Desiccator Volume: A Vital Yet Ignored Parameter in CaCO3 Crystallization by the Ammonium Carbonate Diffusion Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbonate Precipitation
2.3. Characterization
2.4. Carbonate Dissolution Quantification
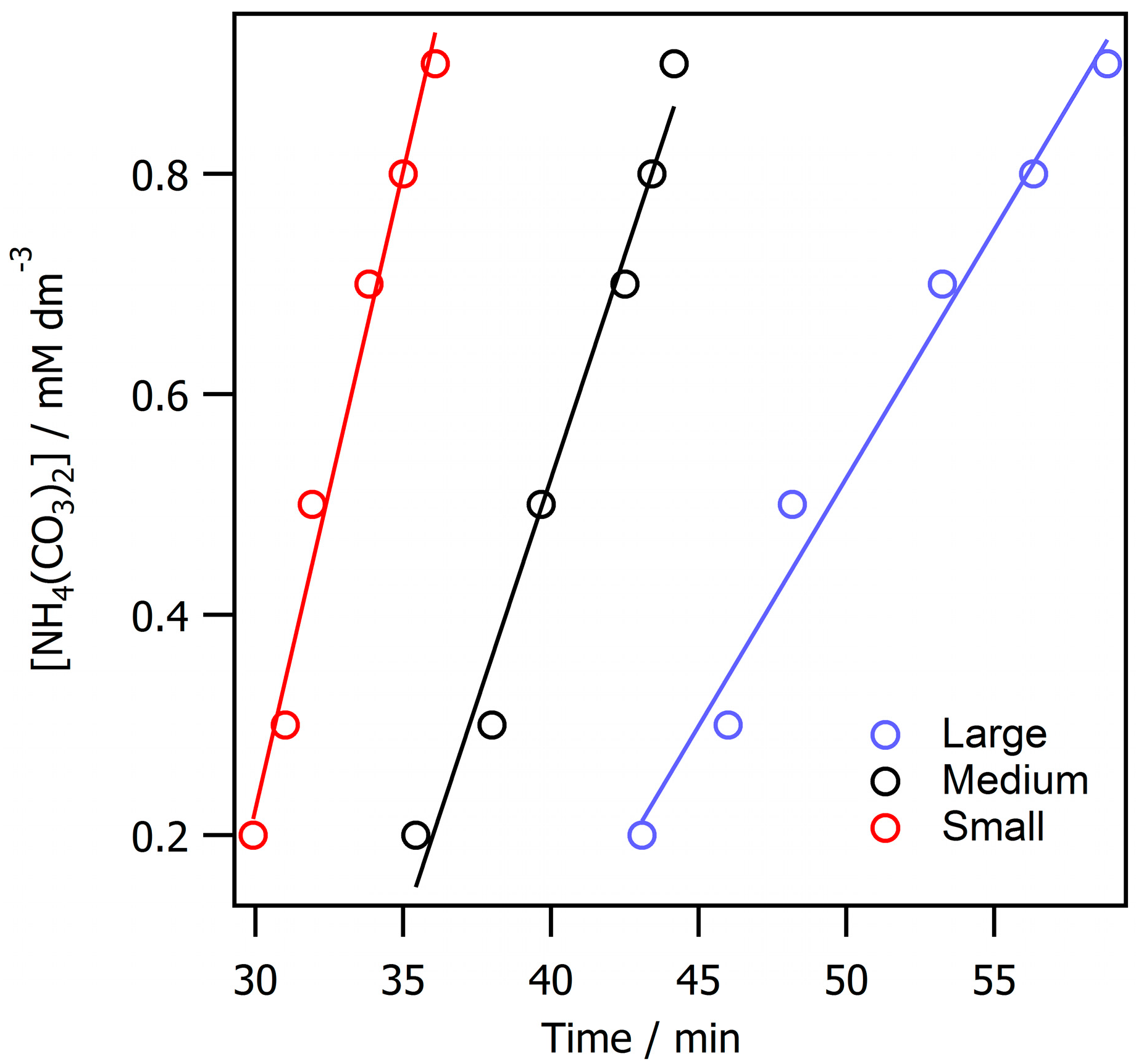
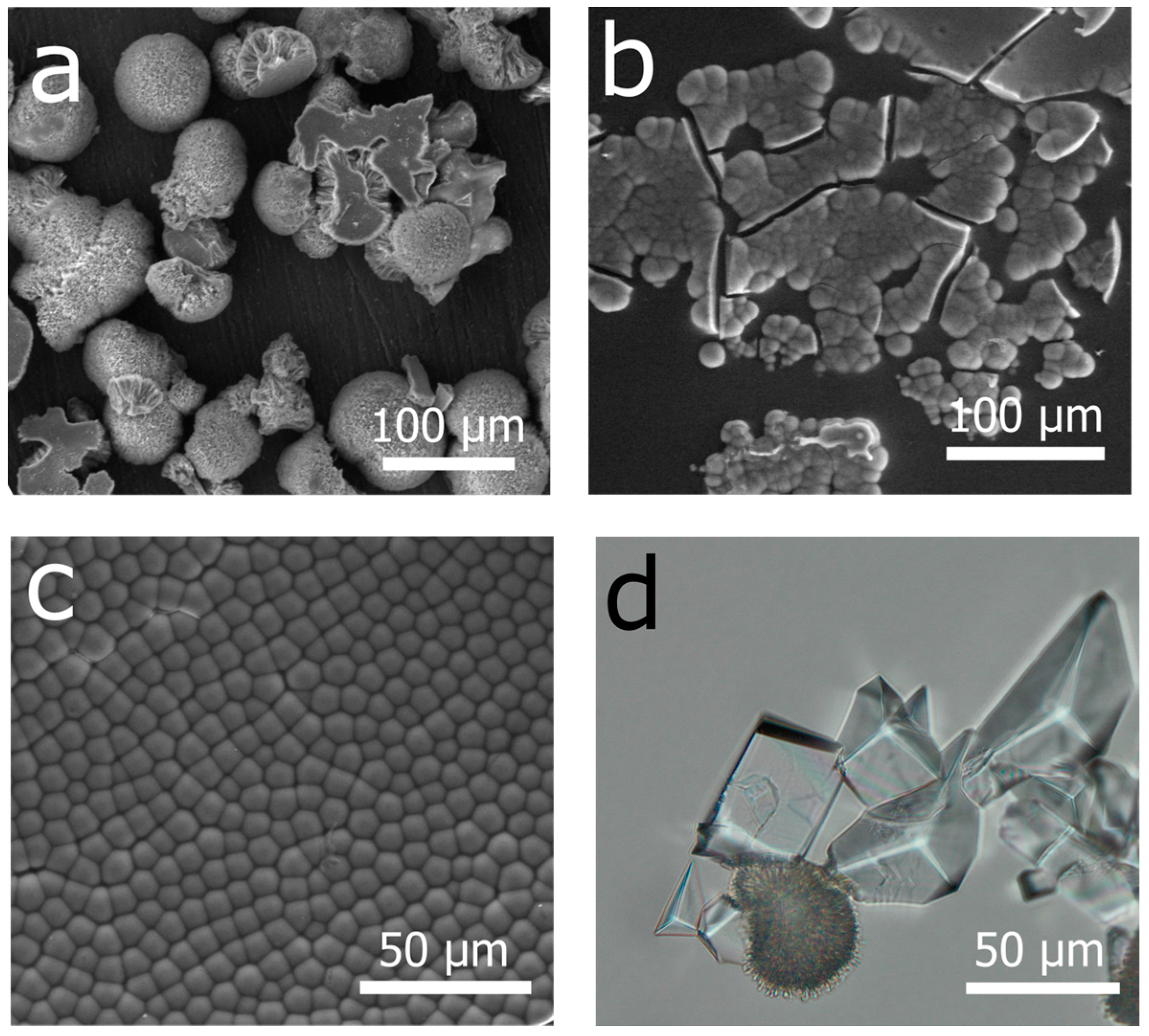
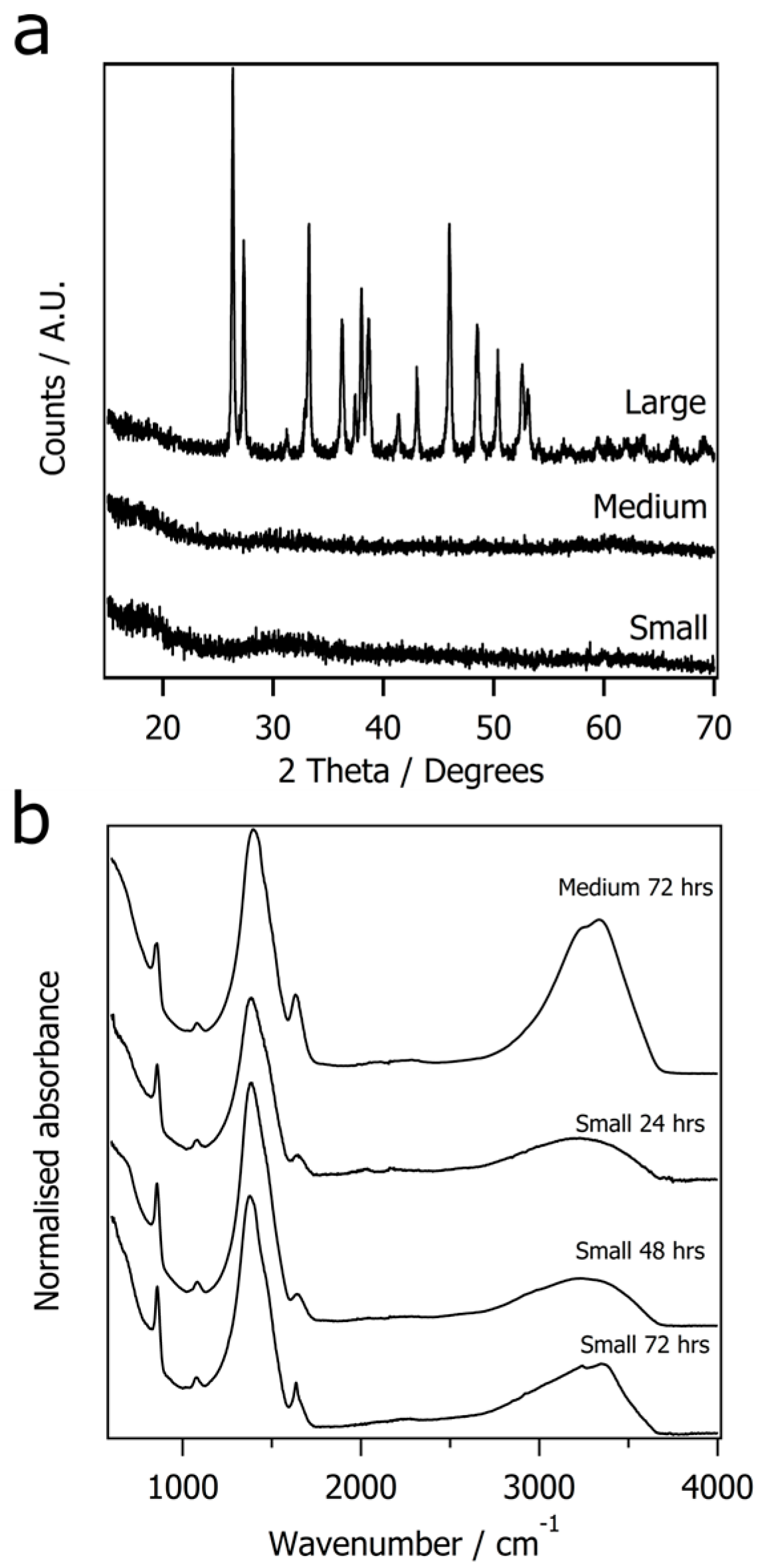
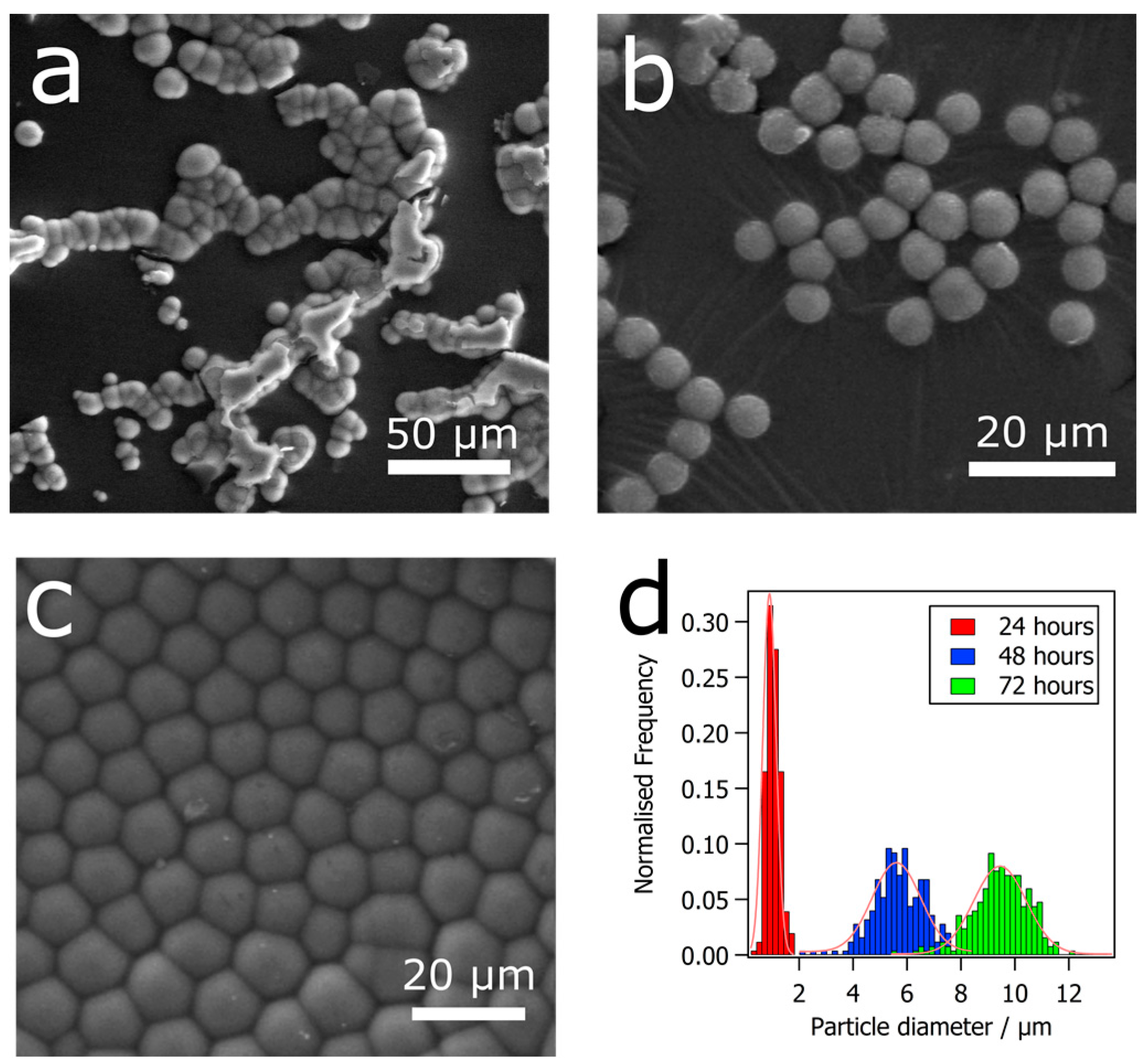
3. Results
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, Y.; Aichmayer, B.; Paris, O.; Fratzl, P.; Meibom, A.; Metzler, R.A.; Politi, Y.; Addadi, L.; Gilbert, P.U.P.A.; Weiner, S. The grinding tip of the sea urchin tooth exhibits exquisite control over calcite crystal orientation and Mg distribution. Proc. Natl. Acad. Sci. USA 2009, 106, 6048–6053. [Google Scholar] [CrossRef] [PubMed]
- Hovden, R.; Wolf, S.E.; Holtz, M.E.; Marin, F.; Muller, D.A.; Estroff, L.A. Nanoscale assembly processes revealed in the nacroprismatic transition zone of Pinna nobilis mollusc shells. Nat. Commun. 2015, 6, 10097. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Milliron, G.W.; Miserez, A.; Evans-Lutterodt, K.; Herrera, S.; Gallana, I.; Mershon, W.J.; Swanson, B.; Zavattieri, P.; DiMasi, E.; et al. The stomatopod dactyl club: A formidable damage-tolerant biological hammer. Science 2012, 336, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Wagermaier, W.; Masic, A.; Kommareddy, K.P.; Bennet, M.; Manjubala, I.; Lee, S.-W.; Park, S.B.; Cölfen, H.; Fratzl, P. Self-assembly of amorphous calcium carbonate microlens arrays. Nat. Commun. 2012, 3, 725. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Mey, I.; Hajir, M.; Mondeshki, M.; Wolf, S.E. Pseudomorphic transformation of amorphous calcium carbonate films follows spherulitic growth mechanisms and can give rise to crystal lattice tilting. CrystEngComm 2015, 17, 6831–6837. [Google Scholar] [CrossRef]
- Marie, B.; Joubert, C.; Tayalé, A.; Zanella-Cléon, I.; Belliard, C.; Piquemal, D.; Cochennec-Laureau, N.; Marin, F.; Gueguen, Y.; Montagnani, C. Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc. Natl. Acad. Sci. USA 2012, 109, 20986–20991. [Google Scholar] [CrossRef] [PubMed]
- Loste, E.; Wilson, R.M.; Seshadri, R.; Meldrum, F.C. The role of magnesium in stabilising amorphous calcium carbonate and controlling calcite morphologies. J. Cryst. Growth 2003, 254, 206–218. [Google Scholar] [CrossRef]
- Ihli, J.; Bots, P.; Kulak, A.; Benning, L.G.; Meldrum, F.C. Elucidating mechanisms of diffusion-based calcium carbonate synthesis leads to controlled mesocrystal formation. Adv. Funct. Mater. 2013, 23, 1965–1973. [Google Scholar] [CrossRef]
- Davis, K.J.; Dove, P.M.; De Yoreo, J.J. The role of Mg2+ as an impurity in calcite growth. Science 2000, 290, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, J.; Wolf, S.E. Desiccator Volume: A Vital Yet Ignored Parameter in CaCO3 Crystallization by the Ammonium Carbonate Diffusion Method. Minerals 2017, 7, 122. https://doi.org/10.3390/min7070122
Harris J, Wolf SE. Desiccator Volume: A Vital Yet Ignored Parameter in CaCO3 Crystallization by the Ammonium Carbonate Diffusion Method. Minerals. 2017; 7(7):122. https://doi.org/10.3390/min7070122
Chicago/Turabian StyleHarris, Joe, and Stephan E. Wolf. 2017. "Desiccator Volume: A Vital Yet Ignored Parameter in CaCO3 Crystallization by the Ammonium Carbonate Diffusion Method" Minerals 7, no. 7: 122. https://doi.org/10.3390/min7070122
APA StyleHarris, J., & Wolf, S. E. (2017). Desiccator Volume: A Vital Yet Ignored Parameter in CaCO3 Crystallization by the Ammonium Carbonate Diffusion Method. Minerals, 7(7), 122. https://doi.org/10.3390/min7070122




