Preliminary Study on Additives for Controlling As, Se, B, and F Leaching from Coal Fly Ash
Abstract
1. Introduction
2. Materials and Methods
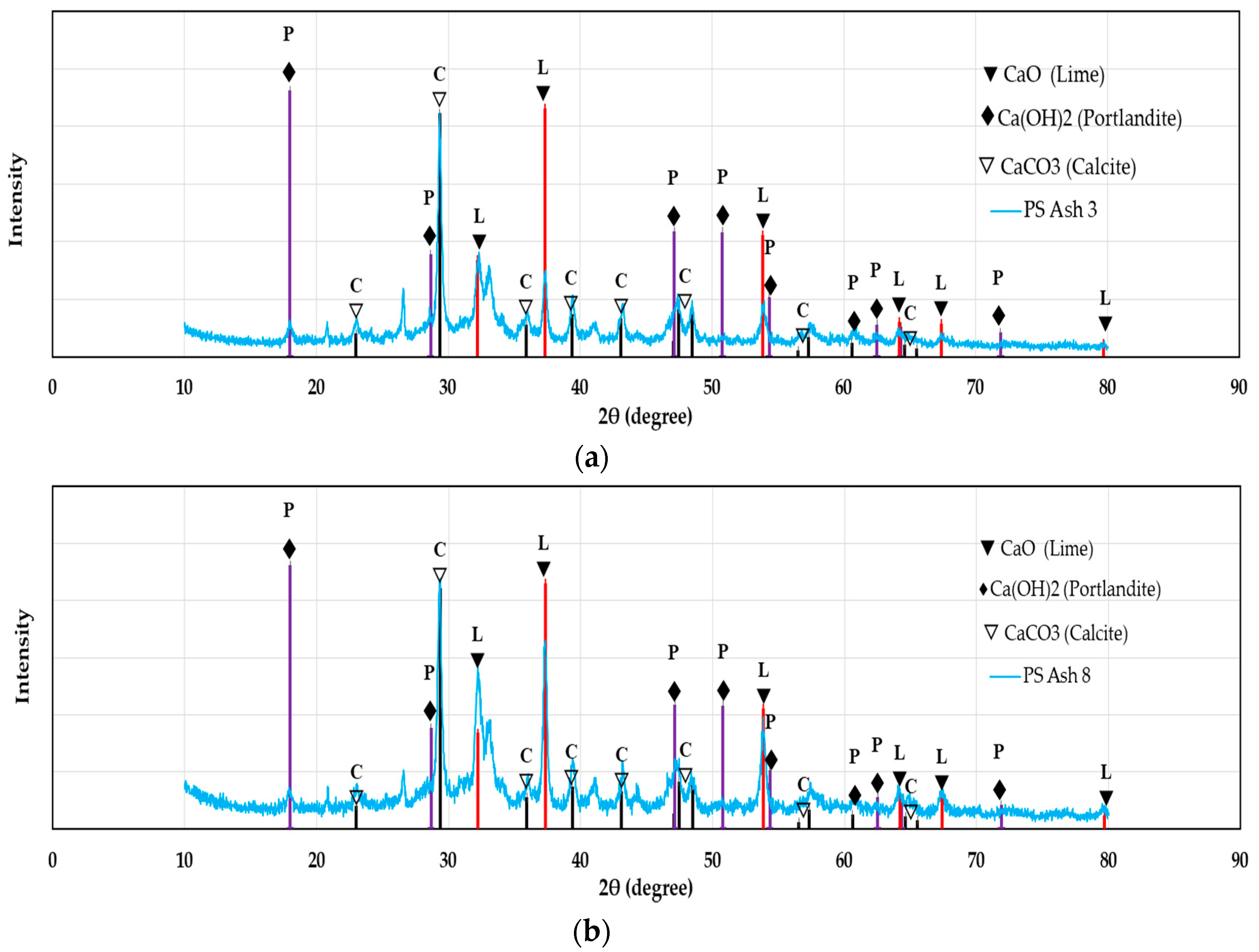
2.1. Coal Fly Ash and Additives
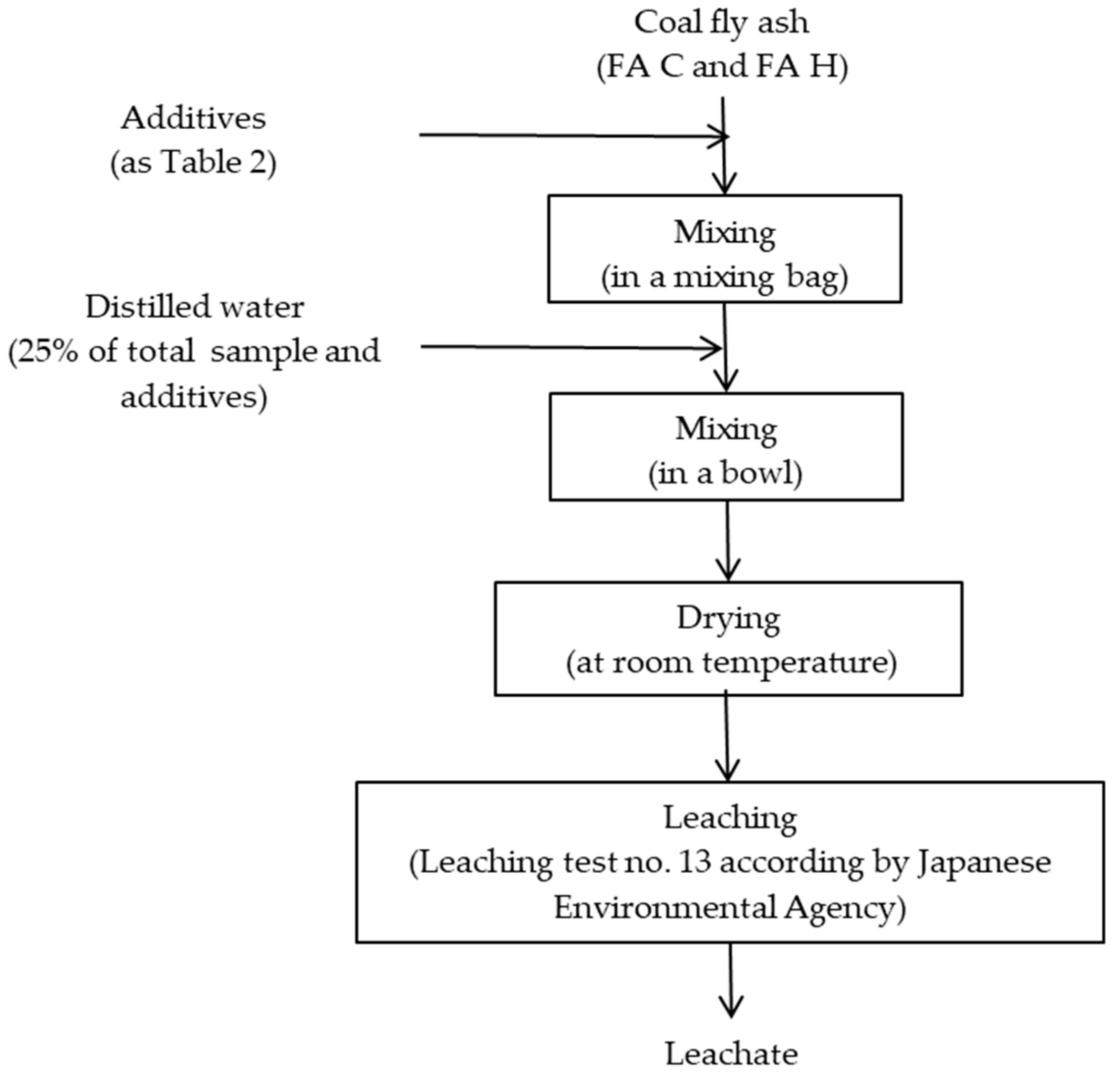
2.2. Sample Preparation and Leaching Test
2.3. Analysis and Instrumentation
3. Results
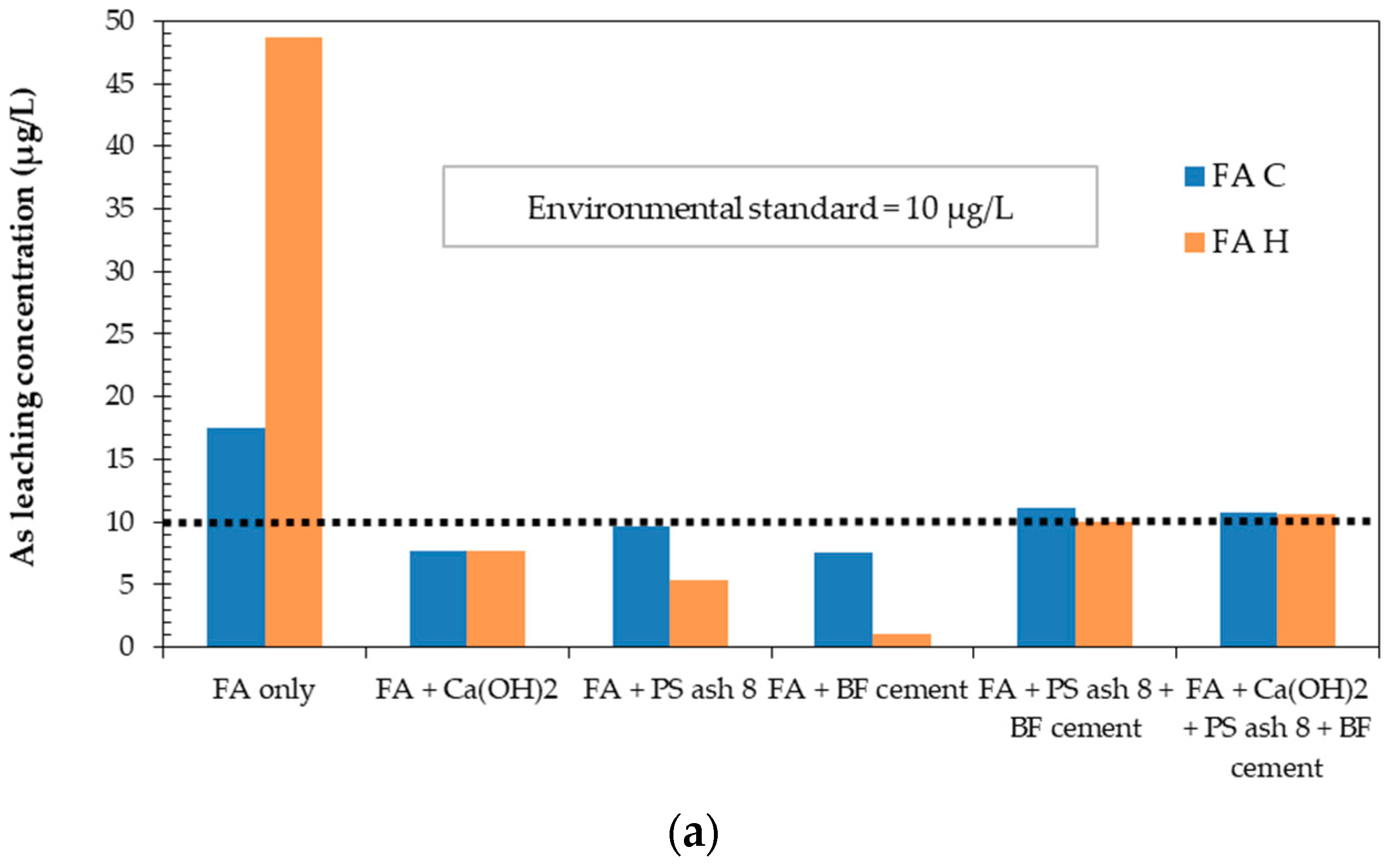
3.1. Ca(OH)2, PS ash 8, and BF Cement as Single Additives
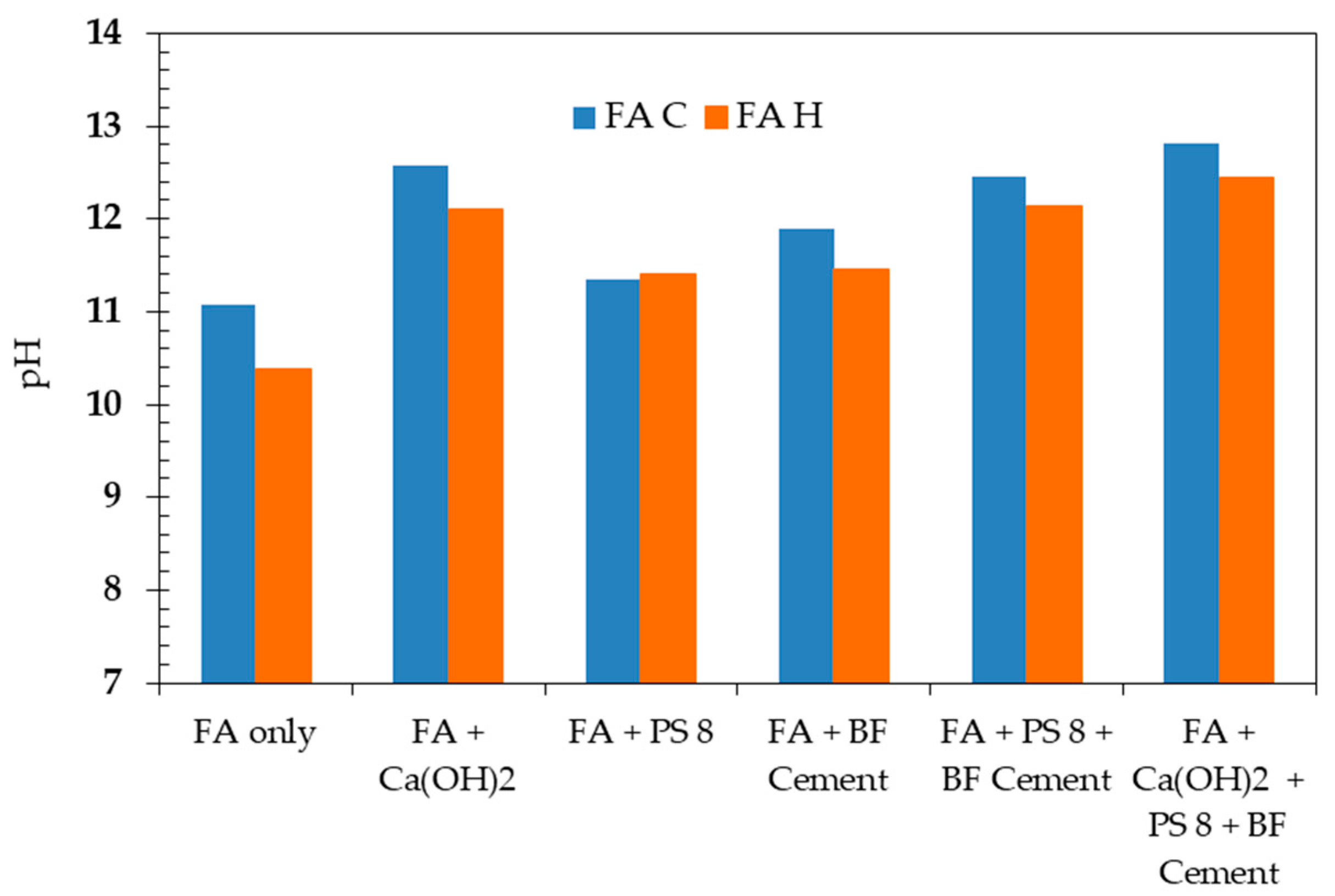
3.2. Ca(OH)2, PS ash 8, and BF Cement as Mixed Additives
4. Conclusions
Author Contributions
Acknowledgements
Conflicts of Interest
References
- World Energy Council. World Energy Resources Report 2016; World Energy Council: London, UK, 2016. [Google Scholar]
- Kalyoncu, R.S.; Olson, D.W. Coal Combustion Products. ACAA International: Last Modified 29 November 2016. Available online: https://pubs.usgs.gov/fs/fs076-01/fs076-01.html (accessed on 14 September 2017).
- Iwashita, A.; Sakaguchi, Y.; Nakajima, T.; Takanashi, H.; Ohki, A.; Kambara, S. Leaching characteristics of boron and selenium for various coal fly ashes. Fuel 2005, 84, 479–485. [Google Scholar] [CrossRef]
- Otero-Rey, J.; Mato-Fernandez, M.; Moreda-Piñeiro, J.; Alonso-Rodríguez, E.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Influences of several experimental parameters on As and Se leaching from coal fly ash samples. Anal. Chim. Acta 2005, 531, 299–305. [Google Scholar] [CrossRef]
- Kadir, A.A.; Hassan, M.I.H.; Abdullah, M.M.A.B. Investigation on leaching behaviour of fly ash and bottom ash replacement in self-compacting concrete. Mater. Sci. Eng. 2016, 133, 012036. [Google Scholar] [CrossRef]
- Akar, G.; Polat, M.; Galecki, G.; Ipekoglu, U. Leaching behavior of selected trace elements in coal fly ash samples from Yenikoy coal-fired power plants. Fuel Process. Technol. 2012, 104, 50–56. [Google Scholar] [CrossRef]
- Yilmaz, H. Characterizations and comparison of leaching behaviors of fly ash samples from three different power plants in Turkey. Fuel Process. Technol. 2015, 137, 240–249. [Google Scholar] [CrossRef]
- Hayashi, S.; Takahashi, T. Chemical state of boron in coal fly ash investigated by focused-ion-beam time-of-flight secondary ion mass spectrometry (FIB-TOF-SIMS) and satellite-transition magic angle spinning Nuclear magnetic resonance (STMAS NMR). Chemosphere 2010, 80, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, J.; Tang, Y.; Shi, H.; Ladwig, K. Leaching characteristics of arsenic and selenium from coal fly ash: Role of calcium. Energy Fuel 2009, 23, 2959–2966. [Google Scholar] [CrossRef]
- Hartuti, S.; Kambara, S.; Takeyama, A. Arsenic leachability of coal fly ashes from different types of coal fired power plants. J. Mater. Sci. Eng. 2017, 169–177. [Google Scholar] [CrossRef]
- Hartuti, S.; Kambara, S.; Takeyama, A.; Hanum, F.F. Leaching characteristic of arsenic in coal fly ash. J. Mater. Sci. Eng. 2017, 7, 19–26. [Google Scholar]
- Hartuti, S.; Fadhillah Hanum, F.; Takeyama, A.; Kambara, S. Effect of additives on arsenic, boron, and selenium leaching from coal fly ash. Minerals 2017, 7, 99. [Google Scholar] [CrossRef]
- Mavroulidou, M.; Bouluki, G.; Unsworth, C. Incorporating Waste Water Paper Sludge Ash As Partial Cement Replacement in Concrete. In Proceedings of the 13th International Conference of Environmental Science and Technology, Athens, Greece, 5–7 September 2013. [Google Scholar]
- Catalfamo, P.; Pasquale, S.D.; Corigliano, F.; Mavilia, L. Influence of the calcium content on the coal fly ash features in some innovative applications. Resour. Conserv. Recycl. 1997, 20, 119–125. [Google Scholar] [CrossRef]
- Palumbo, A.V.; Tarver, J.R.; Fagan, L.A.; McNeilly, M.S.; Ruther, R.; Fisher, L.S.; Amonette, J.E. Comparing metal leaching and toxicity from low pH, high pH and high ammonia fly ash. Fuel 2007, 86, 1623–1630. [Google Scholar] [CrossRef]
- Janskowki, J.; Ward, C.R.; French, D.; Groves, S. Mobility of trace elements from selected Australian fly ashes and its potential impact on aquatic ecosystems. Fuel 2006, 85, 243–256. [Google Scholar] [CrossRef]
- Sun, W.; Renew, J.E.; Zhang, W.; Tang, Y.; Huang, C.H. Sorption of Se (IV) and Se (VI) to coal fly ash/cement composite: Effect of Ca2+ and high ionic strength. Chem. Geol. 2016, 464, 76–83. [Google Scholar] [CrossRef]
- Nabajyoti, S.; Kato, S.; Toshinori, K. Behavior of B, Cr, Se, As, Pb, Cd, and Mo present in waste leachates generated from combustion residues during the formation of ettringite. Environ. Toxicol. Chem. 2006, 25, 1710–1719. [Google Scholar]
- Vhahangwele, M.; Mugera, G.W.; Hlanganani, T. Adsorption of As, B, Cr, Mo and Se from coal fly ash leachate by Fe3+ modified bentonite clay. J. Water Reuse Desalin. 2016, 6, 382–391. [Google Scholar] [CrossRef]
- Harashima, A.; Ito, K. The conditions of ettringite formation by the reaction of a blast furnace slag with aqueous alkaline solutions. ISIJ Int. 2016, 56, 1738–1745. [Google Scholar] [CrossRef]
- Cetin, B.; Aydilek, A.H. pH and fly ash type effect on trace metal leaching from embankment soils. Resour. Conserv. Recycl. 2013, 80, 107–117. [Google Scholar] [CrossRef]
- Guo, Q.; Tian, J. Removal of fluoride and arsenate from aqueous solution by hydrocaluminate via precipitation and anion exchange. Chem. Eng. J. 2013, 231, 121–131. [Google Scholar] [CrossRef]
- Iyer, R. The surface chemistry of the leaching of coal fly ash. J. Hazard. Mater. 2002, 93, 321–329. [Google Scholar] [CrossRef]
- Wang, G.; Lou, Z.; Zhang, J.; Zhao, Y. Modes of occurence of fluorine by extraction and SEM method in a coal-fired power plant from Inner Mongolia China. Mineral 2015, 5, 863–869. [Google Scholar] [CrossRef]
- Radoslaw, S.; Zdzislaw, C. Effect of soil contamination with fluorine on the contents of calcium and magnesium in the biomass of crop plans. J. Elm. 2017, 20, 731–742. [Google Scholar]
- Zhang, M.; Reardon, E.J. Removal of B, Cr, Mo and Se from wastewater by incorporation into Hydrocalumite and Ettringite. Environ. Sci. Technol. 2003, 37, 2947–2952. [Google Scholar] [CrossRef] [PubMed]
- Kumarasathan, P.; McCarthy, G.J.; Hasset, D.J.; Pflughoet-Hassett, D.F. Oxyanion substituted ettringites; synthesis and characterization and their potential role in immobilization of As, B, Cr, Se and V. MRS Proc. 1989, 178, 83. [Google Scholar] [CrossRef]
- Tian, J.; Guo, Q. Thermal decomposition of hydrocalumite over a temperature range 400–1500 °C and its structure reconstruction in water. J. Chem. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, J.; Zhang, S. The mechanism of heavy metal immobilization by cementitious material treatments and thermal treatment: A review. J. Environ. Eng. 2017, 193, 410–422. [Google Scholar] [CrossRef] [PubMed]



| Chemical Composition | Coal Fly Ash | Additives | ||||
|---|---|---|---|---|---|---|
| FA C | FA H | Ca(OH)2 | PS Ash 8 | BF Cement | ||
| SiO2 | (%) | 64.34 | 59.25 | 0.09 | 28.76 | 31.03 |
| Al2O3 | 22.79 | 25.63 | 0.07 | 15.41 | 13.32 | |
| TiO2 | 2.27 | 1.99 | 0.07 | 0.35 | 0.19 | |
| Fe2O3 | 3.71 | 7.49 | BDL | 0.91 | 0.44 | |
| CaO | 2.71 | 2.05 | 99.23 | 51.22 | 48.35 | |
| MgO | 0.85 | 0.79 | 0.36 | 2.76 | 3.77 | |
| Na2O | 1.20 | 0.60 | 0.08 | 0.02 | 0.08 | |
| K2O | 0.80 | 1.56 | 0.01 | 0.15 | 0.36 | |
| P2O5 | 0.07 | 0.18 | 0.05 | 0.10 | BDL | |
| MnO | 0.06 | BDL | BDL | 0.04 | 0.05 | |
| V2O5 | BDL | 0.03 | 0.03 | 0.02 | 0.02 | |
| SO3 | 0.35 | 0.42 | 0.01 | 0.27 | 2.39 | |
| Coal Fly Ash | Additives |
|---|---|
| FA C and FA H | (1) 3% of Calcium Hydroxide / Ca(OH)2 |
| (2) 10% of Paper Sludge Ash / PS ash 8 | |
| (3) 10% of BF cement | |
| (4) 10% PS ash 8 + 10% BF cement | |
| (5) 3% Ca(OH)2 + 10% PS ash 8 + 10% BF cement |
| Leaching Concentration | As (µg/L) | B (mg/L) | Se (µg/L) | F (mg/L) |
|---|---|---|---|---|
| FAC | 17.55 | 3.88 | 90.77 | 1.66 |
| FAH | 48.66 | 5.39 | 86.9 | 0.38 |
| Japanese Environmental Limit | 10 | 1.0 | 10 | 0.8 |
| Chemical Composition | FA C | FA H | ||
|---|---|---|---|---|
| Before Leaching with (Ca(OH)2 + PS 8 + Cement B) | After Leaching with (Ca(OH)2 + PS 8 + Cement B) | Before Leaching with (Ca(OH)2 + PS 8 + Cement B) | After Leaching with (Ca(OH)2 + PS 8 + Cement B) | |
| SiO2 | 56.93 | 46.11 | 54.24 | 48.08 |
| Al2O3 | 22.09 | 17.74 | 22.20 | 18.07 |
| TiO2 | 1.74 | 2.73 | 1.56 | 2.87 |
| Fe2O3 | 2.36 | 11.05 | 5.96 | 5.41 |
| CaO | 13.65 | 18.99 | 12.56 | 22.21 |
| MgO | 1.34 | 0.86 | 1.24 | 1.13 |
| Na2O | 0.80 | 0.18 | 0.46 | 0.58 |
| K2O | 0.57 | 1.39 | 1.14 | 0.78 |
| P2O5 | 0.00 | 0.14 | 0.11 | 0.14 |
| MnO | 0.04 | 0.04 | 0.03 | 0.03 |
| V2O5 | 0.02 | 0.01 | 0.01 | 0.02 |
| SO3 | 0.46 | 0.76 | 0.50 | 0.68 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanum, F.F.; Desfitri, E.R.; Hayakawa, Y.; Kambara, S. Preliminary Study on Additives for Controlling As, Se, B, and F Leaching from Coal Fly Ash. Minerals 2018, 8, 493. https://doi.org/10.3390/min8110493
Hanum FF, Desfitri ER, Hayakawa Y, Kambara S. Preliminary Study on Additives for Controlling As, Se, B, and F Leaching from Coal Fly Ash. Minerals. 2018; 8(11):493. https://doi.org/10.3390/min8110493
Chicago/Turabian StyleHanum, Farrah Fadhillah, Erda Rahmilaila Desfitri, Yukio Hayakawa, and Shinji Kambara. 2018. "Preliminary Study on Additives for Controlling As, Se, B, and F Leaching from Coal Fly Ash" Minerals 8, no. 11: 493. https://doi.org/10.3390/min8110493
APA StyleHanum, F. F., Desfitri, E. R., Hayakawa, Y., & Kambara, S. (2018). Preliminary Study on Additives for Controlling As, Se, B, and F Leaching from Coal Fly Ash. Minerals, 8(11), 493. https://doi.org/10.3390/min8110493




