Ciriottiite, Cu(Cu,Ag)3Pb19(Sb,As)22(As2)S56, the Cu-Analogue of Sterryite from the Tavagnasco Mining District, Piedmont, Italy
Abstract
:1. Introduction
2. Geological Setting and Occurrence of Ciriottiite
3. Mineral Description and Physical Properties
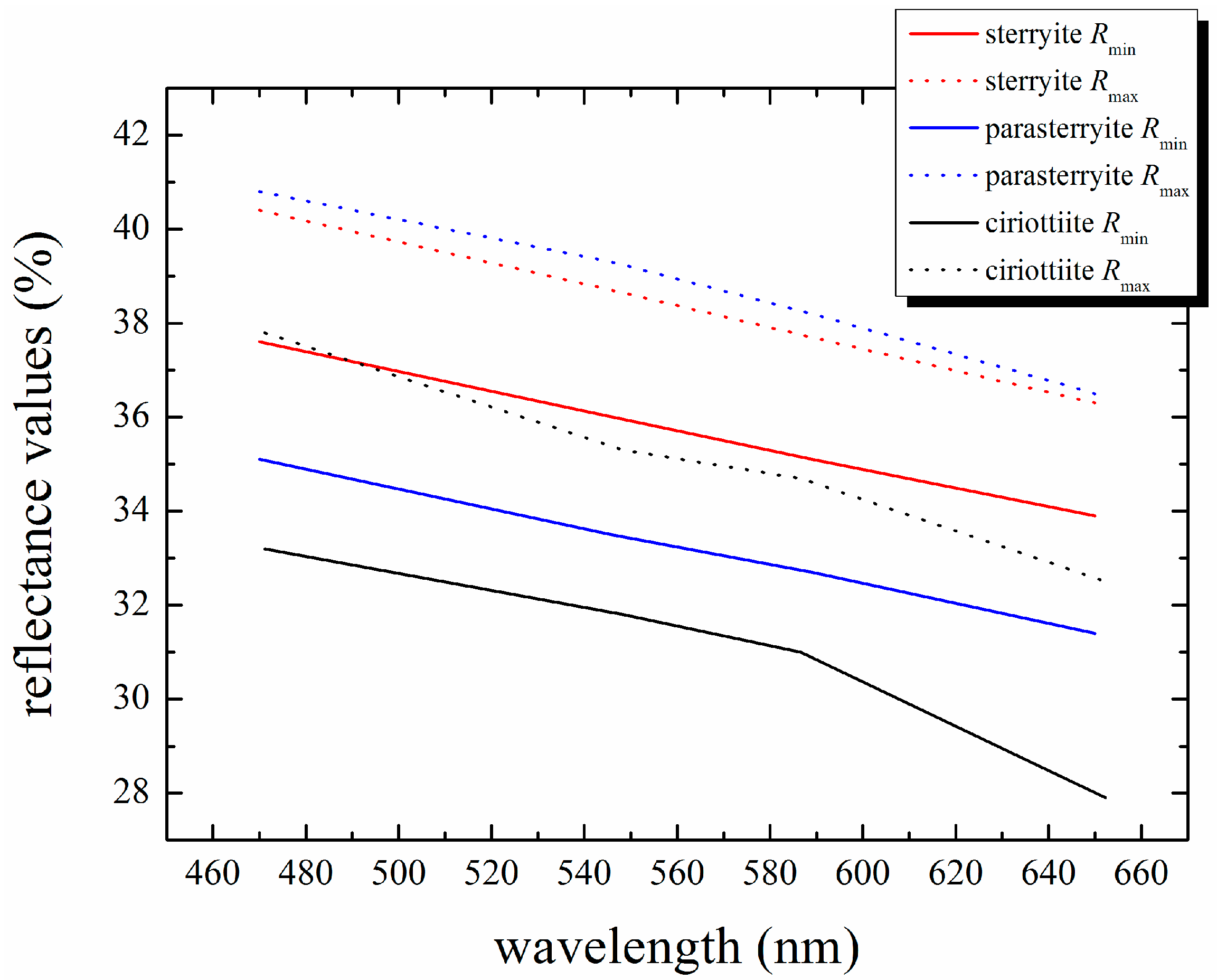
3.1. Chemical Data
| Element | wt (%) (n = 5) | Range | Estimated Standard Deviation |
|---|---|---|---|
| Cu | 2.33 | 2.22–2.41 | 0.08 |
| Ag | 0.53 | 0.48–0.60 | 0.05 |
| Hg | 0.98 | 0.92–1.06 | 0.06 |
| Tl | 0.78 | 0.74–0.81 | 0.03 |
| Pb | 44.06 | 43.89–44.21 | 0.14 |
| As | 4.66 | 4.58–4.75 | 0.07 |
| Sb | 23.90 | 23.77–24.05 | 0.10 |
| Bi | 1.75 | 1.65–1.83 | 0.07 |
| S | 20.37 | 20.22–20.46 | 0.10 |
| Total | 99.38 | 98.94–99.61 | 0.26 |
3.2. Crystallography
| Crystal Data | |
|---|---|
| Crystal size (mm3) | 0.040 × 0.045 × 0.060 |
| Cell setting, space group | Monoclinic, P21/n |
| a, b, c (Å) | 8.178 (2), 28.223 (6), 42.452 (5) |
| β (°) | 93.55 (2) |
| V (Å3) | 9779.5 (5) |
| Z | 4 |
| Data Collection and Refinement | |
| Radiation, wavelength (Å) | Mo Kα, λ = 0.71073 |
| Temperature (K) | 293 |
| 2θmax (°) | 56.13 |
| Measured reflections | 172985 |
| Unique reflections | 22638 |
| Reflections with Fo > 4σ (Fo) | 21304 |
| Rint | 0.0578 |
| Rσ | 0.0704 |
| Range of h, k, l | −10 ≤ h ≤ 10, −37 ≤ k ≤ 37, −55 ≤ l ≤ 55 |
| R (Fo > 4σ (Fo)) | 0.1176 |
| R (all data) | 0.1194 |
| wR (on F2) | 0.3187 |
| GooF | 1.159 |
| Number of least-square parameters | 960 |
| Maximun and minimum residuals (e/Å3) | 9.60 (at 0.87 from As28), −8.26 (at 1.00 from As28) |
| Icalc | dcalc | h k l | Icalc | dcalc | h k l |
|---|---|---|---|---|---|
| 11 | 10.59 | 0 0 4 | 20 | 2.939 | 2 5 6 |
| 13 | 4.237 | 0 0 10 | 54 | 2.936 | 0 8 8 |
| 25 | 4.113 | 0 6 5 | 37 | 2.928 | 2 2 9 |
| 20 | 4.058 | 0 2 10 | 14 | 2.877 | −2 1 11 |
| 15 | 3.920 | 2 2 0 | 20 | 2.871 | −2 5 8 |
| 14 | 3.916 | 0 4 9 | 22 | 2.868 | 2 7 0 |
| 11 | 3.897 | −2 2 2 | 21 | 2.859 | −2 7 2 |
| 12 | 3.816 | 0 1 11 | 27 | 2.854 | 2 6 5 |
| 15 | 3.714 | 0 6 7 | 22 | 2.852 | 2 3 9 |
| 26 | 3.652 | 2 3 2 | 33 | 2.833 | −2 2 11 |
| 100 | 3.641 | 0 7 5 | 12 | 2.823 | 0 8 9 |
| 35 | 3.595 | −2 3 4 | 36 | 2.800 | −2 6 7 |
| 18 | 3.562 | 2 1 5 | 28 | 2.796 | 2 2 10 |
| 20 | 3.531 | 0 0 12 | 16 | 2.764 | −2 3 11 |
| 12 | 3.517 | 0 6 8 | 12 | 2.739 | 2 7 4 |
| 17 | 3.459 | −2 1 7 | 19 | 2.705 | −2 2 12 |
| 14 | 3.442 | 2 0 6 | 20 | 2.698 | 0 9 8 |
| 15 | 3.407 | −2 4 4 | 19 | 2.670 | −2 8 1 |
| 13 | 3.334 | −2 0 8 | 23 | 2.313 | 2 10 1 |
| 12 | 3.299 | 2 4 4 | 22 | 2.303 | −2 10 3 |
| 19 | 3.257 | 0 8 5 | 11 | 2.213 | 0 7 16 |
| 82 | 3.238 | 0 1 13 | 18 | 2.120 | 0 7 17 |
| 57 | 3.208 | 0 7 8 | 78 | 2.043 | −4 0 2 |
| 11 | 3.205 | 2 2 7 | 16 | 2.036 | 0 8 17 |
| 13 | 3.176 | 0 2 13 | 15 | 1.996 | 0 14 3 |
| 27 | 3.030 | 2 5 5 | 12 | 1.895 | 2 6 17 |
| 10 | 3.009 | 0 1 14 | 11 | 1.771 | −4 7 7 |
| 14 | 2.977 | 2 1 9 | 11 | 1.702 | −4 1 15 |
| 24 | 2.966 | −2 5 7 | 12 | 1.692 | 0 1 25 |
4. Crystal Structure Description
4.1. General Features
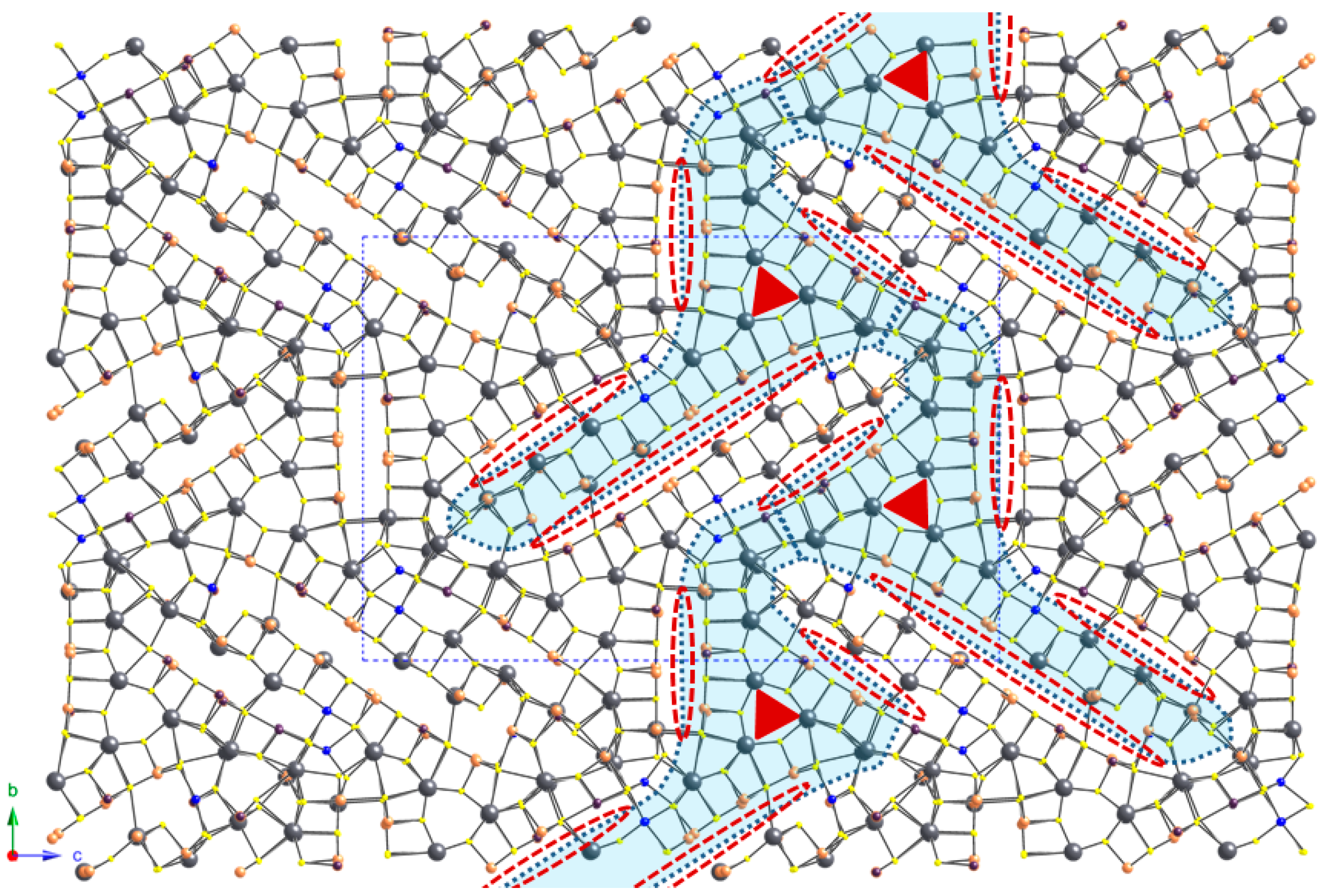

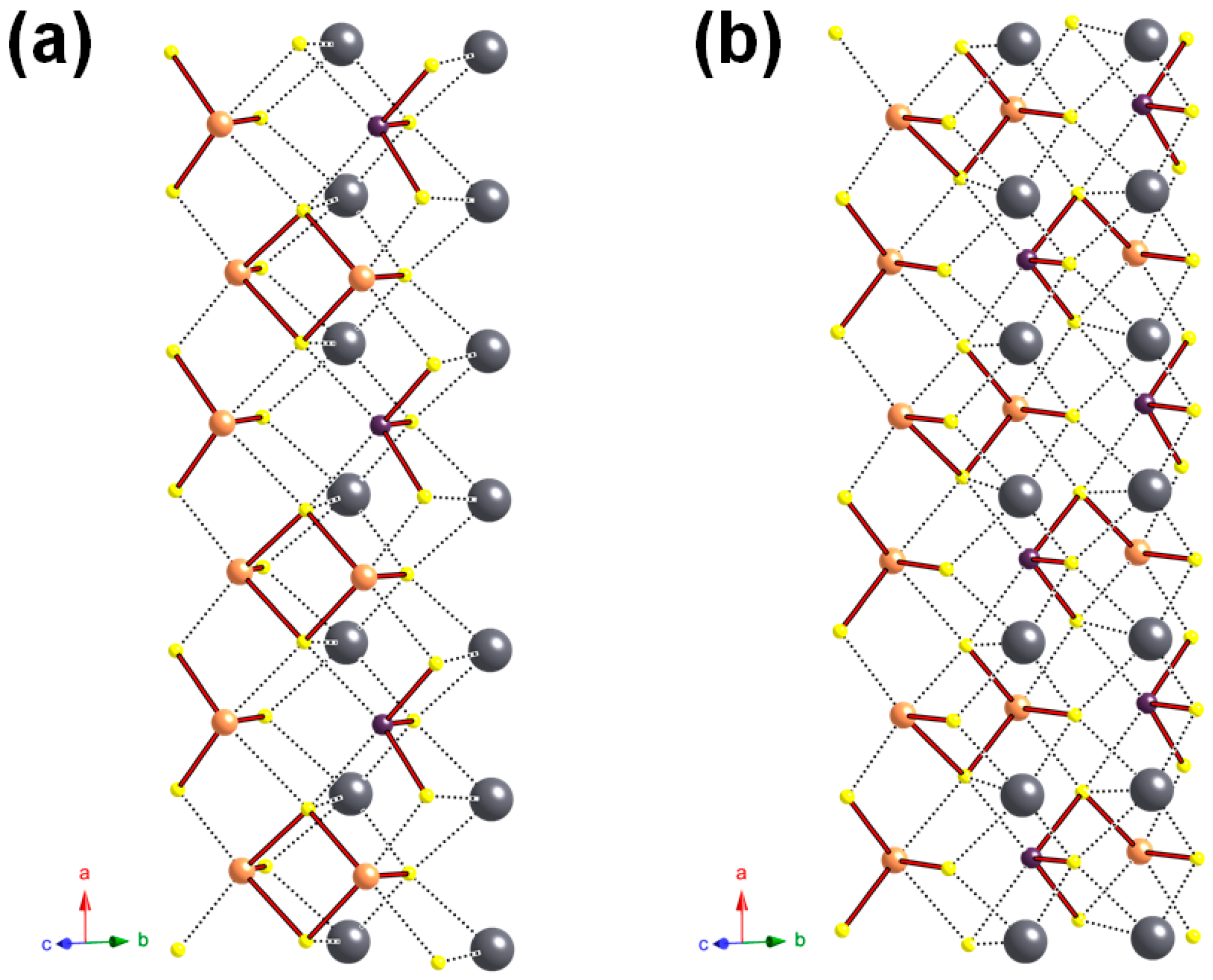
4.2. Cation Coordination and Site Occupancies
4.3. Polymerization of (Sb/As) Sites
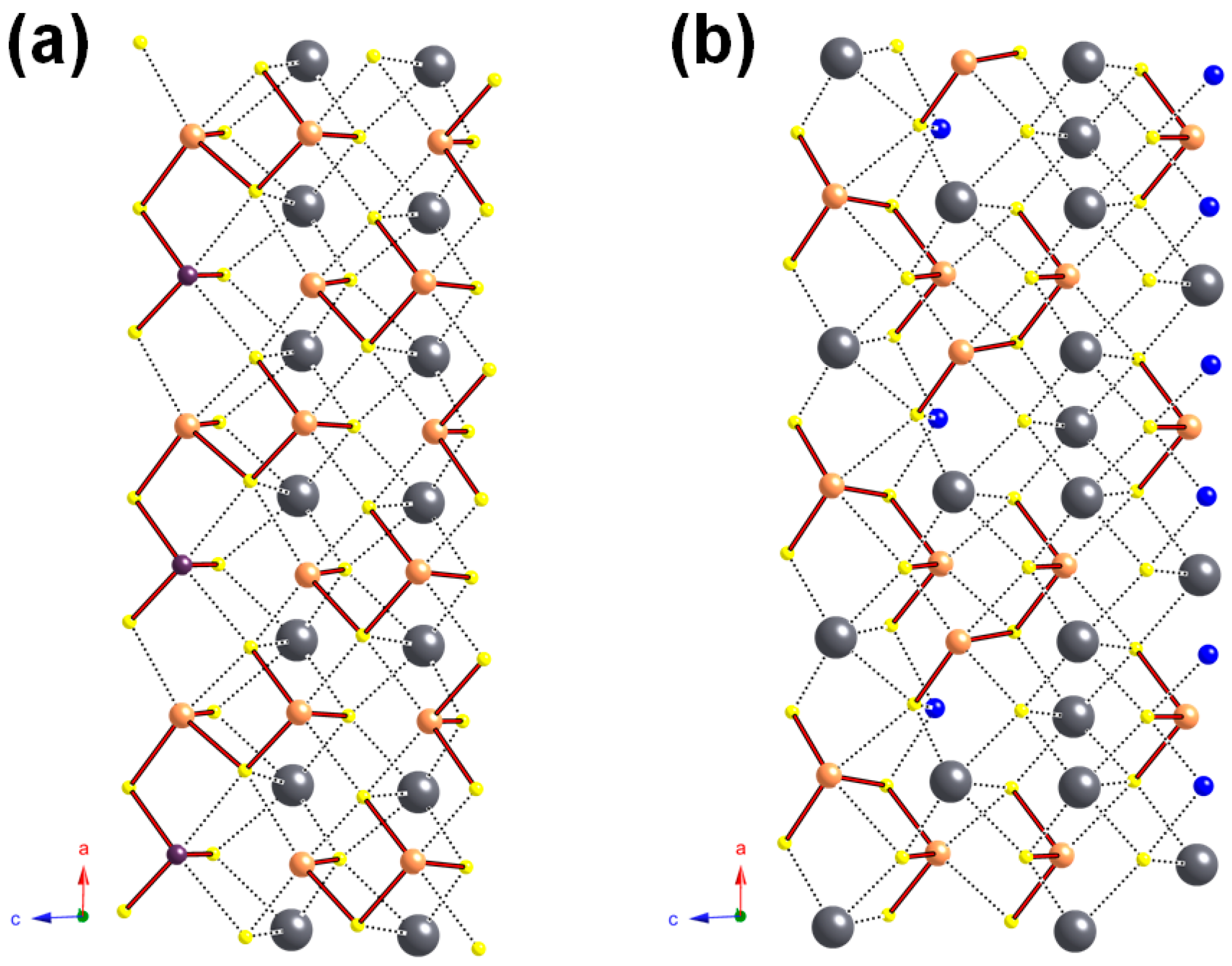
5. Discussion
5.1. Ciriottiite in the Framework of the Owyheeite Group and Its Comparison with Sterryite
| Mineral | a (Å) | b (Å) | c (Å) | β (°) | V (Å3) | Space Group | Ref. |
|---|---|---|---|---|---|---|---|
| Ciriottiite | 8.178 | 28.223 | 42.452 | 93.55 | 9780 | P21/n | This work |
| Meerschautite | 8.2393 | 43.6015 | 28.3688 | 94.128 | 10165 | P21 | [11] |
| Owyheeite | 4.1035 | 27.3144 | 22.3966 | 90.359 | 2571 | P21/c | [19] |
| Parasterryite | 8.3965 | 27.954 | 43.884 | 90.061 | 10300 | P21/c | [8] |
| Sterryite Madoc | 28.4 | 42.6 | 8.26 | 90 | 9921 | Pba2 or Pbam | [20] |
| Sterryite Pollone | 8.1891 | 28.5294 | 42.98 | 94.896 | 10005 | P21/n | [8] |
| Tubulite | 4.132 (×2) | 43.1 | 27.4 | 93.2 | 4872 (×2) | P2/c, Pc or P21/c | [21] |
5.2. The Study of Sulfosalts as a Proxy for Unraveiling Ore Geochemistry
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ciriotti, M.E.; Fascio, L.; Pasero, M. Italian Type Minerals; Edizioni Plus: Pisa, Italy, 2009; p. 360. [Google Scholar]
- Bindi, L.; Biagioni, C.; Martini, B.; Salvetti, A. Ciriottiite, IMA 2015-027. CNMNC Newsletter No. 26, August 2015, page 943. Miner. Mag. 2015, 79, 941–947. [Google Scholar]
- Bindi, L.; Biagioni, C.; Martini, B.; Salvetti, A.; Dalla Fontana, G.; Taronna, M.; Ciriotti, M.E. Tavagnascoite, Bi4O4(SO4)(OH)2, a new oxy-hydroxy bismuth sulfate related to klebelsbergite. Miner. Mag. 2016. [Google Scholar] [CrossRef]
- Jambor, J.L. New lead sulfantimonides from Madoc, Ontario. 1. Can. Miner. 1967, 9, 7–24. [Google Scholar]
- Moëlo, Y.; Orlandi, P.; Guillot-Deudon, C.; Biagioni, C.; Paar, W.; Evain, M. Lead-Anitmony sulfosalts from Tuscany (Italy). XI. The new mineral species parasterryite, Ag4Pb20(Sb14.5As9.5)S24S58, and associated sterryite, Cu(Ag,Cu)3Pb19(Sb,As)22(As–As)S56, from the Pollone mine, Tuscany, Italy. Can. Miner. 2011, 49, 623–638. [Google Scholar] [CrossRef]
- Oxford Diffracxtion. CrysAlis RED (Version 1.171.31.2) and ABSPACK in CrysAlis RED; Oxford Diffraction Ltd.: Oxfordshire, England, UK, 2006. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Moëlo, Y.; Guillot-Deudon, C.; Evain, M.; Orlandi, P.; Biagioni, C. Comparative modular analysis of two complex sulfosalt structures: Sterryite, Cu(Ag,Cu)3Pb19(Sb,As)22(As–As)S56, and parasterryite, Ag4Pb20(Sb,As)24S58. Acta Crystallogr. 2012, B68, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.C. (Ed.) International Tables for Crystallography. Volume C: Mathematical, Physical and Chemical Tables; Kluwer Academic: Dordrecht, The Netherland, 1992.
- Kraus, W.; Nolze, G. PowderCell—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Biagioni, C.; Moëlo, Y.; Orlandi, P.; Stanley, C. Lead-antimony sulfosalts from Tuscany (Italy). XVII. Meerschautite, (Ag,Cu)5.5Pb42.4(Sb,As)45.1S112O0.8, a new expanded derivative of owyheeite from the Pollone mine, Valdicastello Carducci: Occurrence and crystal structure. Miner. Mag. 2016. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Bonazzi, P.; Bindi, L.; Popova, V.; Pratesi, G.; Menchetti, S. Alacranite, As8S9: Structural study of the holotype and re-assignment of the original chemical formula. Am. Miner. 2003, 88, 1796–1800. [Google Scholar] [CrossRef]
- Bindi, L.; Pratesi, G.; Muniz-Miranda, M.; Zoppi, M.; Chelazzi, L.; Lepore, G.O.; Menchetti, S. From ancient pigments to modern optoelectronic applications of arsenic sulfides: Bonazziite, the natural analogue of β-As4S4 from Khaidarkan deposit, Kyrgyzstan. Miner. Mag. 2015, 79, 121–131. [Google Scholar] [CrossRef]
- Mullen, D.J.E.; Nowacki, W. Refinement of the crystal structure of realgar, AsS and orpiment, As2S3. Z. Kristallogr. 1972, 136, 48–65. [Google Scholar] [CrossRef]
- Bindi, L.; Popova, V.; Bonazzi, P. Uzonite, As4S5, from the type locality: Single-crystal X-ray study and effects of exposure to light. Can. Miner. 2003, 41, 1463–1468. [Google Scholar] [CrossRef]
- Bonazzi, P.; Lampronti, G.I.; Bindi, L.; Zanardi, S. Wakabayashilite, [(As,Sb)6S9][As4S5]: Crystal structure, pseudosymmetry, twinning, and revised chemical formula. Am. Miner. 2005, 90, 1108–1114. [Google Scholar] [CrossRef]
- Bindi, L.; Nestola, F.; De Battisti, L.; Guastoni, A. Dervillite, Ag2AsS2, from Lengenbach quarry, Binn valley, Switzerland: Occurrence and crystal structure. Miner. Mag. 2013, 77, 3105–3112. [Google Scholar] [CrossRef]
- Laufek, F.; Pažout, R.; Makovicky, E. Crystal structure of owyheeite, Ag1.5Pb4.43Sb6.07S14: Refinement from powder synchrotron X-ray diffraction. Eur. J. Miner. 2007, 19, 557–566. [Google Scholar] [CrossRef]
- Jambor, J.L. New lead sulfantimonides from Madoc, Ontario. 2. Mineral descriptions. Can. Miner. 1967, 9, 191–213. [Google Scholar]
- Moëlo, Y.; Pecorini, R.; Ciriotti, M.E.; Meisser, N.; Caldes, M.T.; Orlandi, P.; Petit, P.E.; Martini, B.; Salvetti, A. Tubulite—Ag2Pb22Sb20S53, a new Pb–Ag–Sb sulfosalt from Le Rivet quarry, Peyrebrune ore field (Tarn, France) and Biò, Borgofranco mines, Borgofranco d’Ivrea (Piedmont, Italy). Eur. J. Miner. 2013, 25, 1017–1030. [Google Scholar]
- Makovicky, E. Cyclically twinned sulphosalt structures and their approximate analogues. Z. Kristallogr. 1985, 173, 1–23. [Google Scholar] [CrossRef]
- Moëlo, Y.; Makovicky, E.; Mozgova, N.N.; Jambor, J.L.; Cook, N.; Pring, A.; Paar, W.H.; Nickel, E.H.; Graeser, S.; Karup-Møller, S.; et al. Sulfosalt systematics: A review. Report of the sulfosalt sub-committee of the IMA Commission on Ore Mineralogy. Eur. J. Miner. 2008, 20, 7–46. [Google Scholar] [CrossRef]
- Makovicky, E.; Topa, D.; Mumme, W.G. The crystal structure of dadsonite. Can. Miner. 2006, 44, 1499–1512. [Google Scholar] [CrossRef] [Green Version]
- Orlandi, P.; Meerschaut, A.; Palvadeau, P.; Merlino, S. Lead-antimony sulfosalts from Tuscany (Italy). V. Definition and crystal structure of moëloite, Pb6Sb6S14(S3), a new mineral from the Ceragiola marble quarry. Eur. J. Miner. 2002, 14, 599–606. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindi, L.; Biagioni, C.; Martini, B.; Salvetti, A. Ciriottiite, Cu(Cu,Ag)3Pb19(Sb,As)22(As2)S56, the Cu-Analogue of Sterryite from the Tavagnasco Mining District, Piedmont, Italy. Minerals 2016, 6, 8. https://doi.org/10.3390/min6010008
Bindi L, Biagioni C, Martini B, Salvetti A. Ciriottiite, Cu(Cu,Ag)3Pb19(Sb,As)22(As2)S56, the Cu-Analogue of Sterryite from the Tavagnasco Mining District, Piedmont, Italy. Minerals. 2016; 6(1):8. https://doi.org/10.3390/min6010008
Chicago/Turabian StyleBindi, Luca, Cristian Biagioni, Bruno Martini, and Adrio Salvetti. 2016. "Ciriottiite, Cu(Cu,Ag)3Pb19(Sb,As)22(As2)S56, the Cu-Analogue of Sterryite from the Tavagnasco Mining District, Piedmont, Italy" Minerals 6, no. 1: 8. https://doi.org/10.3390/min6010008
APA StyleBindi, L., Biagioni, C., Martini, B., & Salvetti, A. (2016). Ciriottiite, Cu(Cu,Ag)3Pb19(Sb,As)22(As2)S56, the Cu-Analogue of Sterryite from the Tavagnasco Mining District, Piedmont, Italy. Minerals, 6(1), 8. https://doi.org/10.3390/min6010008