Potential of Soil, Sludge and Sediment for Mineral Carbonation Process in Selinsing Gold Mine, Malaysia
Abstract
1. Introduction
1.1. Potential of Mining Waste for Carbon Sequestration
1.2. The Mineral Carbonation Process
1.3. Factors Affecting The Mineral Carbonation Process
2. Materials and Methods
2.1. Site Description
2.2. Field Sampling
2.3. Particle-Size Distribution Analysis
2.4. Mineralogical Analysis
2.5. Morphological and Chemical Analysis
3. Results
3.1. pH and Particle-Size Distribution of the Soil, Sludge and Sediment
3.2. Mineralogy and Chemical Composition of Soil, Sludge and Sediment
4. Discussion
4.1. Properties of Soil, Sludge, and Sediment for Mineral Carbonation
4.2. Availability of Silicate Mineral for Mineral Carbonation
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- IPCC, Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Synthesis Report; IPCC, Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharps, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Manning, D.A.C. Biological enhancement of soil carbonate precipitation: Passive removal of atmospheric CO2. Mineralo. Mag. 2008, 72, 639–649. [Google Scholar] [CrossRef]
- Renforth, P. Mineral Carbonation in Soils Engineering the Soil Carbon Sink. Ph.D. Thesis, Newcastle University, Newcastle, UK, 2011. [Google Scholar]
- Renforth, P.; Washbourne, C.L.; Taylder, J.; Manning, D.A.C. Silicate production and availability for mineral carbonation. Environ. Sci. Technol. 2011, 45, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Manning, D.A.C.; Renforth, P. Passive sequestration of atmospheric CO2 through coupled plant-mineral reactions in urban soils. Environ. Sci. Technol. 2013, 47, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jorat, M.E.; Goddard, M.A.; Kolosz, B.W.; Sohi, S.; Manning, D.A.C. Sustainable Urban Carbon Capture: Engineering Soils for Climate Change (SUCCESS). In Proceedings of the XVIECSMGE Geotechnical Engineering for Infrastructure and Development, Edinburgh, UK, 13–17 September 2015; pp. 2559–2564. [Google Scholar]
- Washbourne, C.L.; Lopez-Capel, E.; Renforth, P.; Ascough, P.L.; Manning, D.A.C. Rapid removal of atmospheric CO2 by urban soils. Environ. Sci. Technol. 2015, 49, 5434–5440. [Google Scholar] [CrossRef] [PubMed]
- Arce, G.L.A.F.; Neto, T.G.S.; Ávila, I.; Luna, C.M.R.; dos Santos, J.C.; Carvalho, J.A. Influence of physicochemical properties of Brazilian serpentinites on the leaching process for indirect CO2 mineral carbonation. Hydrometallurgy 2017, 169, 142–151. [Google Scholar] [CrossRef]
- Jorat, M.E.; Kolosz, B.W.; Sohi, S.P.; Lopez-Capel, E.; Manning, D.A.C. Changes in geotechnical properties of urban soils during carbonation. In Proceedings of the 15th Pan-American Conference on Soil Mechanics and Geotechnical Engineering, Buenos Ares, Argentina, 15–18 November 2015; pp. 912–918. [Google Scholar]
- Jorat, M.E.; Kolosz, B.W.; Goddard, M.A.; Sohi, S.P.; Akgun, N.; Dissanayake, D.; Manning, D.A.C. Geotechnical requirements for capturing CO2 through highways land. Int. J. GEOMATE 2017, 13, 22–27. [Google Scholar] [CrossRef]
- Renforth, P.; Manning, D.A.C.; Lopez-Capel, E. Carbonate precipitation in artificial soils as a sink for atmospheric carbon dioxide. Appl. Geochem. 2009, 24, 1757–1764. [Google Scholar] [CrossRef]
- Moosdorf, N.; Hartmann, J.; Lauerwald, R.; Hagedorn, B.; Kempe, S. Atmospheric CO2 consumption by chemical weathering in North America. Geochim. Cosmochim. Ac. 2011, 75, 7829–7854. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chang, E.E.; Chiang, P.-C. CO2 capture by accelerated carbonation of alkaline wastes: A review on its principles and applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar]
- Power, I.M.; Harrison, A.L.; Dipple, G.M. Carbon mineralization: From natural analogues to engineered systems. Rev. Mineral. Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Li, P.; Pan, S.-Y.; Pei, S.; Lin, Y.J.; Chiang, P.-C. Challenges and perspectives on carbon fixation and utilization technologies: An overview. Aerosol Air Qual. Res. 2016, 16, 1327–1344. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. A review on integrated mineral carbonation process in ultramafic mine deposit. In Proceedings of the 8th International Conference on Sustainable Development in the Minerals Industry, Beijing, China, 25–29 June 2017; pp. 148–154. [Google Scholar]
- Manning, D.A.C.; Renforth, P.; Lopez-Capel, E.; Robertson, S.; Ghazireh, N. Carbonate precipitation in artificial soils produced from basaltic quarry fines and composts: An opportunity for passive carbon sequestration. Int. J. Greenh. Gas Control 2013, 17, 309–317. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Molson, J.; Beaudoin, G. Impact of temperature and oxygen availability on the dynamics of ambient CO2 mineral sequestration by nickel mining residues. Chem. Eng. J. 2014, 240, 394–403. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Molson, J.; Beaudoin, G. Comparative study of five Québec ultramafic mining residues for use in direct ambient carbon dioxide mineral sequestration. Chem. Eng. J. 2014, 245, 56–64. [Google Scholar] [CrossRef]
- Bodénan, F.; Bourgeois, F.; Petiot, C.; Augé, T.; Bonfils, B.; Julcour-Lebigue, C.; Guyot, F.; Boukary, A.; Tremosa, J.; Lassin, A.; et al. Ex situ mineral carbonation for CO2 mitigation: Evaluation of mining waste resources, aqueous carbonation processability and life cycle assessment (Carmex project). Miner. Eng. 2014, 59, 52–63. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Thom, J.M.; Anderson, R.G.; Raudsepp, M.; Gabite, J.E.; Southam, G. Carbon dioxide fixation within mine wastes of ultramafic-hosted ore deposits: Examples from the Clinton Creek and Cassiar chrysotile deposits, Canada. Econ. Geol. 2009, 104, 95–112. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Ultra-fine grinding and mechanical activation of mine waste rock using a high-speed stirred mill for mineral carbonation. Int. J. Miner. Metall. Mater. 2015, 22, 1005–1017. [Google Scholar] [CrossRef]
- Washbourne, C.L.; Renforth, P.; Manning, D.A.C. Investigating carbonate formation in urban soils as a method for capture and storage of atmospheric carbon. Sci. Total Environ. 2012, 431, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. CO2 sequestration in chrysotile mining residues-implication of watering and passivation under environmental conditions. Ind. Eng. Chem. Res. 2012, 51, 8726–8734. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. Dynamics of carbon dioxide uptake in chrysotile mining residues—Effect of mineralogy and liquid saturation. Int. J. Greenh. Gas Control 2013, 12, 124–135. [Google Scholar] [CrossRef]
- Ah-Hyung, A.P.; Liang-Shih, F. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar]
- Vogeli, J.; Reid, D.L.; Becker, M.; Broadhurst, J.; Franzidis, J.P. Investigation of the potential for mineral carbonation of PGM tailings in South Africa. Miner. Eng. 2011, 24, 1348–1356. [Google Scholar] [CrossRef]
- Gras, A.; Beaudoin, G.; Molson, J.; Plante, B.; Bussière, B.; Lemieux, J.M.; Dupont, P.P. Isotopic evidence of passive mineral carbonation in mine wastes from the Dumont Nickel Project (Abitibi, Quebec). Int. J. Greenh. Gas Control 2017, 60, 10–23. [Google Scholar] [CrossRef]
- Jacobs, A.D. Quantifying the Mineral Carbonation Potential of Mine Waste Material: A New Parameter for Geospatial Estimation. Ph.D. Thesis, University of British Columbia, Vancouver, Canada, 2014. [Google Scholar]
- Xie, H.; Yue, H.; Zhu, J.; Liang, B.; Li, C.; Wang, Y.; Xie, L.; Zhou, X. Scientific and engineering progress in CO2 mineralization using industrial waste and natural minerals. Engineering 2015, 1, 150–157. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; Zhao, Y.; Liu, R.; Zheng, C. CO2 sequestration by direct aqueous mineral carbonation under low-medium pressure conditions. J. Chem. Eng. Jpn. 2015, 48, 937–946. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Assima, G.P.; Beaudoin, G.; Larachi, F. Biomass torrefaction and CO2 capture using mining wastes—A new approach for reducing greenhouse gas emissions of co-firing plants. Fuel 2014, 115, 749–757. [Google Scholar] [CrossRef]
- Harrison, A.L.; Power, I.M.; Dipple, G.M. Accelerated carbonation of brucite in mine tailings for carbon sequestration. Environ. Sci. Technol. 2013, 47, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Lechat, K.; Jean-Michel, L.; Molson, J.; Beaudoin, G.; Hébert, R. Field evidence of CO2 sequestration by mineral carbonation in ultramafic milling wastes, Thetford Mines, Canada. Int. J. Greenh. Gas Control 2016, 47, 110–121. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Molson, J.; Beaudoin, G. Emulation of ambient carbon dioxide diffusion and carbonation within nickel mining residues. Miner. Eng. 2014, 59, 39–44. [Google Scholar] [CrossRef]
- Yadav, V.S.; Prasad, M.; Khan, J.; Amritphale, S.S.; Singh, M.; Raju, C.B. Sequestration of carbon dioxide (CO2) using red mud. J. Hazard. Mater. 2010, 176, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Lutpi, N.A.; Zhu, J. Carbonation of bauxite residue: A solution for carbon dioxide capture in alumina industry. In Proceedings of the ICSTIE, Gurney Resort Hotel and Residences, Penang, Malaysia, 16–17 December 2010; pp. 1–8. [Google Scholar]
- Renforth, P.; Mayes, W.M.; Jarvis, A.P.; Burke, I.T.; Manning, D.A.C.; Gruiz, K. Contaminant mobility and carbon sequestration downstream of the Ajka (Hungary) red mud spill: The effects of gypsum dosing. Sci. Total Environ. 2012, 421–422, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.C. Neutralization of Red Mud Using CO2 Sequestration and Their Utilization. Ph.D. Thesis, National Institute of Technology Rourkela, Orissa, India, 2011. [Google Scholar]
- Oskierski, H.C.; Dlugogorski, B.Z.; Jacobsen, G. Sequestration of atmospheric CO2 in chrysotile mine tailings of the Woodsreef Asbestos Mine, Australia: Quantitative mineralogy, isotopic fingerprinting and carbonation rates. Chem. Geol. 2013, 358, 156–169. [Google Scholar] [CrossRef]
- Yeap, E.B. Tin and gold mineralizations in Peninsular Malaysia and their relationships to the tectonic development. J. Southeast Asian Earth 1993, 8, 329–348. [Google Scholar] [CrossRef]
- Makoundi, C.; Zaw, K.; Large, R.R.; Meffre, S.; Chun-Kit, L.; Hoe, T.G. Geology, geochemistry and metallogenesis of the Selinsing gold deposit, central Malaysia. Gondwana Res. 2014, 26, 241–261. [Google Scholar] [CrossRef]
- Pour, A.B.; Hashim, M. Structural mapping using PALSAR data in the Central Gold Belt, Peninsular Malaysia. Ore Geol. Rev. 2015, 64, 13–22. [Google Scholar] [CrossRef]
- Monument Mining Limited Selinsing Gold Mine and Buffalo Reef Project-Malaysia; NI 43-101 Technical Report; Snowden: Perth, Australia, 2016.
- EPA, U.S. Environmental Protection Agency. Guidance on Choosing a Sampling Design for Environmental Data Collection; Report QA/G-5S; Office of Environmental Information: Washington, DC, USA, 2002.
- Smith, K.S.; Hageman, P.L.; Ramsey, C.A.; Wildeman, T.R.; Ranville, J.F. Reconnaissance sampling and characterization of mine-waste material. In Proceedings of the US Environmental Protection Agency Hard Rock Mining 2006 Conference, Tucson, AZ, USA, 14–16 November 2006. [Google Scholar]
- B1377 (9: 1990). British Standard Methods of Test for Soils for Civil Engineering Purposes, Part 3: In-Situ Tests; British Standards Institution: London, UK, 1990. [Google Scholar]
- Shamshuddin, J. Methods in Soil Mineralogy; Universiti Putra Malaysia Press: Serdang, Malaysia, 2011; pp. 14–42. ISBN 978-967-344-198-3. [Google Scholar]
- EPA, U.S. Environmental Protection Agency. EPA and Hardrock Mining: A Source Book for Industry in the Northwest and Alaska, Appendix C: Characterization of Ore, Waste Rock, and Tailings; Office of Solid Waste: Washington, DC, USA, 2003.
- Teh, C.B.S.; Talib, J. Soil Physics Analysis; Universiti Putra Malaysia Press: Serdang, Malaysia, 2006; Volume 1, pp. 1–6. ISBN 983-3455-64-6. [Google Scholar]
- Kandji, E.H.B.; Plante, B.; Bussière, B.; Beaudoin, G.; Pierre-Philippe, D. Geochemical behavior of ultramafic waste rocks with carbon sequestration potential: A case study of the Dumont Nickel Project, Amos, Québec. Environ. Sci. Pollut. Res. 2017, 24, 11734–11751. [Google Scholar] [CrossRef] [PubMed]
- Bell, F.G. Lime stabilization of clay minerals and soils. Eng. Geol. 1996, 42, 223–237. [Google Scholar] [CrossRef]
- Mani, D.; Charan, S.N.; Kumar, B. Assessment of carbon dioxide sequestration potential of ultramafic rocks in the greenstone belts of southern India. Curr. Sci. India 2008, 94, 5–60. [Google Scholar]
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Change 2007, 80, 5–23. [Google Scholar] [CrossRef]
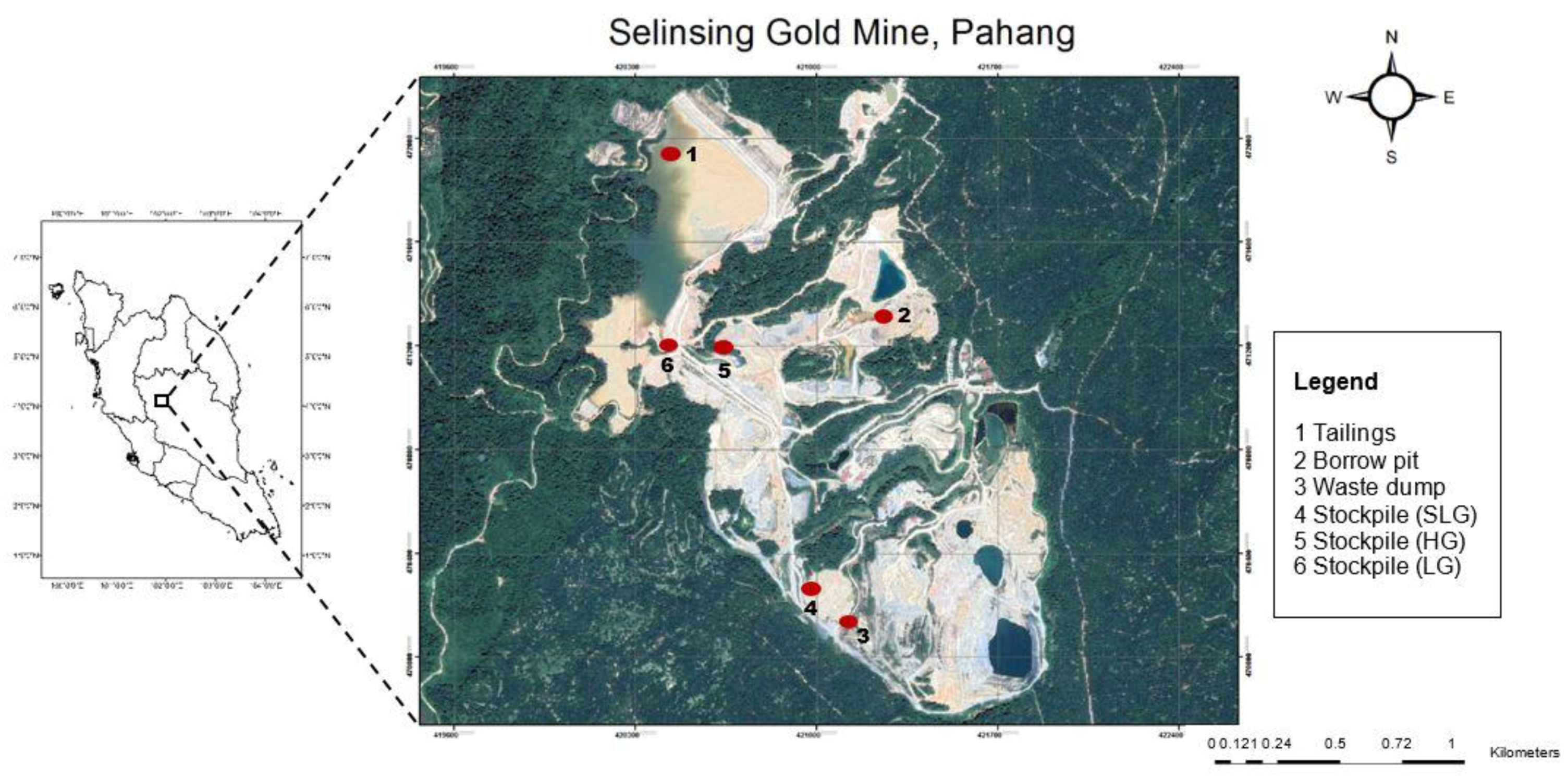
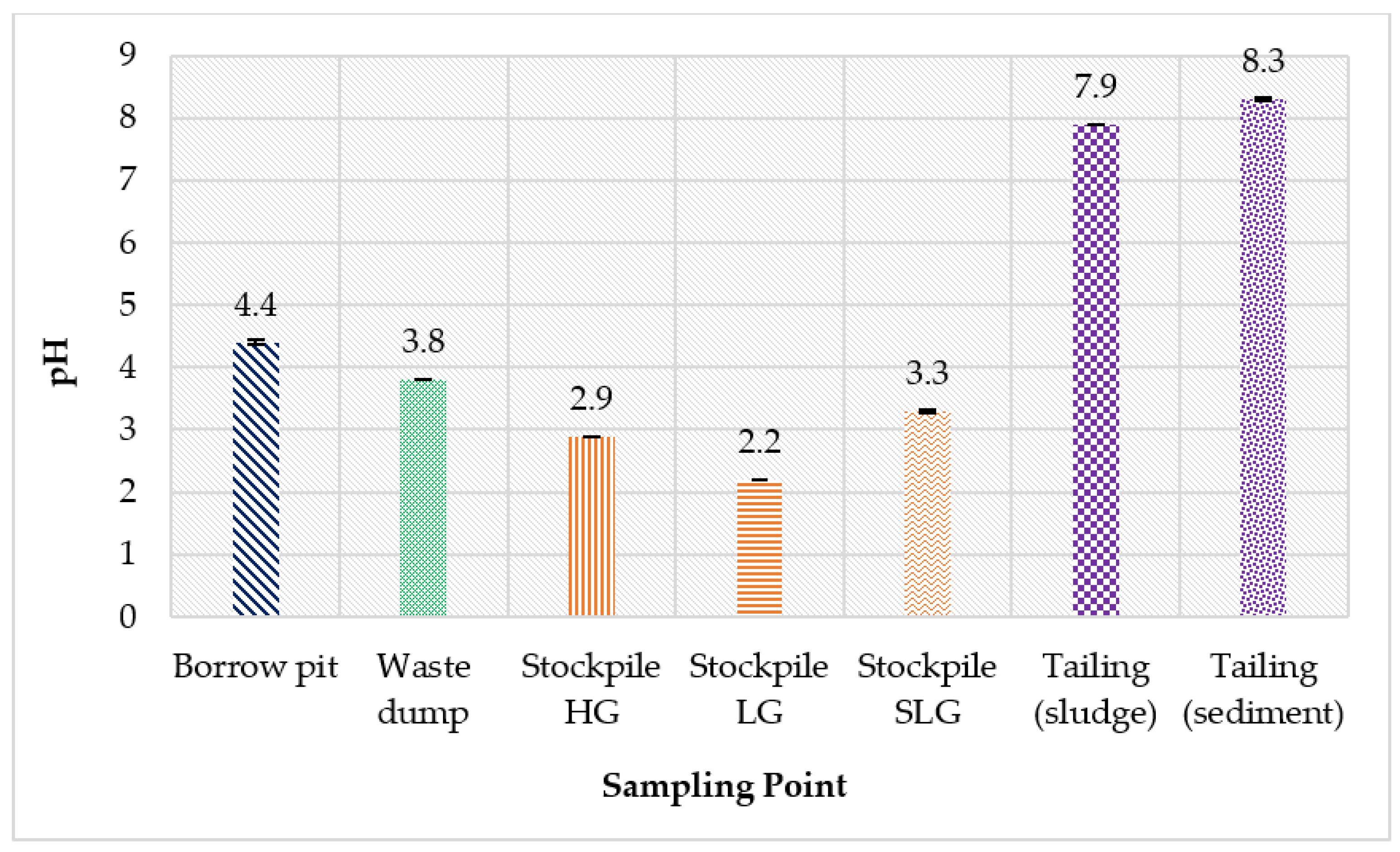
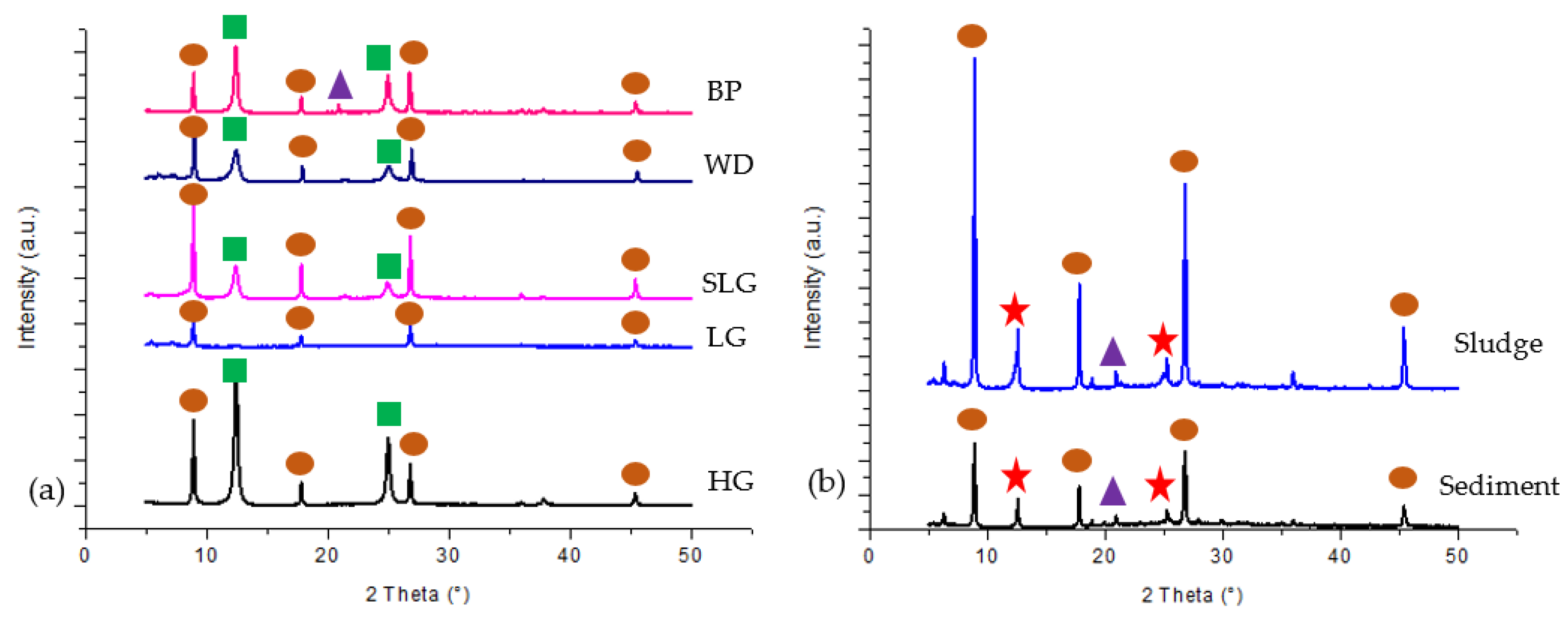
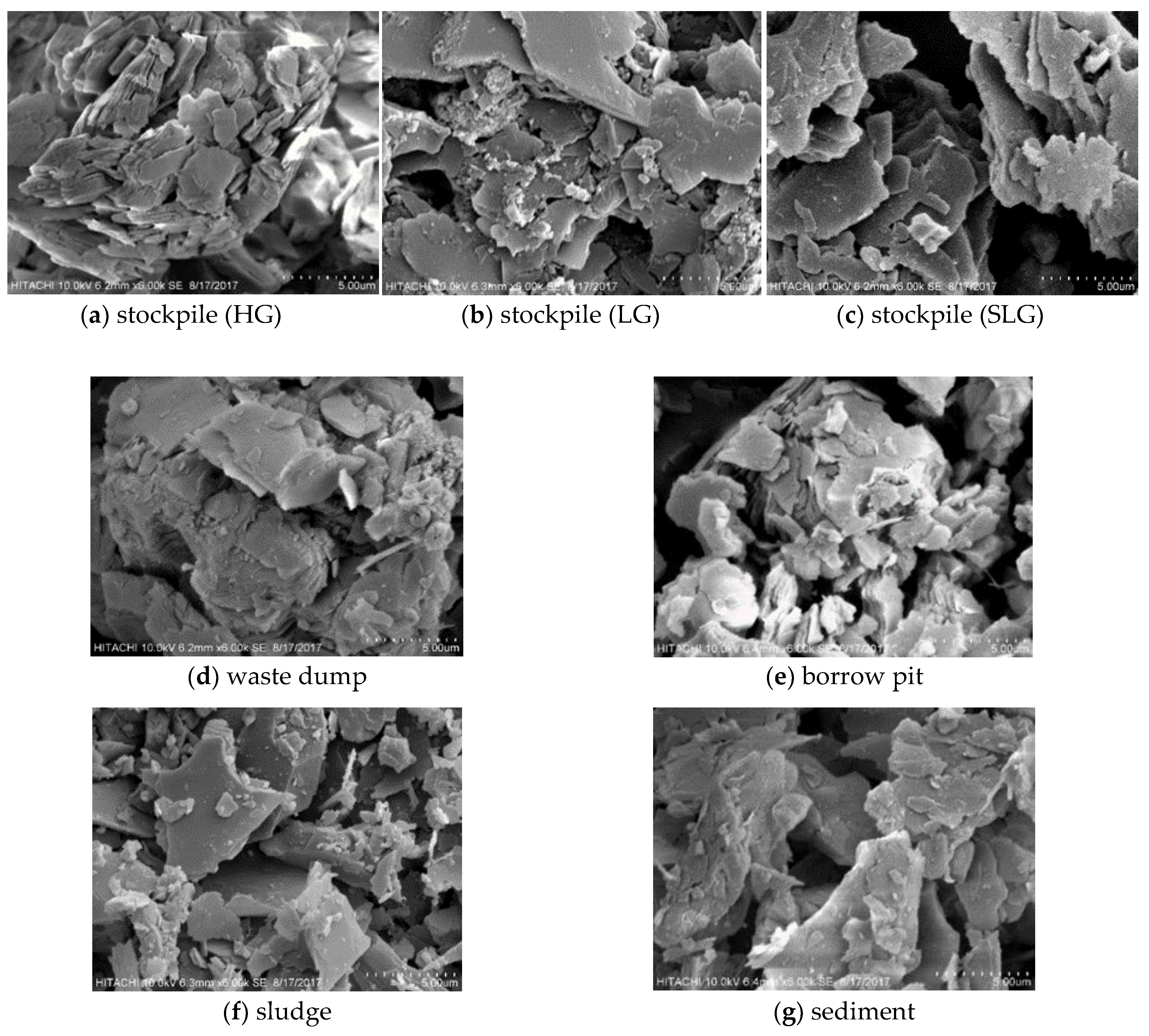
| Sampling Point | Type of Sample | Total Sample Weight | Characteristic |
|---|---|---|---|
| Tailings | Sludge sediment | 2.20 kg 4.60 kg | Waste from treatment plant. |
| Stockpile | Soil | 13.57 kg | |
| High Grade (HG) | Phyllite, conglomerate. | ||
| Lower Grade (LG) | Phyllite, shale. | ||
| Super Lower Grade (SLG) | Tuffaceous, shale. | ||
| Waste Dump | Soil | 17.37 kg | Sedimentary rock, arginite, volcanic. |
| Borrow Pit | Soil | 15.37 kg | Highly silicate clay, argillite, kaolinite, serinite, medium to fine size, highly oxidize. |
| Particle Size Distribution (%) | Soil Texture Class | |||
|---|---|---|---|---|
| Sampling Point | Clay | Silt | Sand | (USDA) |
| (<2 µm) | (2–50 µm) | (>50 µm) | ||
| Borrow pit | 7.81 | 91.05 | 1.19 | Silt |
| Waste dump | 11.16 | 68.06 | 20.66 | Silt loam |
| Stockpile HG | 10.80 | 63.98 | 25.17 | Silt loam |
| Stockpile LG | 19.17 | 18.08 | 62.77 | Sandy loam |
| Stockpile SLG | 7.25 | 60.65 | 32.01 | Silt loam |
| Tailings (sludge) | 17.43 | 78.23 * | 4.28 | Silt loam |
| Tailings (sediment) | 6.96 | 53.21 | 39.74 | Silt loam |
| Minerals | Sampling Point | |||
|---|---|---|---|---|
| Waste Dump | Stockpile | Tailings | Borrow Pit | |
| 1. Quartz SiO2 | √ | √ | √ | √ |
| 2. Kaolinite Al2Si2O5(OH)4 | √ | √ | √ | √ |
| 3. Chlorite-serpentine a (Mg,Al)6(Si,Al)4O10(OH)8 | √ | |||
| 4. Illite b (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)] | √ | √ | √ | √ |
| 5. Aerinite b [(Fe+2,Fe+3,Al)3Mg3(Ca,Na)4(Si13.5Al4.5O42)(OH)6]·12H2O | √ | |||
| 6. Stilpnomelane b Fe2Si3O9 | √ | |||
| 7. Sepiolite a Mg4Si6O15(OH)2·6H2O | √ | |||
| Major Oxide (%) | Sampling Point | ||||||
|---|---|---|---|---|---|---|---|
| Stockpile HG | Stockpile LG | Stockpile SLG | Waste Dump | Borrow Pit | Tailings (Sludge) | Tailings (Sediment) | |
| MgO * | - | 2.72 * | - | - | - | 1.74 * | - |
| SiO2 | 65.61 | 48.46 | 67.90 | 59.53 | 75.26 | 60.39 | 64.70 |
| Fe2O3 * | - | 11.79 * | 3.15 * | 3.60 * | - | 3.20 * | 3.04 * |
| Al2O3 | 25.84 | 20.18 | 22.82 | 29.93 | 19.25 | 18.22 | 14.60 |
| K2O | 3.00 | 7.24 | 5.72 | 6.36 | 4.11 | 7.06 | 6.63 |
| SO3 | 2.53 | 8.83 | - | - | - | 1.37 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed Hasan, S.N.M.; Mohd Kusin, F.; Jusop, S.; Mohamat Yusuff, F. Potential of Soil, Sludge and Sediment for Mineral Carbonation Process in Selinsing Gold Mine, Malaysia. Minerals 2018, 8, 257. https://doi.org/10.3390/min8060257
Syed Hasan SNM, Mohd Kusin F, Jusop S, Mohamat Yusuff F. Potential of Soil, Sludge and Sediment for Mineral Carbonation Process in Selinsing Gold Mine, Malaysia. Minerals. 2018; 8(6):257. https://doi.org/10.3390/min8060257
Chicago/Turabian StyleSyed Hasan, Sharifah Nur Munirah, Faradiella Mohd Kusin, Shamshuddin Jusop, and Ferdius Mohamat Yusuff. 2018. "Potential of Soil, Sludge and Sediment for Mineral Carbonation Process in Selinsing Gold Mine, Malaysia" Minerals 8, no. 6: 257. https://doi.org/10.3390/min8060257
APA StyleSyed Hasan, S. N. M., Mohd Kusin, F., Jusop, S., & Mohamat Yusuff, F. (2018). Potential of Soil, Sludge and Sediment for Mineral Carbonation Process in Selinsing Gold Mine, Malaysia. Minerals, 8(6), 257. https://doi.org/10.3390/min8060257





