Removal of Uranium and Associated Contaminants from Aqueous Solutions Using Functional Carbon Nanotubes-Sodium Alginate Conjugates
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Investigation Tools
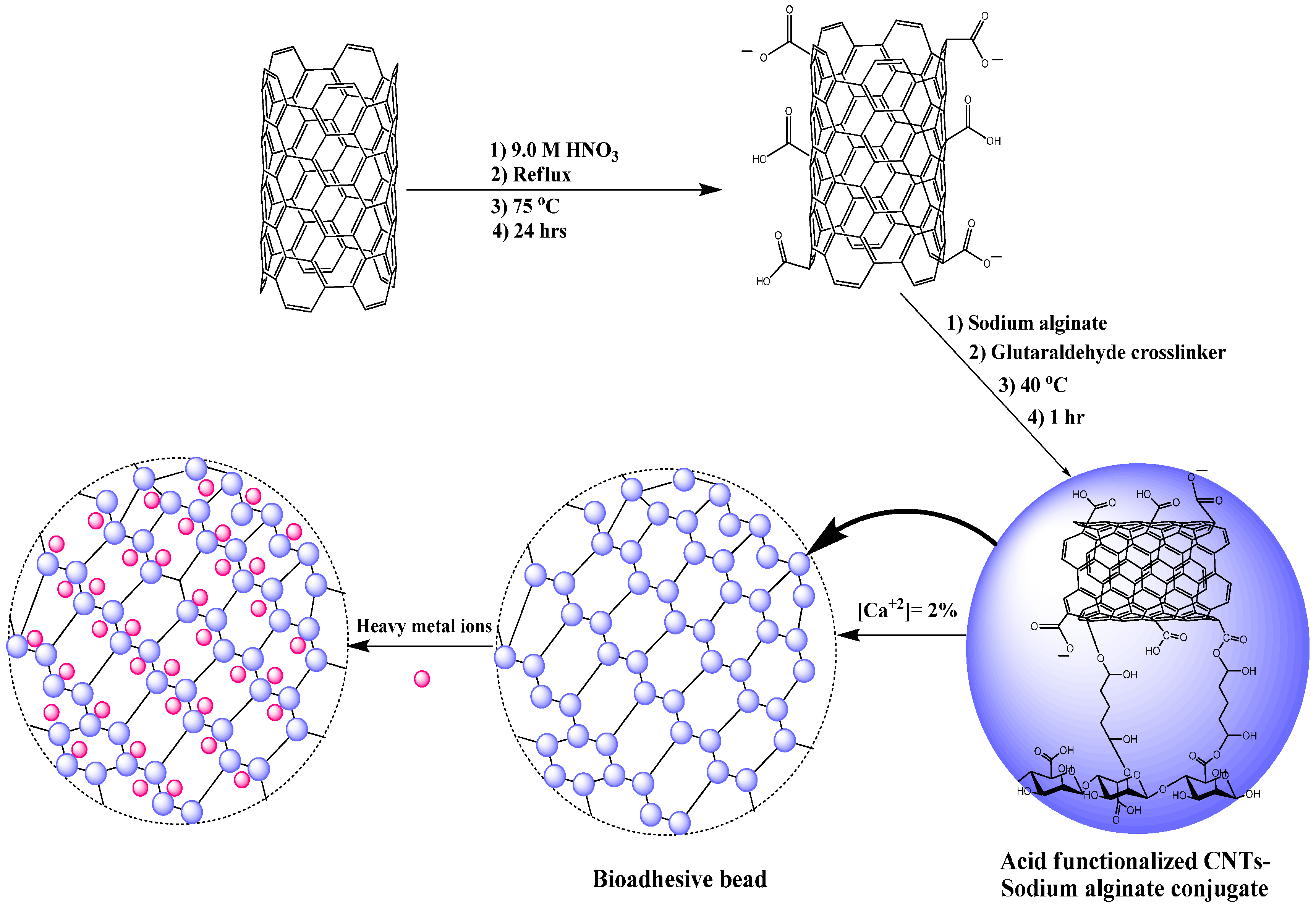
2.3. Acid Functionalized Carbon Nanotubes (CNTs)
2.4. Functional CNT-Alginate Beads
| Sample | Functional CNTs (%) |
|---|---|
| I | 17 |
| II | 33 |
| III | 50 |
| IV | 100 |
2.5. Removal of Uranium and Other Contaminants
3. Results and Discussion
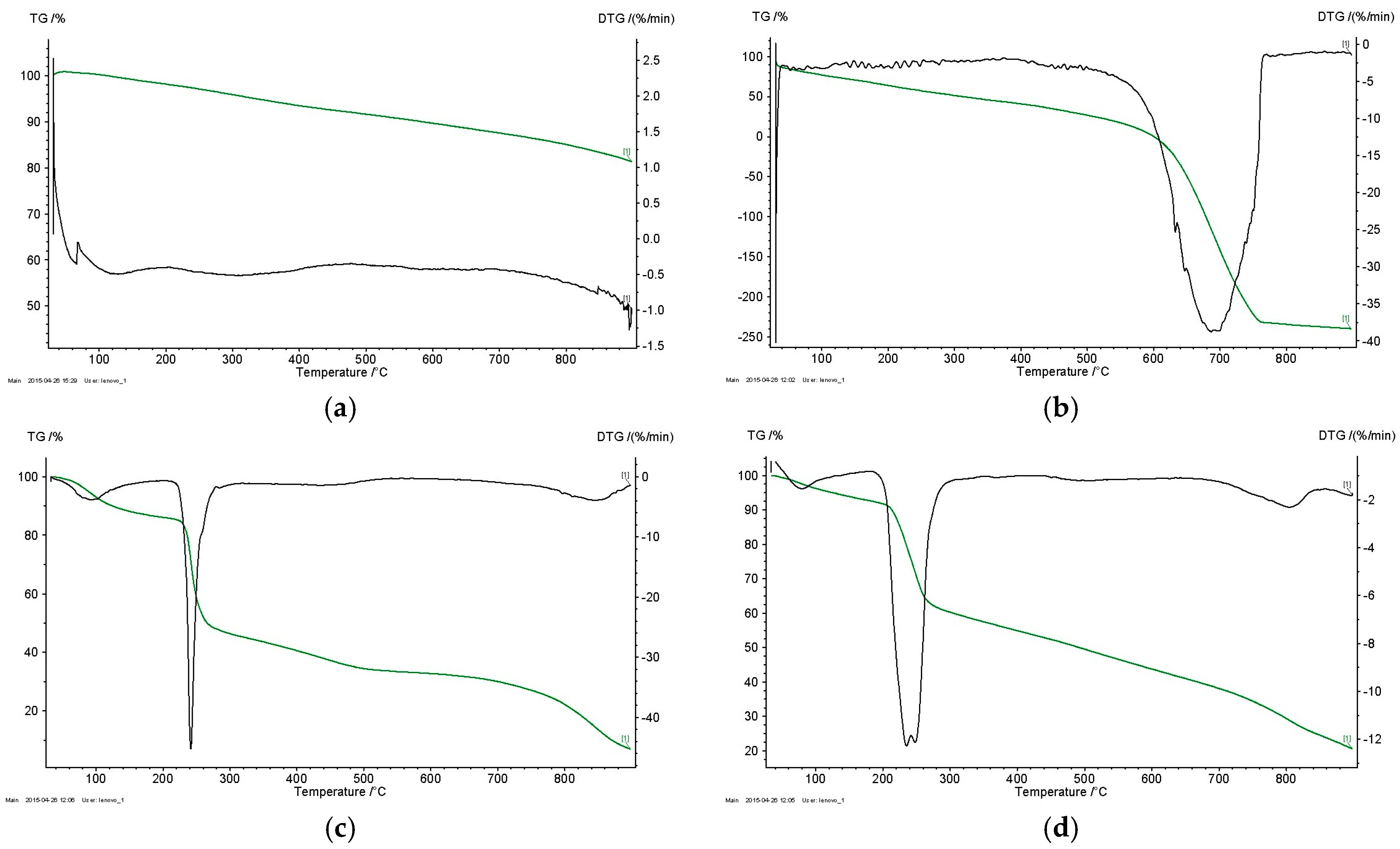
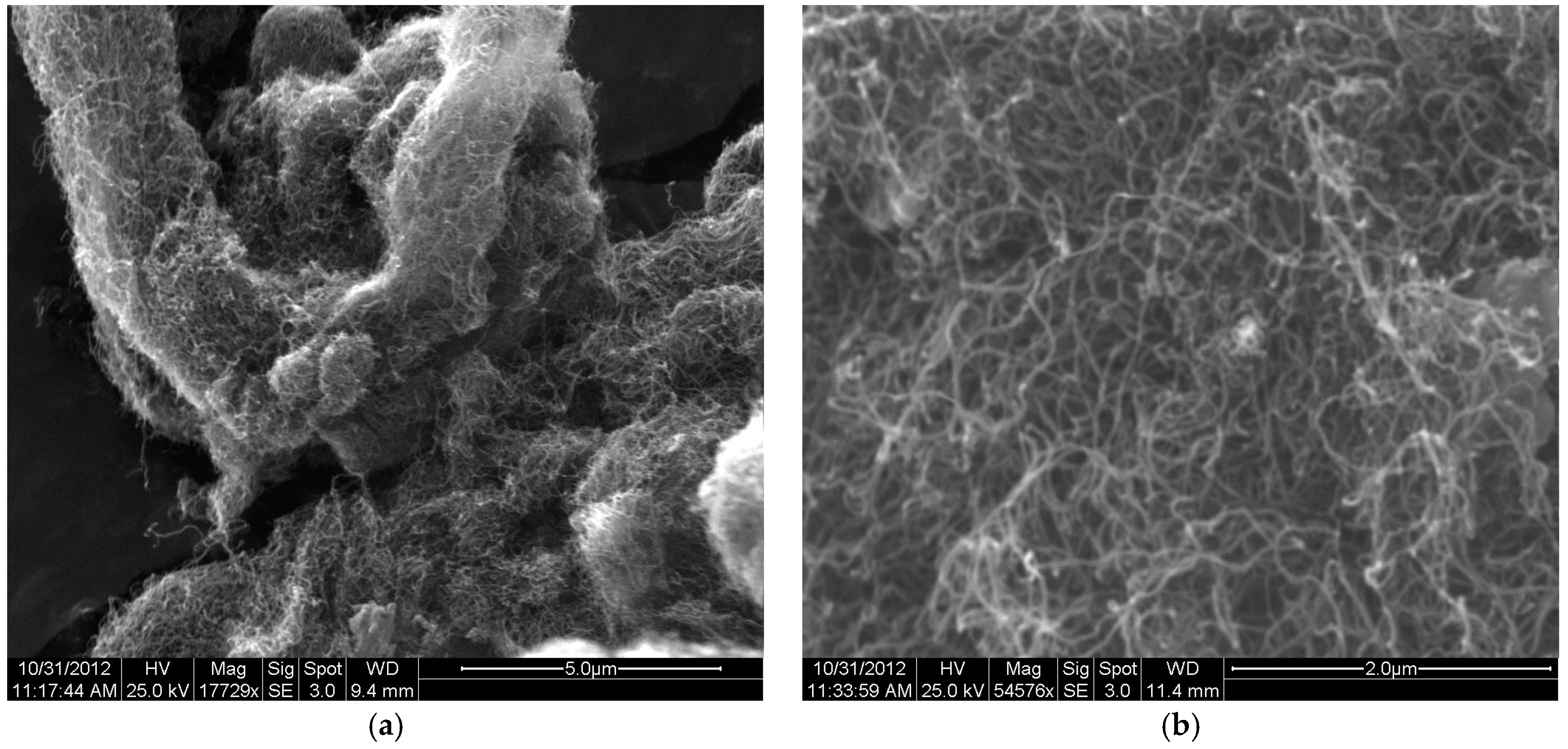
3.1. Structural Identification of Functional CNT-Alginate Conjugates
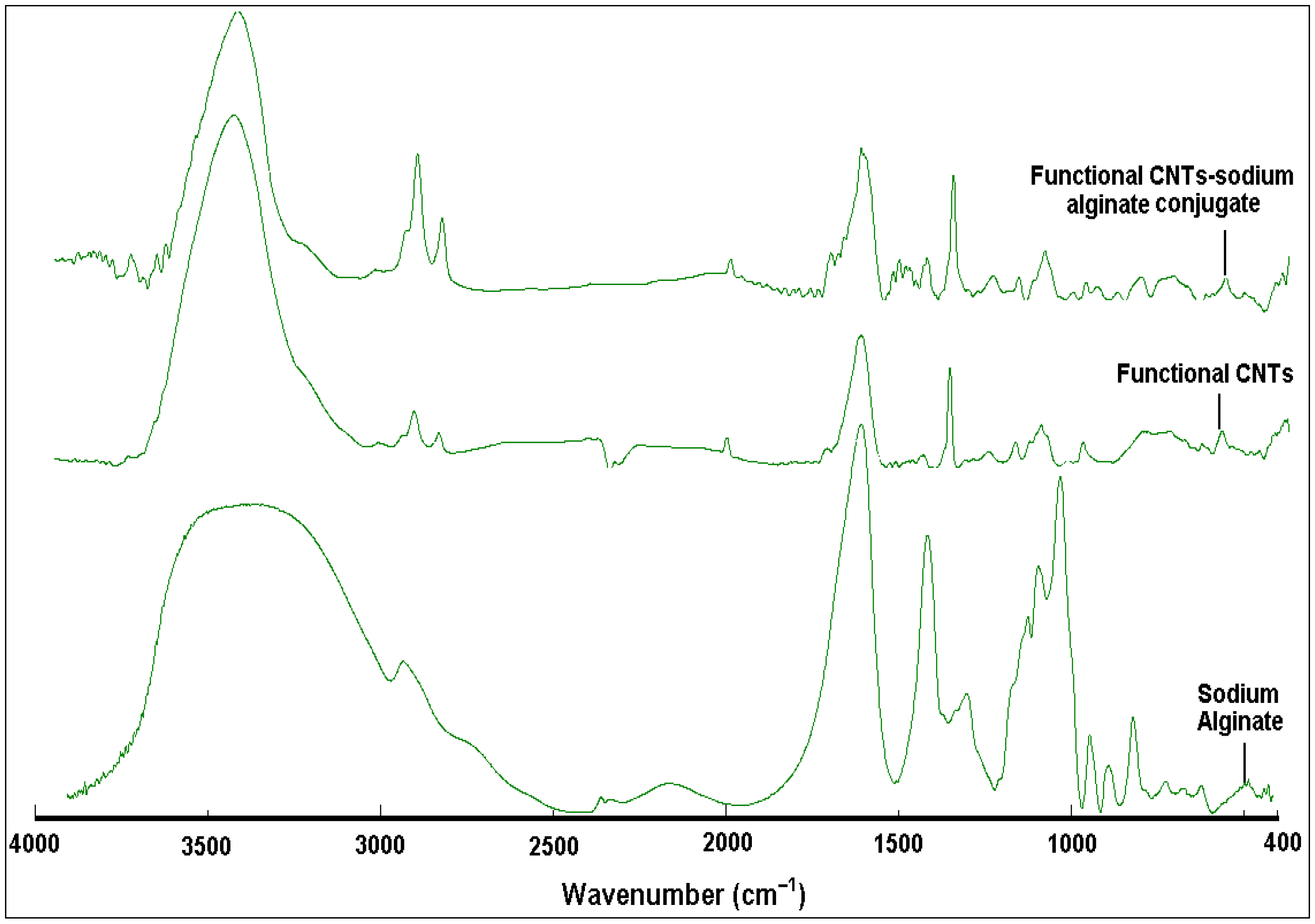
| Composite | Wavenumber (cm−1) | Absorbance | Functional Group | Reference |
|---|---|---|---|---|
| Functional CNTs | 3443 | 0.003 | OH stretching | [47,48] |
| 2960 | 0.074 | Asymmetric CH stretching of methyl groups | [47,48] | |
| 2922 | 0.073 | Asymmetric CH stretching of methyl bounded to aromatic groups | [49,50] | |
| 2851 | 0.077 | Symmetric CH stretching of methyl and methylene groups | [47,48,49,50] | |
| 1735 | 0.087 | C=O stretching | - | |
| 1638 | 0.069 | Asymmetric COO– stretching | - | |
| 1557 | 0.089 | C=C stretching | [51] | |
| 1387 | 0.072 | Symmetric COO– stretching | [52] | |
| Functional CNTs-alginate conjugates | 3443 | 0.096 | OH stretching | - |
| 2960 | 0.160 | Asymmetric CH stretching of methyl groups | - | |
| 2922 | 0.149 | Asymmetric CH stretching of methyl bounded to aromatic groups | - | |
| 2851 | 0.159 | Symmetric CH stretching of methyl and methylene groups | - | |
| 1735 | 0.164 | C=O stretching | - | |
| 1638 | 0.144 | Asymmetric COO stretching | - | |
| 1557 | 0.157 | C=C stretching | - | |
| 1387 | 0.152 | Symmetric COO stretching | - |
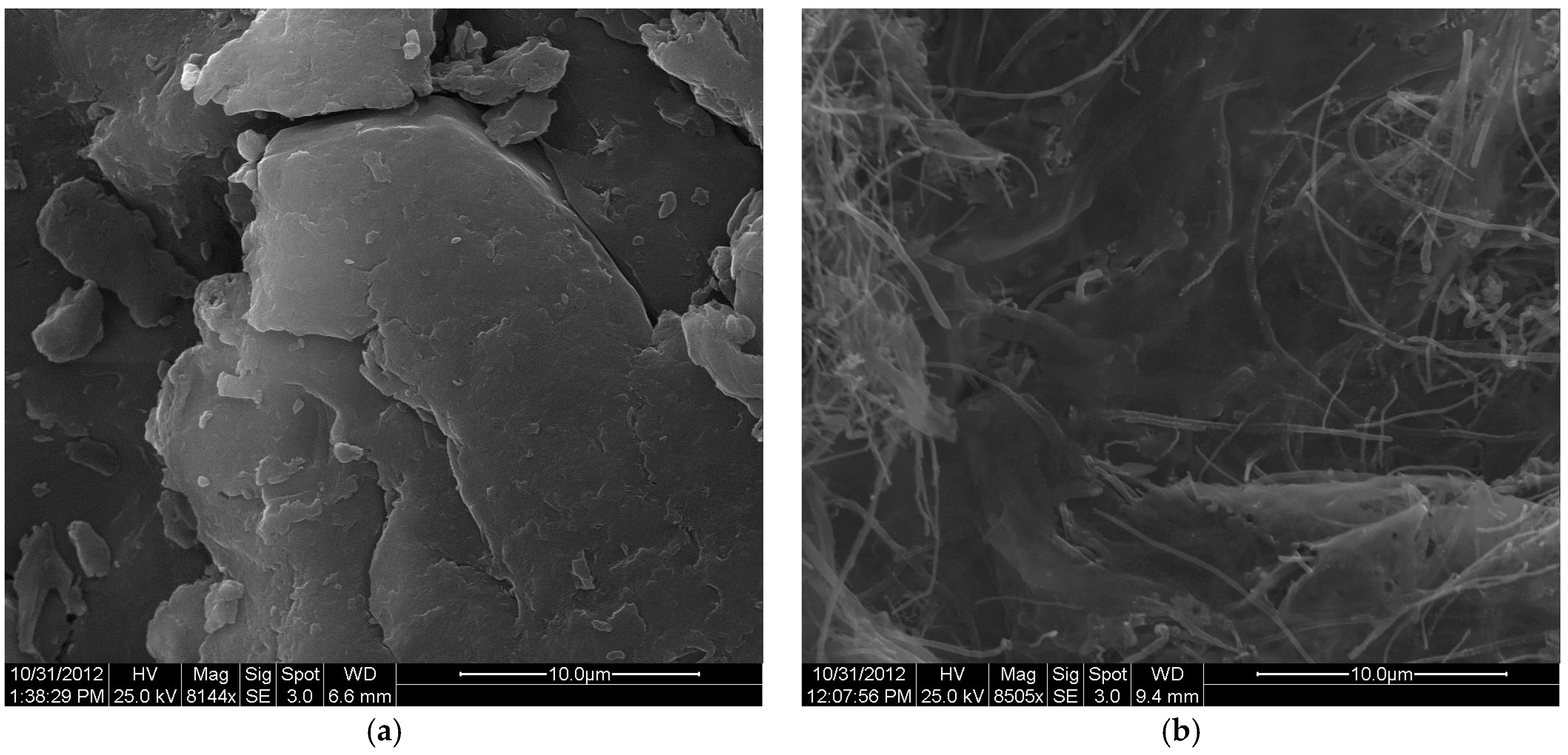

3.2. Removal of Uranium and Associated Contaminants Using the Synthesized Biosorbent
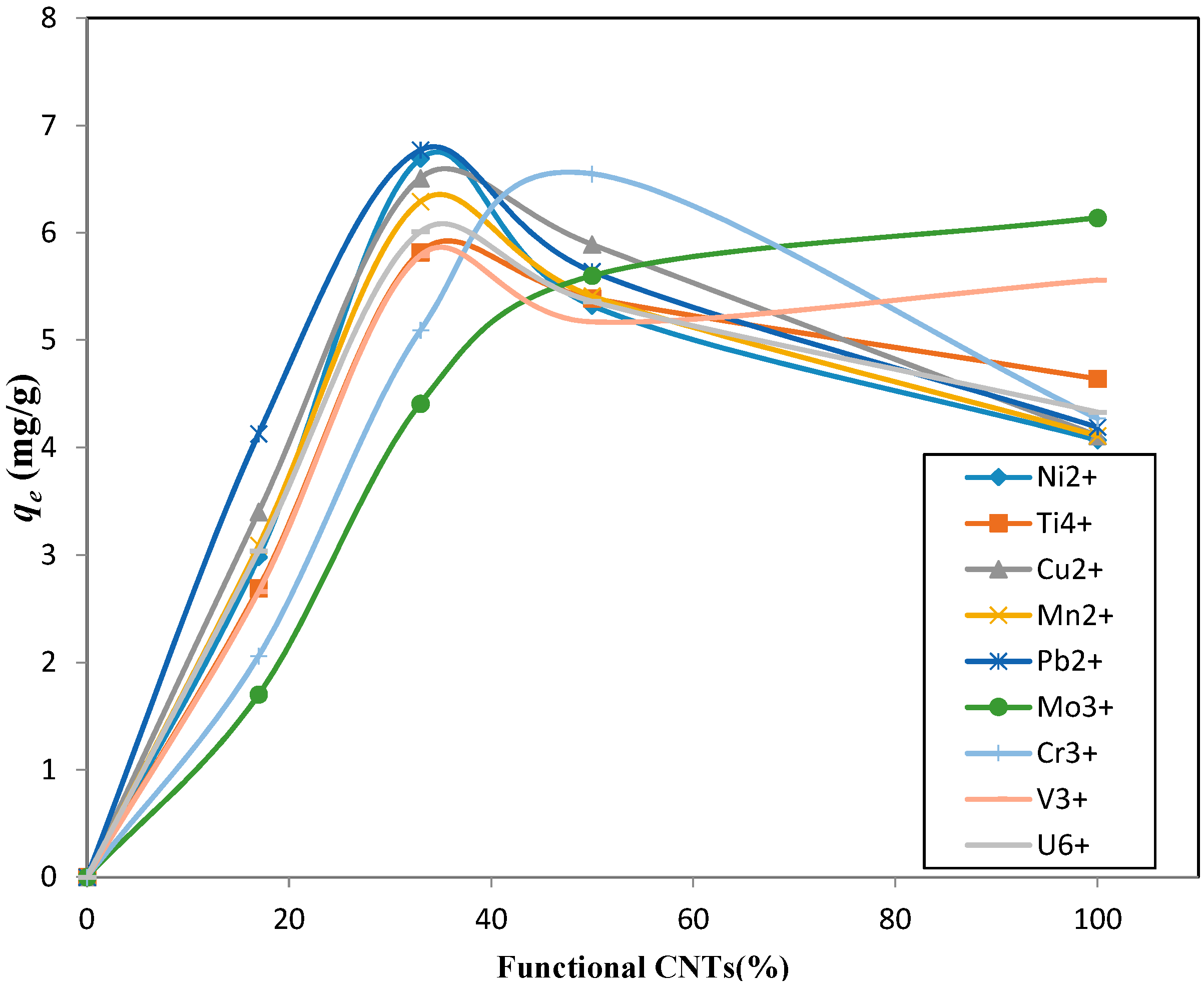
| Functional CNTs (%) | Ni2+ | Ti4+ | Cu2+ | Mn2+ | Pb2+ | Mo3+ | Cr3+ | V3+ | U6+ |
|---|---|---|---|---|---|---|---|---|---|
| 17 | 2.98 | 2.69 | 3.40 | 3.09 | 4.13 | 1.70 | 2.06 | 2.66 | 3.04 |
| 33 | 6.69 | 5.82 | 6.51 | 6.29 | 6.77 | 4.41 | 5.09 | 5.79 | 6.01 |
| 50 | 5.32 | 5.39 | 5.89 | 5.41 | 5.64 | 5.60 | 6.55 | 5.17 | 5.36 |
| 100 | 4.07 | 4.64 | 4.11 | 4.11 | 4.19 | 6.14 | 4.27 | 5.56 | 4.33 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ames Technology Capabilities and Facilities. Available online: http://www.nasa.gov/centers/ames/research/technology-onepagers/gas_detection.html (accessed on 28 January 2016).
- Cao, Q.; Han, S.-J.; Tulevski, G.S.; Zhu, Y.; Lu, D.D.; Haensch, W. Arrays of single-walled carbon nanotubes with full surface coverage for high-performance electronics. Nat. Nanotechnol. 2013, 8, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Franklin, A.D.; Tulevski, G.S.; Han, S.-J.; Shahrjerdi, D.; Cao, Q.; Chen, H.-Y.; Wong, H.-S.P.; Haensch, W. Variability in carbon nanotube transistors: Improving device-to-device consistency. ACS NANO 2012, 6, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Prasher, R.; Tong, T.; Majumdar, A. Approximate analytical models for phonon specific heat and ballistic thermal conductance of nanowires. Nano Lett. 2008, 8, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Gautier, S.; Gaucher, H.; Bonnamy, S.; Beguin, F. Electrochemical storage of lithium in multiwalled carbon nanotubes. Carbon 1999, 37, 61–69. [Google Scholar] [CrossRef]
- Kurt, R.; Bonard, J.-M.; Karimi, A. Morphology and field emission properties of nano-structured nitrogenated carbon films produced by plasma enhanced hot filament CVD. Carbon 2001, 39, 1723–1730. [Google Scholar] [CrossRef]
- Kannan, A.M.; Kanagala, P.; Veedu, V. Development of carbon nanotubes based gas diffusion layers by in situ chemical vapor deposition process for proton exchange membrane fuel cells. J. Power Sour. 2009, 192, 297–303. [Google Scholar] [CrossRef]
- Sharma, T.; Reddy, A.L.M.; Chandra, T.S.; Ramaprabhu, S. Development of carbon nanotubes and nanofluids based microbial fuel cell. Inter. J. Hydrog. Energy 2008, 33, 6749–6754. [Google Scholar] [CrossRef]
- Chang, J.; Lee, J.-H.; Najeeb, C.K.; Nam, G.-H.; Lee, M.; Kim, J.-H. Area-selective growth of ZnO nanorod arrays on single-walled carbon nanotube patterns. Scr. Mater. 2010, 63, 520–523. [Google Scholar] [CrossRef]
- Lyu, S.C.; Zhang, Y.; Ruh, H.; Lee, H.J.; Lee, C.J. Synthesis of high-purity GaP nanowires using a vapor deposition method. Chem. Phys. Lett. 2003, 67, 717–722. [Google Scholar] [CrossRef]
- Hong, S.Y.; Tobias, G.; Al-Jamal, K.T.; Ballesteros, B.; Ali-Boucetta, H.; Lozano-Perez, S.; Nellist, P.D.; Sim, R.B.; Finucane, C.; Mather, S.J.; et al. Filled and glycosylated carbon nanotubes for in vivo radioemitter localization and imaging. Nat. Mater. 2010, 9, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Welsher, K.; Sherlock, S.P.; Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl. Acad. Sci. USA 2011, 108, 8943–8949. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Malhotra, M.; Shao, W.; Tomaro-Duchesneau, C.; Abbasi, S. Polymeric nanohybrids and functionalized carbon nanotubes as drug delivery carriers for cancer therapy. Adv. Drug Deliv. Rev. 2011, 63, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Arsawang, U.; Saengsawang, O.; Rungrotmongkol, T.; Sornmee, P.; Wittayanarakul, K.; Remsungnen, T.; Hannongbua, S. How do carbon nanotubes serve as carriers for gemcitabine transport in a drug delivery system? J. Mol. Graph. Model. 2011, 29, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Foldvari, M.; Bagonluri, M. Carbon nanotubes as functional excipients for nanomedicines: II. Drug delivery and biocompatibility issues. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Karchemski, F.; Zucker, D.; Barenholz, Y.; Regev, O. Carbon nanotubes-liposomes conjugate as a platform for drug delivery into cells. J. Control. Release 2012, 160, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, G.; Wang, X.; Zhang, J.; Chen, Y.; Shi, J.; Yang, H.; Hu, H.; Yang, S. Solvothermal synthesis of cobalt ferrite nanoparticles loaded on multiwalled carbon nanotubes for magnetic resonance imaging and drug delivery. Acta Biomater. 2011, 7, 3496–3504. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Lin, G.; Lu, Q.; Meng, L.; Shen, X.; Dong, L.; Fu, C.; Zhang, X. Targeted therapy of SMMC-7721 liver cancer in vitro and in vivo with carbon nanotubes based drug delivery system. J. Colloid Interface Sci. 2012, 365, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ju, H. Rapid detection of ssDNA and RNA using multi-walled carbon nanotubes modified screen-printed carbon electrode. Biosens. Bioelectron. 2005, 21, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Shakhmaeva, I.I.; Bulatov, E.R.; Bondar, O.V.; Saifullina, D.V.; Culha, M.; Rizvanov, A.A.; Abdullin, T.I. Binding and purification of plasmid DNA using multi-layered carbon nanotubes. J. Biotech. 2011, 152, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, K.; Singh, D.P.; Singh, S.K.; Dash, D.; Srivastava, O.N. Attachment of biomolecules (protein and DNA) to amino-functionalized carbon nanotubes. New Carbon Mater. 2009, 24, 301–306. [Google Scholar] [CrossRef]
- Krajcik, R.; Jung, A.; Hirsch, A.; Neuhuber, W.; Zolk, O. Functionalization of carbon nanotubes enables non-covalent binding and intracellular delivery of small interfering RNA for efficient knock-down of genes. Biochem. Biophys. Res. Commun. 2008, 369, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, W.; Jing, Q.; Zhu, L. Aqueous adsorption of aniline, phenol, and their substitutes by multi-walled carbon nanotubes. Environ. Sci. Technol. 2008, 42, 7931–7936. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.P.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Global Carbon Nanotubes Market—Industry Beckons. Available online: http://www.nanowerk.com/spotlight/spotid=23118.php (accessed on 28 January 2016).
- Fares, M.M.; Al-Shboul, A.M. Stimuli pH-responsive (N-vinylimidazole-coacryloylmorpholine) hydrogels; meso- and nano-porous scaffolds. J. Biomed. Mater. Res. Part A 2012, 100, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.M.; Othman, A.A. Smart pH-sensitive alternating copolymers of (methylacrylamide-hydroxyethylmethacrylate); kinetic and physical properties. J. Macromol. Sci. Part A 2009, 47, 61–70. [Google Scholar] [CrossRef]
- Fares, M.M.; El-Faqeeh, A.S. Thermal and thermoxidative degradations of starch and thermosensitive starch-g-BAM copolymers. J. Therm. Anal. Calorim. 2005, 82, 161–166. [Google Scholar] [CrossRef]
- Fares, M.M.; Othman, A.A. Lower critical solution temperature determination of smart, thermosensitive N-isopropylacrylamide-alt-2-hydroxyethyl methacrylate copolymers: Kinetics and physical properties. J. Appl. Polym. Sci. 2008, 110, 2815–2825. [Google Scholar] [CrossRef]
- Fares, M.M. Graft copolymerization onto Chitosan-II. Grafting of acrylic acid and hydrogel formation. J. Polym. Mater. 2003, 20, 75–82. [Google Scholar]
- Fares, M.M.; Abul-Haija, Y.; Assaf, S. Pectin grafted poly(vinylpyrrolidone); optimization and in vitro controllable theophylline drug release. J. Appl. Polym. Sci. 2010, 117, 1945–1954. [Google Scholar] [CrossRef]
- Fares, M.M.; Sheikh Salem, M.; Khanfar, M. Inulin and poly(acrylic acid) grafted inulin for dissolution enhancement and preliminary controlled release of poorly water-soluble irbesartan drug. Int. J. Pharm. 2011, 410, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.M.; Salem, M.S. Dissolution enhancement of curcumin via curcumin-prebiotic inulin nanoparticles. Drug Dev. Ind. Pharm. 2015, 41, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.; Fares, M.M.; Salem, M.S.; Qandil, A. Mesoporous silica based macromolecule for dissolution enhancement of Irbesartan drug using pre-adjusted pH method. Microporous Mesoporous Mater. 2013, 123, 22–28. [Google Scholar] [CrossRef]
- Fares, M.M.; Abu Al-Rub, F.A.; Kandah, M.; Allaboun, H. Environmentally-friendly copolymeric beads of chlorella vulgaris and poly(methacrylamide) gfted aligic acid di-block copolymers for biosorption of zinc ions. Polym. Int. 2013, 62, 1179–1186. [Google Scholar]
- Fares, M.M.; Tahboub, Y.R.; Khatatbeh, S.T.; Abul-Haija, Y.M. Eco-friendly, vascular shape and interpenetrating poly(acrylic acid) grafted pectin hydrogels; biosorption and desorption investigations. J. Polym. Environ 2011, 19, 431–439. [Google Scholar] [CrossRef]
- Fares, M.M.; Maayta, A.K.; Al-Mustafa, J.A. Synergistic corrosion inhibition of aluminum by poly(ethylene glycol) and ciprofloxacin in acidic Media. Adhes. Sci. Technol. 2013, 27, 2495–2506. [Google Scholar] [CrossRef]
- Fares, M.M.; Maayta, A.K.; Al-Qudah, M.M. Polysorbate-20 adsorption layers below and above critical micelle concentration; cloud point and inhibitory role investigations at the solid/liquid interfaces. Surf. Interface Anal. 2013, 45, 906–912. [Google Scholar] [CrossRef]
- Fares, M.M.; Maayta, A.K.; Al-Mustafa, J.A. Corrosion inhibition of iota-carrageenan natural polymer on aluminum in presence of zwitterion mediator in HCl media. Corros. Sci. 2012, 65, 223–230. [Google Scholar] [CrossRef]
- Fares, M.M.; Maayta, A.K.; Al-Qudah, M.M. Pectin as promising green inhibitor of aluminum in hydrochloric acid solution. Corros. Sci. 2012, 60, 112–117. [Google Scholar] [CrossRef]
- Wiltsey, C.; Christiani, T.; Williams, J.; Scaramazza, J.; van Sciver, C.; Toomer, K.; Sheehan, J.; Branda, A.; Nitzl, A.; England, E.; et al. Thermogelling bioadhesive scaffolds for intervertebral disk tissue engineering: Preliminary in vitro comparison of aldehyde-based versus alginate microparticle-mediated adhesion. Acta Biomater. 2015, 16, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.; Katsoyiannis, I.A. Arsenic removal using iron oxide loaded alginate beads. Ind. Eng. Chem. Res. 2002, 41, 6149–6155. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A. Removal of uranium from contaminated drinking water: A mini review of available treatment methods. Desalination Water Treat. 2013, 51, 2915–2925. [Google Scholar] [CrossRef]
- Rasheed, A.; Howe, J.Y.; Dadmun, M.D.; Britt, P.F. The efficiency of the oxidation of carbon nanofibers with various oxidizing agents. Carbon 2007, 45, 1072–1080. [Google Scholar] [CrossRef]
- Papajová, E.; Bujdos, M.; Chorvát, D.; Stach, M.; Lacík, I. Method for preparation of planar alginate hydrogels by external gelling using an aerosol of gelling solution. Carbohydr. Polym. 2012, 90, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liang, B.; Ogino, A.; Wang, X.; Nagatsu, M. Oxygen functionalization of multiwall carbon nanotubes by microwave-excited surface-wave plasma treatment. J. Phys. Chem. C 2009, 113, 7659–7665. [Google Scholar] [CrossRef]
- Scheibe, B.; Borowiak-Palen, E.; Kalenczuk, R.J. Oxidation and reduction of multiwalled carbon nanotubes—Preparation and characterization. Mater. Charact. 2010, 61, 185–191. [Google Scholar] [CrossRef]
- Zhao, C.; Ji, L.; Liu, H.; Hu, G.; Zhang, S.; Yang, M.; Yang, Z. Functionalized carbon nanotubes containing isocyanate groups. J. Solid State Chem. 2004, 177, 4394–4398. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Miralles, P.; Johnson, E.; Church, T.L.; Harris, A.T. Multiwalled carbon nanotubes in alfalfa and wheat: Toxicology and uptake. J. R. Soc. Interface 2012, 9, 3514–3527. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Ghosh, D.; Chen, W.; Pradhan, S.; Chang, X.; Chen, S. Nanosized carbon particles from natural gas soot. Chem. Mater. 2009, 21, 2803–2809. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaboun, H.; Fares, M.M.; Abu Al-Rub, F.A. Removal of Uranium and Associated Contaminants from Aqueous Solutions Using Functional Carbon Nanotubes-Sodium Alginate Conjugates. Minerals 2016, 6, 9. https://doi.org/10.3390/min6010009
Allaboun H, Fares MM, Abu Al-Rub FA. Removal of Uranium and Associated Contaminants from Aqueous Solutions Using Functional Carbon Nanotubes-Sodium Alginate Conjugates. Minerals. 2016; 6(1):9. https://doi.org/10.3390/min6010009
Chicago/Turabian StyleAllaboun, Hussein, Mohammad M. Fares, and Fahmi A. Abu Al-Rub. 2016. "Removal of Uranium and Associated Contaminants from Aqueous Solutions Using Functional Carbon Nanotubes-Sodium Alginate Conjugates" Minerals 6, no. 1: 9. https://doi.org/10.3390/min6010009
APA StyleAllaboun, H., Fares, M. M., & Abu Al-Rub, F. A. (2016). Removal of Uranium and Associated Contaminants from Aqueous Solutions Using Functional Carbon Nanotubes-Sodium Alginate Conjugates. Minerals, 6(1), 9. https://doi.org/10.3390/min6010009






