Recovery and Purification of Tin from Tailings from the Penouta Sn–Ta–Nb Deposit
Abstract
1. Introduction
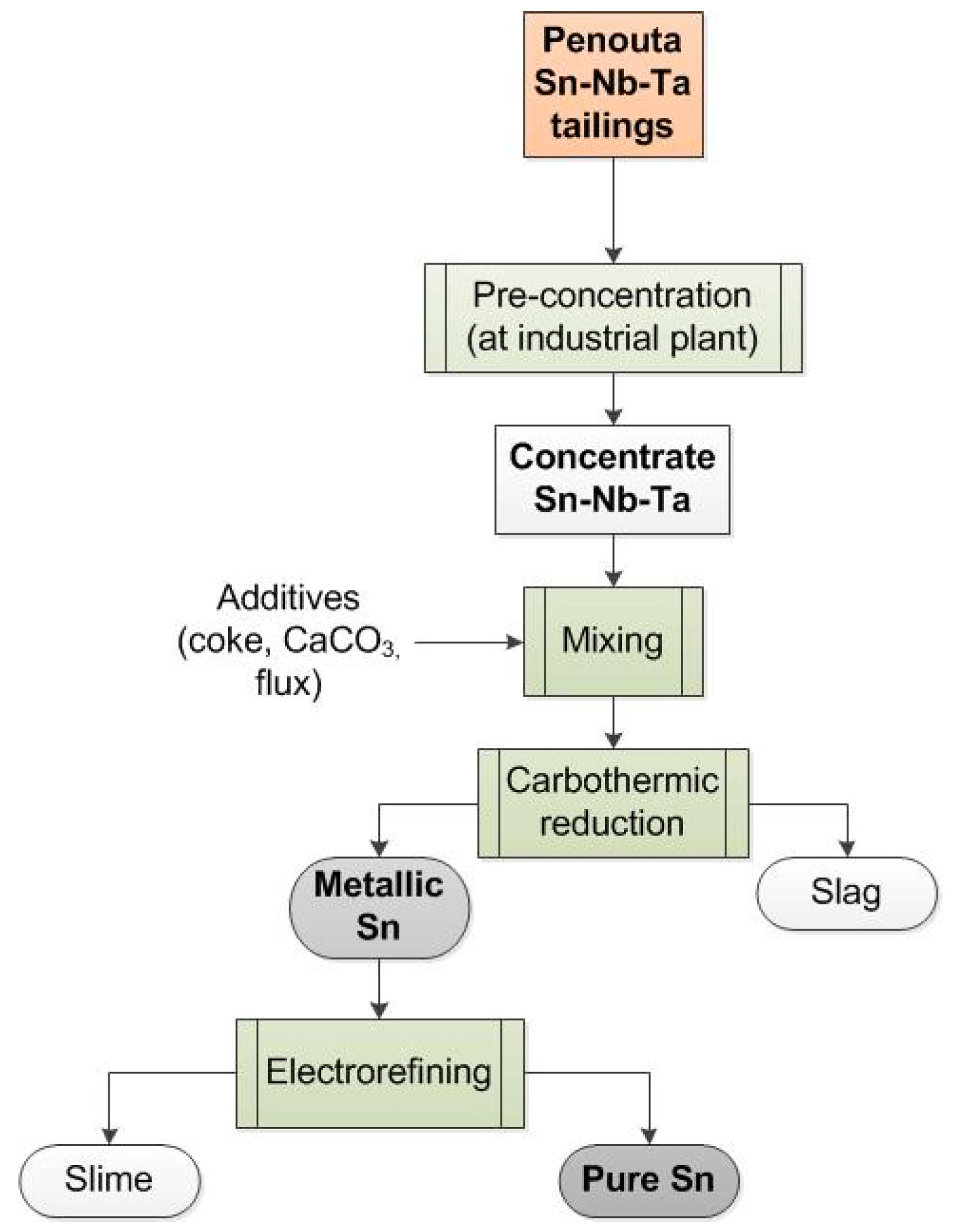
2. Materials and Methods
- Milling. The tailings are milled in a mineral ball mill to a particle size of approximately 300 µm.
- Cyclonic separation. Very small-grained material (<75 µm) is removed; the remaining material is retained for gravimetric concentration.
- Gravimetric concentration. This involves several stages of gravity-assisted concentration made possible by density differences:
- Spirals. Rougher, cleaner, recleaner and scavenger spirals remove the sterile material of lower density (gangue minerals such as silicates), concentrating the those of interest. The product then passes to the next stage.
- Shaking tables. Rougher, cleaner, recleaner and scavenger stage shaking tables separate the minerals on the basis of their density. The product then passes to the next stage.
- Falcon treatment. Gravimetric concentration, using centripetal force for the concentration of the finest metallic particles.
- Low-intensity magnetic separation. This separates out the non-mineralogical iron (not associated with columbo-tantalite) as well as that released during milling. The aim is to improve the purity of the Sn to be obtained in later processing.
- Drying. The gravimetric concentration process occurs under 30%/70% solid/liquid conditions. The resulting materials must, therefore, be dried before further processing.
3. Results
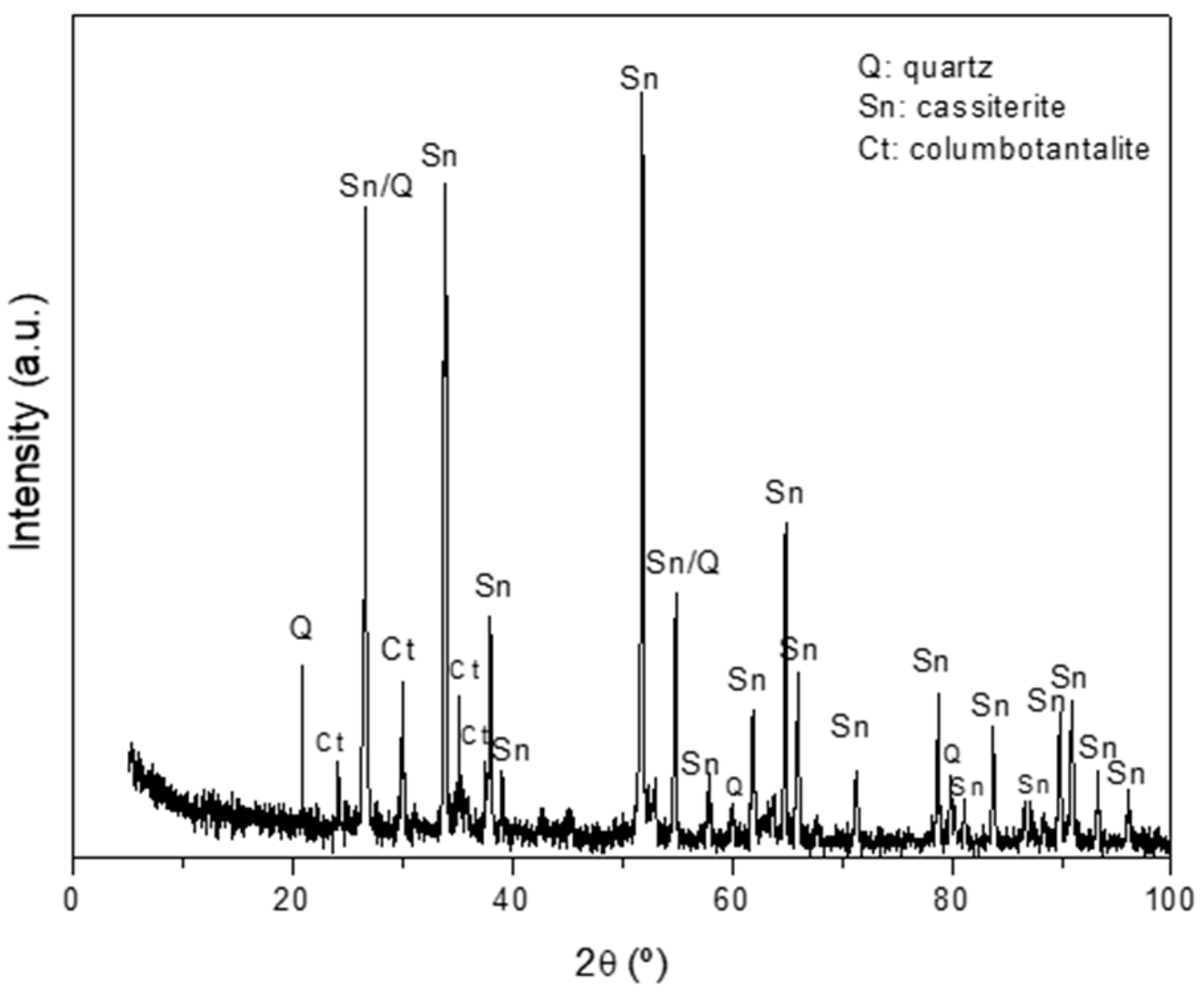
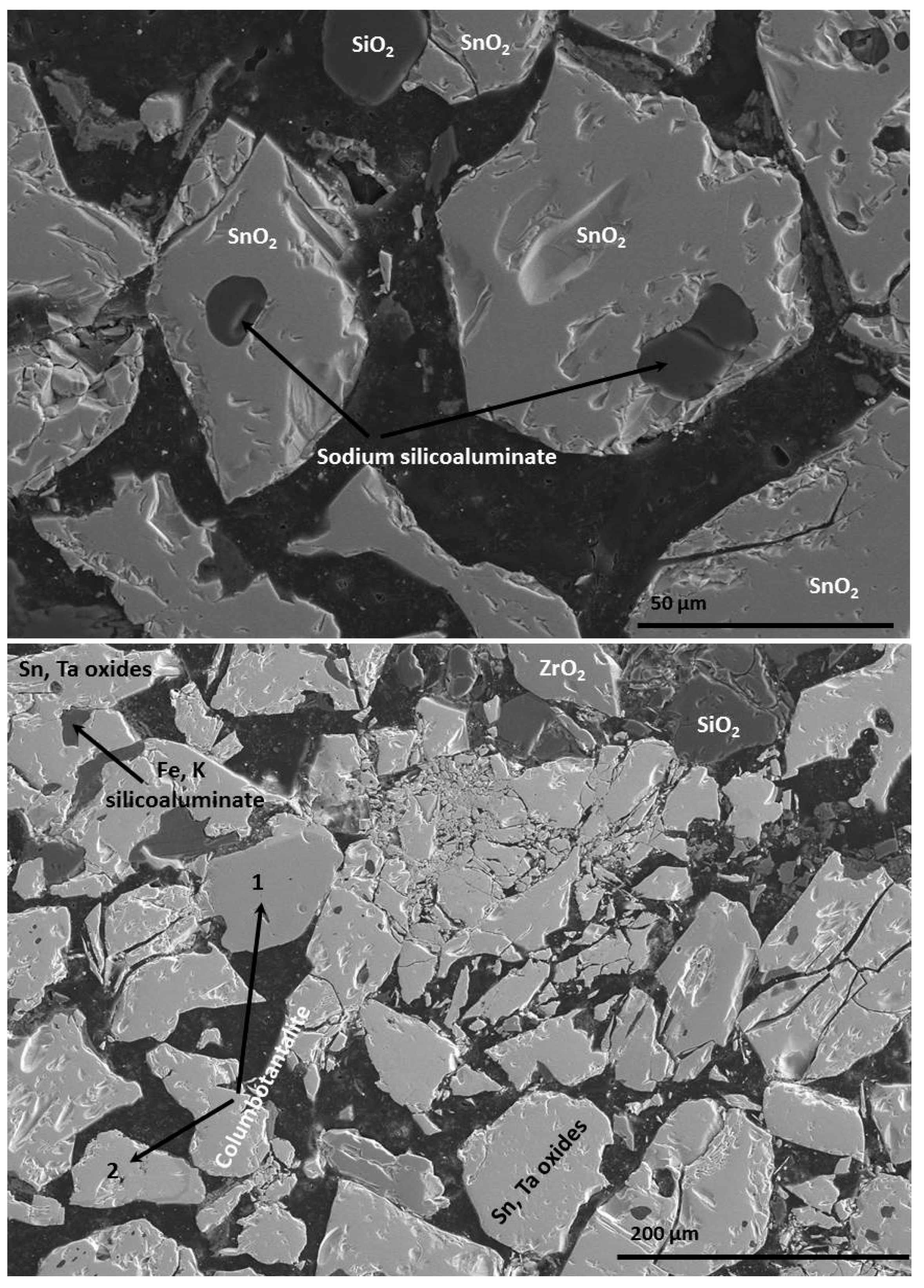
3.1. Chemical and Mineralogical Characterization of the Concentrate
3.2. Obtaining Sn from the Mineral Concentrate
3.3. Electrorefining
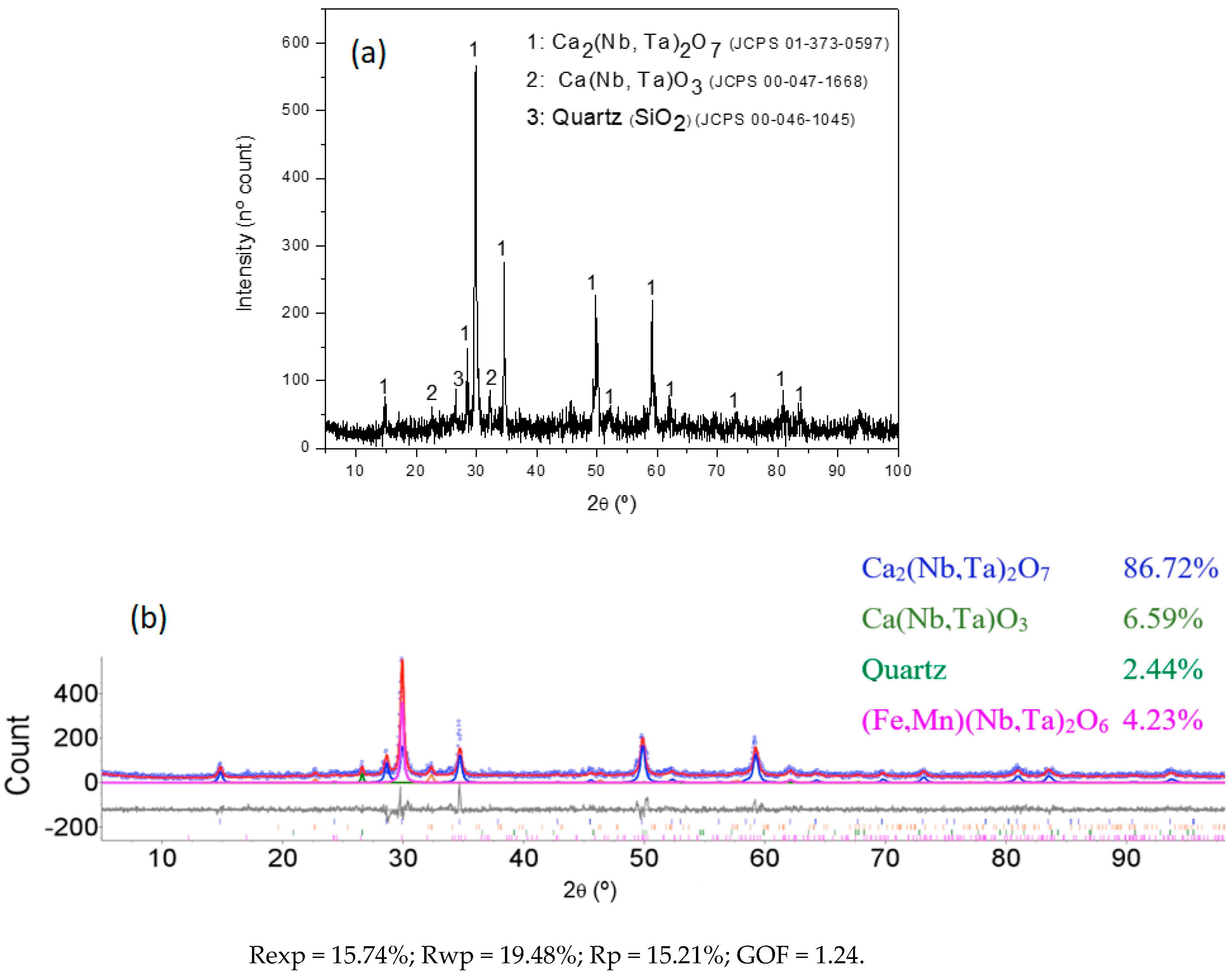
3.4. Characterization of the Slag Produced by the Pyrometallurgical Reduction Smelting Process
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ITRI. 2016 Report on Global Tin Resources and Reserves. Available online: https://www.itri.co.uk/index.php?option=com_mtree&task=att_download&link_id=55516&cf_id=24 (accessed on 12 January 2018).
- Angadi, S.I.; Sreenivas, T.; Jeon, H.-S.; Baek, S.-H.; Mishra, B.K. A review of cassiterite beneficiation fundamentals and plant practices. Miner. Eng. 2015, 70, 178–200. [Google Scholar] [CrossRef]
- Leistner, T.; Embrechts, M.; Leißner, T.; Chehreh Chelgani, S.; Osbahr, I.; Möckel, R.; Peuker, U.A.; Rudolph, M. A study of the reprocessing of fine and ultrafine cassiterite from gravity tailing residues by using various flotation techniques. Miner. Eng. 2016, 96–97, 94–98. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Sun, L.; Hu, Y.; Sun, W. Effect and mechanism of octanol in cassiterite flotation using benzohydroxamic acid as collector. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2016, 26, 3253–3257. [Google Scholar] [CrossRef]
- López-Moro, F.J.; García Polonio, F.; Llorens González, T.; Sanz Contreras, J.L.; Fernández Fernández, A.; Moro Benito, M.C. Ta and Sn concentration by muscovite fractionation and degassing in a lens-like granite body: The case study of the Penouta rare-metal albite granite (NW Spain). Ore Geol. Rev. 2017, 82, 10–30. [Google Scholar] [CrossRef]
- Johan, V.; Johan, Z. Accessory minerals of the Cínovec (Zinnwald) granite cupola, Czech Republic Part 1: Nb-, Ta- and Ti-bearing oxides. Mineral. Petrol. 1994, 51, 323–343. [Google Scholar] [CrossRef]
- Raimbault, L.; Cuney, M.; Azencott, C.; Duthou, J.-L.; Joron, J.L. Geochemical evidence for a multistage magmatic genesis of Ta-Sn-Li mineralization in the granite at Beauvoir, French Massif Central. Econ. Geol. 1995, 90, 548–576. [Google Scholar] [CrossRef]
- McLellan, B.; Yamasue, E.; Tezuka, T.; Corder, G.; Golev, A.; Giurco, D. Critical Minerals and Energy–Impacts and Limitations of Moving to Unconventional Resources. Resources 2016, 5, 19. [Google Scholar] [CrossRef]
- Llorens González, T.; García Polonio, F.; López Moro, F.J.; Fernández Fernández, A.; Sanz Contreras, J.L.; Moro Benito, M.C. Tin-tantalum-niobium mineralization in the Penouta deposit (NW Spain): Textural features and mineral chemistry to unravel the genesis and evolution of cassiterite and columbite group minerals in a peraluminous system. Ore Geol. Rev. 2017, 81, 79–95. [Google Scholar] [CrossRef]
- Peng, Z.; Mackey, P.J. New Developments in Pyrometallurgy. JOM 2013, 65, 1550–1551. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Li, G.H.; Jiang, T.; Guo, Y.F.; Huang, Z.C. Reduction behavior of tin-bearing iron concentrate pellets using diverse coals as reducers. Int. J. Miner. Process. 2012, 110–111, 109–116. [Google Scholar] [CrossRef]
- Ha, H.; Yoo, M.; An, H.; Shin, K.; Han, T.; Sohn, Y.; Kim, S.; Lee, S.-R.; Han, J.H.; Kim, H.Y. Design of Reduction Process of SnO2 by CH4 for Efficient Sn Recovery. Sci. Rep. 2017, 7, 14427. [Google Scholar] [CrossRef] [PubMed]
- Komkov, V.G.; Gostishchev, V.V.; Ri, E.K. Physicochemical aspects of carbothermic reduction of cassiterite in the ionic melt. Russ. J. Non-Ferrous Met. 2009, 50, 596–599. [Google Scholar] [CrossRef]
- You, Z.; Li, G.; Wen, P.; Peng, Z.; Zhang, Y.; Jiang, T. Reduction of Sn-Bearing Iron Concentrate with Mixed H2/CO Gas for Preparation of Sn-Enriched Direct Reduced Iron. Metall. Mater. Trans. B 2017, 48, 1486–1493. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J. The effect of admixtures on the reduction of cassiterite by graphite. Thermochim. Acta 1988, 124, 109–118. [Google Scholar] [CrossRef]
- Cetinkaya, S.; Eroglu, S. Thermodynamic analysis and reduction of tin oxide with methane. Int. J. Miner. Process. 2012, 110, 71–73. [Google Scholar] [CrossRef]
- Rimaszeki, G.; Kulcsar, T.; Kekesi, T. Application of HCl solutions for recovering the high purity metal from tin scrap by electrorefining. Hydrometallurgy 2012, 125–126, 55–63. [Google Scholar] [CrossRef]
- Rimaszeki, G.; Kulcsar, T.; Kekesi, T. Investigation and optimization of tin electrorefining in hydrochloric acid solutions. J. Appl. Electrochem. 2012, 42, 573–584. [Google Scholar] [CrossRef]
- Kekesi, T. Electrorefining in aqueous chloride media for recovering tin from waste materials. Acta Metall. Slovaca 2013, 19, 196–205. [Google Scholar] [CrossRef]
- López, F.A.; Alguacil, F. Recovery of high-purity Sn from Sn alloys containing Pb by means of electro refining. Patent WO 2016/075350-A1, 19 May 2016. [Google Scholar]
- Deposita, M. Magnetism in Cassiterite. Miner. Depos. 1966, 171, 148–171. [Google Scholar] [CrossRef]
- Nagase, K.; Shimodaira, T.; Itoh, M.; Zheng, Y. Kinetics and mechanisms of the reverse Boudouard reaction over metal carbonates in connection with the reactions of solid carbon with the metal carbonates. Phys. Chem. Chem. Phys. 1999, 1, 5659–5664. [Google Scholar] [CrossRef]
- Mitchell, A.R.; Parker, R.H. The reduction of SnO2 and Fe2O3 by solid carbon. Miner. Eng. 1988, 1, 53–66. [Google Scholar] [CrossRef]
- Padilla, R.; Sohn, H.Y. The reduction of stannic oxide with carbon. Metall. Trans. B 1979, 10, 109–115. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, Y.; Liu, B.; Lu, M.; Li, G.; Jiang, T. Extraction and Separation of Tin from Tin-Bearing Secondary Resources: A Review. JOM 2017, 69, 2364–2372. [Google Scholar] [CrossRef]
- Zhang, Y.B. Research on Physicochemical Fundamental and New Technology of Tin, Zinc-Bearing Complex Iron Concentrate Pellets by Weak Reduction Roasting. Ph.D. Thesis, Central South University, Changsha, China, 2006. [Google Scholar]
- Su, Z.; Zhang, Y.; Chen, J.; Liu, B.; Li, G.; Jiang, T. Selective separation and recovery of iron and tin from high calcium type tin- and iron-bearing tailings using magnetizing roasting followed by magnetic separation. Sep. Sci. Technol. 2016, 51, 1900–1912. [Google Scholar] [CrossRef]
- Li, G.; You, Z.; Zhang, Y.; Rao, M.; Wen, P.; Guo, Y.; Jiang, T. Synchronous Volatilization of Sn, Zn, and As, and Preparation of Direct Reduction Iron (DRI) from a Complex Iron Concentrate via CO Reduction. JOM 2014, 66, 1701–1710. [Google Scholar] [CrossRef]
- Leitner, J.; Nevřiva, M.; Sedmidubský, D.; Voňka, P. Enthalpy of formation of selected mixed oxides in a CaO–SrO–Bi2O3–Nb2O5 system. J. Alloys Compd. 2011, 509, 4940–4943. [Google Scholar] [CrossRef]
- Ibrahim, M.; Bright, N.F.H.; Rowland, J.F. The Binary System CaO-Nb2O5. J. Am. Ceram. Soc. 1962, 45, 329–334. [Google Scholar] [CrossRef]
- Cores, A.; Mochón, J.; Ruiz-Bustinza, I.; Parra, R. Control of the flame front advance in a sintering bed of iron ores. Rev. Metal. 2010, 46, 249–259. [Google Scholar] [CrossRef]
- Nete, M.; Purcell, W.; Nel, J.T. Hydrometallurgical Separation of Niobium and Tantalum: A Fundamental Approach. JOM 2016, 68, 556–566. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.J. A review of developments in the electrodeposition of tin. Surf. Coat. Technol. 2016, 288, 79–94. [Google Scholar] [CrossRef]




| % (Weight) | Blend A | Blend B | Blend C |
|---|---|---|---|
| Concentrate | 85.5 | 80.0 | 74.6 |
| CaCO3 | 8.6 | - | 7.5 |
| Borax | - | 5.5 | - |
| Na2CO3 | - | 6.5 | 7.5 |
| K2CO3 | - | - | 5.2 |
| Graphite | 6.0 | 8.0 | 5.2 |
| C/SnO2 (mol ratio) | 1/0.76 | 1/0.54 | 1/1.16 |
| Current (A) | Electrolyte Flow Rate (L/h) | Anode-Cathode Separation (cm) | Reaction Time (h) |
|---|---|---|---|
| 1.20 (190.1 A/m2) | 5 | 4 | 148 |
| Blend | Sn Recovery (wt %) |
|---|---|
| Blend A | 95.2 ± 3.8 |
| Blend B | 87.6 ± 0.6 |
| Blend C | 54.0 ± 0.3 |
| Element | Content |
|---|---|
| Sn | 96.06 wt % |
| Fe | 3.46 wt % |
| Mn | 0.19 wt % |
| Nb | 998 ppm |
| Cr | 899 ppm |
| Ti | 385 ppm |
| Cu | 276 ppm |
| Metal | Sn | Fe | Ag | Cu | Pb |
|---|---|---|---|---|---|
| Pure Sn | 99.97 | 0.005 | 0.007 | 0.010 | 0.009 |
| Anode Slime | 53.28 | 0.44 | 0.16 | 44.49 | 1.63 |
| Component | Content (wt %) |
|---|---|
| Ta2O5 | 25.48 |
| Nb2O5 | 21.43 |
| CaO | 15.79 |
| SiO2 | 10.00 |
| MnO | 7.23 |
| SnO2 | 5.66 |
| ZrO2 | 3.68 |
| Al2O3 | 3.23 |
| Fe2O3 | 0.73 |
| TiO2 | 0.62 |
| K2O | 0.51 |
| Na2O | 0.30 |
| MgO | 0.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, F.A.; García-Díaz, I.; Rodríguez Largo, O.; Polonio, F.G.; Llorens, T. Recovery and Purification of Tin from Tailings from the Penouta Sn–Ta–Nb Deposit. Minerals 2018, 8, 20. https://doi.org/10.3390/min8010020
López FA, García-Díaz I, Rodríguez Largo O, Polonio FG, Llorens T. Recovery and Purification of Tin from Tailings from the Penouta Sn–Ta–Nb Deposit. Minerals. 2018; 8(1):20. https://doi.org/10.3390/min8010020
Chicago/Turabian StyleLópez, Félix Antonio, Irene García-Díaz, Olga Rodríguez Largo, Francisco García Polonio, and Teresa Llorens. 2018. "Recovery and Purification of Tin from Tailings from the Penouta Sn–Ta–Nb Deposit" Minerals 8, no. 1: 20. https://doi.org/10.3390/min8010020
APA StyleLópez, F. A., García-Díaz, I., Rodríguez Largo, O., Polonio, F. G., & Llorens, T. (2018). Recovery and Purification of Tin from Tailings from the Penouta Sn–Ta–Nb Deposit. Minerals, 8(1), 20. https://doi.org/10.3390/min8010020







