The Distribution, Character, and Rhenium Content of Molybdenite in the Aitik Cu-Au-Ag-(Mo) Deposit and Its Southern Extension in the Northern Norrbotten Ore District, Northern Sweden
Abstract
:1. Introduction
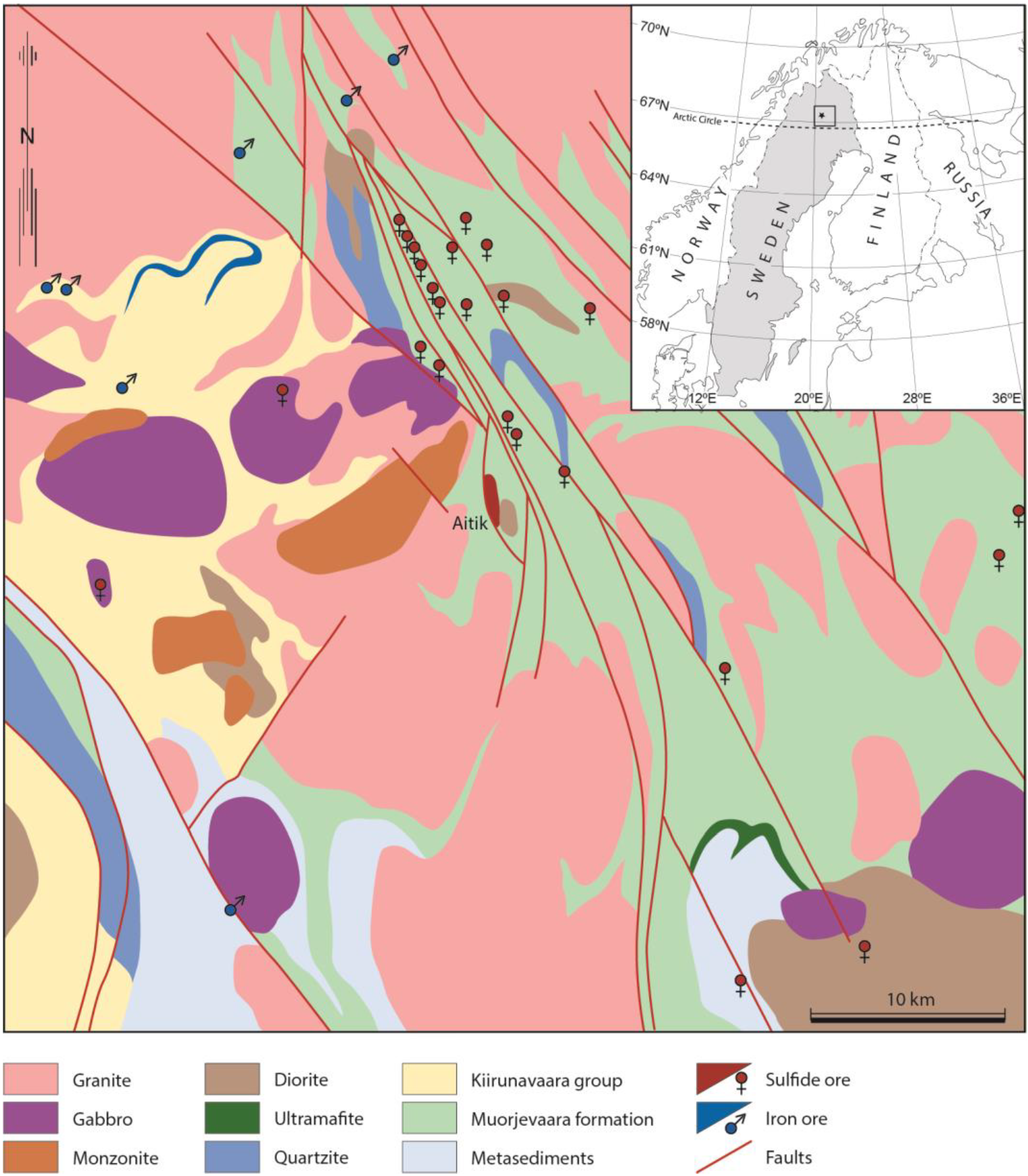

2. Geology
Deposit Geology
3. Sampling and Methods
3.1. QEMSCAN Analysis
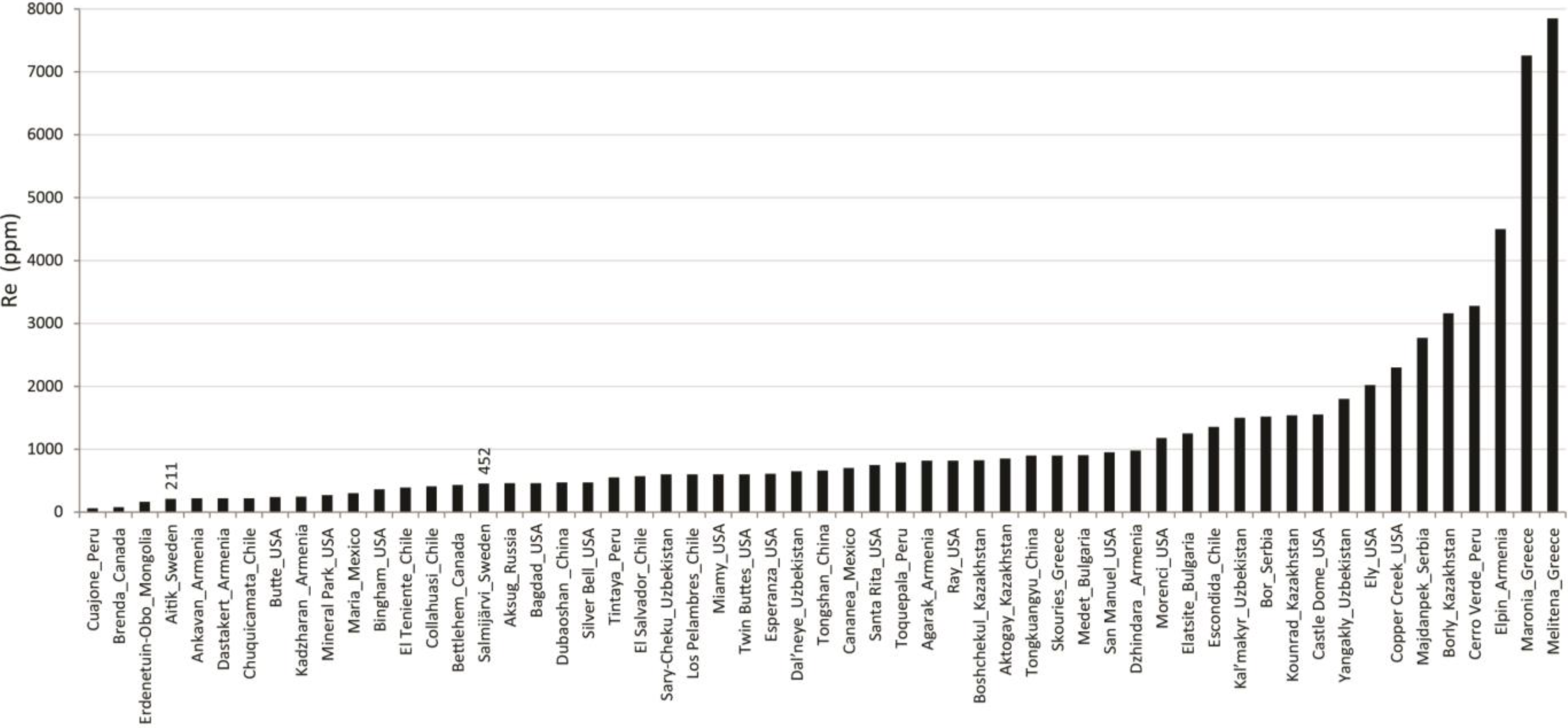
3.2. Molybdenite Re Abundance Determination
3.3. Laboratory Flotation Tests
4. Results
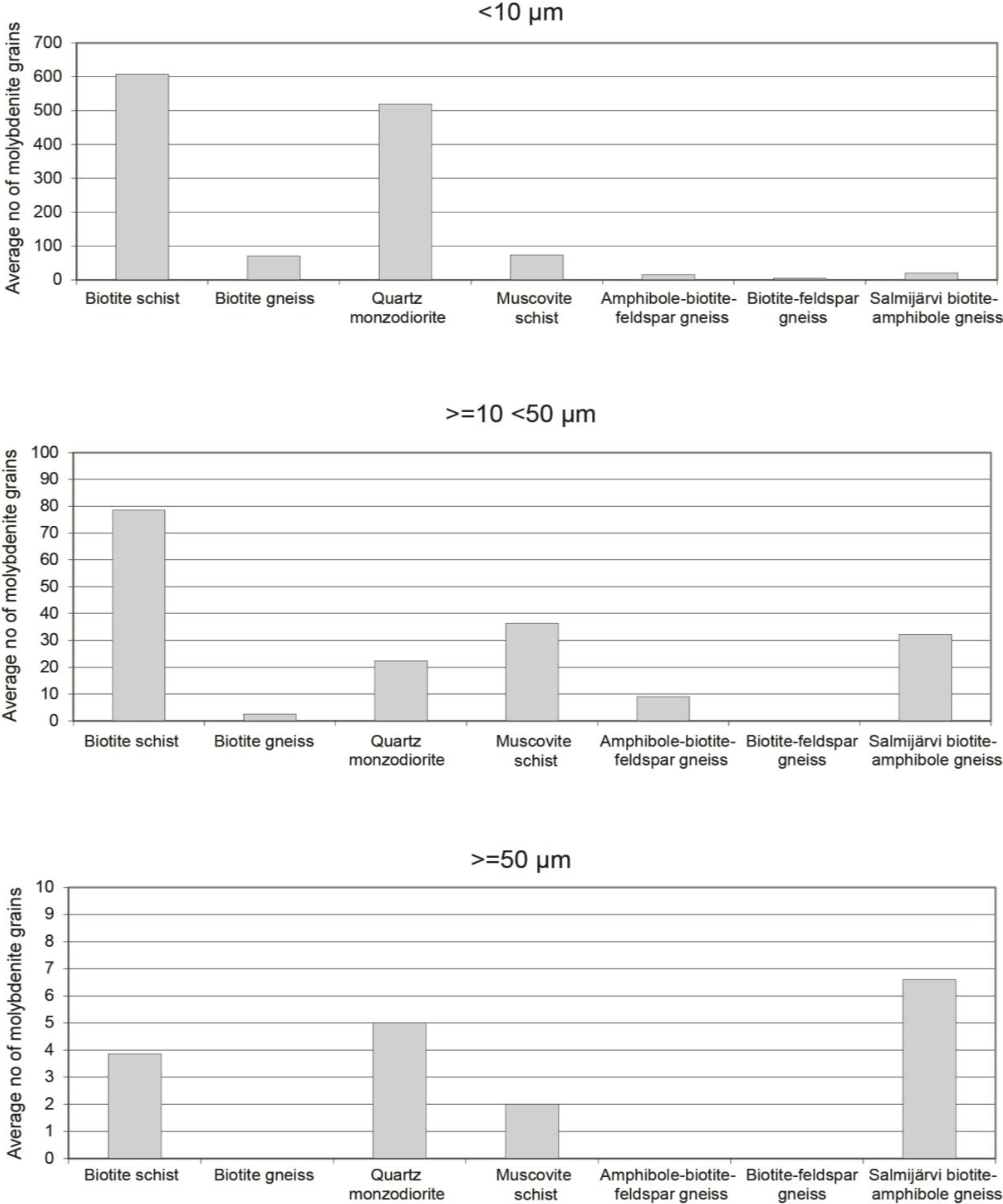
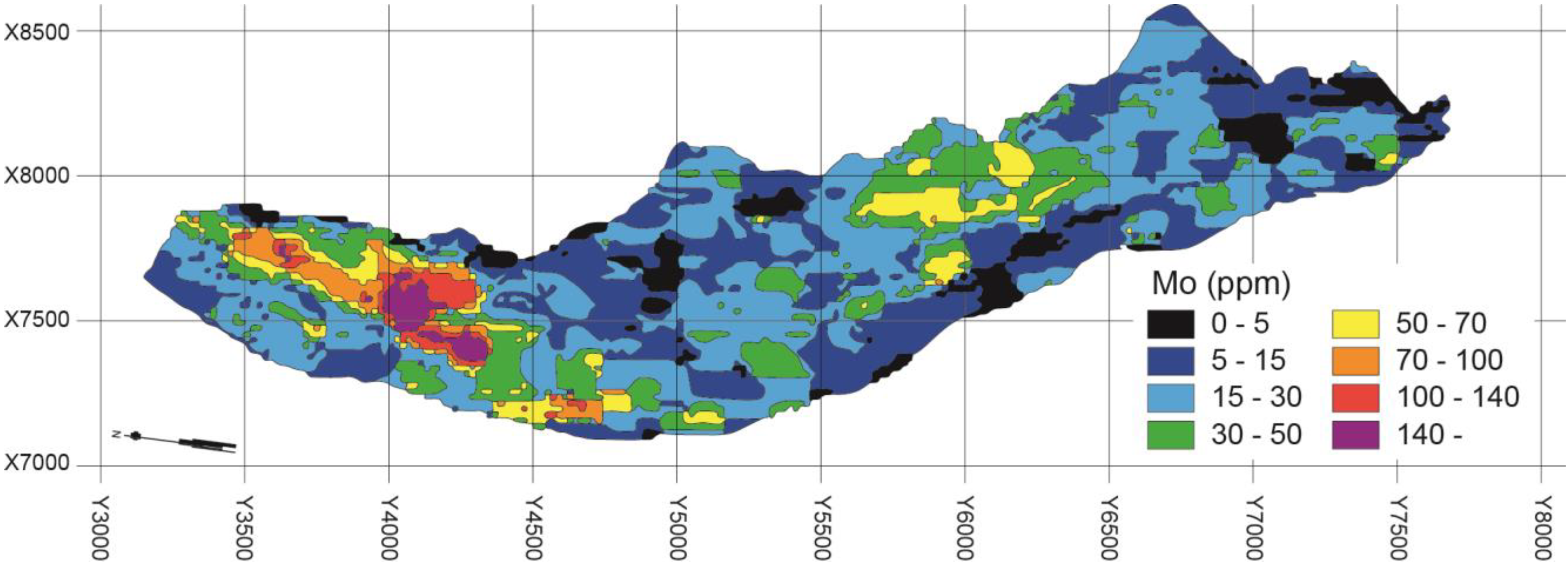
4.1. QEMSCAN Analysis of the Distribution and Character of Molybdenite


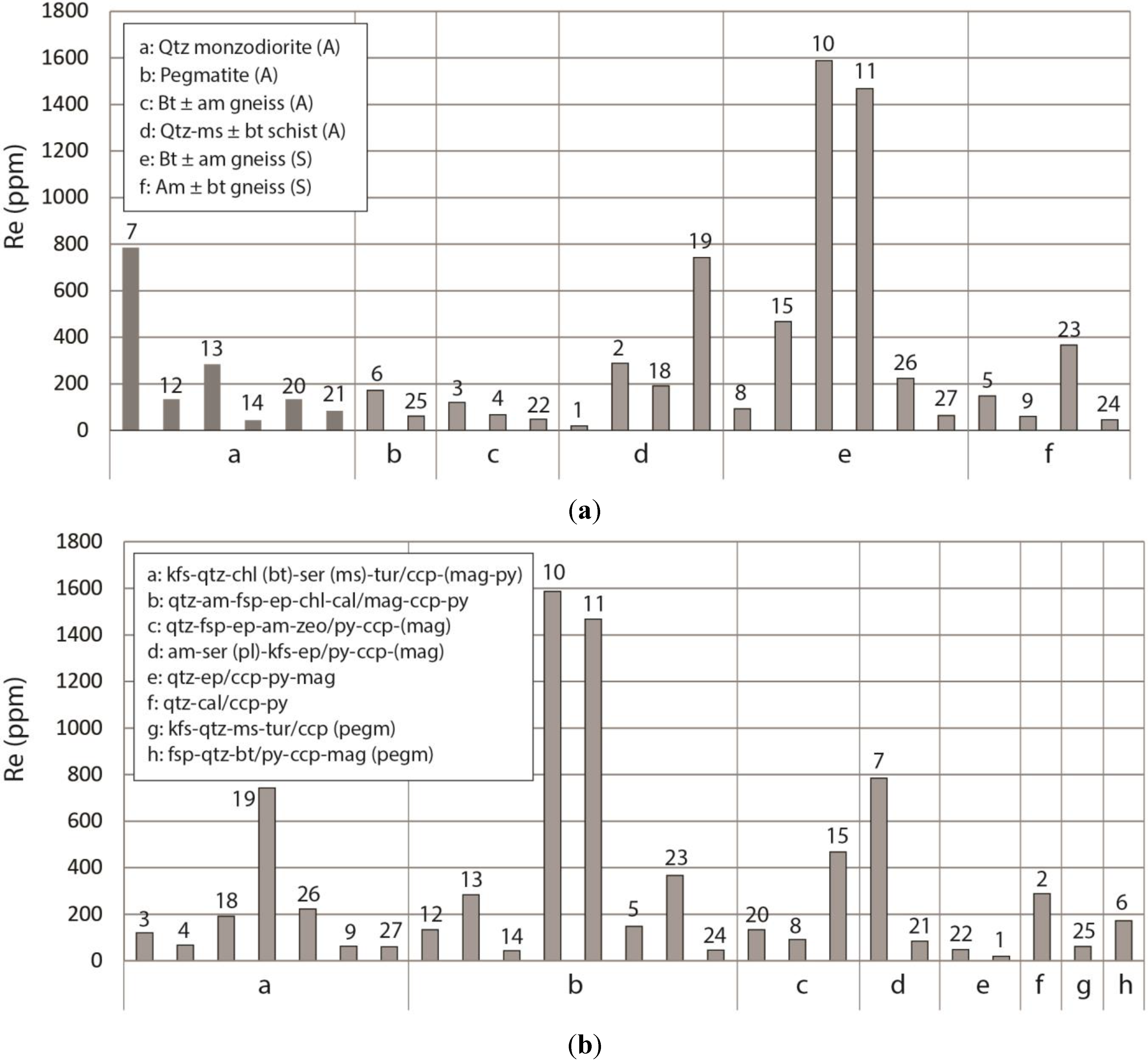
4.2. Rhenium Abundance of Molybdenite
| Sample code (Mo) | Host rock | Sample description | Sample code (Re) | MoS2 weight (mg) | Calc Re abundance (ppm) |
|---|---|---|---|---|---|
| Agm806-61.6 | Qtz-ms schist | Qtz-ep-alteration with rich dissem of ccp, py, mag, and with scattered Mo grains assoc with matrix-qtz | 1 | 0.012 | 20 ± 1 |
| Agm1041-111.5 | Qtz-ms schist | 4 m wide qtz-(cal) vein. Mo, ccp, py scattered in qtz | 2 | 0.037 | 289 ± 5 |
| Am969-493.0 | Bt gneiss | 1m wide vein of qtz, fsp, bt, ms, chl, tur, and few ccp grains. Mo between fsp and bt-ms-chl clot | 3 | 0.041 | 120 ± 2 |
| Bt gneiss | 1m wide vein of qtz, fsp, bt, ms, chl, tur, and few ccp grains. Mo within bt-ms-chl clot | 4 | 0.018 | 67 ± 2 | |
| Sgm1021-119.5 | Am-(bt) gneiss | Sporadic am-fsp-qtz-cal-chl-mag-py-(ccp)-clots with Mo. Mo assoc with am-fsp | 5 | 0.054 | 148 ± 2 |
| P-EW | Pegmatite in bt-fsp-am gneiss | 6 cm wide, undeformed, E-W trending (cutting main foliation). Composed of fsp-qtz-(bt) with accessory py, ccp, mag, Mo. Mo assoc with fsp-qtz | 6 | 0.026 | 172 ± 4 |
| Am1042-542.3 | Qtz monzo-diorite | 5 m section of strong ser-ep-am-alteration with dissem py, ccp, (mag). Mo assoc with ser (pl)-ep-am | 7 | 0.058 | 784 ± 8 |
| Sm1022-140.8 | Bt-(am) gneiss | 5 cm wide qtz-fsp-ep-zeo-vein rich in mag-py-ccp and with inclusions of ccp and Mo in coarse py | 8 | 0.010 | 92 ± 5 |
| Bt-(am) gneiss | 5 cm wide qtz-fsp-ep-zeo-vein rich in mag-py-ccp and with inclusions of ccp and Mo in coarse py | 15 | 0.005 | 467 ± 53 | |
| Sm1012-128.9 | Am-bt gneiss | Qtz-fsp-altered with 3 mm wide kfs-qtz-vein containing sparse ccp, mag, Mo. Mo assoc with kfs | 9 | 0.006 | 60 ± 6 |
| Sm1022-138.3 | Bt-(am) gneiss | 1 dm of qtz-am-ep-mag-ccp-py-alteration rich in fine grained Mo. Mo assoc with py | 10 | 0.037 | 1587 ± 25 |
| Bt-(am) gneiss | 1 dm of qtz-am-ep-mag-ccp-py-alteration rich in fine grained Mo. Mo assoc with am-qtz and intergrown with ccp | 11 | 0.086 | 1468 ± 119 | |
| Am947-352.0 | Qtz monzo-diorite | Am-ep-qtz-alteration with mag-py-ccp dissem Mo assoc with ep-(am-qtz-ccp) | 12 | 0.006 | 133 ± 13 |
| Qtz monzo-diorite | Am-ep-qtz-alteration with mag-py-ccp dissem Mo assoc with qtz-(am-ep-py) | 13 | 0.034 | 284 ± 5 | |
| Qtz monzo-diorite | Am-ep-qtz-alteration with mag-py-ccp dissem Mo assoc with am-(ep-qtz-ccp) | 14 | 0.002 | 43 ± 13 | |
| Am652-518.4 | Bt-ms schist | Qtz-kfs-ser-(tur)-alteration with a weak dissem of ccp-(py,mag), sporadic cm-wide qtz-fsp-veins (brittle, drusy), and a rich Mo-impreg. Mo assoc with qtz-kfs-ser | 18 | 0.007 | 191 ± 15 |
| Bt-ms schist | Qtz-kfs-ser-(tur)-alteration with a weak dissem of ccp-(py,mag), sporadic 0.5 cm wide qtz-fsp-veins (brittle, drusy), and a rich Mo-impreg. Mo assoc with qtz-kfs-ser | 19 | 0.018 | 742 ± 23 | |
| Agm751-246.7 | Qtz monzo-diorite | 3 cm wide am-zeo-ccp-py-Mo-bearing qtz-clot. Mo assoc with am-qtz-zeo-ccp | 20 | 0.018 | 133 ± 4 |
| Am947-356.9 | Qtz monzo-diorite | Am-kfs-(ser)-alteration with ccp, py, Mo. Mo assoc with am-(kfs) | 21 | 0.001 | 84 ± 49 |
| Agm878-436.6 | Bt-(am) schist | 0.5 cm wide qtz-ccp-py-mag-Mo-vein. Mo grain situated between qtz and py | 22 | 0.005 | 48 ± 6 |
| Sgm1021-153.2 | Am-bt gneiss | 1 dm am-qtz-ccp-py-mag-clot with Mo. Mo assoc with am-(ccp?) | 23 | 0.001 | 367 ± 208 |
| Am-bt gneiss | 1 dm am-qtz-ccp-py-mag-clot with Mo. Mo assoc with am-(mag?) | 24 | 0.003 | 46 ± 9 | |
| P-NS | Pegmatite in ms schist | Folded, N-S trending (parallel to main foliation). Composed of kfs-qtz-ms-(tur) with accessory ccp, Mo. Mo assoc with coarsegrained ms and kfs | 25 | 0.088 | 61 ± 0.4 |
| Sg1025-112.4 | Bt gneiss | Kfs-qtz-chl-alteration with dissem and intergrown ccp, mag, py, dissem Mo, and ccp-mag-bearing qtz-veins. Mo assoc with kfs in matrix | 26 | 0.039 | 223 ± 3 |
| Bt gneiss | Kfs-qtz-chl-alteration with dissem and intergrown ccp, mag, py, dissem Mo, and ccp-mag-bearing qtz-veins. Mo assoc with bt in matrix | 27 | 0.013 | 63 ± 3 |
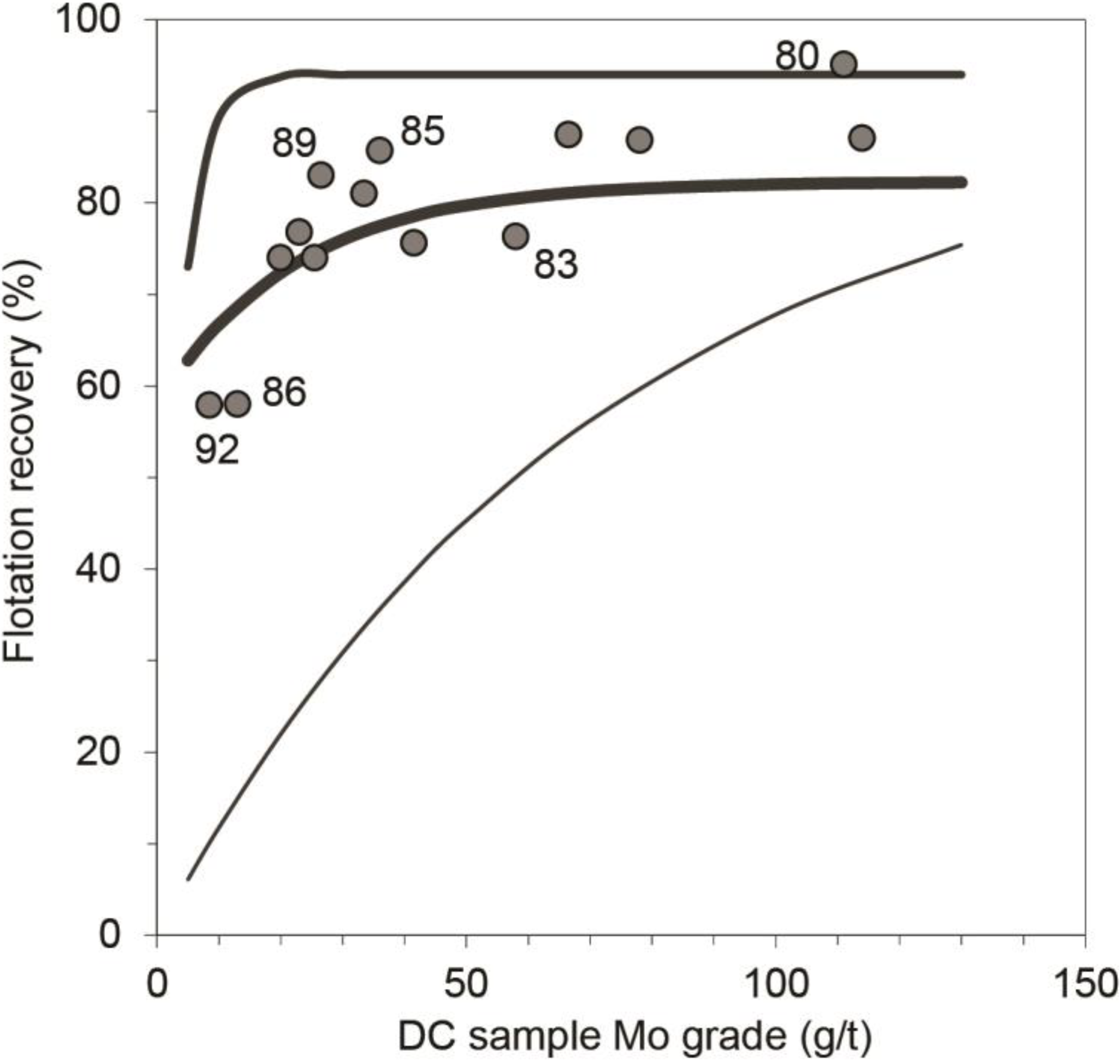
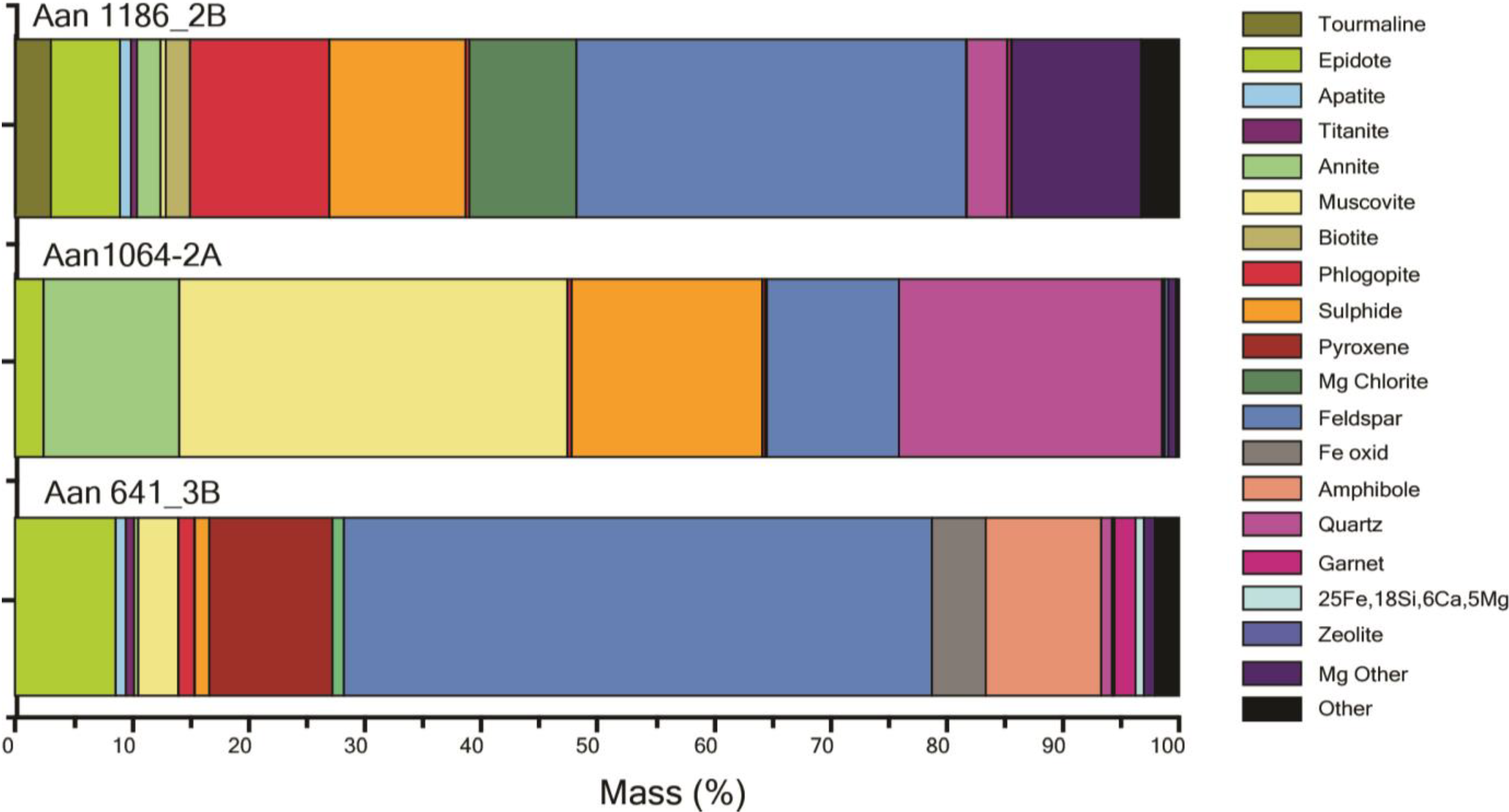
4.3. Molybdenum Flotation
| Sample | Mo DC-grade g/t | Mo recovery difference | Sample description | |
|---|---|---|---|---|
| Name | TMP No. | |||
| Aan953-1B | S-6780 | 111 | 13% | Slightly am-qtz-ep-altered qtz-monzodiorite with ccp-py-mag, ccp sometimes intergrown with py |
| Aan1055-3A | S-6785 | 36 | 8% | Unaltered bt gneiss with ccp-mag |
| Aan641-3B | S-6789 | 26 | 8% | Strongly ep-chl-ttn-ser-altered am-fsp-bt gneiss with mag-py-(ccp), ccp often as inclusions in py, which is bordered by mag |
| Aan1110-1B | S-6781 | 66 | 6% | Strongly kf-ser-chl-altered qtz-monzodiorite with thin qtz-veins and ccp-mag-py, commonly intergrown with each other |
| Aan953-1A | S-6779 | 78 | 5% | Slightly ser-altered qtz-monzodiorite with ccp-mag-py, ccp commonly intergrown with mag |
| Aan1080-3C | S-6791 | 114 | 5% | Slightly qtz-tur-altered bt gneiss with ccp-py-mag |
| Aan990-3C | S-6790 | 33 | 4% | Slightly kfs-altered bt gneiss with rich ccp-py-po dissemination |
| Aan1045-3B | S-6787 | 23 | 3% | Strongly kfs-qtz-ep-tur-altered bt schist with po-ccp-(py) |
| Aan1064-2A | S-6782 | 20 | 2% | Moderately grt-mag-qtz-altered ms-(bt) schist with mag-ccp-py |
| Aan1064-3B | S-6788 | 26 | −1% | Moderately am-ep-qtz-altered bt schist with py-po-ccp |
| Aan1069-2B | S-6784 | 42 | −3% | Moderately chl-qtz-ser-altered ms-bt-qtz schist with py-ccp-po, ccp intergrown with po |
| Aan1186-2B | S-6783 | 58 | −4% | Strongly ser-chl (1) and kfs-qtz-ep (2) altered ser-bt-chl schist with py-ccp |
| Aan1084-4 | S-6792 | 8 | −7% | Strongly ser-chl-kfs-qtz-altered fsp-bt gneiss with py-ccp-mag, intergrown py + ccp commonly rimmed by mag |
| Aan732-3A | S-6786 | 13 | −11% | Moderately qtz-altered bt gneiss with mag-py-ccp |
5. Discussion
Processing Implications
Mo Extraction
Re Extraction
| Product | Weight ktonne | Grade Cu | [%] Mo | Distribution Cu | [%] Mo |
|---|---|---|---|---|---|
| Ore feed | 36,000 | 0.25 | 0.0025 | 100 | 100 |
| Cu conc. | 324 | 25 | 0.2479 | 90 | 85 |
| Mo conc. | 0.849 | 1.6 | 53 | 0.02 | 50 |
| Cu final conc. | 323 | 25.06 | 0.1093 | 89.98 | 35 |
| Final tailing | 35,676 | 0.025 | 0.00027 | 10 | 15 |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fleischer, M. The geochemistry of rhenium, with special reference to its occurence in molybdenite. Econ. Geol. 1959, 54, 1406–1413. [Google Scholar] [CrossRef]
- Martinsson, O.; Wanhainen, C. Fe oxide and Cu-Au deposits in the northern Norrbotten ore district. In Proceedings of the 12th Biennial SGA Meeting, Uppsala, Sweden, 12–15 August 2013.
- Wanhainen, C.; Broman, C.; Martinsson, O.; Magnor, B. Modification of a Palaeoproterozoic porphyry system in northern Sweden: Integration of structural, geochemical, petrographic, and fluid inclusion data from the Aitik Cu-Au-Ag deposit, northern Sweden. Ore Geol. Rev. 2012, 48, 306–331. [Google Scholar] [CrossRef]
- Wanhainen, C.; Kontturi, M.; Martinsson, O. Copper and gold distribution at the Aitik deposit, Gällivare area, northern Sweden. Appl. Earth Sci. Trans. Inst. Min. Metall. B 2003, 112, 260–267. [Google Scholar] [CrossRef]
- Sammelin-Kontturi, M.; Wanhainen, C.; Martinsson, O. Gold mineralogy at the Aitik Cu-Au-Ag deposit, Gällivare area, northern Sweden. GFF 2011, 133, 1–12. [Google Scholar] [CrossRef]
- Jakobsson, P.; Joslin, G.; Knipfer, S.; Nordin, R.; Wasström, A.; Wanhainen, C. The Aitik porphyry Cu-Au-Ag-(Mo) deposit in Sweden. In Proceedings of the Eleventh Biennal SGA Meeting, Antofagasta, Chile, 26–29 September 2011; pp. 346–347.
- Wanhainen, C.; Billström, K.; Martinsson, O. Age, petrology and geochemistry of the porphyritic Aitik intrusion, and its relation to the disseminated Aitik Cu-Au-Ag deposit, Northern Sweden. GFF 2006, 128, 273–286. [Google Scholar] [CrossRef]
- Wanhainen, C.; Martinsson, O. Geochemical characteristics of host rocks to the Aitik Cu-Au deposit, Gällivare area, northern Sweden. In Proceeding of the Fifth Biennial SGA Meeting and the Tenth Quadrennial IAGOD Meeting, London, UK, 22–25 August 1999; pp. 1443–1446.
- Wanhainen, C.; Billström, K.; Martinsson, O.; Stein, H.; Nordin, R. 160 Ma of magmatic/hydrothermal and metamorphic activity in the Gällivare area: Re-Os dating of molybdenite and U-Pb dating of titanite from the Aitik Cu-Au-Ag deposit, northern Sweden. Miner. Deposita 2005, 40, 435–447. [Google Scholar] [CrossRef]
- Wanhainen, C.; Johansson, B. Character of gold within the Aitik Ore body: Preliminary results from a geometallurgical study. In Proceedings of the Conference in Minerals Engineering, Luleå University of Technology, Luleå, Sweden, 5–6 February 2008; pp. 143–150.
- Monro, D. The Geology and Genesis of the Aitik Copper-Gold Deposit, Arctic Sweden. Ph.D. Thesis, University of Wales, College of Cardiff, Cardiff, United Kingdom, 1988. [Google Scholar]
- Nordin, R. Geologisk beskrivning. In Ansökan om Bearbetningskoncession Aitik K nr 4; Gällivare Kommun: Norrbottens län, Sweden, 2005; p. 7. (In Swedish) [Google Scholar]
- Sarlus, Z. Geology of the Salmijärvi Cu-Au Deposit. Master’s Thesis, Luleå University of Technology, Luleå, Sweden, 2012; p. 75. [Google Scholar]
- Stein, H.; Markey, R.; Hannah, J.; Schersten, A. The remarkable Re-Os Chronometer in molybdenite: How and why it works. Terra Nova 2001, 13, 479–486. [Google Scholar] [CrossRef]
- Kosler, J.; Simonetti, A.; Sylvester, P.; Cox, R.; Tubrett, M.N.; Wilton, D. Laser ablation ICP-MS measurements of Re/Os in molybdenites and implications for Re-Os geochronology. Can. Mineral. 2003, 41, 307–320. [Google Scholar] [CrossRef]
- Selby, D.; Creaser, R. Macroscale NTIMS and microscale LA-MC-ICP-MS Re-Os isotopic analysis of molybdenite: Testing spatial restrictions for reliable Re-Os age determinations, and implications for the decoupling of Re and Os within molybdenite. Geochim. Cosmochim. Acta 2004, 68, 3897–3908. [Google Scholar] [CrossRef]
- Charlier, B.A.; Ginibre, C.; Morgan, D.; Nowell, G.M.; Pearson, D.G.; Davidson, J.P.; Ottley, C.J. Methods for the microsampling and high-precision analysis of strontium and rubidium isotopes at single crystal scale for petrological and geochronological applications. Chem. Geol. 2006, 232, 114–133. [Google Scholar] [CrossRef]
- Siivola, J.; Schmid, R. Recommendations by the IUGS Subcommission on the Systematics of Metamorphic Rocks: List of Mineral Abbreviations. IUGS Commission on the Systematics in Petrology. Available online: http://www.bgs.ac.uk/scmr/docs/papers/paper_12.pdf (accessed on 24 November 2014).
- Sinclair, W.D. Porphyry Deposits. Geol. Surv. Can. Spec. Publ. 2007, 5, 223–243. [Google Scholar]
- Kirkham, R.V.; Sinclair, W.D. Porphyry copper, gold, molybdenum, tungsten, tin, silver. In Geology of Canadian Mineral Deposit Types; Eckstrand, O.R., Sinclair, W.D., Thorpe, R.I., Eds.; Geological Survey of Canada: Ottawa, ON, Canada, 1995; pp. 421–446. [Google Scholar]
- Singer, D.A.; Berger, V.I.; Moring, B.C. Porphyry Copper Deposits of the World: Database and Grade and Tonnage Models; US Geological Survey Open-File Report; US Geological Survey: Reston, VA, USA, 2008; p. 45. [Google Scholar]
- Berzina, A.; Sotnikov, V.I.; Economou-Eliopoulos, M.; Eliopoulos, D.G. Distribution of rhenium in molybdenite from porphyry Cu-Mo and Mo-Cu deposits of Russia (Siberia) and Mongolia. Ore Geol. Rev. 2005, 26, 91–113. [Google Scholar] [CrossRef]
- Millensifer, T.A.; Sinclair, D.; Jonasson, I.; Lipmann, A. Chapter 14: Rhenium. In Critical Metals Handbook, 1st ed.; Gunn, G., Ed.; Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 340–360. [Google Scholar]
- Giles, D.L.; Shilling, J.H. Variation in rhenium content of molybdenite. In Proceedings of the 24th International Geological Congress Section, Montreal, QC, Canada, 21–29 August 1972; pp. 145–152.
- Sinclair, W.D.; Jonasson, I.R.; Kirkham, R.V.; Soregaroli, A.E. Rhenium and Other Platinum-Group Metals in Porphyry Deposits; Geological Survey of Canada Open-File; Geological Survey of Canada: Ottawa, ON, Canada, 2009. [Google Scholar]
- Bergman, S.; Kubler, L.; Martinsson, O. Description of Regional Geological and Geophysical Maps of Northern Norrbotten County; Sveriges Geologiska Undersökning: Uppsala, Sweden, 2001; pp. 5–100. [Google Scholar]
- Stein, H. Low-rhenium molybdenite by metamorphism in northern Sweden: Recognition, genesis, and global implications. Lithos 2006, 87, 300–327. [Google Scholar] [CrossRef]
- Zweifel, H. Aitik: Geological Documentation of a Disseminated Copper Deposit—A Preliminary Investigation; Sveriges Geologiska Undersökning: Uppsala, Sweden, 1976. [Google Scholar]
- Sillitoe, R. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Seo, J.; Guillong, M.; Heinrich, C. Separation of Molybdenum and Copper in Porphyry Deposits: The Roles of Sulfur, Redox, and pH in Ore Mineral Deposition at Bingham Canyon. Econ. Geol. 2012, 107, 333–356. [Google Scholar] [CrossRef]
- Vokes, F.M.; Spry, P.G.; Marshall, B. Ores and metamorphism: Introduction and historical perspectives. Rev. Econ. Geol. 2000, 11, 1–18. [Google Scholar]
- Marshall, B.; Gilligan, L.B. An introduction to remobilisation: Information from orebody geometry and experimental considerations. Ore Geol. Rev. 1987, 2, 87–131. [Google Scholar] [CrossRef]
- Kirkham, R.V.; Pilote, P.; Sinclair, W.D.; Robert, F.; Daigneault, R. Merrill Island Cu-Au veins and Clark Lake Cu-(Mo) porphyry deposit, Doré Lake mining camp, Chibougamau. In Geology and Metallogeny of the Chapais-Chibougamau Mining District: A New Vision of the Discovery Potential: Proceedings of the Chapais-Chibougamau 1998 Symposium; Pilote, P., Ed.; Quebec Ministry of Communications: Quebec City, QC, Canada, 1998; pp. 85–92. [Google Scholar]
- Aminzadeh, B.; Shahabpour, J.; Maghami, M. Variation of Rhenium Contents in Molybdenites from the Sar Cheshmeh Porphyry Cu-Mo Deposit in Iran. Res. Geol. 2011, 61, 290–295. [Google Scholar]
- Xiong, Y.; Wood, S. Experimental determination of the hydrothermal solubility of ReS2 and the Re–ReO2 buffer assemblage and transport of rhenium under supercritical conditions. Geochem. Trans. 2002, 3, 1–10. [Google Scholar] [CrossRef]
- Voudouris, P.C.; Melfos, V.; Spry, P.G.; Bindi, L.; Kartal, T.; Arikas, K.; Moritz, R.; Ortelli, M. Rhenium-rich molybdenite and rheniite in the Pagoni Rachi Mo-Cu-Te-Ag-Au prospect, Northern Greece: Implications for the Re Geochemistry of porphyry style Cu-Mo and Mo mineralization. Can. Mineral. 2009, 47, 1013–1036. [Google Scholar] [CrossRef]
- Selby, D.; Creaser, R. Re-Os Geochronology and systematic in molybdenite from the Endako porphyry molybdenum deposit, British Columbia, Canada. Econ. Geol. 2001, 96, 197–204. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Kelson, C.R.; Guerin, R.; Kalleske, N.; Danyushevsky, L. Trace element heterogeneity in molybdenite fingerprints stages of mineralization. Chem. Geol. 2013, 347, 175–189. [Google Scholar] [CrossRef]
- Naumov, A. Rhythms of Rhenium. Russ. J. Non-Ferr. Met. 2007, 48, 418–423. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Zou, X.; Zhang, L.; Liu, S. Extraction of molybdenum from molybdenite concentrates with hydrometallurgical processing. JOM 2012, 64, 1285–1289. [Google Scholar] [CrossRef]
- Lan, X.; Liang, S.; Song, Y. Recovery of rhenium from molybdenite calcine by a resine-in-pulp process. Hydrometallurgy 2006, 82, 133–136. [Google Scholar] [CrossRef]
- Fatai, I. Purification of Molybdenite Concentrates. Master’s Thesis, Luleå University of Technology, Luleå, Sweden, 2008; p. 89. [Google Scholar]
- Bulatovic, S.M. Handbook of Flotation Reagents—Chemistry, Theory and Practice: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Triffett, B.; Veloo, C.; Adair, B.; Badshaw, D. An investigation of the factors affecting the recovery of molybdenite in the Kennecott Utah Copper blulk flotation circuit. Miner. Eng. 2008, 21, 832–840. [Google Scholar] [CrossRef]
- Zanin, M.; Ametov, I.; Grano, S.; Zhou, L.; Skinner, W. A study of mechanisms affecting molybdenite recovery in a bulk copper/molybdenum flotation circuit. Int. J. Miner. Process. 2009, 93, 256–266. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanhainen, C.; Nigatu, W.; Selby, D.; McLeod, C.L.; Nordin, R.; Bolin, N.-J. The Distribution, Character, and Rhenium Content of Molybdenite in the Aitik Cu-Au-Ag-(Mo) Deposit and Its Southern Extension in the Northern Norrbotten Ore District, Northern Sweden. Minerals 2014, 4, 788-814. https://doi.org/10.3390/min4040788
Wanhainen C, Nigatu W, Selby D, McLeod CL, Nordin R, Bolin N-J. The Distribution, Character, and Rhenium Content of Molybdenite in the Aitik Cu-Au-Ag-(Mo) Deposit and Its Southern Extension in the Northern Norrbotten Ore District, Northern Sweden. Minerals. 2014; 4(4):788-814. https://doi.org/10.3390/min4040788
Chicago/Turabian StyleWanhainen, Christina, Wondowossen Nigatu, David Selby, Claire L. McLeod, Roger Nordin, and Nils-Johan Bolin. 2014. "The Distribution, Character, and Rhenium Content of Molybdenite in the Aitik Cu-Au-Ag-(Mo) Deposit and Its Southern Extension in the Northern Norrbotten Ore District, Northern Sweden" Minerals 4, no. 4: 788-814. https://doi.org/10.3390/min4040788




