(10.4) Face of Ordered and Disordered Dolomite, MgCa(CO3)2: A Computational Study to Reveal the Growth Mechanism
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Bulk Structures of Dolomite
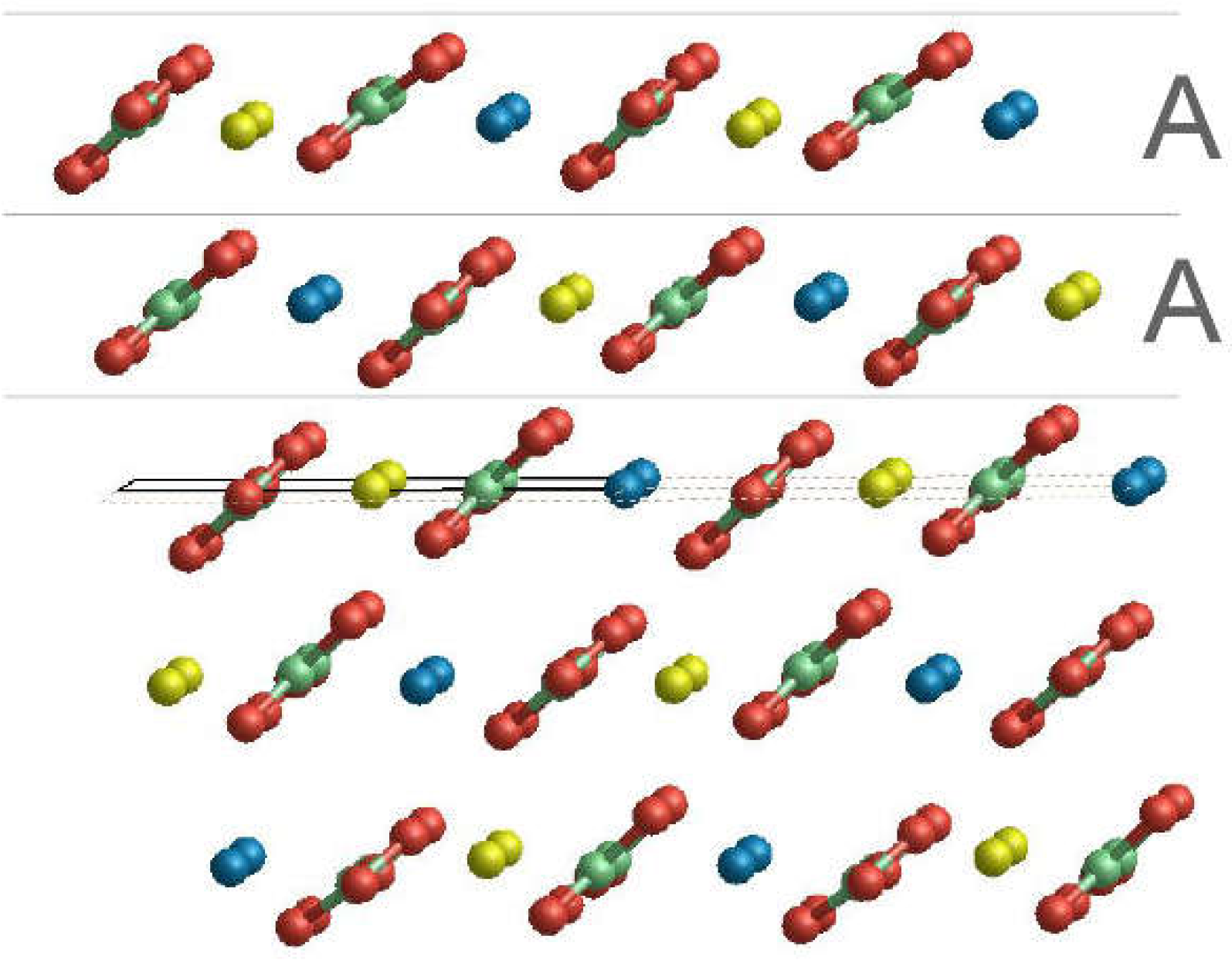
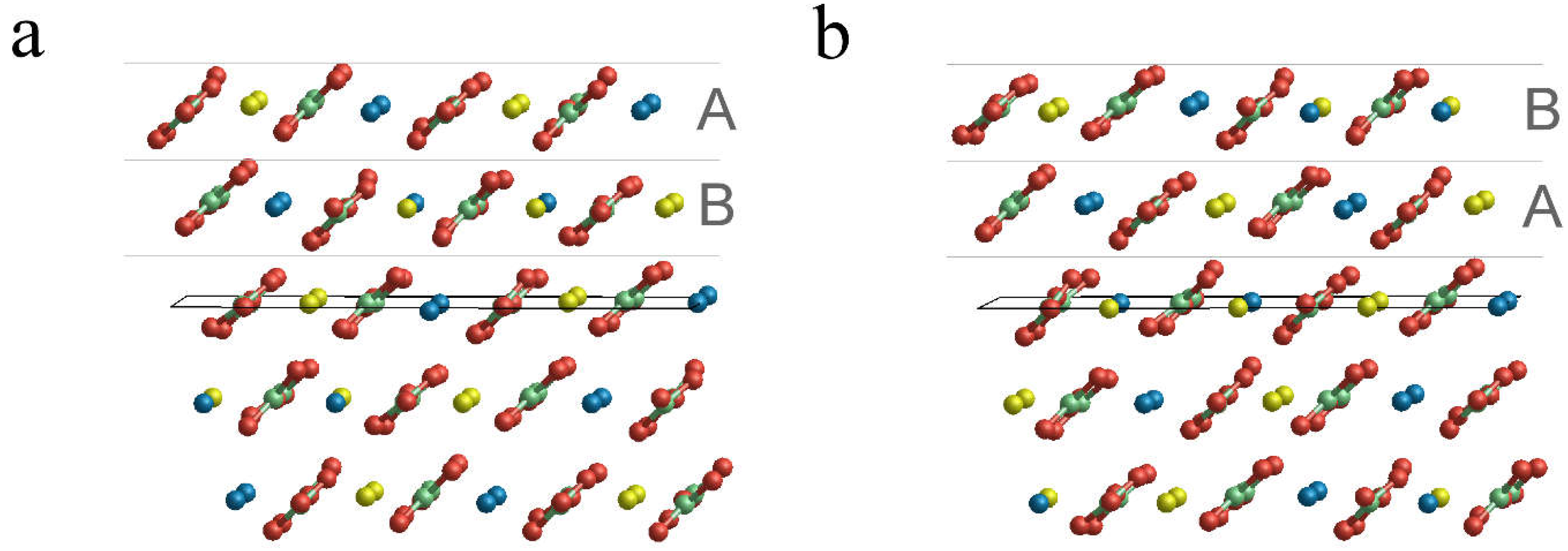
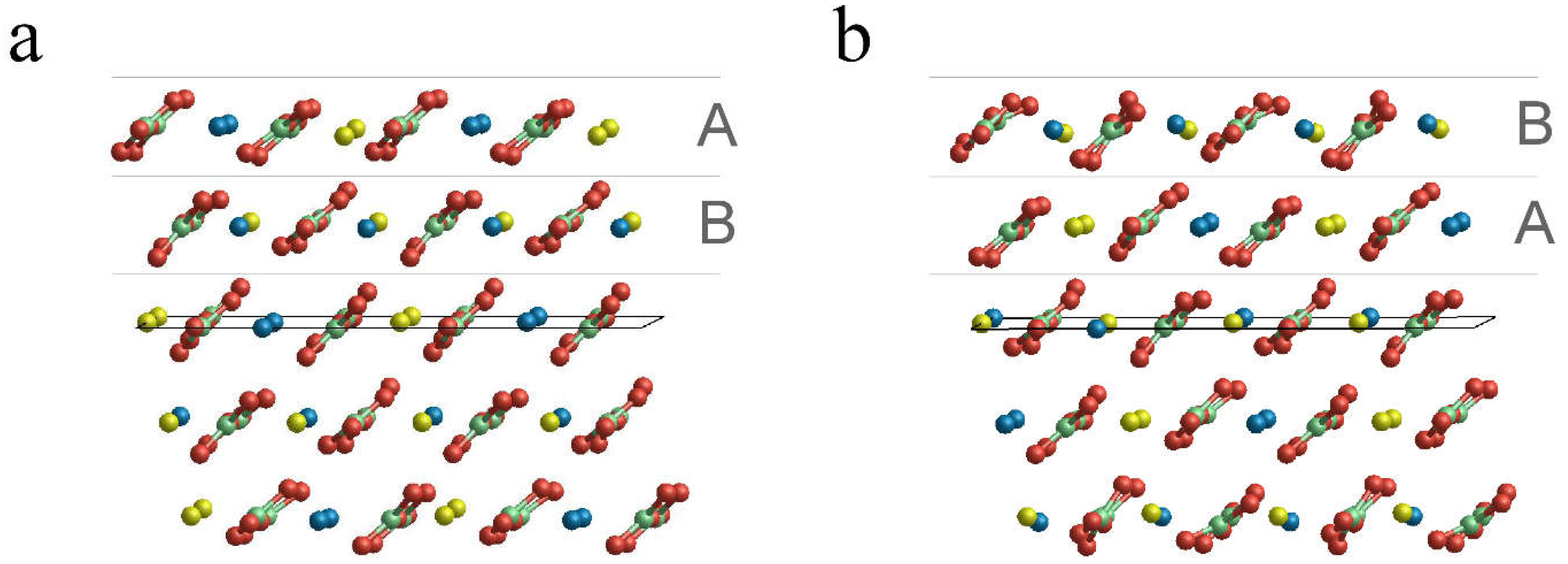
3.2. Surface Terminations and Surface Energies of the (10.4) Faces of Dolomite
- The surface energy of the (10.4) face of calcite (0.507 J/m2) is slightly higher than that of magnesite (0.493 J/m2). Previous surface energy calculations on the (10.4) face of calcite and magnesite were performed by our research group, always using the B3LYP Hamiltonian but considering a poorer basis sets: 0.508 and 0.501 J/m2 for calcite and magnesite, respectively [39]. These small variations in the surface energy values suggest that our estimates are barely affected by the Basis Set Superposition Error (BSSE) [23]. Indeed, if the use of richer basis sets does not produce significant variations in the surface energy value, then it is licit to suppose that our estimates are reliable.
- The surface energies of the , and faces are lower than those of calcite and magnesite: 0.489, 0.437 and 0.450 J/m2. This clearly highlights that the (10.4) face is strongly stabilized when there is a mixing of Ca2+ and Mg2+. Furthermore, such stabilization is also a function of the ordering degree of Ca2+ and Mg2+. Indeed, the surface energies of the and faces are decreased by 10.6% and 8.0%, respectively, compared to that of the face. Our calculations suggest that a disordered surface is more stable of an ordered one, in contrast with the findings on the bulk of the crystal [16], where the more stable structure is the ordered one (SC1).
- The two surface terminations obtained by cutting SC4895 have very similar surface energy. This implies an analogous probability to be observed when a crystal grows.
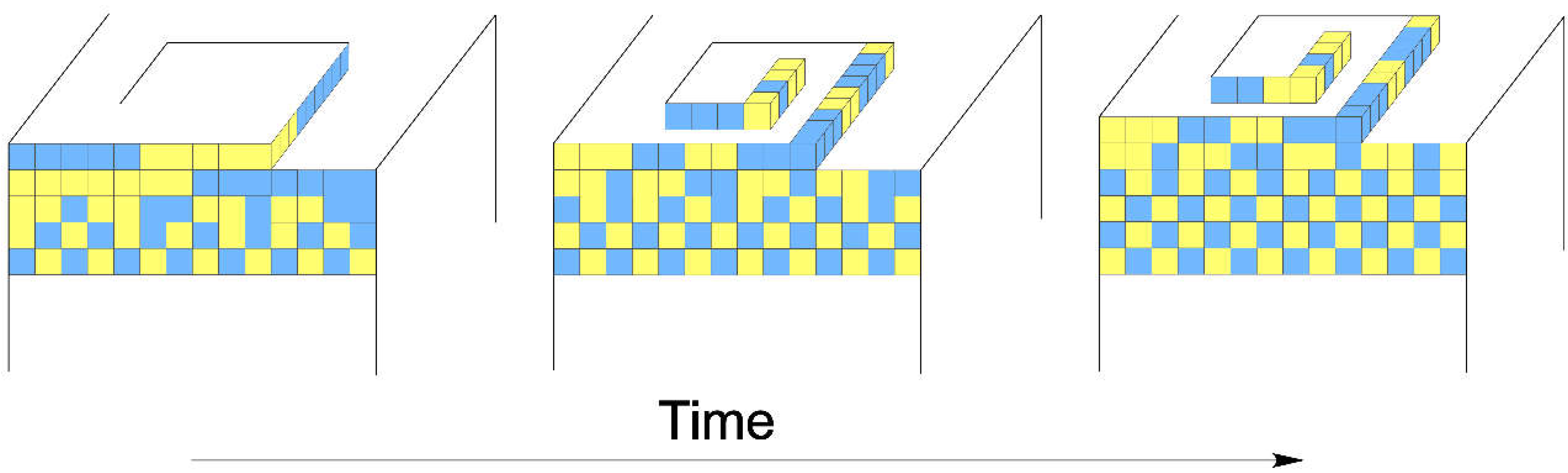
3.3. A Growth Model for Dolomite
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Saussure, H.B. Analyse de la dolomite. Obs. Physi. l’Hist. Nat. Arts 1792, 40, 161–173. [Google Scholar]
- Drits, V.A.; McCarty, D.K.; Sakharov, B.; Milliken, K.L. New insight into structural and compositional variability in some ancient excess-Ca dolomite. Can. Miner. 2005, 43, 1255–1290. [Google Scholar] [CrossRef]
- Busenberg, E.; Plummer, L.N. The kinetics of dissolution of dolomite in CO2-H2O systems at 1.5 to 65 °C and 0 to 1 atm PCO2. Am. J. Sci. 1982, 282, 45–78. [Google Scholar] [CrossRef]
- Veizer, J. Trace elements and isotopes in sedimentary carbonates. Rev. Miner. Geochem. 1983, 11, 265–299. [Google Scholar]
- Shirasaka, M.; Takahashi, E.; Nishihara, Y.; Matsukage, K.; Kikegawa, T. In situ X-ray observation of the reaction dolomite = aragonite + magnesite at 900–1300 K. Am. Miner. 2002, 87, 922–930. [Google Scholar] [CrossRef]
- Poli, S.; Franzolin, E.; Molina, J.-F. High-pressure behaviour of carbonates and hydrates, and devolatilization of the subducted oceanic crust. Geochim. Cosmochim. Acta 2009, S73, A1038. [Google Scholar]
- Collettini, C.; Viti, C.; Smith, S.A.; Holdsworth, R.E. Development of interconnected talc network sand weakening of continent allow-angle normal faults. Geology 2009, 37, 567–570. [Google Scholar] [CrossRef]
- Hammouda, T.; Andrault, D.; Koga, K.; Katsura, T.; Martin, A. Ordering in double carbonates and implications for processes at subduction zones. Contrib. Miner. Pet. 2011, 161, 439–450. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Heard, H.C. Subsolidus phase relations in the system CaCO3-MgCO3. J. Geol. 1961, 69, 45–74. [Google Scholar] [CrossRef]
- Reeder, R.J.; Wenk, H. Structure refinements of some thermally disordered dolomites. Am. Miner. 1983, 68, 769–776. [Google Scholar]
- Antao, S.M.; Mulder, W.H.; Hassan, I.; Crichton, W.A.; Parise, J.B. Cation disorder in dolomite, CaMg(CO3)2, and its influence on the aragonite + magnesite → dolomite reaction boundary. Am. Miner. 2004, 89, 1142–1147. [Google Scholar] [CrossRef]
- Parise, J.B.; Antao, S.M.; Martin, C.D.; Crichton, W. Diffraction studies of order–disorder at high pressures and temperatures. Powder Diffr. 2005, 20, 80–86. [Google Scholar] [CrossRef]
- Vinograd, V.L.; Winkler, B.; Putnis, A.; Gale, J.D.; Sluiter, M.H. Static lattice energy calculations of mixing and ordering enthalpy in binary carbonate solid solutions. Chem. Geol. 2006, 225, 304–313. [Google Scholar] [CrossRef]
- Vinograd, V.L.; Burton, B.P.; Gale, J.D.; Allan, N.L.; Winkler, B. Activity-composition relations in the system CaCO3-MgCO3 predicted from static structure energy calculations and Monte Carlo simulations. Geochim. Cosmochim. Acta 2007, 71, 974–983. [Google Scholar] [CrossRef]
- Vinograd, V.L.; Sluiter, M.; Winkler, B. Subsolidus phase relations in the CaCO3-MgCO3 system predicted from the excess enthalpies of super cell structures with single and double defects. Phys. Rev. B 2009, 79, 104201. [Google Scholar] [CrossRef]
- Zucchini, A.; Prencipe, M.; Comodi, P.; Frondini, F. Ab initio study of cation disorder in dolomite. Calphad 2012, 38, 177–184. [Google Scholar] [CrossRef]
- Zucchini, A.; Comodi, P.; Nazzareni, S.; Hanfland, M. The effect of cation ordering and temperature on the high-pressure behaviour of dolomite. Phys. Chem. Miner. 2014, 41, 783–793. [Google Scholar] [CrossRef]
- Zucchini, A.; Prencipe, M.; Belmonte, D.; Comodi, P. Ab initio study of the dolomite to dolomite-II high-pressure phase transition. Eur. J. Miner. 2017, 29, 227–238. [Google Scholar] [CrossRef]
- Solomatova, N.V.; Asimow, P.D. Ab initio study of the structure and stability of CaMg(CO3)2 at high pressure. Am. Miner. 2017, 102, 210–215. [Google Scholar] [CrossRef]
- Dovesi, R.; Orlando, R.; Civalleri, B.; Roetti, C.; Saunders, V.R.; Zicovich-Wilson, C.M. CRYSTAL: A computational tool for the ab initio study of the electronic properties of crystals. Z. Krist. 2005, 220, 571–573. [Google Scholar] [CrossRef]
- Dovesi, R.; Saunders, V.R.; Roetti, C.; Orlando, R.; Zicovich-Wilson, C.M.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.M.; Bush, I.J.; et al. CRYSTAL09 User’s Manual; University of Torino: Torino, Italy, 2009. [Google Scholar]
- Wu, Z.; Cohen, R.E. More accurate generalized gradient approximation for solids. Phys. Rev. 2006, B73, 235116. [Google Scholar] [CrossRef]
- Dovesi, R.; Civalleri, B.; Orlando, R.; Roetti, C.; Saunders, V.R. Ab Initio Quantum Simulation in Solid State Chemistry. In Reviews in Computational Chemistry; Lipkowitz, B.K., Larter, R., Cundari, T.R., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2005; Volume 21, pp. 1–125. [Google Scholar]
- Pisani, C.; Dovesi, R.; Roetti, C. Hartree-Fock Ab Initio Treatment of Crystalline Systems; Lecture Notes in Chemistry; Springer: Berlin, Germany, 1988; Volume 48. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Bruno, M.; Massaro, F.R.; Prencipe, M.; Aquilano, D. Surface reconstructions and relaxation effects in a centre-symmetrical crystal: The {00.1} form of calcite (CaCO3). CrystEngComm 2010, 12, 3626–3633. [Google Scholar] [CrossRef]
- Peintinger, M.F.; Oliveira, D.V.; Bredow, T.J. Consistent Gaussian basis sets of triple-zeta valence with polarization quality for solid-state calculations. Comput. Chem. 2013, 34, 451459. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Häser, M.; Patzelt, H.; Ahlrichs, R. RI-MP2: Optimized auxiliary basis sets and demonstration of efficiency. Chem. Phys. Lett. 1998, 294, 143–152. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Monkhrost, H.J.; Pack, J.D. Special points for Brillouin-zone integration. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Civalleri, B.; D’Arco, P.; Orlando, R.; Saunders, V.R.; Dovesi, R. Hartree−Fock geometry optimization of periodic systems with the CRYSTAL code. Chem. Phys. Lett. 2001, 348, 131–138. [Google Scholar] [CrossRef]
- Doll, K. Implementation of analytical Hartree-Fock gradients for periodic systems. Comput. Phys. Commun. 2001, 137, 74–88. [Google Scholar] [CrossRef]
- Doll, K.; Saunders, V.R.; Harrison, N.M. Analytical Hartree-Fock gradients for periodic systems. Int. J. Quantum Chem. 2001, 82, 1–13. [Google Scholar] [CrossRef]
- Available online: http://mabruno.weebly.com/download (accessed on 27 July 2018).
- Bruno, M. The reconstruction of dipolar surfaces: A preliminary step for adsorption modeling. Cryst. Res. Technol. 2013, 48, 811–818. [Google Scholar] [CrossRef]
- Bruno, M.; Rubbo, M.; Massaro, F.R. Behaviour of the chemical potential in calcite and magnesite crystals: A damped harmonic oscillation. Cryst. Growth Des. 2016, 16, 2671–2677. [Google Scholar] [CrossRef]
- Hartman, P.; Perdok, W.G. On the relations between structure and morphology of crystals. III. Acta Cryst. 1955, 8, 525–529. [Google Scholar] [CrossRef]
- Bruno, M. A revised thermodynamic model for crystal surfaces. I. Theoretical aspects. CrystEngComm 2017, 19, 6314–6324. [Google Scholar] [CrossRef]
- Banerjee, A. Estimation of dolomite formation: Dolomite precipitation and dolomitization. J. Geol. Soc. India 2016, 87, 1–12. [Google Scholar] [CrossRef]
- Wang, G.; Li, P.; Hao, F.; Zou, H.; Yu, X. Dolomitization process and its implications for porosity development in dolostones: A case study from the Lower Triassic Feixianguan Formation, Jiannan area, Eastern Sichuan Basin, China. J. Petrol. Sci. Eng. 2015, 131, 184–199. [Google Scholar] [CrossRef]
- Hans, G.M. Concepts and models of dolomitization: A critical reappraisal. Geol. Soc. Sp. 2004, 235, 7–63. [Google Scholar]
| This Work | Zucchini et al. [16] | ||||
|---|---|---|---|---|---|
| Space Group | a (Å) | c (Å) | a (Å) | c (Å) | |
| calcite | 5.062 | 17.351 | - | - | |
| SC1 | 4.855 | 16.210 | 4.825 | 15.971 | |
| SC8 | 4.857 | 16.227 | 4.827 | 16.000 | |
| SC4895 | 4.866 | 16.249 | 4.838 | 15.992 | |
| magnesite | 4.668 | 15.108 | - | - | |
| Slab | Space Group | a (Å) | b (Å) | a^b (°) | γ (J/m2) |
|---|---|---|---|---|---|
| 5.0426 | 8.1526 | 90.0 | 0.507 | ||
| 4.8551 | 7.7862 | 90.0 | 0.489 | ||
| 9.6602 | 15.4230 | 90.0 | 0.437 | ||
| 9.6743 | 15.3506 | 89.8 | 0.450 | ||
| 4.6682 | 7.3769 | 90.0 | 0.493 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, M.; Bittarello, E. (10.4) Face of Ordered and Disordered Dolomite, MgCa(CO3)2: A Computational Study to Reveal the Growth Mechanism. Minerals 2018, 8, 323. https://doi.org/10.3390/min8080323
Bruno M, Bittarello E. (10.4) Face of Ordered and Disordered Dolomite, MgCa(CO3)2: A Computational Study to Reveal the Growth Mechanism. Minerals. 2018; 8(8):323. https://doi.org/10.3390/min8080323
Chicago/Turabian StyleBruno, Marco, and Erica Bittarello. 2018. "(10.4) Face of Ordered and Disordered Dolomite, MgCa(CO3)2: A Computational Study to Reveal the Growth Mechanism" Minerals 8, no. 8: 323. https://doi.org/10.3390/min8080323
APA StyleBruno, M., & Bittarello, E. (2018). (10.4) Face of Ordered and Disordered Dolomite, MgCa(CO3)2: A Computational Study to Reveal the Growth Mechanism. Minerals, 8(8), 323. https://doi.org/10.3390/min8080323





