Potential Occurrence of Accessory Minerals in the Lower Mantle
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
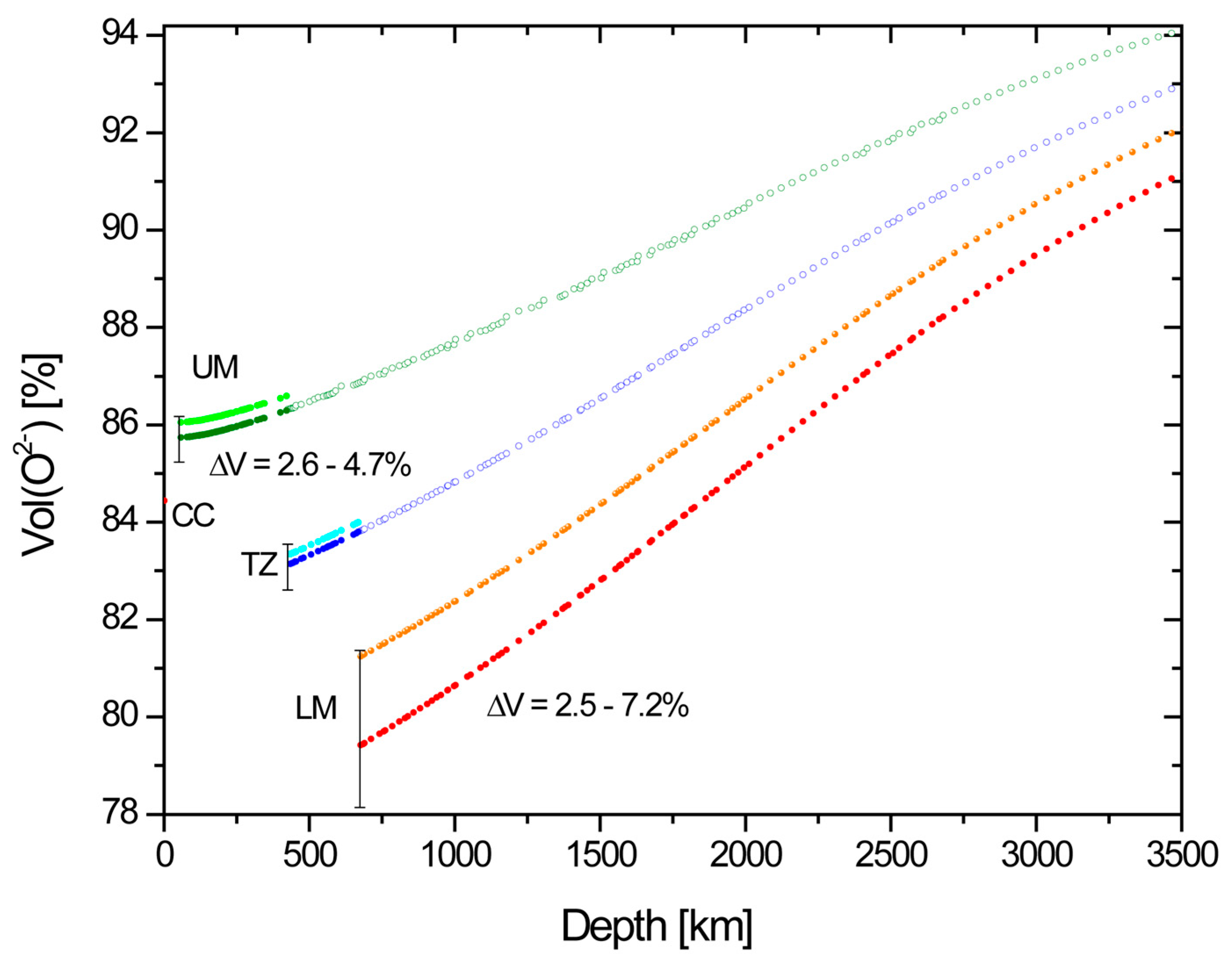
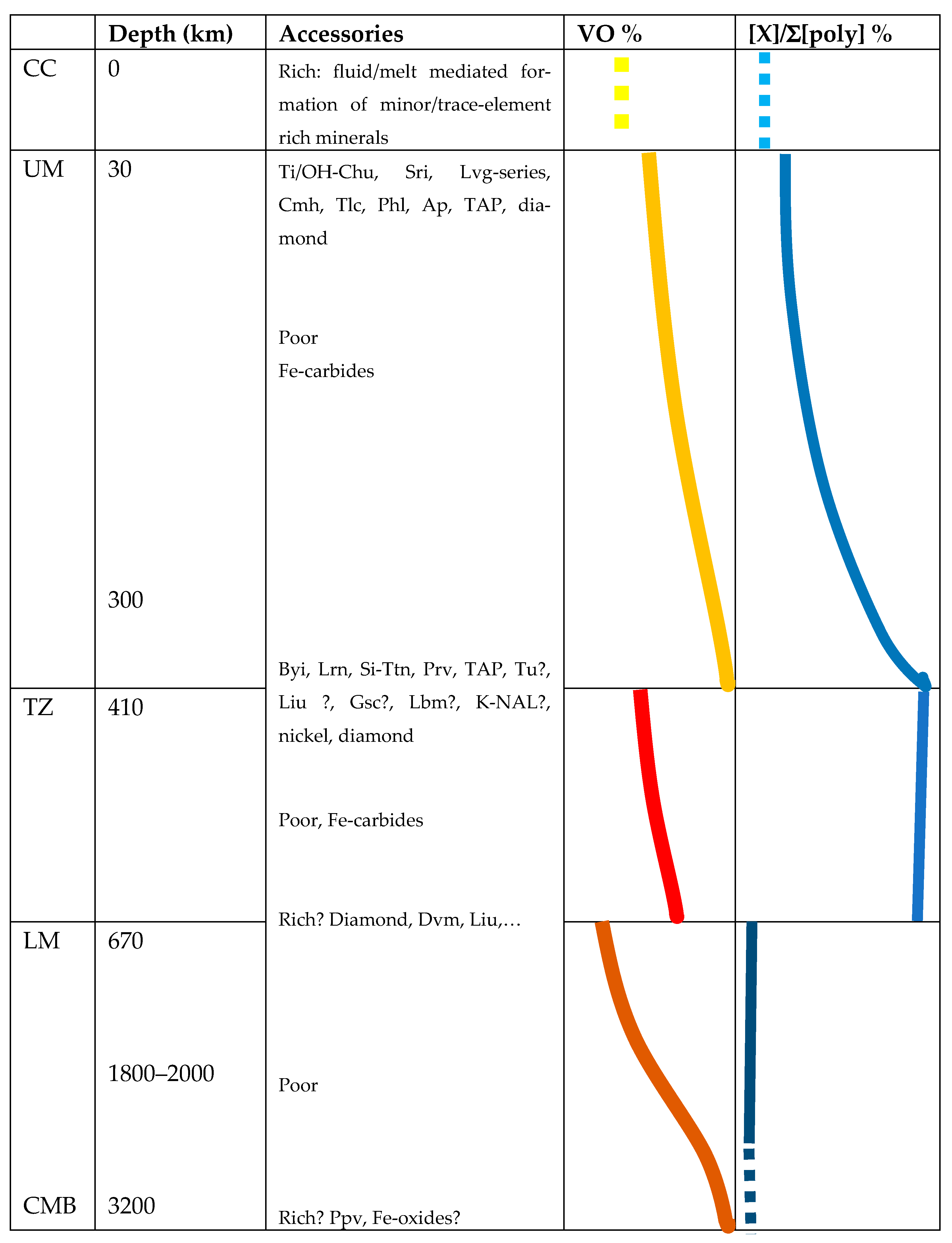
- VO, the volume fraction of O2− ions of the total ionic volume of BSE, increases monotonously with pressure for each of the three mantle zones, such that in extrapolation from the top to the bottom of the whole mantle, VO gains 6-7% to 91.0 ± 1.0% (Figure 1).
- VO drops at the UM-TZ boundary by between 2.6 and 4.7% and at the TZ-LM boundary by another 2.5–7.2%. These values compare to a 5.1 and 9.6% increase in rock density at the 420 and 670 km discontinuities [4]. Thus, the reduction in VO is a substantial component of the densification at both discontinuities but at 670 km the change in cation coordination accounts for at least a third of the density increase. The enstatite chondrite model [6] remains within uncertainties for UM and TZ, but for the LM, it gives values of VO slightly higher than the initial assessment of uncertainties, which therefore are adjusted to encompass the EC model.
- The comparison between the results for pyrolitic and enstatite chondritic mantle shows that differences between upper and lower mantle affect VO, which, however, does not respond sensitively to compositional differences less marked than those between these two models, at least not with the given uncertainties.
4.1. Geophysical Implications
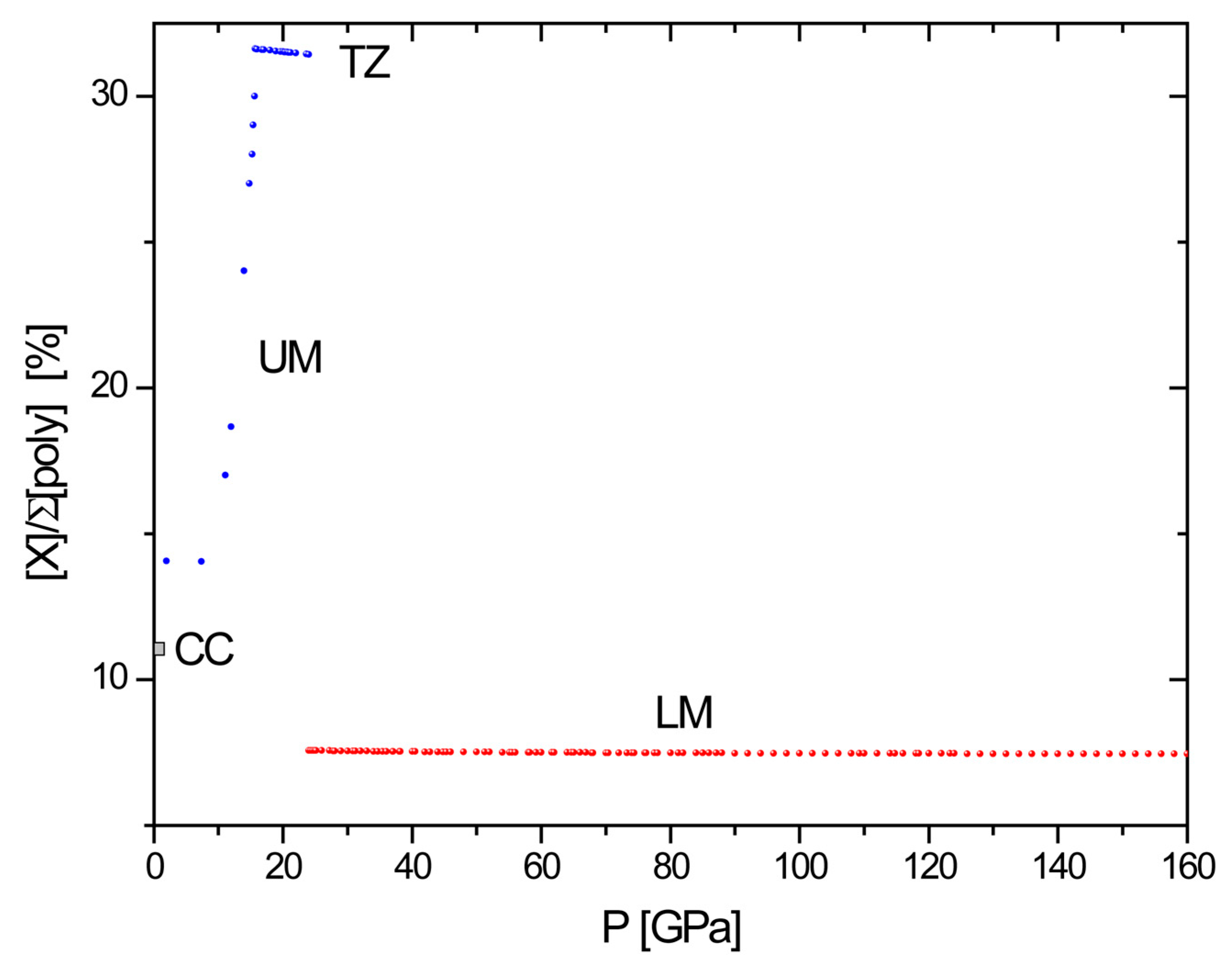
4.2. Geochemical Implications
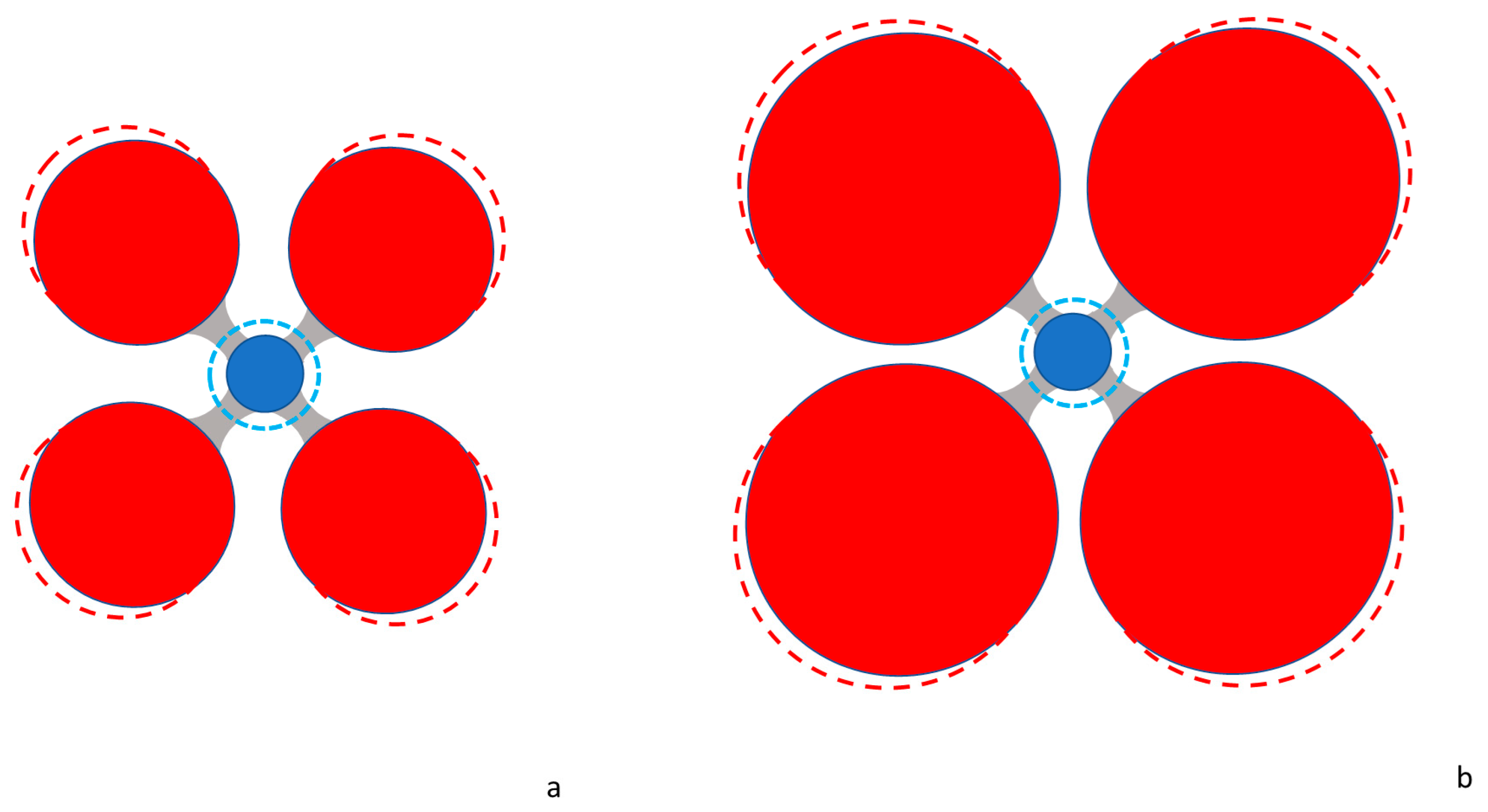
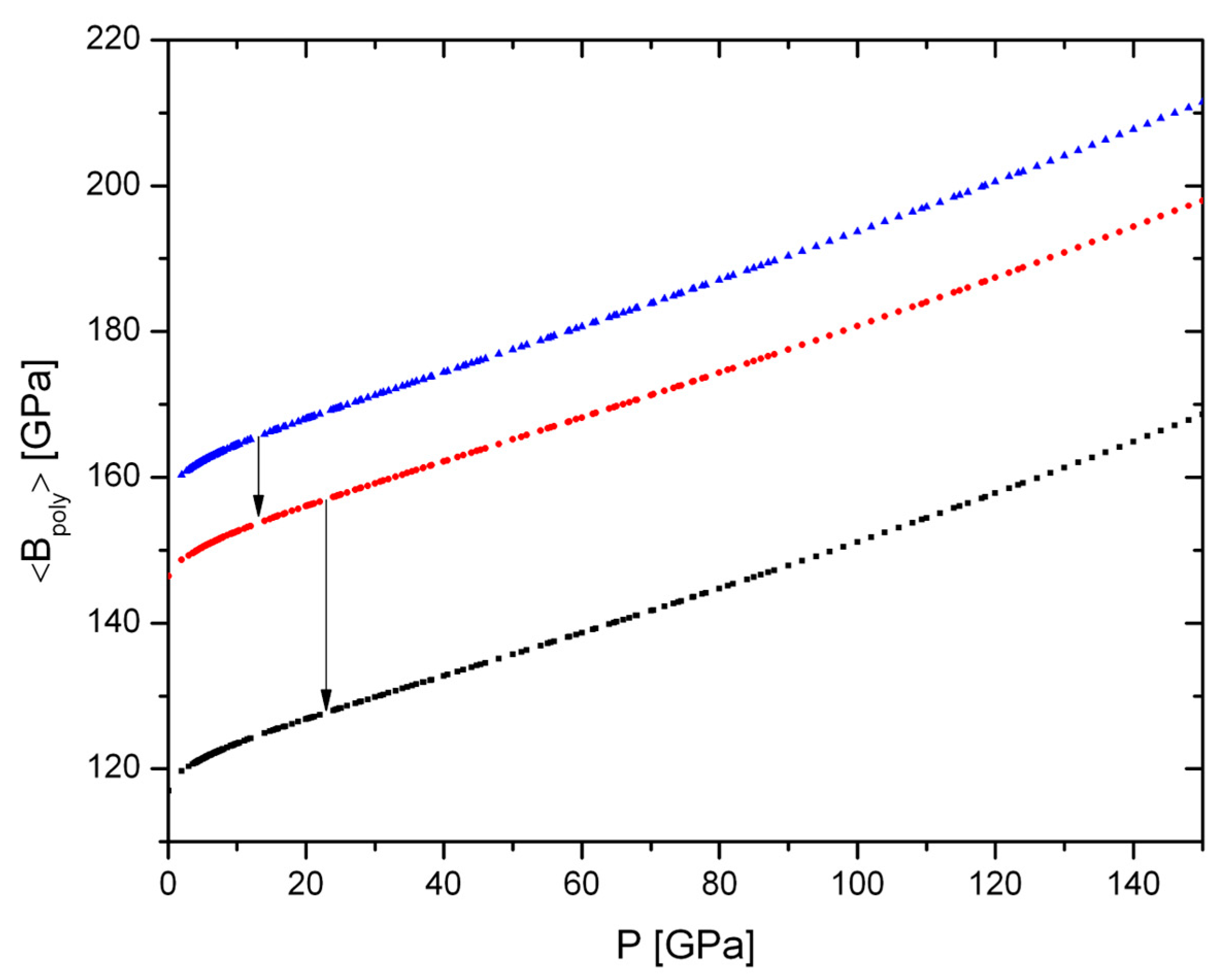
Geochemical Implications–Polyhedral Volumes
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goldschmidt, V.M. Geochemische Verteilungsgesetze und kosmische Häufigkeit der Elemente. Die Naturwiss. 1930, 49, 1001–1013. [Google Scholar]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Die Naturwiss. 1926, 21, 477–485. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-s. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Dziewonski, A.; Anderson, D.L. Preliminary Reference Earth Model. Phys. Earth Planet. Int. 1981, 25, 297–356. [Google Scholar] [CrossRef]
- Ringwood, A.E. Phase transformations and their bearing on the constitution and dynamics of the mantle. Geochim. Cosmochim. Acta 1991, 55, 2083–2110. [Google Scholar] [CrossRef]
- Javoy, M.; Kaminski, E.; Guyot, F.; Andrault, D.; Sanloup, C.; Moreira, M.; Labrosse, S.; Jambon, A.; Agrinier, P.; Davaille, A.; et al. The chemical composition of the Earth: Enstatite chondrite models. Earth Planet. Sci. Lett. 2010, 293, 259–268. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Rudnick, R.L., Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 3, pp. 1–64, 659p. ISBN 0-08-043751-6. [Google Scholar]
- Shannon, R.D. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Tschauner, O. Pressure-Dependent Crystal Radii. Solids 2023, 4, 235–253. [Google Scholar] [CrossRef]
- Gibbs, G.V.; Cox, D.F.; Ross, N.L. The incompressibility of atoms at high pressures. Am. Min. 2020, 105, 1761–1768. [Google Scholar] [CrossRef]
- Tschauner, O. Systematics of Crystalline Oxide and Framework Compression. Crystals 2024, 14, 140. [Google Scholar] [CrossRef]
- Rahm, M.; Cammi, R.; Ashcroft, N.W.; Hoffmann, R. Squeezing All Elements in the Periodic Table: Electron Configuration and Electronegativity of the Atoms under Compression. J. Am. Chem. Soc. 2019, 141, 10253–10271. [Google Scholar] [CrossRef]
- Tschauner, O. Towards an Extended Concept of Tolerance Factors for Postspinel Phases. Minerals 2025, 15, 309. [Google Scholar] [CrossRef]
- Holzapfel, W.B. Equations of state for solids under strong compression. High Press. Res. 1998, 16, 81–126. [Google Scholar] [CrossRef]
- Prewitt, C.T.; Downs, R.T. High-pressure crystal chemistry. In Ultrahigh-Pressure Mineralogy: Physics and Chemistry of the Earth’s Deep Interior; Hemley, R.J., Ed.; Mineralogical Society of America: Washington, DC, USA, 1998; Volume 37, pp. 283–317. [Google Scholar]
- Sturhahn, W.; Jackson, J.M.; Lin, J.F. The spin state of iron in minerals of Earth’s lower mantle. Geophys. Res. Lett. 2005, 32, L12307. [Google Scholar] [CrossRef]
- Hirose, K.; Labrosse, S.; Hernlund, J. Composition and State of the Core. Ann. Rev. Earth Planet. Sci. 2013, 41, 657–691. [Google Scholar] [CrossRef]
- Hirose, K.; Shimizu, N.; van Westrenen, W.; Fei, Y. Trace element partitioning in Earth’s lower mantle and implications for geochemical consequences of partial melting at the core–mantle boundary. Phys. Earth Planet Inter. 2004, 146, 249–260. [Google Scholar] [CrossRef]
- Corgne, A.; Liebske, C.; Wood, B.J.; Rubie, D.C.; Frost, D.J. Silicate perovskite-melt partitioning of trace elements and geochemical signature of a deep perovskitic reservoir. Geochim. Cosmochim. Acta 2005, 69, 485–496. [Google Scholar] [CrossRef]
- Blundy, J.; Wood, B. Prediction of crystal-melt partition coefficients from elastic moduli. Nature 1994, 372, 452–454. [Google Scholar] [CrossRef]
- Nagasawa, H. Trace Element Partition Coefficient in Ionic Crystals. Science 1966, 152, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Hazen, R.M.; Finger, L.W. Bulk modulus—Volume relationship for cation-anion polyhedra. J. Geophys. Res. 1979, 84, 6723–6728. [Google Scholar] [CrossRef]
- Ma, C.; Tschauner, O.; Beckett, J.R.; Prakapenka, V.B. New high-pressure Fe-Ti oxide minerals in the Shergotty Martian meteorite: Feiite, Fe 2+2(Fe2+Ti4+)O5, liuite, FeTiO3, and tschaunerite, (Fe2+)( Fe2+Ti4+)O4. Meteor. Planet. Sci. 2025, 60, 375–391. [Google Scholar] [CrossRef]
- Meyer, N.A.; Wenz, M.D.; Walsh, J.P.S.; Jacobsen, S.D.; Locock, A.J.; Harris, J.W. Goldschmidtite, (K,REE,Sr)(Nb,Cr)O3: A new perovskite supergroup mineral found in diamond from Koffiefontein, South Africa. Am. Min. 2019, 104, 1345–1350. [Google Scholar] [CrossRef]
- Bercovici, D.; Karato, S. Whole-mantle convection and the transition-zone water filter. Nature 2003, 425, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tschauner, O.; Yang, S.; Humayun, M.; Liu, W.; Gilbert Corder, S.N.; Bechtel, H.A.; Tischler, J. HIMU Geochemical Signature Originating from the Transition Zone. Earth Planet. Sci. Lett. 2020, 542, 116323. [Google Scholar] [CrossRef]
| Pressure [GPa] | r(OIII) [Å] | r(OIV) [Å] |
|---|---|---|
| 0.1 | 1.21 | 1.24 |
| 0.5 | 1.209 | 1.237 |
| 1.0 | 1.208 | 1.235 |
| 2.0 | 1.206 | 1.233 |
| 3.0 | 1.205 | 1.231 |
| 4.0 | 1.203 | 1.230 |
| 5.0 | 1.202 | 1.229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tschauner, O. Potential Occurrence of Accessory Minerals in the Lower Mantle. Minerals 2026, 16, 9. https://doi.org/10.3390/min16010009
Tschauner O. Potential Occurrence of Accessory Minerals in the Lower Mantle. Minerals. 2026; 16(1):9. https://doi.org/10.3390/min16010009
Chicago/Turabian StyleTschauner, Oliver. 2026. "Potential Occurrence of Accessory Minerals in the Lower Mantle" Minerals 16, no. 1: 9. https://doi.org/10.3390/min16010009
APA StyleTschauner, O. (2026). Potential Occurrence of Accessory Minerals in the Lower Mantle. Minerals, 16(1), 9. https://doi.org/10.3390/min16010009





