Adsorption Mechanism of Pb2+ Activator for the Flotation of Rutile
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
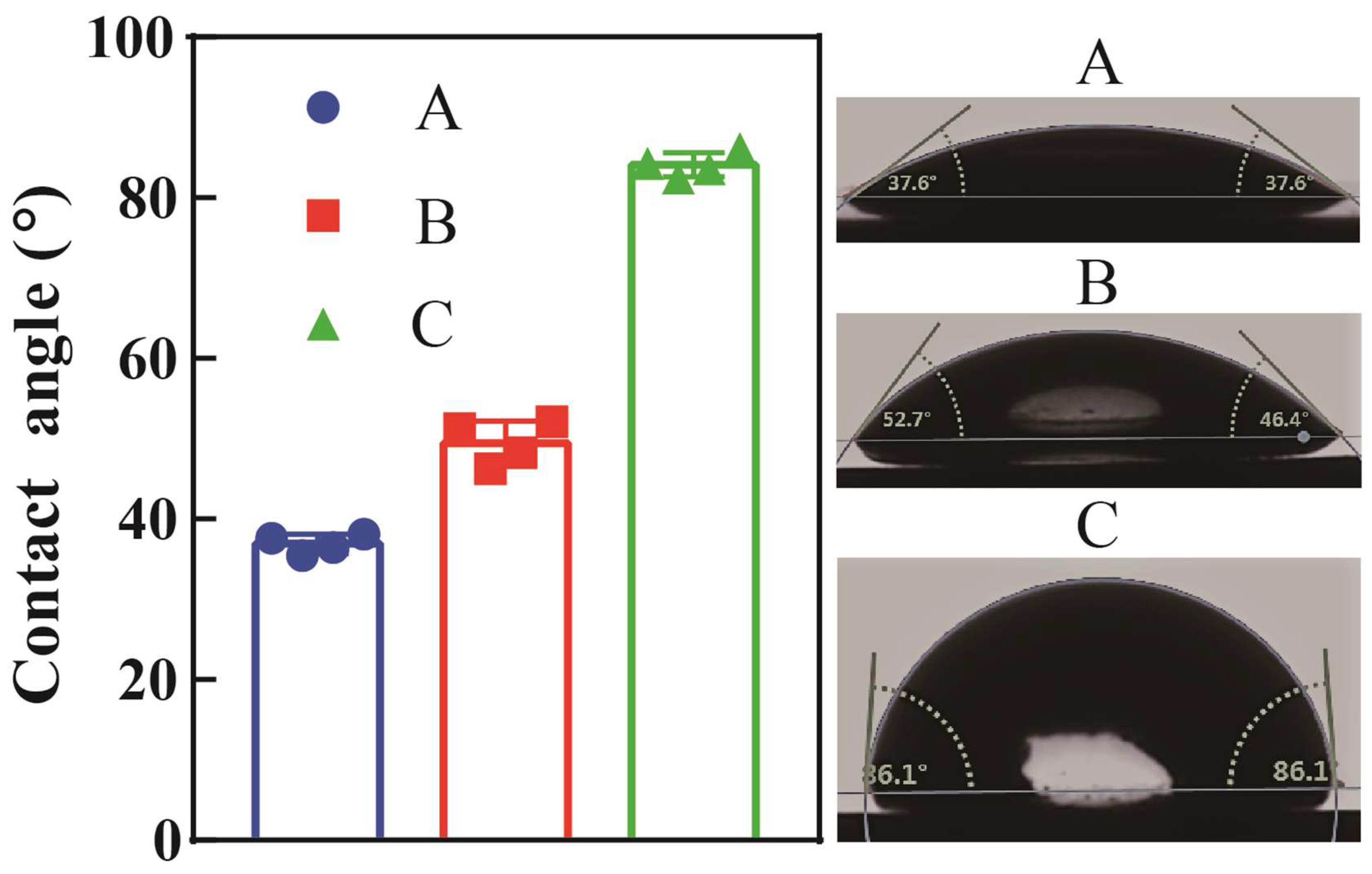
2.2.1. Contact Angle Measurements
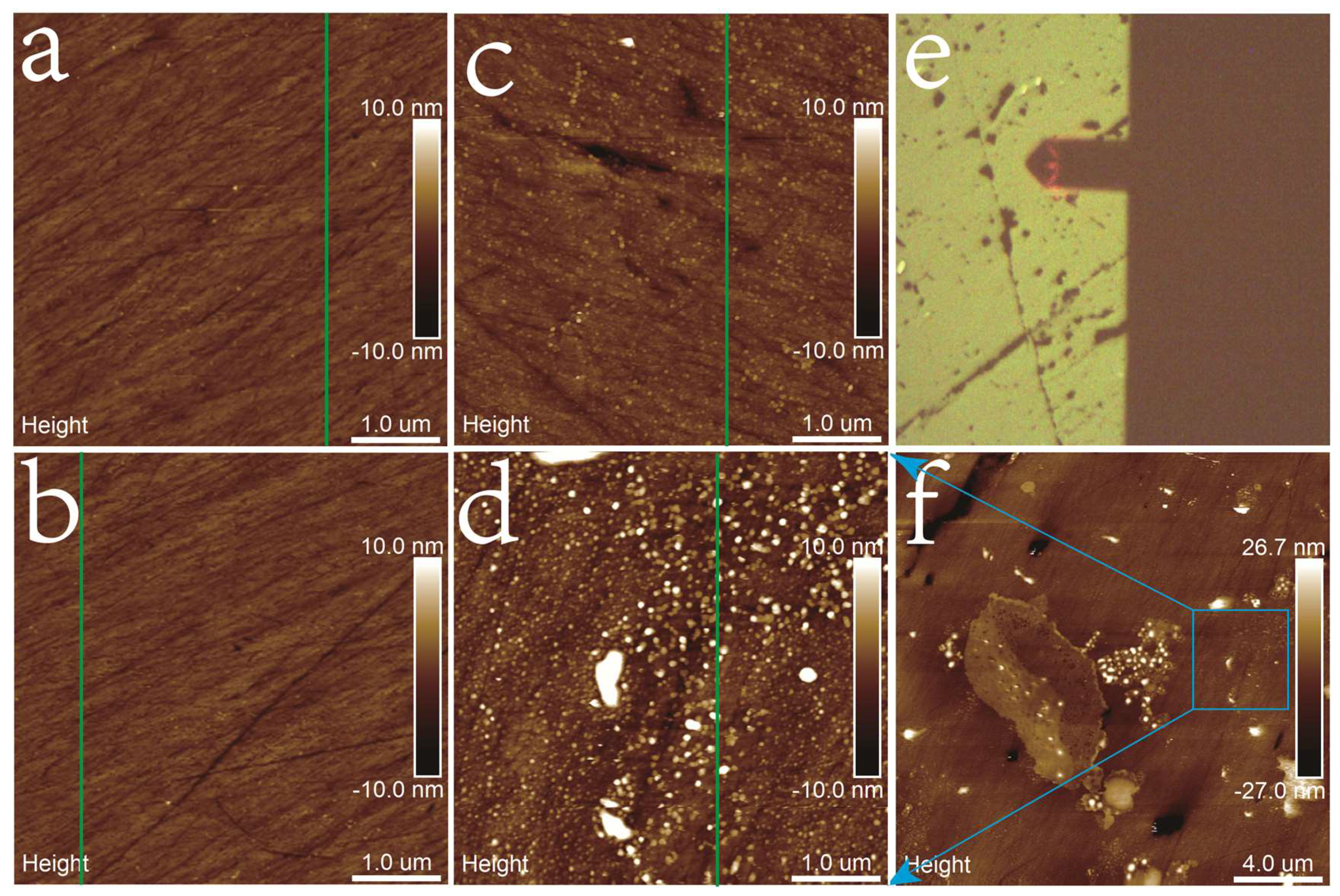
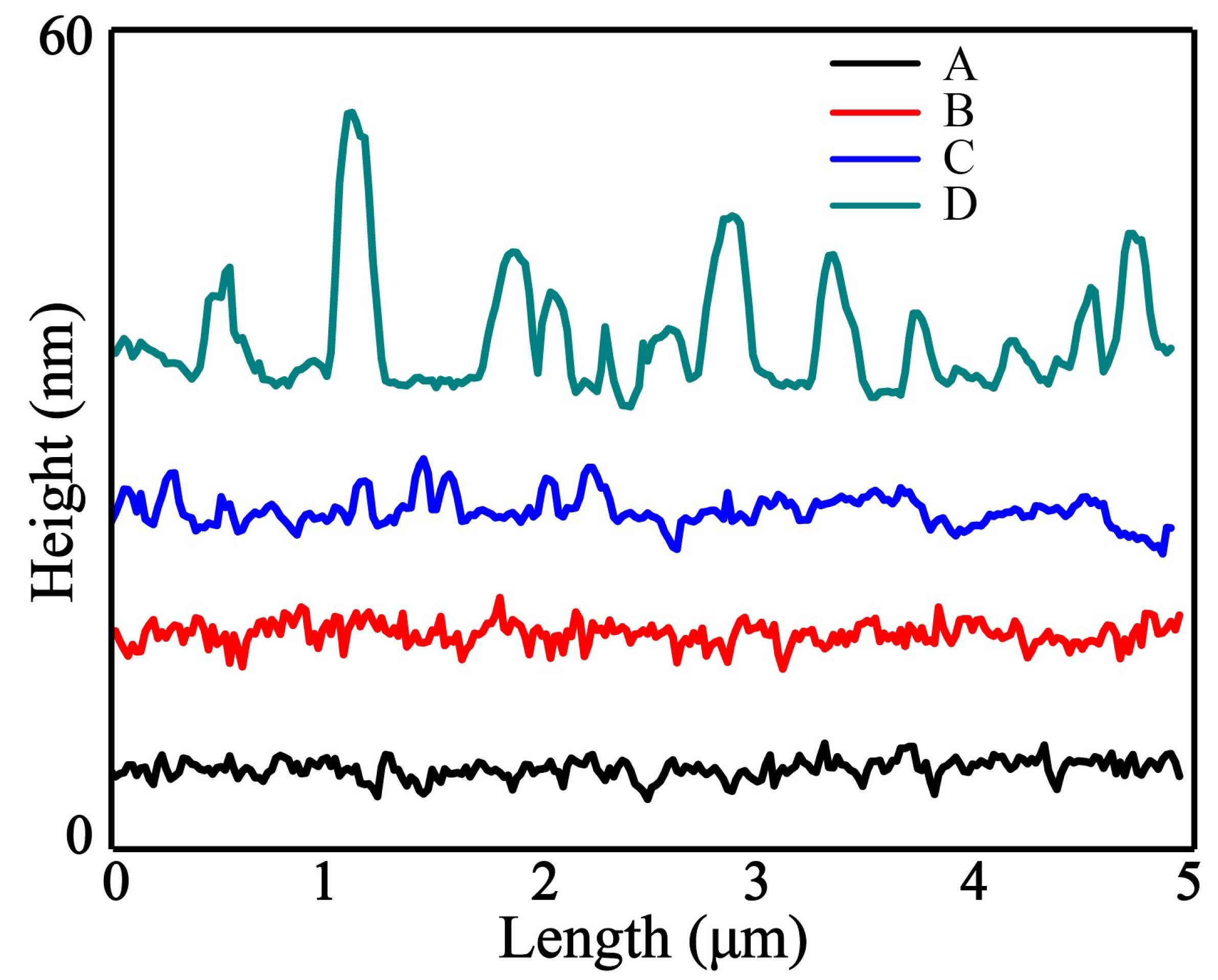
2.2.2. PF-QNM Imaging
3. Results and Discussions
3.1. Contact Angle Measurements
3.2. AFM Imaging
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De la Rosa, J.M.; Miller, A.Z.; Santiago Pozo-Antonio, J.; Gonzalez-Perez, J.A.; Jimenez-Morillo, N.T.; Dionisio, A. Assessing the effects of UVA photocatalysis on soot-coated TiO2-containing mortars. Sci. Total Environ. 2017, 605, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, Y.; Wang, Q.; Zhang, D.; Zhang, A.; Miao, M. TiO2 crystalline structure and electrochemical performance in two-ply yarn CNT/TiO2 asymmetric supercapacitors. J. Mater. Sci. 2017, 52, 7733–7743. [Google Scholar] [CrossRef]
- Nam, I.; Park, J.; Park, S.; Bae, S.; Yoo, Y.G.; Han, J.W.; Yi, J. Observation of crystalline changes of titanium dioxide during lithium insertion by visible spectrum analysis. Phys. Chem. Chem. Phys. 2017, 19, 13140–13146. [Google Scholar] [CrossRef] [PubMed]
- Zaghdoudi, M.; Fourcade, F.; Soutrel, I.; Floner, D.; Amrane, A.; Maghraoui-Meherzi, H.; Geneste, F. Direct and indirect electrochemical reduction prior to a biological treatment for dimetridazole removal. J. Hazard. Mater. 2017, 335, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z. The activation mechanism of lead ions in the flotation of ilmenite using sodium oleate as a collector. Min. Eng. 2017, 111, 100–107. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z.; Lai, X. Adsorption mechanism of lead ions at ilmenite/water interface and its influence on ilmenite flotability. J. Ind. Eng. Chem. 2017, 53, 285–293. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Yu, L.; Xu, Y.; Du, Y. Study on the activation mechanism of lead ions in the flotation of ilmenite using benzyl hydroxamic acid as collector. J. Ind. Eng. Chem. 2018, 62, 209–216. [Google Scholar] [CrossRef]
- Li, H.; Mu, S.; Weng, X.; Zhao, Y.; Song, S. Rutile Flotation with Pb2+ Ions as Activator: Adsorption of Pb2+ at Rutile/Water Interface. Colloid Surf. A Physicochem. Eng. Asp. 2016, 506, 431–437. [Google Scholar] [CrossRef]
- Xiao, W.; Cao, P.; Liang, Q.; Peng, H.; Zhao, H.; Qin, W.; Qiu, G.; Wang, J. The Activation Mechanism of Bi3+ Ions to Rutile Flotation in a Strong Acidic Environment. Minerals 2017, 7, 113. [Google Scholar] [CrossRef]
- Xiao, W.; Cao, P.; Liang, Q.; Huang, X.; Li, K.; Zhang, Y.; Qin, W.; Qiu, G.; Wang, J. Adsorption behavior and mechanism of Bi(III) ions on rutile-water interface in the presence of nonyl hydroxamic acid. Trans. Nonferrous Met. Soc. China 2018, 28, 348–355. [Google Scholar] [CrossRef]
- Xiao, W.; Fang, C.; Wang, J.; Liang, Q.; Cao, P.; Wang, X.; Zhang, L.; Qiu, G.; Hu, J. The role of EDTA on rutile flotation using Al3+ ions as an activator. Rsc Adv. 2018, 8, 4872–4880. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Wang, S.; Liu, G. The activation mechanism of Cu(II) to ilmenite and subsequent flotation response to α-hydroxyoctyl phosphinic acid. J. Ind. Eng. Chem. 2016, 37, 123–130. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Larachi, F.; Hart, B. Collector attachment to lead-activated sphalerite—Experiments and DFT study on pH and solvent effects. Appl. Surf. Sci. 2016, 367, 459–472. [Google Scholar] [CrossRef]
- Xing, Y.; Li, C.; Gui, X.; Cao, Y. Interaction Forces between Paraffin/Stearic Acid and Fresh/Oxidized Coal Particles Measured by Atomic Force Microscopy. Energy Fuels 2017, 31, 3305–3312. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Yang, Y.; Sun, W.; Hu, Y. Effect of Pb2+ ions on ilmenite flotation and adsorption of benzohydroxamic acid as a collector. Appl. Surf. Sci. 2017, 425, 796–802. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Feng, Q.; Zhang, G.; Chang, Z. The role of calcium and carbonate ions in the separation of pyrite and talc. Min. Eng. 2018, 119, 205–211. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Li, Q.; Ou, L.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161. [Google Scholar] [CrossRef]
- Xiao, W.; Ke, S.; Quan, N.; Zhou, L.; Wang, J.; Zhang, L.; Dong, Y.; Qin, W.; Qiu, G.; Hu, J. The Role of Nanobubbles in the Precipitation and Recovery of Organic-Phosphine-Containing Beneficiation Wastewater. Langmuir 2018. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Zhao, H.; Qin, W.; Qiu, G.; Wang, J. Adsorption Mechanism of Pb2+ Activator for the Flotation of Rutile. Minerals 2018, 8, 266. https://doi.org/10.3390/min8070266
Xiao W, Zhao H, Qin W, Qiu G, Wang J. Adsorption Mechanism of Pb2+ Activator for the Flotation of Rutile. Minerals. 2018; 8(7):266. https://doi.org/10.3390/min8070266
Chicago/Turabian StyleXiao, Wei, Hongbo Zhao, Wenqing Qin, Guanzhou Qiu, and Jun Wang. 2018. "Adsorption Mechanism of Pb2+ Activator for the Flotation of Rutile" Minerals 8, no. 7: 266. https://doi.org/10.3390/min8070266
APA StyleXiao, W., Zhao, H., Qin, W., Qiu, G., & Wang, J. (2018). Adsorption Mechanism of Pb2+ Activator for the Flotation of Rutile. Minerals, 8(7), 266. https://doi.org/10.3390/min8070266






