A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite)
Abstract
1. Introduction
2. Particle Size Effect
3. Surface Properties
3.1. Electrical Charge
3.2. Contact Angle
4. Solubility
5. Flotation
5.1. Direct Flotation
5.1.1. Collectors
5.1.2. Depressants and Modifiers
5.2. Depression of Mg Carbonates
Bio Depression
5.3. Flotation Separation of Dolomite from Magnesite
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Kozhevnikov, E.K.; Kropanev, S.I.; Baranovskii, N.I. Beneficiation of Dolomites. Raw Mater. 1973, 3, 19–21. [Google Scholar] [CrossRef]
- Luo, X.M.; Yin, W.Z.; Wang, Y.F.; Sun, C.Y.; Ma, Y.Q.; Liu, J. Effect and mechanism of dolomite with different size fractions on hematite flotation using sodium oleate as collector. J. Cent. South Univ. 2016, 23, 529–534. [Google Scholar] [CrossRef]
- Zheng, X.; Arps, P.J.; Smith, R.W. Adsorption of Bacillus subtilis to minerals: Effect on the flotation of dolomite and apatite. Process Metall. 1999, 62, 127–136. [Google Scholar]
- Zheng, X.; Arps, P.J.; Smith, R.W. Adhesion of two bacteria onto dolomite and apatite: Their effect on dolomite depression in anionic flotation. Int. J. Miner. Process. 2001, 62, 159–172. [Google Scholar] [CrossRef]
- Ding, K.; Laskowski, J.S. Application of a Modified Water Glass in a Cationic Flotation of Calcite and Dolomite. Can. Metall. Q. 2006, 45, 199–206. [Google Scholar] [CrossRef]
- Elmahdy, A.M.; El-Midany, A.A.; Abdel-Khalek, N.A. Application of amphoteric collector for dolomite separation by statistically designed experiments. Miner. Process. Extr. Metall. 2007, 116, 72–76. [Google Scholar] [CrossRef]
- Zheng, X.; Smith, R.W. Dolomite Depressants in the Flotation of Apatite and Collophane from Dolomite. Miner. Eng. 1997, 10, 537–545. [Google Scholar] [CrossRef]
- Birkena, I.; Bertuccib, M.; Chappelinb, J.; Jordab, E. Quantification of impurities, including carbonates speciation for phosphates beneficiation by flotation. Procedia Eng. 2016, 138, 72–84. [Google Scholar] [CrossRef]
- Lawver, J.E.; Weigel, R.L.; Snow, R.E.; Hwang, C.L. Phosphate reserves enhanced by beneficiation. Min. Congr. 1982, 68, 27–31. [Google Scholar]
- Liu, Y.; Liu, Q. Flotation separation of carbonate from sulfide minerals, I: Flotation of single minerals and mineral mixtures. Miner. Eng. 2004, 17, 855–863. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, L.; Xu, Z.; Liu, Q.; Chi, R. Reactive oily bubble technology for flotation of apatite, dolomite and quartz. Int. J. Miner. Process. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. Effect of solution chemistry on floatability of magnesite and dolomite. Int. J. Miner. Process. 2004, 74, 343–357. [Google Scholar] [CrossRef]
- Gence, N.; Ozbay, N. pH dependence of electrokinetic behavior of dolomite and magnesite in aqueous electrolyte solutions. Appl. Surf. Sci. 2006, 252, 8057–8061. [Google Scholar] [CrossRef]
- Moudgil, B.M.; Ince, D.E. Flotation Separation of Apatite from Dolomite Using Dodecylamineand Sodium Chloride. In Particle Technology and Surface Phenomena in Minerals and Petroleum; Springer: Boston, MA, USA, 1991; pp. 191–197. [Google Scholar]
- Matis, K.A.; Balabanidis, T.H.N.; Gallios, G.P. Processing of Magnesium Carbonate Fines by Dissolved-Air Flotation. Colloids Surf. 1988, 29, 191–203. [Google Scholar] [CrossRef]
- Ni, X.; Liu, Q. Adsorption behaviour of Sodium Hexametaphosphate on Pyrochlore and Calcite. Can. Metall. Q. 2013, 52, 473–478. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Wen, S.; Ma, M.; Sun, C.; Yin, W.; Ma, Y. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores. Int. J. Miner. Process. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Li, D.; Yin, W.; Xue, J.; Yao, J.; Fu, Y.; Liu, Q. Solution chemistry of carbonate minerals and its effects on the flotation of hematite with sodium oleate. Int. J. Miner. Metall. Mater. 2017, 7, 736–744. [Google Scholar] [CrossRef]
- Nunes, A.P.L.; Peres, A.E.C.; De Araujo, A.C.; Valadão, G.E.S. Electrokinetic properties of wavellite and its floatability with cationic and anionic collector. J. Colloid Interface Sci. 2011, 361, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chi, R.; Xu, Z. Solution chemistry study of salt-type mineral flotation systems: Role of inorganic dispersants. Ind. Eng. Chem. Res. 2003, 42, 1641–1647. [Google Scholar]
- Rahnemaie, R.; Hiemstra, T.; van Riemsdijk, W.H. Carbonate adsorption on goethite in competition with phosphate. J. Colloid Interface Sci. 2007, 315, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, H.; Wang, X.; Miller, J.D. Surface chemistry considerations in the flotation of rare-earth and other semi-soluble salt minerals. Miner. Metall. Process. 2013, 30, 24–37. [Google Scholar]
- Yao, J.; Yin, W.; Gong, E. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Process. 2016, 149, 84–93. [Google Scholar] [CrossRef]
- Hanna, H.S.; Somasundaran, P. Flotation. In Gaudin Memorial Volume, Flotation of Salt Type Minerals; Fuerstenau, M.C., Ed.; AIME: New York, NY, USA, 1976; Volume 1, pp. 197–272. [Google Scholar]
- Gharabaghi, M.; Irannajad, M.; Noaparast, M. A review of the beneficiation of calcareous phosphate ore using organic acid leaching. Hydrometallurgy 2010, 103, 96–107. [Google Scholar] [CrossRef]
- Wills, B.A.; Napier-Munn, T. Froth flotation–flowsheet design. In Wills’ Mineral Processing Technology (Seventh Edition): An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Elsevier: New York, NY, USA, 2005; pp. 293–302. [Google Scholar]
- Soto, H.; Iwasaki, L. Selective flotation of phosphates from dolomite using cationic collectors. part II effect of particle size, abrasion and pH. Int. J. Miner. Process. 1986, 16, 17–27. [Google Scholar] [CrossRef]
- Lima, P.; Thiago, C.; Aline, C.; Jenni, S. The entrainment effect on the performance of iron ore reverse flotation. Miner. Eng. 2016, 96, 53–58. [Google Scholar] [CrossRef]
- Wang, L.; Runge, K.; Peng, Y.; Vos, C. An empirical model for the degree of entrainment in froth flotation based on particle size and density. Miner. Eng. 2016, 98, 187–193. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Luo, H.; Cheng, R.; Liu, F. Selective reverse flotation of apatite from dolomite in collophanite ore using saponified gutter oil fatty acid as a collector. Int. J. Miner. Process. 2017, 165, 20–27. [Google Scholar] [CrossRef]
- Duzyol, S.; Ozkan, A. Correlation of Flocculation and Agglomeration of Dolomite with its Wettability. Sep. Sci. Technol. 2011, 46, 876–881. [Google Scholar] [CrossRef]
- Matis, K.A.; Gallios, G.P. Anionic Flotation of Magnesium Carbonates by Modifiers. Int. J. Miner. Process. 1989, 25, 261–274. [Google Scholar] [CrossRef]
- Zheng, X.P.; Smith, R.W.; Misra, M.; Mehta, R.K.; Raichur, A.M. Effect of a Water Soluble Fraction Derived from Mycobacterium phlei on the Surface Characteristics and Flotation of Apatite and Dolomite. Miner. Process. Extr. Metall. Rev. Int. J. 1998, 19, 355–368. [Google Scholar] [CrossRef]
- Espiritu, E.R.L.; Waters, K.E. Flotation studies of monazite and dolomite. Miner. Eng. 2018, 116, 101–106. [Google Scholar] [CrossRef]
- Moudgil, B.M.; Ince, D. Role of pH and Collector Concentration in Separation of Phosphates from Dolomitic Gangue Using DDA-HCL. In Surfactants in Solution; Springer: Boston, MA, USA, 1989; pp. 457–465. [Google Scholar]
- Soto, H.; Iwasaki, I. Selective flotation of phosphates from dolomite using cationic collectors. Part I. effect of collector and nonpolar hydrocarbons. Int. J. Miner. Process. 1986, 16, 3–16. [Google Scholar] [CrossRef]
- Khalek, A.M.A. Separation of dolomite from phosphate minerals by flotation with a new amphoteric surfactant as collector. Miner. Process. Extr. Metall. 2001, 110, 89–93. [Google Scholar] [CrossRef]
- Yu, J.; Ge, Y.; Guo, X.; Guo, W. The depression effect and mechanism of NSFC on dolomite in the flotation of phosphate ore. Sep. Purif. Technol. 2016, 161, 88–95. [Google Scholar] [CrossRef]
- Elmahdy, A.; El-Mofty, S.; Abdel-Khalek, M.; Abdel-Khalek, N.; El-Midany, A. Dolomite-apatite separation by amphoteric collector in presence of bacteria. J. Cent. South Univ. 2013, 20, 1645–1652. [Google Scholar]
- Elmahdy, A.M.; El-Mofty, S.E.; Abdel-Khalek, M.A.; Abdel-Khalek, N.A.; El-Midany, A.A. Bacterially induced phosphate–dolomite separation using amphoteric collector. Sep. Purif. Technol. 2013, 102, 94–102. [Google Scholar] [CrossRef]
- Moudgil, B.M.; Vasudevan, T.V. Effect of Solution Chemistry of Sodium Oleate on Adsorption and Surface Wettability of Apatite and Dolomite. In Surfactants in Solution; Springer: Boston, MA, USA, 1990; pp. 351–358. [Google Scholar]
- Gence, N. Wetting behavior of magnesite and dolomite surfaces. Appl. Surf. Sci. 2006, 252, 3744–3750. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Forsberg, C.W.; Doyle, R.J. Major sites of metal binding in Bacillus licheniformis walls. J. Bacteriol. 1982, 150, 1438–1448. [Google Scholar] [PubMed]
- Ozdemir, O.; Karaguzel, C.; Nguyen, A.V.; Celik, M.S.; Miller, J.D. Contact angle and bubble attachment studies in the flotation of Trona and other soluble carbonate salts. Miner. Eng. 2009, 22, 168–175. [Google Scholar] [CrossRef]
- Shafrin, E.G.; Zisman, W.A. Constitutive relations in the wetting of low energy surfaces and the theory of the retraction method of preparing monolayers. J. Phys. Chem. 1960, 64, 519–524. [Google Scholar] [CrossRef]
- Yarar, B.; Kaoma, J. Estimation of the critical surface tension of wetting of hydrophobic solids by flotation. Colloids Surf. 1984, 11, 429–436. [Google Scholar] [CrossRef]
- Al-Fariss, T.F.; Ozbelge, H.O.; Abdulrazik, A.M. Flotation of a carbonate rich sedimentary phosphate rock. Fertil. Res. 1991, 29, 203–208. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Wada, Y.; Hiaki, T.; Onoe, K.; Matsumoto, M. Effects of CO2 fine bubble injection on reactive crystallization of dolomite from concentrated brine. J. Cryst. Growth 2017, 469, 36–41. [Google Scholar] [CrossRef]
- El-Midany, A.A.; El-Shall, H.; Svoronos, S. Modeling the PVA-coated dolomite floatability in acidic media. Powder Technol. 2011, 209, 25–28. [Google Scholar] [CrossRef]
- Abouzeid, A.-Z.M. Physical and thermal treatment of phosphate ores—An overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Kiersznicki, T.; Majewski, J.; Mzyk, J. 5-Alkylsalicylaldoximes as Collectors in Flotation of Sphalerite, Smithsonite and Dolomite in a Hallimond Tube. Int. J. Miner. Process. 1981, 7, 311–318. [Google Scholar] [CrossRef]
- Chen, G.L.; Tao, D. Reverse Flotation of Magnesite by Dodecyl Phosphate from Dolomite in the Presence of Sodium Silicate. Sep. Sci. Technol. 2005, 39, 377–390. [Google Scholar] [CrossRef]
- Ge, Y.Y.; Gan, S.P.; Zeng, X.B.; Yu, Y.F. Double reverse flotation process of collophanite and regulating froth action. Trans. Nonferr. Met. Soc. China 2008, 18, 449–453. [Google Scholar] [CrossRef]
- Hernáinz, F.; Calero, M.; Blázquez, G. Flotation of low-grade phosphate ore. Adv. Powder Technol. 2004, 15, 421–433. [Google Scholar] [CrossRef]
- Ozkan, S. Beneficiation of magnesite slimes with ultrasonic treatment. Miner. Eng. 2002, 15, 99–101. [Google Scholar] [CrossRef]
- Yin, W.Z.; Li, D.; Luo, X.M.; Yao, J.; Sun, Q.Y. Effect and mechanism of siderite on reverse flotation of hematite. Int. J. Miner. Metall. Mater. 2016, 23, 373–379. [Google Scholar] [CrossRef]
- Smith, R.W.; Misra, M. Recent developments in the bioprocessing of minerals. Miner. Process. Extr. Metall. 1993, 12, 37–60. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.M.; Lyklema, J.; Norde, W.; Schraa, G.; Zehnder, A.J.B. The role of bacterial wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 1987, 53, 1893–1897. [Google Scholar] [PubMed]
- Marinakis, K.I.; Shergold, H.L. The mechanism of fatty acid adsorption in the presence of fluorite, calcite and barite. Int. J. Miner. Process. 1985, 14, 161–176. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhang, Z.; Luo, H.; Xiao, C.; Zhou, F.; Chi, R. Effects of Metal Ions on the Flotation of Apatite, Dolomite and Quartz. Mineral 2018, 8, 141. [Google Scholar] [CrossRef]
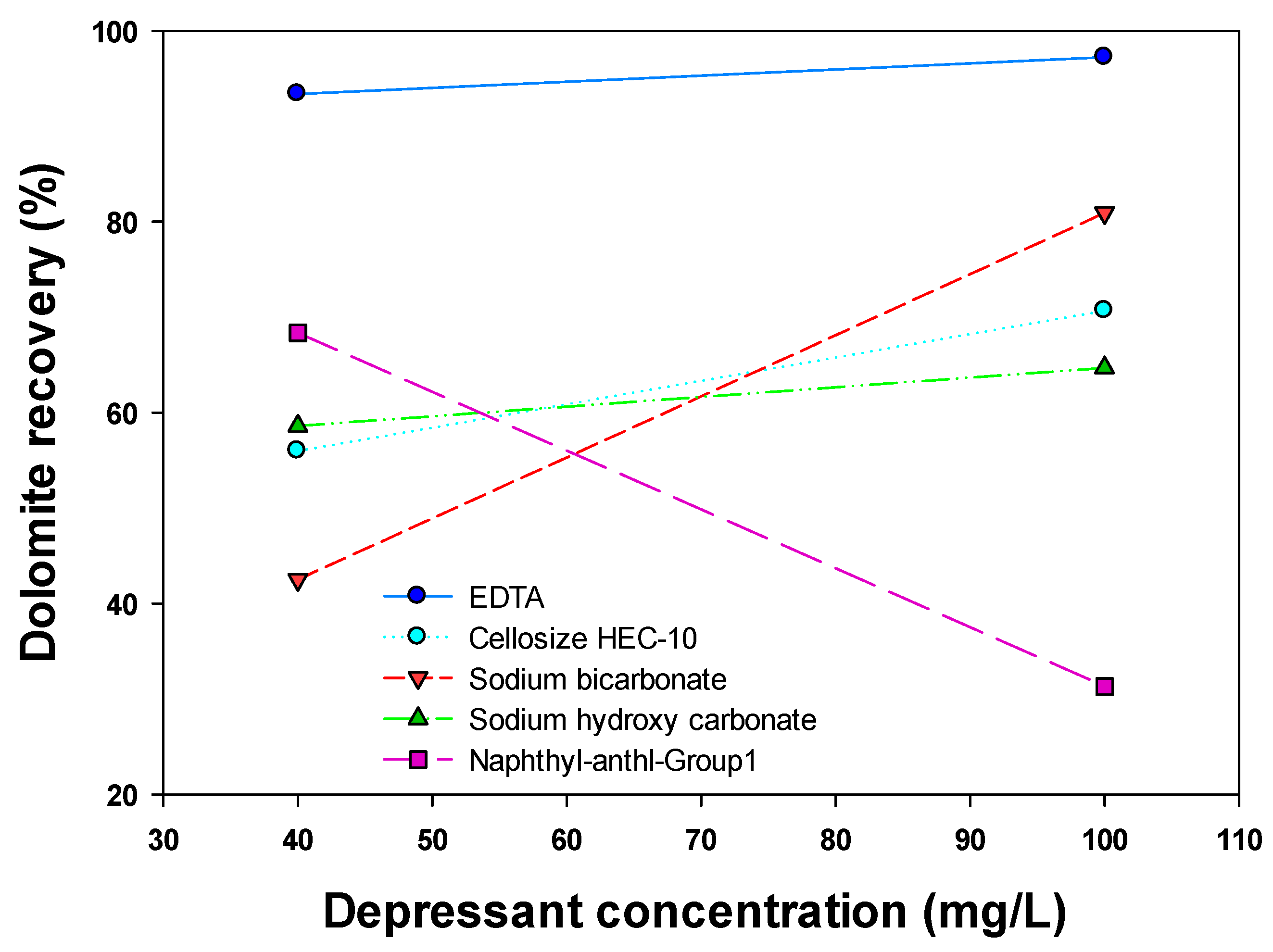

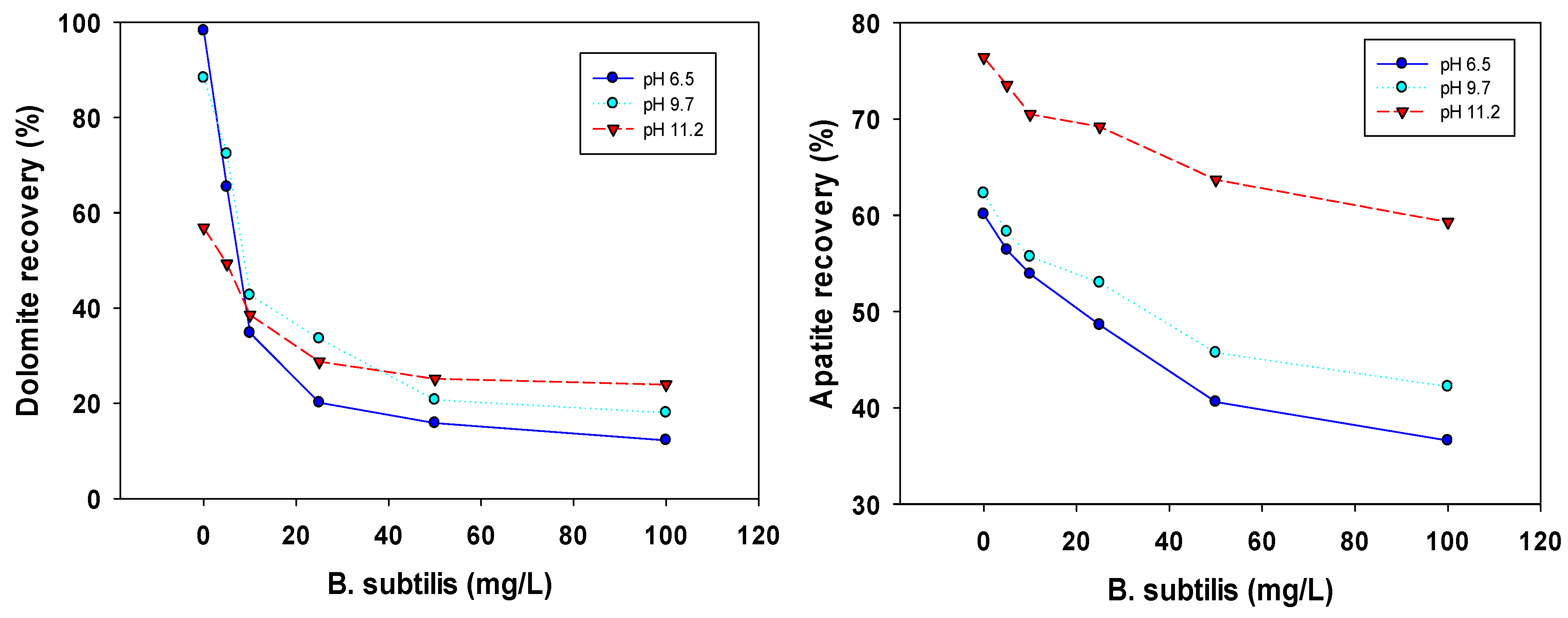
| Particle Size Fraction (μm) | Flotation Type | Dolomite Recovery (%) | Associated Mineral Recovery (%) | Mineral Type | References |
|---|---|---|---|---|---|
| −180 + 125 | Direct | ~27 | - | Single pure | [32] |
| −74 | Direct | 98.30 | Calcite: 93.9 | Single pure | [5] |
| −100 + 65 | Reverse | ~49 | Quartz: 96.66 | Single pure | [23] |
| −212 + 150 | Reverse | 11.37–8.91 | Apatite: 86.86–91.86 | Single pure | [7] |
| 4.45 | Apatite: 87.10 | Pure mixture | |||
| −74 + 38 | Reverse | 31.37 | Apatite: 64.89 | Single pure | [33] |
| −106 + 38 | Reverse | ~10 | Monazite: 37 | Single pure | [34] |
| −106 + 38 | Direct | 66 | Monazite: 29 | Single pure | |
| −212 + 150 | Reverse | 18 | Apatite: 87.7 | Single pure | [14] |
| −212 + 150 | Reverse | ~20 | Apatite: 80 | Pure mixture | |
| −106 + 38 | Direct | ~90 | Gold Bearing Chalcopyrite: ~20 | Single pure | [10] |
| −212 + 150 | Reverse | ~10 | Apatite: ~80 | Single pure | [35] |
| −212 + 150 | Reverse | 37.60 | Apatite: 53.3 | Pure mixture | |
| −100 + 74 | Reverse | ~15 | Apatite: ~85 | Pure mixture | [27] |
| −500 + 100 | Reverse | ~20 | Apatite: ~95 | Pure mixture | |
| −100 + 74 | Direct | 80 | ~95 | Single pure | [36] |
| −74 | Reverse | 12.57 | Apatite: 87.20 | Ore | [30] |
| −106 + 45 | Direct | ~80 | ~90 | Single pure | [18] |
| –106 | Reverse | ~10 | Apatite: 88 | Pure mixture | [37] |
| –106 | Reverse | ~10 | Apatite: 82 | Ore | |
| −74 | Reverse | 9.80 | Apatite: 86.01 | Ore | [38] |
| IEP (Pure Minerals) | IEP (Presence of Reagents) | Reagents | References | ||
|---|---|---|---|---|---|
| Associated Mineral | Carbonates | Associated Mineral | Carbonates | ||
| Apatite: 5.5 | Dolomite: 7 | 7.1 | 9.3 | Ca(NO3)2 | [3] |
| Apatite: 5.5 | Dolomite: 7 | 7.2 | 8.4 | Mg(NO3)2 | |
| Magnesite: 6.8 | Dolomite: 7.6 | 5.5 | 5.5 | Sodium Silicate (NaSiO3) | [12] |
| Apatite: 6.8 | Dolomite: 8.5 | 6.8 (ZPC) | 8.6 (ZPC) | Sodium Chloride (NaCl) | [37] |
| Apatite: 6.5 | Dolomite: 4.4 | 6.4 | 3.6 | B-naphthyl sulfonate formaldehyde condensate (NSFC) | [38] |
| Magnesite: 6.7 | Dolomite: 6 | 9.8 | 9.1 | Dodecylamine (DDA) | [23] |
| Monazite: 5 | Dolomite: 5 | 5.5 | 4.2 | Benzohydroxamic acid | [34] |
| Magnesite: 6.5 | Dolomite: ~ 7 | 7 | 7 | Sodium hexametaphosphate (SHMP) | [15] |
| Magnesite: 6.5 | Dolomite: ~ 7 | 7 | 6.5 | Carboxymethyl cellulose (CMC) | |
| Monazite: 5 | Dolomite: 5 | 3.5 | Negative ZP (pH 3–11) | Sodium oleate (NaOl) | [34] |
| Apatite: 4.2 | Dolomite: 6.2 | 5.4 | Negative ZP (pH 5.5–11) | Potassium Chloride (KCl) + Sodium Silicate (Na2SiO3) | [11] |
| Apatite: 5.3 | Dolomite: 11 | Negative ZP (pH 4–11) | 5.5 | Sodium Chloride (NaCl) | [14] |
| Calcite: 11 | Dolomite: 11.5 | Negative ZP (pH 6.5–11.5) | Negative ZP (pH 6.5–11.5) | Water Glass/ modify water glass (ferric silicate hydrosols) | [5] |
| Monazite: 5 | Dolomite: 5 | Negative ZP (pH 3–11) | Negative ZP (pH 3–11) | Flotinor 1682 (organic phosphoric acid) | [34] |
| Magnesite: 6.5 | Dolomite: ~7 | Negative ZP (pH 7–12) | Negative ZP (pH 7.5–12) | Tetrasodium pyrophosphate (TSPP) | [15] |
| Apatite: 5.5 | Dolomite: 10.3 | - | - | - | [41] |
| Dolomite: 6.3 | Magnesite: 6.7 | - | - | - | [12] |
| Dolomite: 6.3 | Magnesite: 6.8 | - | - | - | [42] |
| Apatite: 4.5 | Dolomite: 6.8 | - | - | - | [11] |
| Collector | Carbonate Recovery (%) | Reference |
|---|---|---|
| Sodium Oleate (NaOl) | 90 | [32] |
| 95 | [41] | |
| ~70 | [34] | |
| ~90 | [10] | |
| ~80 | [18] | |
| ~80 | [38] | |
| Flotinor 1682 (organic phosphoric acid) | ~70 | [34] |
| Saponified Gutter Oil Fatty Acid (GOFA) | 12.57 | [30] |
| 5-propylsalicylaldoxime | ~50 | [52] |
| Dodecyltrimethyl ammonium bromide (DTAB) | ~60 | [5] |
| Dodecylamine (DDA) | ~49 | [23] |
| Dodecyl phosphate | 98 | [12] |
| 80 | [53] | |
| Dodecylamine hydrochloride (DDA-HC1) | 37.60 | [35] |
| 18.50 | [14] | |
| Dodecylamine hydrochloride (DDA-HC1) | 10 | [35] |
| Octadecylamine-kerosene Emulsion (1:3) | ~15 | [36] |
| Dodecyl-N-carboxyethyl-N-hydroxyethyl-imidazoline | ~98 | [37] |
| Associated Mineral | Collector (mg/L) | Depressant (mg/L) | pH | Recovery (%) | Reference |
|---|---|---|---|---|---|
| Hematite | NaOI (120) | - | 9–12.5 | 75.25 | [2] |
| Hematite | NaOI (120) | CaCl2 (15) | 9 | 15 | [2] |
| Hematite | NaOI (120) | CaCl2 (50 & 100) | 9.5–12.5 | 0 | [2] |
| Pyrite | NaOI (60.8) | - | 10 | 80 | [10] |
| Pyrite | NaOI (91.2) | Thioglycollic acid (9.2) | 9–10.5 | 12.5 | [10] |
| Pyrite | NaOI (91.2) | Citric acid (19.2) | 10 | 8.53 | [10] |
| Collector | Dosage (mg/L) | pH | Depressants | Dosage (mg/L) | Recovery Dolomite (%) | Recovery Magnesite (%) | Reference |
|---|---|---|---|---|---|---|---|
| NaOl | 20 | 8.5 | - | - | ~90 | ~20 | [32] |
| NaOl | 20 | 7 | Sodium silicate | 240 | ~25 | ~70 | |
| NaOl | 30 | 10.5–11.5 | Calcon | 30 | ~30 | ~75 | |
| Acintol FA-1 tall oil fatty acid | 300 | 9.5 | Sodium hexametaphosphate | 100 | ~60 | ~20 | [15] |
| Acintol FA-1 tall oil fatty acid | 40 | 7.1 | Tetrasodium pyrophosphate | 600 | ~75 | ~20 | |
| Acintol FA-1 tall oil fatty acid | 30 | 9.5 | CMC | 100 | ~60 | ~30 | |
| Acintol FA-1 tall oil fatty acid | 40 | 9.5 | CMC | 100 | ~66 | ~38 | |
| Dodecyl phosphate | 100 | 6.5 | Sodium silicate | 75 | ~80 | ~10 | [53] |
| Dodecyl phosphate | 100 | 5.5 | Sodium silicate | 75 | ~76.64 | ~23.36 | |
| Dodecyl phosphate | 100 | 5.5 | Sodium silicate | 100 | ~82.97 | ~17.03 | |
| DDA | 159.57 | 9.2–9.5 | - | - | ~49 | ~5 | [23] |
| Dodecyl phosphate | 100 mg/L | 9 | - | - | 98% | 95% | [12] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wonyen, D.G.; Kromah, V.; Gibson, B.; Nah, S.; Chelgani, S.C. A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite). Minerals 2018, 8, 354. https://doi.org/10.3390/min8080354
Wonyen DG, Kromah V, Gibson B, Nah S, Chelgani SC. A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite). Minerals. 2018; 8(8):354. https://doi.org/10.3390/min8080354
Chicago/Turabian StyleWonyen, Darius G., Varney Kromah, Borbor Gibson, Solomon Nah, and Saeed Chehreh Chelgani. 2018. "A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite)" Minerals 8, no. 8: 354. https://doi.org/10.3390/min8080354
APA StyleWonyen, D. G., Kromah, V., Gibson, B., Nah, S., & Chelgani, S. C. (2018). A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite). Minerals, 8(8), 354. https://doi.org/10.3390/min8080354







