Significant Improvement in the Scheelite Heating Flotation with Sodium Sulfide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
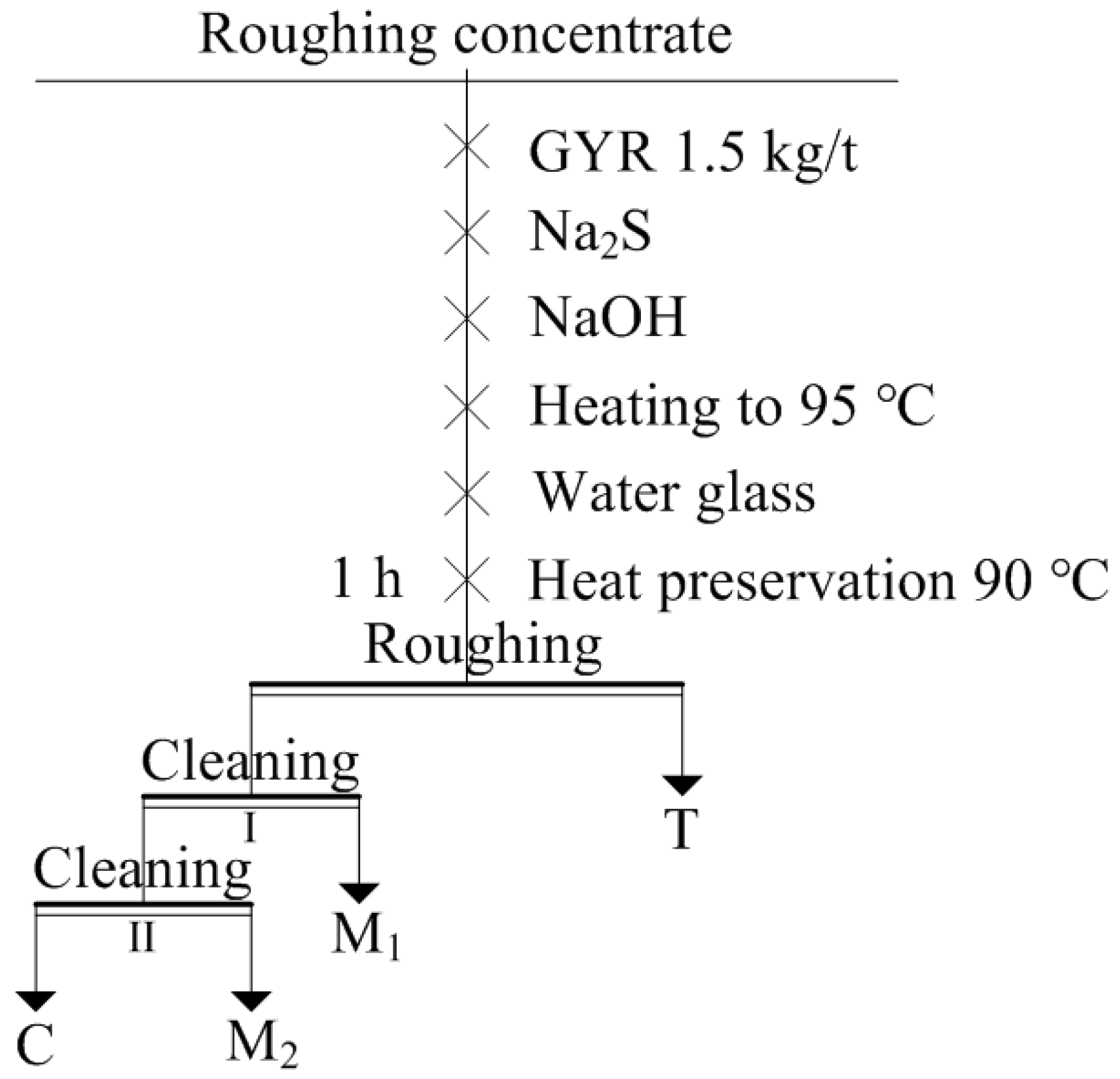
2.2.1. Scheelite Heating Flotation Experiments
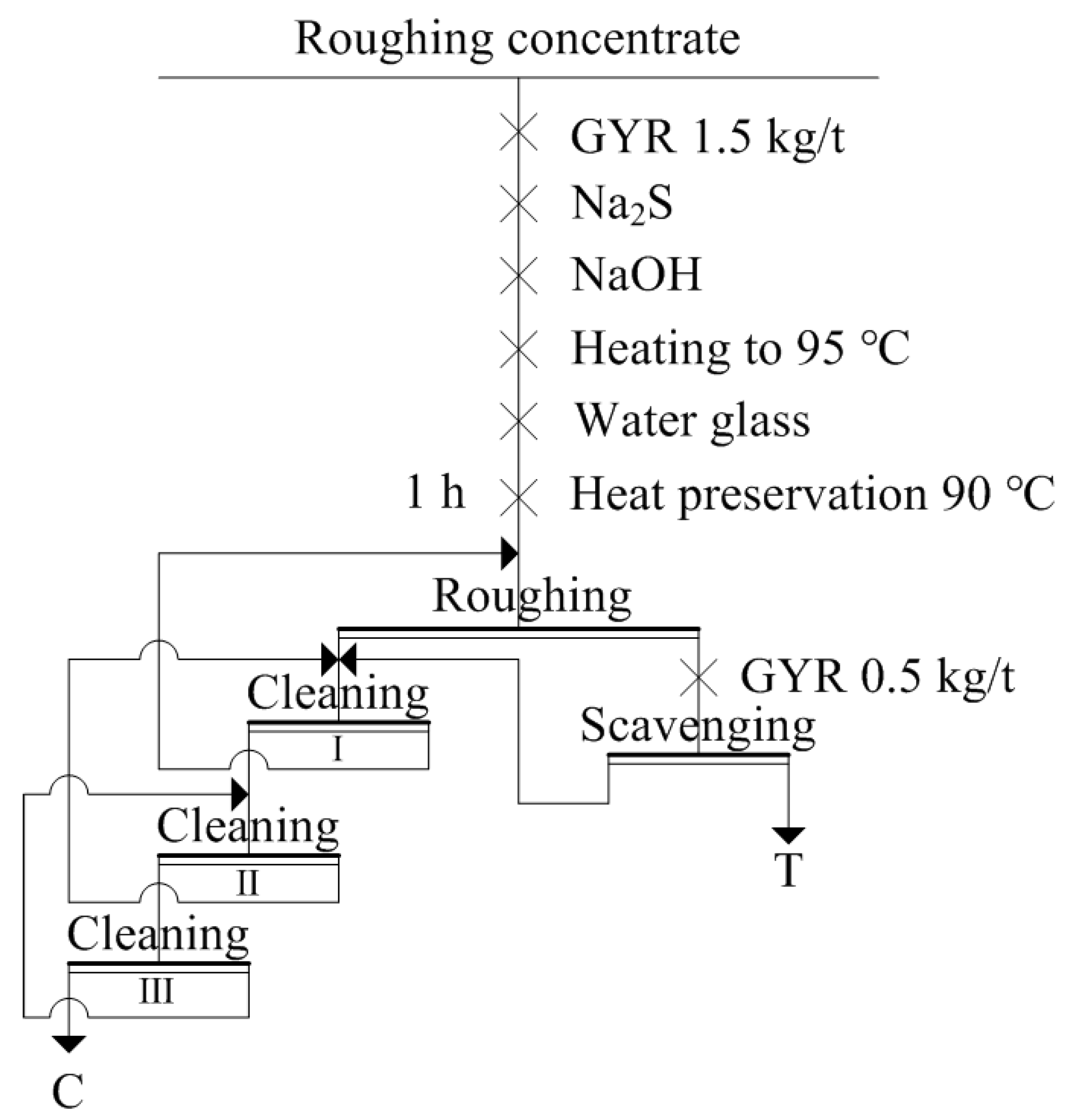
2.2.2. Industrial Tests
2.2.3. Adsorption Amount Analysis
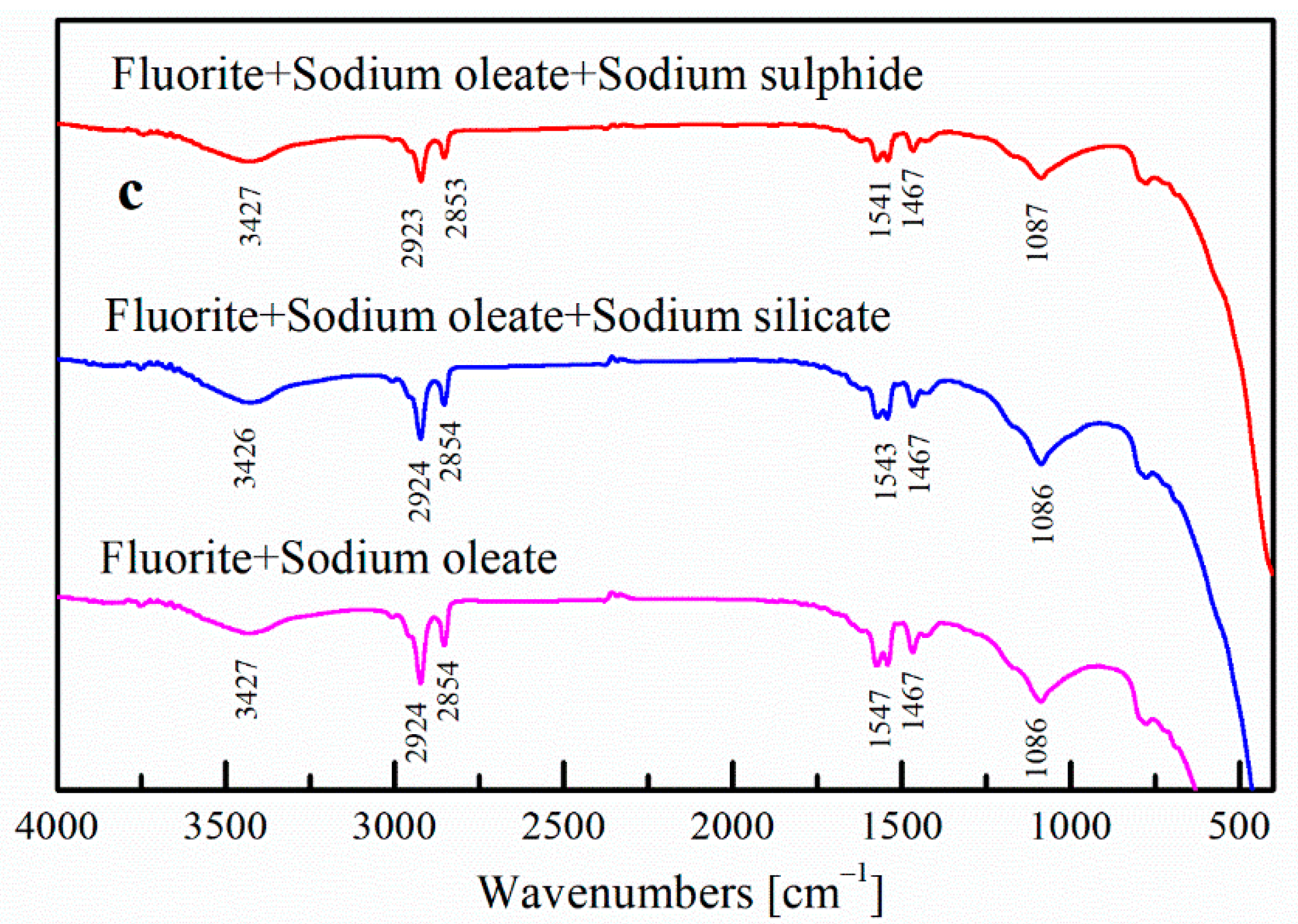
2.2.4. FTIR Analysis
3. Results and Discussion
3.1. Scheelite Heating Flotation Experiments
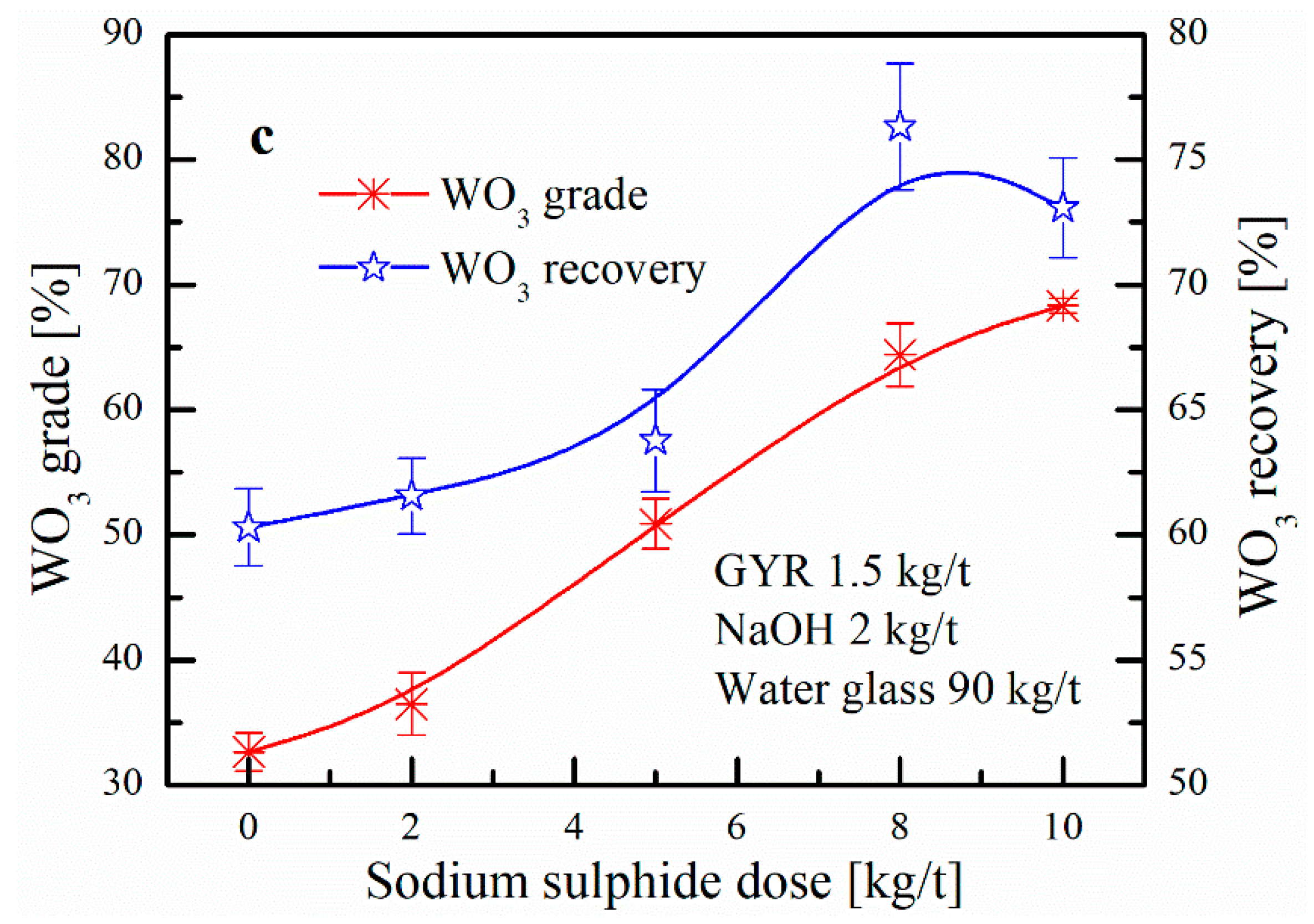
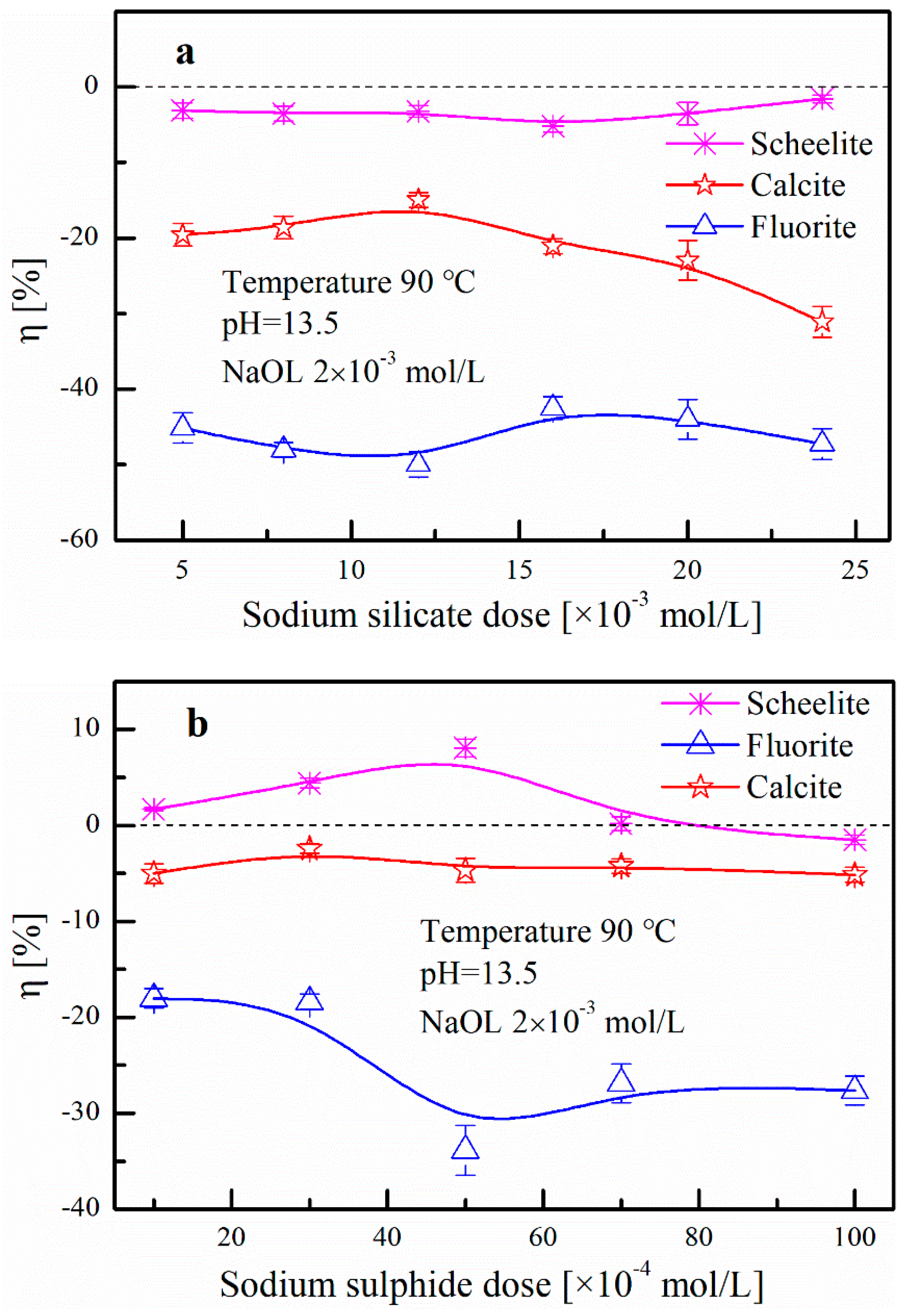
3.1.1. Effect of Desorption Reagent Dose on Scheelite Heating Flotation
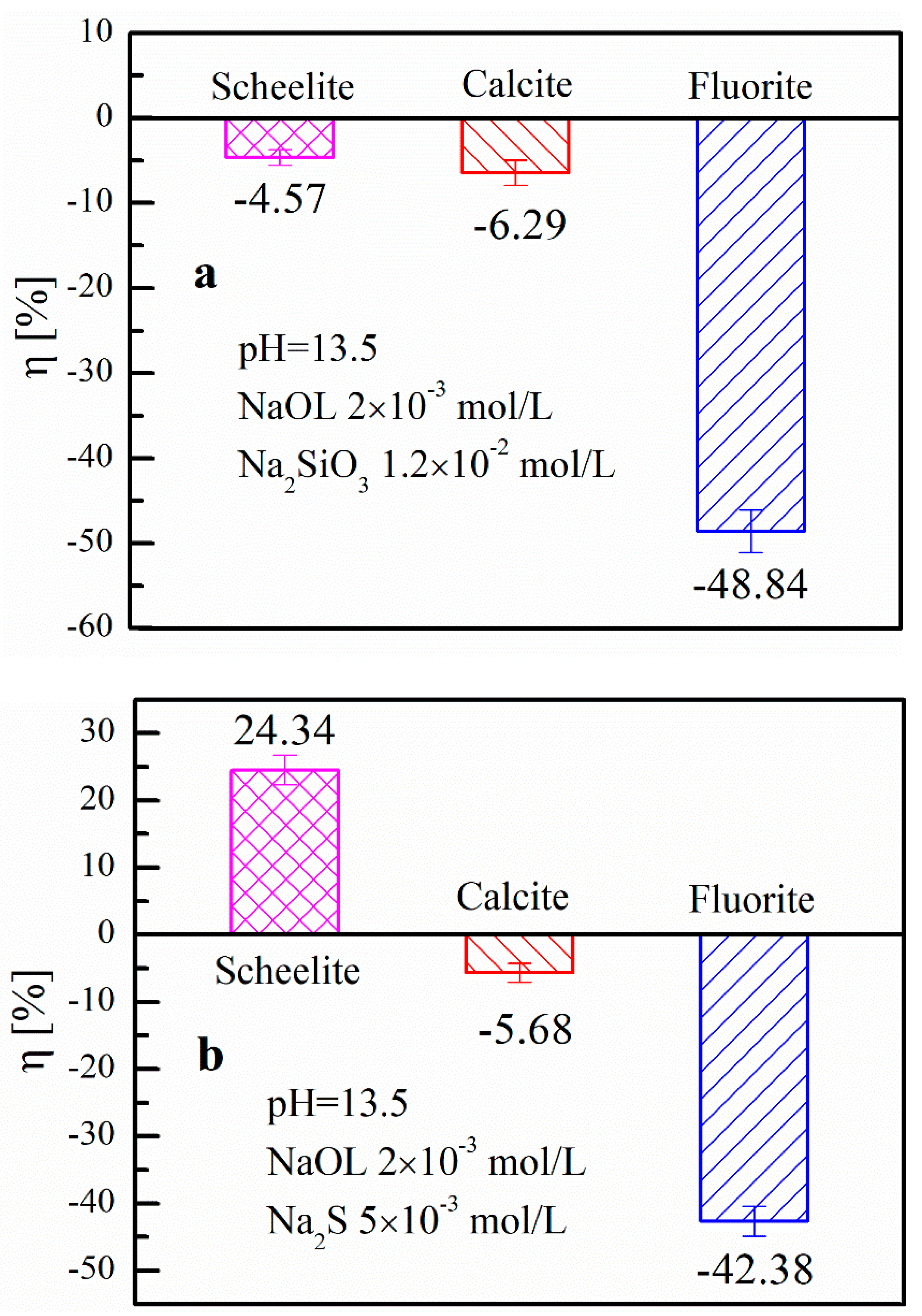
3.1.2. Closed-Circuit Experiment Results
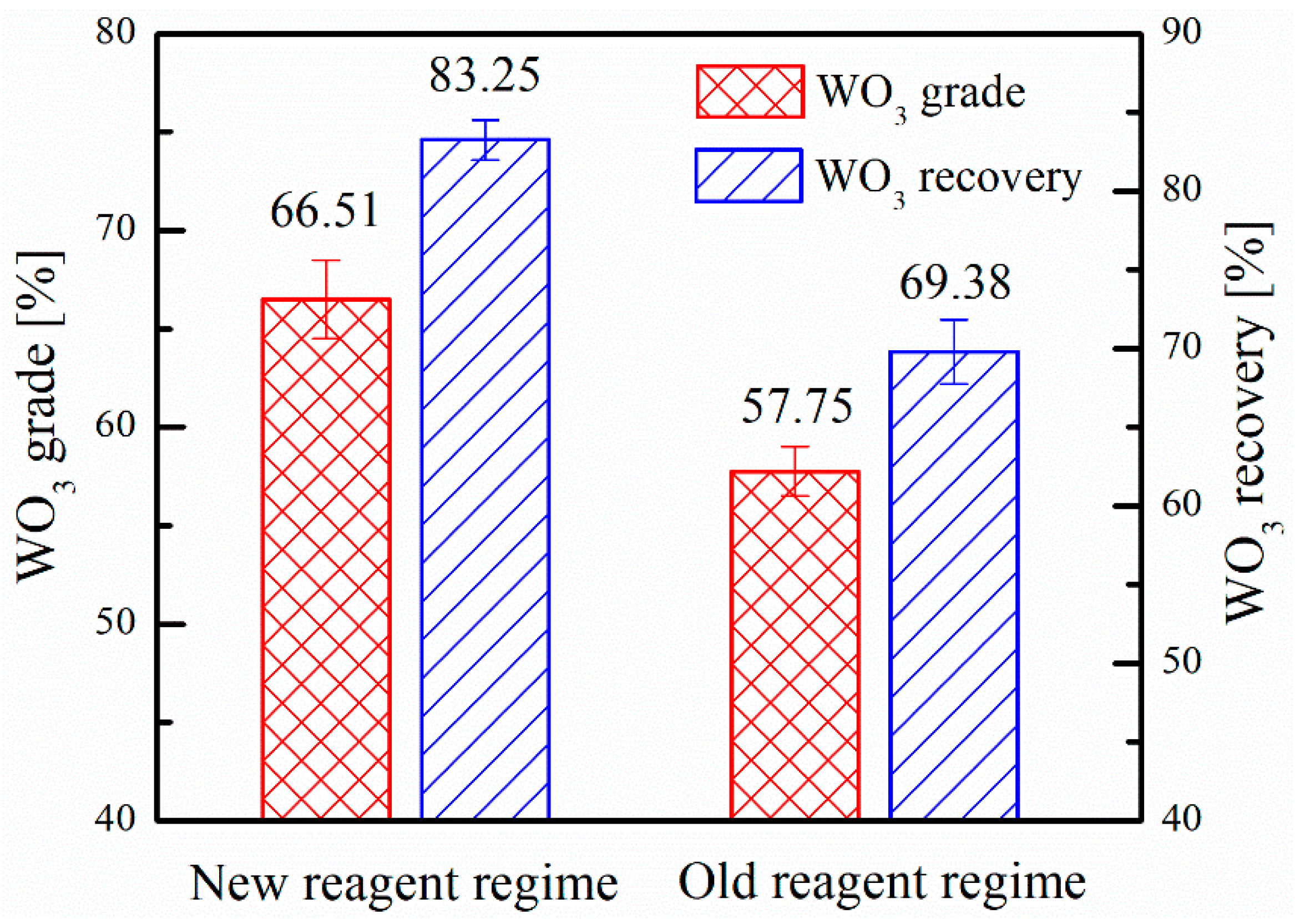
3.2. Industrial Tests
3.3. Adsorption Amount Analysis
3.3.1. Effect of Desorption Reagents Dose on the Change in the Adsorption Amount
3.3.2. Effect of Temperature on the Change in the Adsorption Amount
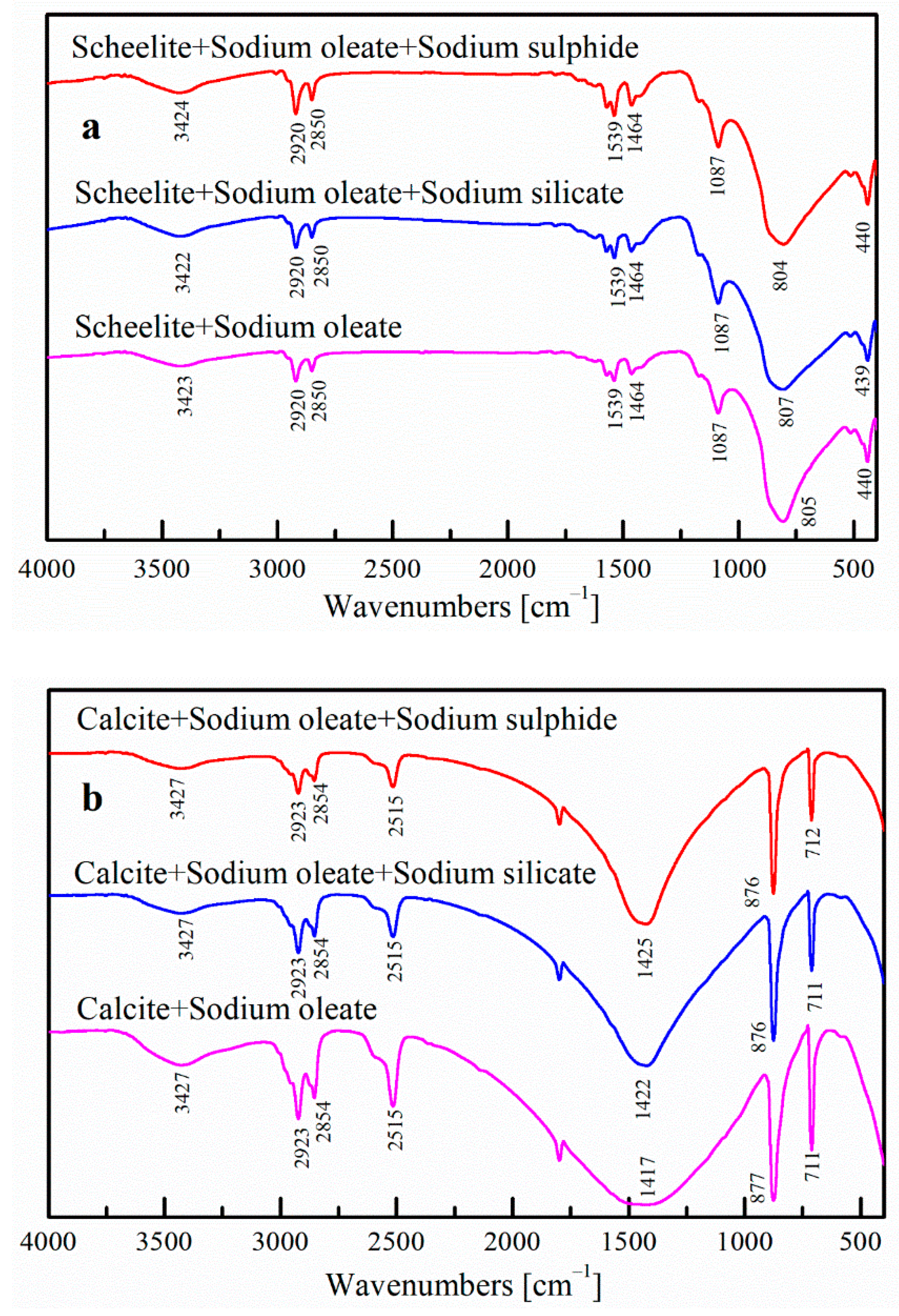
3.4. FTIR Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rao, N.K. Beneficiation of tungsten ores in India: A review. Bull. Mater. Sci. 1996, 19, 201–265. [Google Scholar]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, H.; Ou, L.; Shi, Q. Aggregation of ultra-fine scheelite particles induced by hydrodynamic cavitation. Int. J. Miner. Process. 2016, 157, 236–240. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Cao, C.; Liu, R.; Yue, T.; Meng, X.; Guo, Y.; Wang, J.; et al. Fatty acid flotation versus bha flotation of tungsten minerals and their performance in flotation practice. Int. J. Miner. Process. 2017, 159, 22–29. [Google Scholar] [CrossRef]
- Kang, J.; Chen, C.; Sun, W.; Tang, H.; Yin, Z.; Liu, R.; Hu, Y.; Nguyen, A.V. A significant improvement of scheelite recovery using recycled flotation wastewater treated by hydrometallurgical waste acid. J. Clean. Prod. 2017, 151, 419–426. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Hu, Y. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Yang, F.; Sun, W.; Hu, Y.; Long, S. Cationic flotation of scheelite from calcite using quaternary ammonium salts as collector: Adsorption behavior and mechanism. Miner. Eng. 2015, 81, 18–28. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model. Miner. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z. Interactions of amphoteric amino phosphoric acids with calcium-containing minerals and selective flotation. Int. J. Miner. Process. 2003, 72, 87–94. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.; Gao, Y.; Hu, Y.; Sun, W. Flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Miner. Eng. 2016, 98, 261–263. [Google Scholar] [CrossRef]
- Yan, W.; Liu, C.; Ai, G.; Feng, Q.; Zhang, W. Flotation separation of scheelite from calcite using mixed collectors. Int. J. Miner. Process. 2017, 169, 106–110. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. Effect of acidified water glass on the flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Appl. Surf. Sci. 2018, 444, 747–756. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.; Wang, J.; Wang, P. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Li, Y.; Li, C. Selective flotation of scheelite from calcium minerals with sodium oleate as a collector and phosphates as modifiers. I. Selective flotation of scheelite. Int. J. Miner. Process. 1983, 10, 205–218. [Google Scholar]
- Sarquís, P.E.; Menéndez-Aguado, J.M.; Mahamud, M.M.; Dzioba, R. Tannins: The organic depressants alternative in selective flotation of sulfides. J. Clean. Prod. 2014, 84, 723–726. [Google Scholar] [CrossRef]
- Kang, J.; Hu, Y.; Sun, W.; Liu, R.; Yin, Z.; Tang, H.; Meng, X.; Zhang, Q.; Liu, H. A significant improvement of scheelite flotation efficiency with etidronic acid. J. Clean. Prod. 2018, 180, 858–865. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Liu, D.; Li, L. Selective flotation of scheelite from calcite using calcium lignosulphonate as depressant. Miner. Eng. 2018, 119, 73–75. [Google Scholar] [CrossRef]
- Shepeta, E.D.; Samatova, L.A.; Kondrat’ev, S.A. Kinetics of calcium minerals flotation from scheelite carbonate ores. J. Min. Sci. 2012, 48, 746–753. [Google Scholar] [CrossRef]
- Samatova, L.A.; Shepeta, E.D.; Gvozdev, V.I. Poor scheelite ores from primorye deposits mineralogy and processing characteristics and dressing flowsheets. J. Min. Sci. 2012, 48, 565–573. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, F.; Zeng, Q.; Chen, Z.; Zhang, X. Experimental research on beneficiation of a Yunnan scheelite mine. Met. Mine 2011, 3, 74–77. [Google Scholar]
- Xin, Y.; Jian, J.; Kong, L. Study on dereagent from high temperature thickened slurry in cleaning flotation of scheelite. Min. Mach. 2011, 2, 105–109. [Google Scholar]
- Chen, W. Research on application of sodium sulfide in heating concentration of wolframite and scheelite. China Tungsten Ind. 2002, 17, 26–28. [Google Scholar]
- Yu, Y.; Sun, C.; Wang, Z.; Shang, Y. The influence of different gangue mineral types on scheelite cleaning separation. Non-Ferr. Met. (Miner. Process. Part) 2016, 1, 47–51. [Google Scholar]
- Tian, J.; Xu, L.; Deng, W.; Jiang, H.; Gao, Z.; Hu, Y. Adsorption mechanism of new mixed anionic/cationic collectors in a spodumene-feldspar flotation system. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Lyu, F.; Gao, J.; Sun, N.; Liu, R.; Sun, X.; Cao, X.; Wang, L.; Sun, W. Utilization of propyl gallate as a novel selective collector for diaspore flotation. Miner. Eng. 2019, 131, 66–72. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C. The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Kang, J.; Fan, R.; Hu, Y.; Sun, W.; Liu, R.; Zhang, Q.; Liu, H.; Meng, X. Silicate removal from recycled wastewater for the improvement of scheelite flotation performance. J. Clean. Prod. 2018, 195, 280–288. [Google Scholar] [CrossRef]

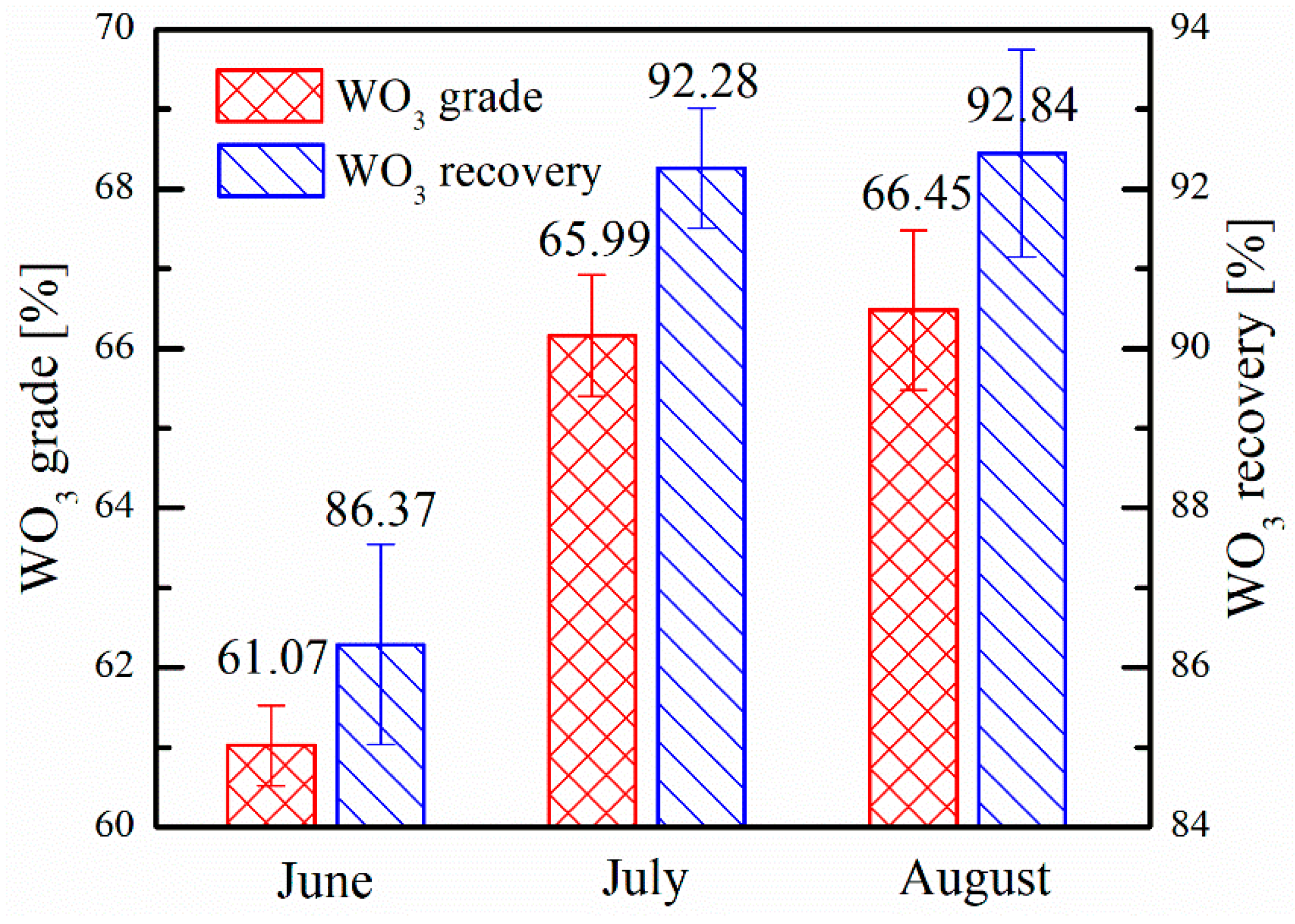
| Items | Price [$/t] | June | July | August | ||||
|---|---|---|---|---|---|---|---|---|
| Mass [t] | Total [$] | Mass [t] | Total [$] | Mass [t] | Total [$] | |||
| Product profit | WO3 | 20,000 | 181 | 3,620,000 | 194 | 3,880,000 | 195 | 3,900,000 |
| Reagent cost | Na2S | 350 | 19 | 6650 | 30 | 10,500 | 32 | 11,200 |
| Water glass | 120 | 370 | 44,400 | 350 | 42,000 | 345 | 41,400 | |
| Profit [$] | 3,568,950 | 3,827,500 | 3,847,400 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Liu, Y.; Ahmed Khoso, S.; Hu, Y.; Sun, W.; Liu, R. Significant Improvement in the Scheelite Heating Flotation with Sodium Sulfide. Minerals 2018, 8, 587. https://doi.org/10.3390/min8120587
Kang J, Liu Y, Ahmed Khoso S, Hu Y, Sun W, Liu R. Significant Improvement in the Scheelite Heating Flotation with Sodium Sulfide. Minerals. 2018; 8(12):587. https://doi.org/10.3390/min8120587
Chicago/Turabian StyleKang, Jianhua, Yuanchao Liu, Sultan Ahmed Khoso, Yuehua Hu, Wei Sun, and Runqing Liu. 2018. "Significant Improvement in the Scheelite Heating Flotation with Sodium Sulfide" Minerals 8, no. 12: 587. https://doi.org/10.3390/min8120587
APA StyleKang, J., Liu, Y., Ahmed Khoso, S., Hu, Y., Sun, W., & Liu, R. (2018). Significant Improvement in the Scheelite Heating Flotation with Sodium Sulfide. Minerals, 8(12), 587. https://doi.org/10.3390/min8120587








