Hydrogen from Radiolysis of Aqueous Fluid Inclusions during Diagenesis
Abstract
:1. Introduction
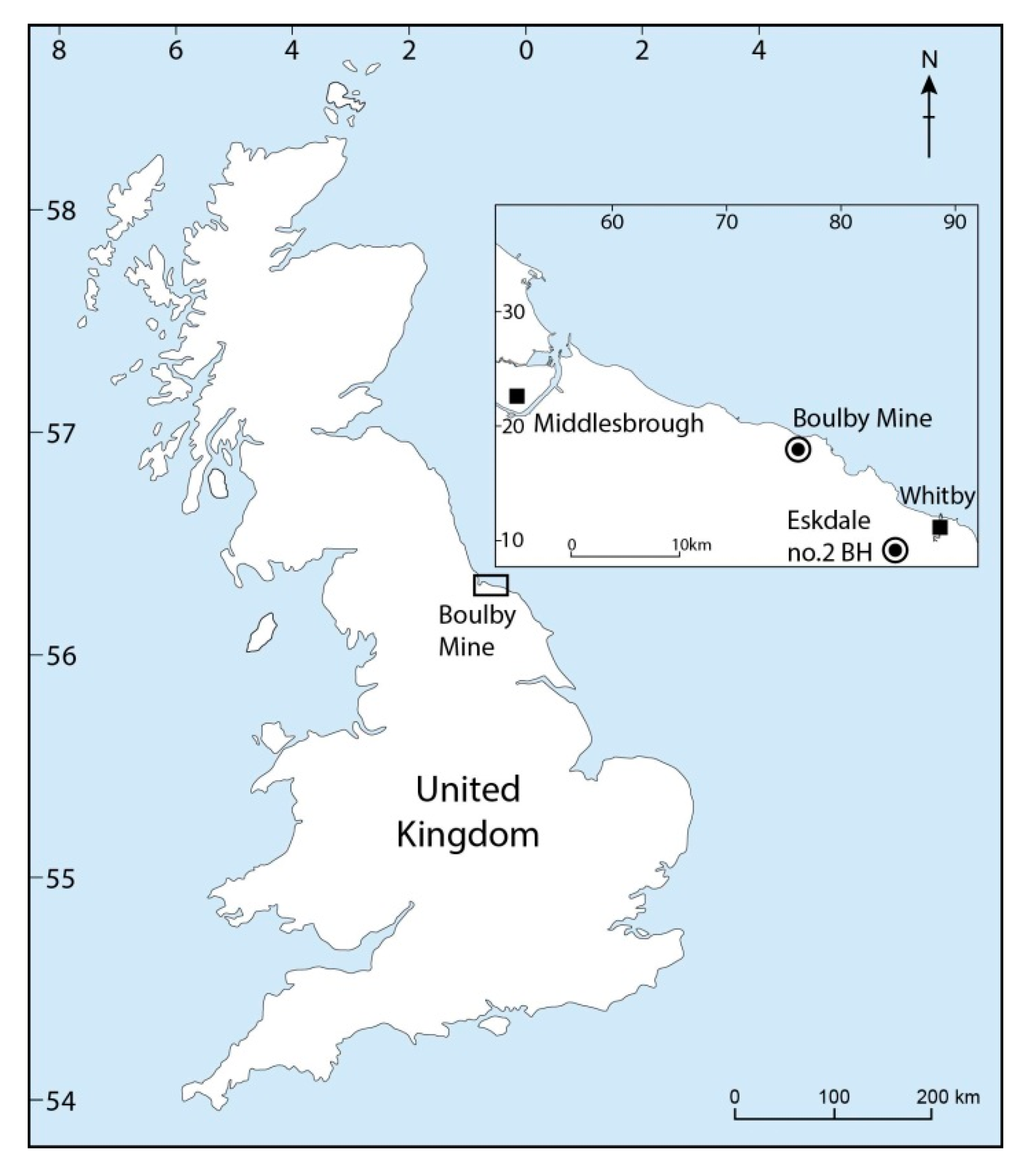
2. Materials and Methods
3. Results
4. Discussion
4.1. Gas Composition
4.2. Subsurface Hydrogen
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dubessy, J.; Pagel, M.; Beny, J.-M.; Christensen, H.; Hickel, B.; Kosztolanyi, C.; Poty, B. Radiolysis evidenced by H2-O2 and H2-bearing fluid inclusions in three uranium deposits. Geochim. Cosmochim. Acta 1988, 52, 1155–1167. [Google Scholar] [CrossRef]
- Savary, V.; Pagel, M. The effects of water radiolysis on local redox conditions in the Oklo, Gabon, natural fission reactors 10 and 16. Geochim. Cosmochim. Acta 1997, 61, 4479–4494. [Google Scholar] [CrossRef]
- Nesmelova, Z.N.; Travnikova, L.G. Radiogenic gases in ancient salt deposits. Geochem. Int. 1973, 5, 554–559. [Google Scholar]
- Smetannikov, A.F. Hydrogen generation during the radiolysis of crystallization water in carnallite and possible consequences of this process. Geochem. Int. 2011, 49, 916–924. [Google Scholar] [CrossRef]
- Vovk, I.F. Radiolytic salt enrichment and brines in the crystalline basement of the East European Platform. In Saline Water and Gases in Crystalline Basement; Fritz, P., Frape, S.K., Eds.; Geological Association of Canada Special Paper: St. John’s, NL, Canada, 1987; Volume 33, pp. 197–210. [Google Scholar]
- Lin, L.H.; Hall, J.; Lippmann-Pipke, J.; Ward, J.A.; Sherwood Lollar, B.; DeFlaun, M.; Rothmel, R.; Moser, D.; Gihring, T.M.; Mislowack, B.; et al. Radiolytic H2 in continental crust: Nuclear power for deep subsurface microbial communities. Geochem. Geophys. Geosyst. 2005, 6, Q07003. [Google Scholar] [CrossRef]
- Parry, W.T.; Blamey, N.J.F. Fault fluid composition from fluid inclusion measurements, Laramide Age Uinta Thrust Fault, Utah. Chem. Geol. 2010, 278, 105–119. [Google Scholar] [CrossRef]
- Blamey, N.J.F. Composition and evolution of crustal, geothermal and hydrothermal fluids interpreted using quantitative fluid inclusion gas analysis. J. Geochem. Explor. 2012, 116–117, 17–27. [Google Scholar] [CrossRef]
- McMahon, S.; Parnell, J.; Blamey, N.J.F. Evidence for seismogenic hydrogen gas, a potential microbial energy source on Earth and Mars. Astrobiology 2016, 16, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.C.; D’Hondt, S.; Spivack, A.J.; Kingsley, R.H. Radiolytic hydrogen and microbial respiration in subsurface sediments. Astrobiology 2007, 7, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Dzaugis, M.E.; Spivack, A.J.; Dunlea, A.G.; Murray, R.W.; D’Hondt, S. Radiolytic hydrogen production in the subseafloor basaltic aquifer. Front. Microbiol. 2016, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.H. The petrology of the evaporites of the Eskdale No. 2 boring, east Yorkshire. Mineral. Mag. 1949, 28, 621–675. [Google Scholar] [CrossRef]
- Woods, P.J.E. The geology of Boulby Mine. Econ. Geol. 1979, 74, 409–418. [Google Scholar] [CrossRef]
- Talbot, C.J.; Tully, C.P.; Woods, P.J.E. The structural geology of Boulby (potash) mine, Cleveland, United Kingdom. Tectonophysics 1982, 85, 167–204. [Google Scholar] [CrossRef]
- Cockell, C.S.; Payler, S.; Paling, S.; McLuckie, D. Boulby International Subsurface Astrobiology Laboratory. Astron. Geophys. 2013, 54, 2.25–2.27. [Google Scholar] [CrossRef]
- Payler, S.J.; Biddle, J.F.; Coates, A.J.; Cousins, C.R.; Cross, R.E.; Cullen, D.C.; Downs, M.T.; Direito, S.O.L.; Edwards, T.; Gray, A.L.; et al. Planetary science and exploration in the deep subsurface: Results from the MINAR Program, Boulby Mine, UK. Int. J. Astrobiol. 2017, in press. [Google Scholar] [CrossRef]
- Blamey, N.J.F.; Parnell, J.; McMahon, S.M.; Mark, D.F.; Tomkinson, T.; Lee, M.; Shivak, J.; Izawa, M.R.M.; Banerjee, N.R.; Flemming, R.L. Evidence for methane in martian meteorites. Nat. Commun. 2015, 6, 7399. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.I.; Blamey, N.J.F. Quantitative gas analysis of fluid inclusion volatiles by a two mass spectrometer system. In European Current Research on Fluid Inclusions; No. XVI, Abstracts; University of Porto: Porto, Portugal, 2001; pp. 341–344. [Google Scholar]
- Wardlaw, N.C. Carnallite-sylvite relationships in the Middle Devonian Prairie Evaporite Formation, Saskatchewan. Geol. Soc. Am. Bull. 1968, 79, 1273–1294. [Google Scholar] [CrossRef]
- Garrett, D.E. Potash Deposits, Processing, Properties and Uses; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Parnell, J.; Boyce, A.J.; Blamey, N.J.F. Follow the methane: The search for a deep biosphere, and the case for sampling serpentinites, on Mars. Int. J. Astrobiol. 2010, 9, 193–200. [Google Scholar] [CrossRef]
- Panno, S.V.; Czyscinski, K.V. Expected brine chemistry in a nuclear waste repository in salt. In Proceedings of the First Canadian/American Conference on Hydrogeology: Practical Applications of Ground Water Geochemistry, Banff, AL, Canada, 22–26 June 1984. [Google Scholar]
- Gaudez, M.T.; Akram, N.; Toulhoat, P.; Toulhoat, N.; Palut, J.M. Analyses of radiolytic gases resulting from gamma irradiation of Asse rocksalt performed at Saclay. In The Effects of Gamma Irradiation in Salt; Celma, A.G., Ed.; Office for Official Publications of the European Communities: Luxembourg, 1996; pp. 209–256. [Google Scholar]
- Blamey, N.J.F.; Boston, P.J.; Rosales-Lagarde, L. High-resolution signatures of oxygenation and microbiological activity in speleothem fluid inclusions. Int. J. Speleol. 2016, 45, 231–241. [Google Scholar] [CrossRef]
- Blamey, N.J.F.; Brand, U.; Parnell, J.; Spear, N.; Benison, K.; Lécuyer, C.; Meng, F.; Ni, P. Paradigm shift in determining Neoproterozoic oxygen. Geology 2016, 44, 651–654. [Google Scholar] [CrossRef]
- Ward, J.A.; Slater, G.F.; Moser, D.P.; Lin, L.H.; Lacrampe-Couloume, G.; Bonin, A.S.; Davidson, M.; Hall, J.A.; Mislowack, B.; Bellamy, R.E.S.; et al. Microbial hydrocarbon gases in the Witwatersrand Basin, South Africa: Implications for the deep biosphere. Geochim. Cosmochim. Acta 2004, 68, 3239–3250. [Google Scholar] [CrossRef]
- Piestrzyński, A. Uranium and thorium in the Kupferschiefer formation, Lower Zechsten, Poland. Miner. Depos. 1990, 25, 146–151. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Li, S.; Jin, K.; Lin, M. Mechanism of uranium accumulation in the Kupferschiefer from Poland and Germany. Energy Explor. Exploit. 2005, 23, 463–473. [Google Scholar] [CrossRef]
- Smedley, P.L.; Smith, B.; Abesser, C.; Lapworth, D. Uranium occurrences and behaviour in British groundwater. In Groundwater Systems & Water Quality Programme Commissioned Report; CR/06/050N; British Geological Survey: Nottingham, UK, 2006. [Google Scholar]
- Duff, M.C.; Coughlin, J.U.; Hunter, D.B. Uranium co-precipitation with iron oxide minerals. Geochim. Cosmochim. Acta 2002, 66, 3533–3547. [Google Scholar] [CrossRef]
- Sleep, N.H.; Bird, D.K. Niches of the pre-photosynthetic biosphere and geologic preservation of Earth’s earliest ecology. Geobiology 2007, 5, 101–117. [Google Scholar] [CrossRef]
- Sherwood Lollar, B.; Voglesonger, K.; Lin, L.-H.; Lacrampe-Couloume, G.; Telling, J.; Abrajano, T.A.; Onstott, T.C.; Pratt, L.M. Hydrogeologic controls on episodic H2 release from Precambrian fractured—Energy for deep subsurface life on Earth and Mars. Astrobiology 2007, 7, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Kawagucci, S.; Suzuki, K. Mechanoradical H2 generation during simulated faulting: Implications for an earthquake-driven subsurface biosphere. Geophys. Res. Lett. 2011, 38, L17303. [Google Scholar] [CrossRef]
- Wankel, S.D.; Germanovich, L.N.; Lilley, M.D.; Genc, G.; DiPerna, C.J.; Bradley, A.S.; Olson, E.J.; Girguis, P.R. Influence of subsurface biosphere on geochemical fluxes from diffuse hydrothermal fluids. Nat. Geosci. 2011, 4, 461–468. [Google Scholar] [CrossRef]
- Jones, A.A.; Bennett, P.C. Mineral microniches control the diversity of subsurface microbial populations. Geomicrobiol. J. 2014, 31, 246–261. [Google Scholar] [CrossRef]
- Ishibashi, J.; Okino, K.; Sunamura, M. (Eds.) Subseafloor Biosphere Linked to Hydrothermal Systems; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Sterniczuk, M.; Bartels, D.M. Source of molecular hydrogen in high-temperature water radiolysis. J. Phys. Chem. A 2016, 120, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.J.; Green, P.F.; Duddy, I.R. Thermal history reconstruction using apatite fission track analysis and vitrinite reflectance: A case study from the UK East Midlands and Southern North Sea. In Exploration Britain: Geological Insights for the Next Decade; Hardman, R.F.P., Ed.; Geological Society Special Publication: London, UK, 1992; Volume 67, pp. 3–25. [Google Scholar]
- Słowakiewicz, M.; Tucker, M.E.; Vane, C.H.; Harding, R.; Collins, A.; Pancost, R.D. Shale-gas potential of the Mid-Carboniferous Bowland-Hodder Unit in the Cleveland Basin (Yorkshire), Central Britain. J. Pet. Geol. 2015, 38, 59–76. [Google Scholar] [CrossRef]
- Warren, J.K. Evaporites through time: Tectonic, climatic and eustatic controls in marine and nonmarine deposits. Earth Sci. Rev. 2010, 98, 217–268. [Google Scholar] [CrossRef]
- Osterloo, M.M.; Hamilton, V.E.; Bandfield, J.L.; Glotch, T.D.; Baldridge, A.M.; Christensen, P.R.; Tornabene, L.L.; Anderson, F.S. Chloride-bearing materials in the Southern Highlands of Mars. Science 2008, 319, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Tosca, N.J.; McLennan, S.M. Chemical divides and evaporite assemblages on Mars. Earth Planet. Sci. Lett. 2006, 241, 21–31. [Google Scholar] [CrossRef]
- Elwood Madden, M.E.; Bodnar, R.J.; Rimstidt, J.D. Jarosite as an indicator of water-limited chemical weathering on Mars. Nature 2004, 431, 821–823. [Google Scholar] [CrossRef] [PubMed]
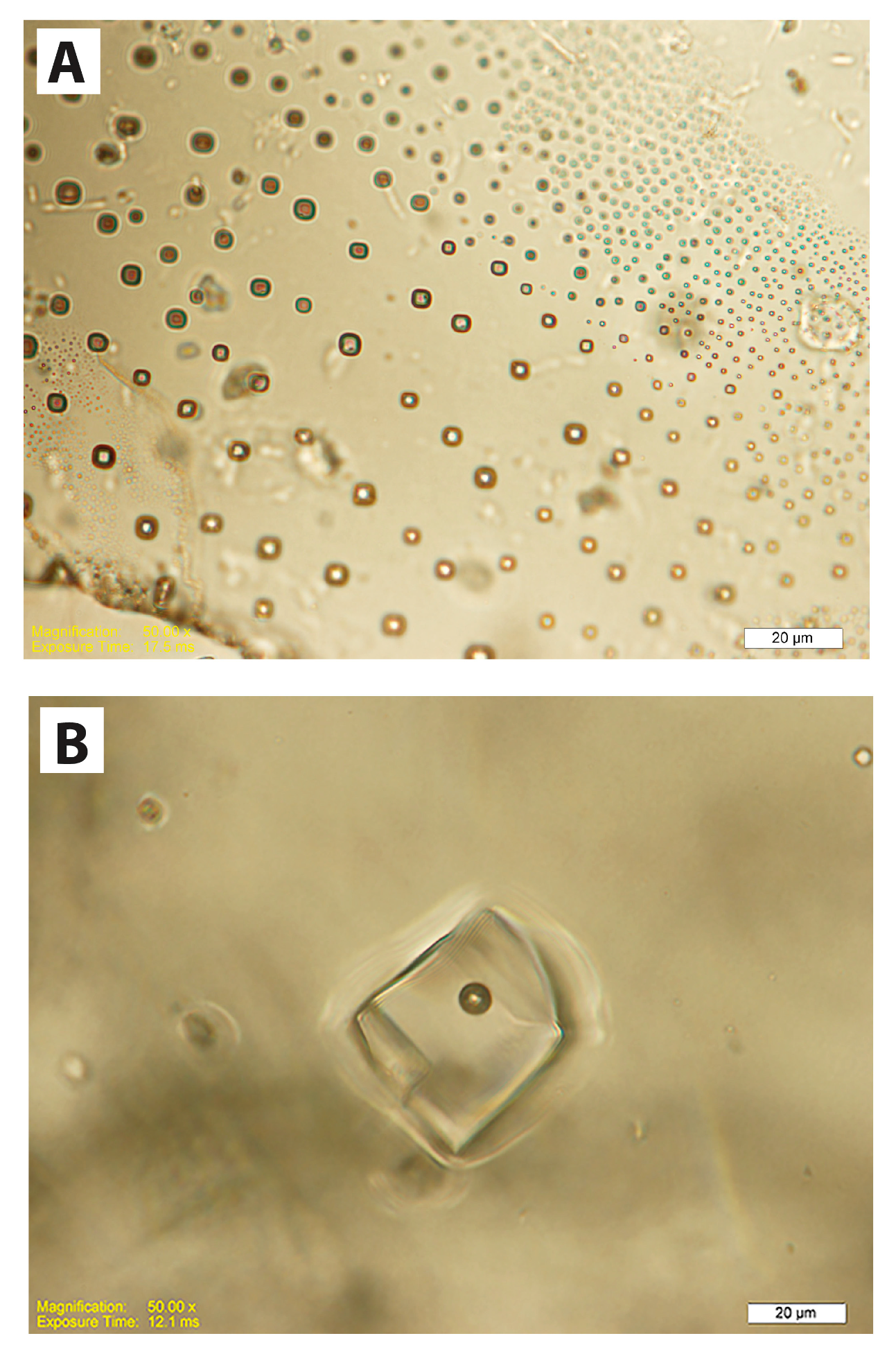
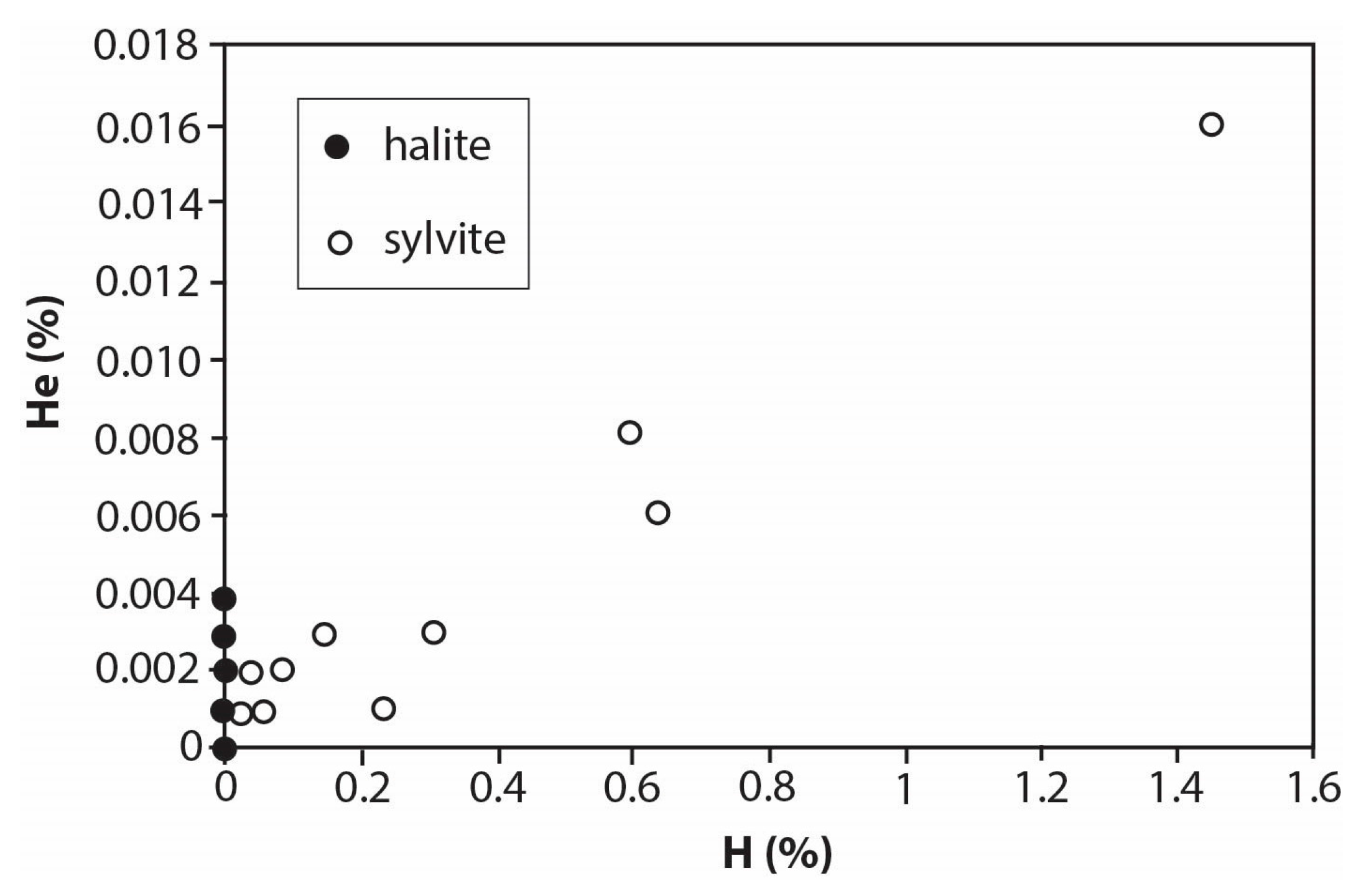
| Lab No. | Mineral | Crushes | Mine Panel | Total Gas (mols) | H2 (%) | He (%) | O (%) |
|---|---|---|---|---|---|---|---|
| 140 | sylvite | 8 | 7.6 × 10−10 | 0.028 | 0.002 | 0.089 | |
| 186 | sylvite | 5 | 6.2 × 10−10 | 0.018 | 0.001 | 0.055 | |
| 187 | sylvite | 8 | 3.0 × 10−9 | 0.023 | 0.001 | 0.055 | |
| 188 | sylvite | 4 | 4.0 × 10−9 | 0.045 | 0.001 | 0.096 | |
| 189 | sylvite | 8 | 3.7 × 10−9 | 0.028 | 0.001 | 0.553 | |
| 194 | sylvite | 9 | 240 | 3.7 × 10−9 | 0.303 | 0.003 | 0.111 |
| 195 | sylvite | 8 | 2008 | 2.1 × 10−9 | 0.230 | 0.001 | 0.103 |
| 196 | sylvite | 10 | 240 | 4.0 × 10−9 | 0.145 | 0.003 | 0 |
| 197 | sylvite | 10 | 2008 | 5.7 × 10−9 | 0.633 | 0.006 | 0 |
| 281 | sylvite | 8 | 1.2 × 10−9 | 0.020 | 0.001 | 0.278 | |
| 283 | sylvite | 9 | 3.3 × 10−9 | 0.080 | 0.002 | 0.278 | |
| 287 | sylvite | 10 | 2008 | 5.6 × 10−9 | 0.598 | 0.008 | 0.094 |
| 288 | sylvite | 10 | 2008 | 2.2 × 10−9 | 1.451 | 0.016 | 0.186 |
| 146 | halite | 7 | 4.2 × 10−9 | 0 | 0.001 | 0.039 | |
| 190 | halite | 6 | 2.0 × 10−8 | 0 | 0.003 | 0.065 | |
| 191 | halite | 9 | 1.9 × 10−8 | 0 | 0.004 | 0.201 | |
| 192 | halite | 4 | 2001 | 2.5 × 10−11 | 0 | 0 | 0.039 |
| 193 | halite | 6 | 2001 | 1.2 × 10−10 | 0 | 0.001 | 0.206 |
| 263 | halite | 9 | E2/4010ft | 2.2 × 10−10 | 0 | 0 | 0.16 |
| 264 | halite | 10 | E2/3816ft | 1.6 × 10−10 | 0 | 0 | 0.373 |
| 265 | halite | 6 | E2/3962ft | 1.6 × 10−10 | 0 | 0 | 0.119 |
| 271 | halite | 9 | E2/3962ft | 2.4 × 10−10 | 0.005 | 0 | 0.348 |
| 282 | halite | 9 | 3.5 × 10−9 | 0.001 | 0.002 | 0.104 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parnell, J.; Blamey, N. Hydrogen from Radiolysis of Aqueous Fluid Inclusions during Diagenesis. Minerals 2017, 7, 130. https://doi.org/10.3390/min7080130
Parnell J, Blamey N. Hydrogen from Radiolysis of Aqueous Fluid Inclusions during Diagenesis. Minerals. 2017; 7(8):130. https://doi.org/10.3390/min7080130
Chicago/Turabian StyleParnell, John, and Nigel Blamey. 2017. "Hydrogen from Radiolysis of Aqueous Fluid Inclusions during Diagenesis" Minerals 7, no. 8: 130. https://doi.org/10.3390/min7080130
APA StyleParnell, J., & Blamey, N. (2017). Hydrogen from Radiolysis of Aqueous Fluid Inclusions during Diagenesis. Minerals, 7(8), 130. https://doi.org/10.3390/min7080130




