, 2922–2926. [Google Scholar] [CrossRef]
- Kullerud, G.; Yoder, H.S., Jr. Sulfide-Silicate Relations: Carnegie Institution of Washington Year Book, v. 62; Carnegie Institution of Washington: Washington, DC, USA, 1963; pp. 215–218. [Google Scholar]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Sobolev, N.V. Sulfidation of silicate mantle by reduced S-bearing metasomatic fluids and melts. Geology 2016, 44, 271–274. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F. Fluid-bearing alkaline–carbonate melts as the medium for the formation of diamonds in the Earth’s mantle: An experimental study. Lithos 2002, 60, 145–159. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Bayukov, O.A.; Zdrokov, E.V. Iron carbide as a source of carbon for graphite and diamond formation under lithospheric mantle P-T parameters. Lithos 2017, 286–287, 151–161. [Google Scholar] [CrossRef]
- Dasgupta, R.; Buono, A.; Whelan, G.; Walker, D. High-pressure melting relations in Fe–C–S systems: Implications for formation, evolution, and structure of metallic cores in planetary bodies. Geochim. Cosmochim. Acta 2009, 73, 6678–6691. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Bataleva, Y.V.; Sokol, A.G.; Palyanova, G.A.; Kupriyanov, I.N. Reducing role of sulfides and diamond formation in the Earth’s mantle. Earth Planet. Sci. Lett. 2007, 260, 242–256. [Google Scholar] [CrossRef]
- Cartigny, P.; Boyd, S.R.; Harris, J.W.; Javoy, M. Nitrogen isotopes in peridotitic diamonds from Fuxian, China: The mantle signature. Terra Nova 1997, 9, 175–179. [Google Scholar] [CrossRef]
- Brazhkin, V.V.; Popova, S.V.; Voloshin, R.N. Pressure-temperature phase diagram of molten elements: Selenium, sulfur and iodine. Phys. B Condens. Matter 1999, 265, 64–71. [Google Scholar] [CrossRef]
- Sharp, W.E. Melting curves of sphalerite, galena, and pyrrhotite and the decomposition curve of pyrite between 30 and 65 kilobars. J. Geophys. Res. 1969, 74, 1645–1652. [Google Scholar] [CrossRef]
- Zhang, Z.; Lentsch, N.; Hirschmann, M.M. Carbon-saturated monosulfide melting in the shallow mantle: Solubility and effect on solidus. Contrib. Mineral. Petrol. 2015, 170, 47. [Google Scholar] [CrossRef]
- Papike, J.J.; Spilde, M.N.; Fowler, G.W.; Layne, G.D.; Shearer, C.K. The Lodran primitive achondrite: Petrogenetic insights from electron and ion microprobe analysis of olivine and orthopyroxene. Geochim. Cosmochim. Acta 1995, 59, 3061–3070. [Google Scholar] [CrossRef]
- Fleet, M.E.; MacRae, N.D. Sulfidation of Mg-rich olivine and the stability of niningerite in enstatite chondrites. Geochim. Cosmochim. Acta 1987, 51, 1511–1521. [Google Scholar] [CrossRef]
- Zajacz, Z.; Candela, P.A.; Piccoli, P.M.; Sanchez-Valle, C.; Waelle, M. Solubility and partitioning behavior of Au, Cu, Ag and reduced S in magmas. Geochim. Cosmochim. Acta 2013, 112, 288–304. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Henderson, C.M.B.; Pacheco, H. Primary Ca-rich carbonatite magma and carbonate-silicate-sulphide liquid immiscibility in the upper mantle. Contrib. Mineral. Petrol. 1995, 121, 267–274. [Google Scholar] [CrossRef]
- Gunn, S.C.; Luth, R.W. Carbonate reduction by Fe-S-O melts at high pressure and high temperature. Am. Mineral. 2006, 91, 1110–1116. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Kupriyanov, I.N.; Sokol, A.G. Synthesis of diamonds with mineral, fluid and melt inclusions. Lithos 2016, 265, 292–303. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M. The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett. 2010, 298, 1–13. [Google Scholar] [CrossRef]
| Phase or System | xS, mol % | Mass Concentrations, wt % | ||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | FeO | MgO | CaO | NiO | SO3 | Total | ||
| Olivine | - | 41.05 | 9.25 | 49.25 | - | 0.45 | - | 100.0 |
| Anhydrite | - | - | - | - | 46.7 | - | 53.3 | 100.0 |
| Magnesium sulfate | - | - | - | 38.5 | - | - | 61.5 | 100.0 |
| Olivine-anhydrite-C system | 2 | 40.5 | 9.1 | 48.1 | 0.9 | 0.4 | 1.0 | 100.0 |
| 5 | 39.4 | 8.8 | 46.7 | 2.2 | 0.4 | 2.5 | 100.0 | |
| Olivine-magnesium sulfate-C system | 5 | 39.6 | 8.9 | 48.6 | - | 0.4 | 2.5 | 100.0 |
| 10 | 37.9 | 8.5 | 48.2 | - | 0.4 | 5.0 | 100.0 | |
| Run N | T, °C | t, h | System | xS, mol % | Final Phases |
|---|---|---|---|---|---|
| 933-2 | 1050 | 60 | Ol–CaSO4–C | 2 | OlR, Cpx, Po |
| 933-5 | 1050 | 60 | Ol–CaSO4–C | 5 | OlR, Cpx, Po |
| 934-2 | 1450 | 23 | Ol–CaSO4–C | 2 | OlR, Lsulf, Lsil-ox |
| 934-5 | 1450 | 23 | Ol–CaSO4–C | 5 | OlR, Lsulf, Lsil-ox |
| 1809-5 | 1450 | 40 | Ol–MgSO4–C | 5 | OlR, Lsulf, Lcarb-sil |
| 1809-10 | 1450 | 40 | Ol–MgSO4–C | 10 | OlR, Lsulf, Lcarb-sil |
| 571-M | 1450 | 2 | MgSO4–C | - | Ms, LS |
| 571-C | 1450 | 2 | CaSO4–C | - | Lcarb, LS |
| Run N | T, °C | xS,mol % | Phase | NA | Composition, wt % | n(O) | Cations per Formula Unit | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | FeO | MgO | CaO | NiO | CO2 | Total | Si | Fe | Mg | Ca | Ni | Sum | ||||||
| Ol–CaSO4–C system | ||||||||||||||||||
| 933-2 | 1050 | 2 | Ol | 14 | 41.2(6) | 7.5(28) | 50.0(54) | 1.0(7) | 0.2(2) | - | 99.9 (4) | 4 | 1.00(1) | 0.15(6) | 1.82(17) | 0.03(2) | - | 2.99(1) |
| Cpx | 8 | 54.8(14) | 2.1(5) | 20.9(39) | 22.0(28) | 0.1(1) | - | 99.9(3) | 6 | 1.98(5) | 0.06(2) | 1.13(20) | 0.85(11) | - | 4.02(2) | |||
| 933-5 | 1050 | 5 | Ol | 27 | 40.7(4) | 8.6(6) | 49.7(7) | 0.2(2) | 0.3(1) | - | 99.6(3) | 4 | 1.00(19) | 0.17(2) | 1.82(3) | - | 0.01(0) | 3.00(4) |
| Cpx | 10 | 56.5(5) | 3.1(6) | 23.6(66) | 16.2(78) | 0.1(1) | - | 99.5(3) | 6 | 2.02(1) | 0.09(1) | 1.12(4) | 0.75(3) | - | 3.98(1) | |||
| 934-2 | 1450 | 2 | Ol | 9 | 41.1(1) | 8.4(1) | 50.6(3) | 0.2(0) | - | - | 100.3(3) | 4 | 0.99(0) | 0.17(0) | 1.84(1) | 0.01(0) | - | 3.01(0) |
| Liq1 | 19 | 41.8(8) | 14.6(9) | 29.6(4) | 13.6(11) | - | - | 99.6(5) | - | - | - | - | - | - | - | |||
| 934-5 | 1450 | 5 | Ol | 8 | 41.7(3) | 6.1(1) | 51.6(2) | 0.2(1) | - | - | 99.5(3) | 4 | 1.00(0) | 0.12(0) | 1.86(1) | 0.01(0) | - | 3.00(0) |
| Liq1 | 15 | 38.0(1) | 12.8(1) | 22.2(18) | 26.4(20) | - | - | 99.4(3) | - | - | - | - | - | - | - | |||
| Ol–MgSO4–C system | ||||||||||||||||||
| 1809-5 | 1450 | 5 | Ol | 16 | 41.2(2) | 6.7(1) | 51.6(3) | - | 0.1(1) | - | 99.6(3) | 4 | 1.00(0) | 0.14(0) | 1.87(1) | - | - | 3.00(0) |
| Fmsq | 4 | - | 5.2(2) | 42.3(2) | 0.9(1) | - | 51.6(4) | 100 | 3 | - | 0.06(0) | 0.91(0) | 0.01(0) | - | 0.98(0) | |||
| Opxq | 10 | 57.6(3) | 6.9(2) | 35.4(3) | - | - | - | 99.9 | 6 | 1.99(0) | 0.20(0) | 1.83(0) | - | - | 4.01(0) | |||
| Liq2 | 20 | 32.0(20) | 24.1(48) | 36.9(31) | - | - | 6.5(5) | 99.5(2) | - | - | - | - | - | - | - | |||
| 1809-10 | 1450 | 10 | Ol | 12 | 41.8(2) | 4.4(8) | 53.4(6) | - | - | - | 99.8(3) | 4 | 1.00(0) | 0.09(2) | 1.91(2) | - | - | 3.00(0) |
| Liq2 | 21 | 31.7(36) | 15.2(77) | 44.3(78) | - | - | 8.1(1) | 99.3(3) | - | - | - | - | - | - | - | |||
| Run N | T, °C | xS, mol % | Phase | NA | Composition, wt % | n(S) | Cations per Formula Unit | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ni | S | Total | Fe | Ni | ||||||
| Ol–CaSO4–C system | |||||||||||
| 933-2 | 1050 | 2 | Ni-Po | 9 | 56(3) | 6.3(27) | 37.0(3) | 99.6 | 1 | 0.87(5) | 0.09(4) |
| 933-5 | 1050 | 5 | Ni-Po | 8 | 50(2) | 11(2) | 38.4(10) | 99.3 | 1 | 0.74(6) | 0.16(3) |
| 934-2 | 1450 | 2 | LiqA | 9 | 41(11) | 24(14) | 35(3) | 99.5 | 1 | 0.66(13) | 0.4(3) |
| 934-5 | 1450 | 5 | LiqА | 16 | 45(9) | 21(10) | 33(1) | 99.6 | 1 | 0.78(14) | 0.34(19) |
| LiqB | 14 | 56(1) | 7(1) | 36.0(7) | 99.5 | 1 | 0.89(4) | 0.12(1) | |||
| Ol–MgSO4–C system | |||||||||||
| 1809-5 | 1450 | 5 | LiqA | 11 | 46.1(8) | 17(1) | 36.4(1) | 99.7 | 1 | 0.72(1) | 0.26(2) |
| LiqB | 10 | 53.1(5) | 8.5(4) | 37.9(2) | 99.5 | 1 | 0.80(1) | 0.12(1) | |||
| 1809-10 | 1450 | 10 | LiqA | 14 | 47(1) | 16(2) | 36.5(2) | 99.6 | 1 | 0.74(2) | 0.24(3) |
| LiqB | 10 | 56.6(4) | 5.5(4) | 37.5(4) | 99.5 | 1 | 0.86(1) | 0.08(1) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataleva, Y.; Palyanov, Y.; Borzdov, Y. Sulfide Formation as a Result of Sulfate Subduction into Silicate Mantle (Experimental Modeling under High P,T-Parameters). Minerals 2018, 8, 373. https://doi.org/10.3390/min8090373
Bataleva Y, Palyanov Y, Borzdov Y. Sulfide Formation as a Result of Sulfate Subduction into Silicate Mantle (Experimental Modeling under High P,T-Parameters). Minerals. 2018; 8(9):373. https://doi.org/10.3390/min8090373
Chicago/Turabian StyleBataleva, Yuliya, Yuri Palyanov, and Yuri Borzdov. 2018. "Sulfide Formation as a Result of Sulfate Subduction into Silicate Mantle (Experimental Modeling under High P,T-Parameters)" Minerals 8, no. 9: 373. https://doi.org/10.3390/min8090373
APA StyleBataleva, Y., Palyanov, Y., & Borzdov, Y. (2018). Sulfide Formation as a Result of Sulfate Subduction into Silicate Mantle (Experimental Modeling under High P,T-Parameters). Minerals, 8(9), 373. https://doi.org/10.3390/min8090373






