Colour Stability of Light-Sensitive Minerals Under UVA340nm Irradiation: Implications for the Conservation of Cultural Heritage and Museum Display Conditions
Abstract
1. Introduction
- (1)
- Possibly reversible light-induced colour changes without any other physical or chemical alteration (e.g., the faded colour of blue celestine SrSO4 may return to its original colour if stored in the dark [16]).
- (2)
- Irreversible light-induced decompositions producing significant bulk physical or chemical changes. Good instances of this second class include red α-HgS (trigonal, cinnabar), where light causes a phase transition of its black polymorph β-HgS (cubic, metacinnabar) at room temperature [20]. Similarly, red–orange coloured realgar (AsS) converts permanently to yellow pararealgar (As4S4) at low temperature [21].
- (3)
- Irreversible light-accelerated surface reactions with air, moisture, and/or pollutants. Instances for this last class are vivianite (Fe2+3(PO4)2 8H2O) darkening with possible crystal decohesion upon light exposure [16].
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. XRD Analyses
2.2.2. SEM-EDS Analyses
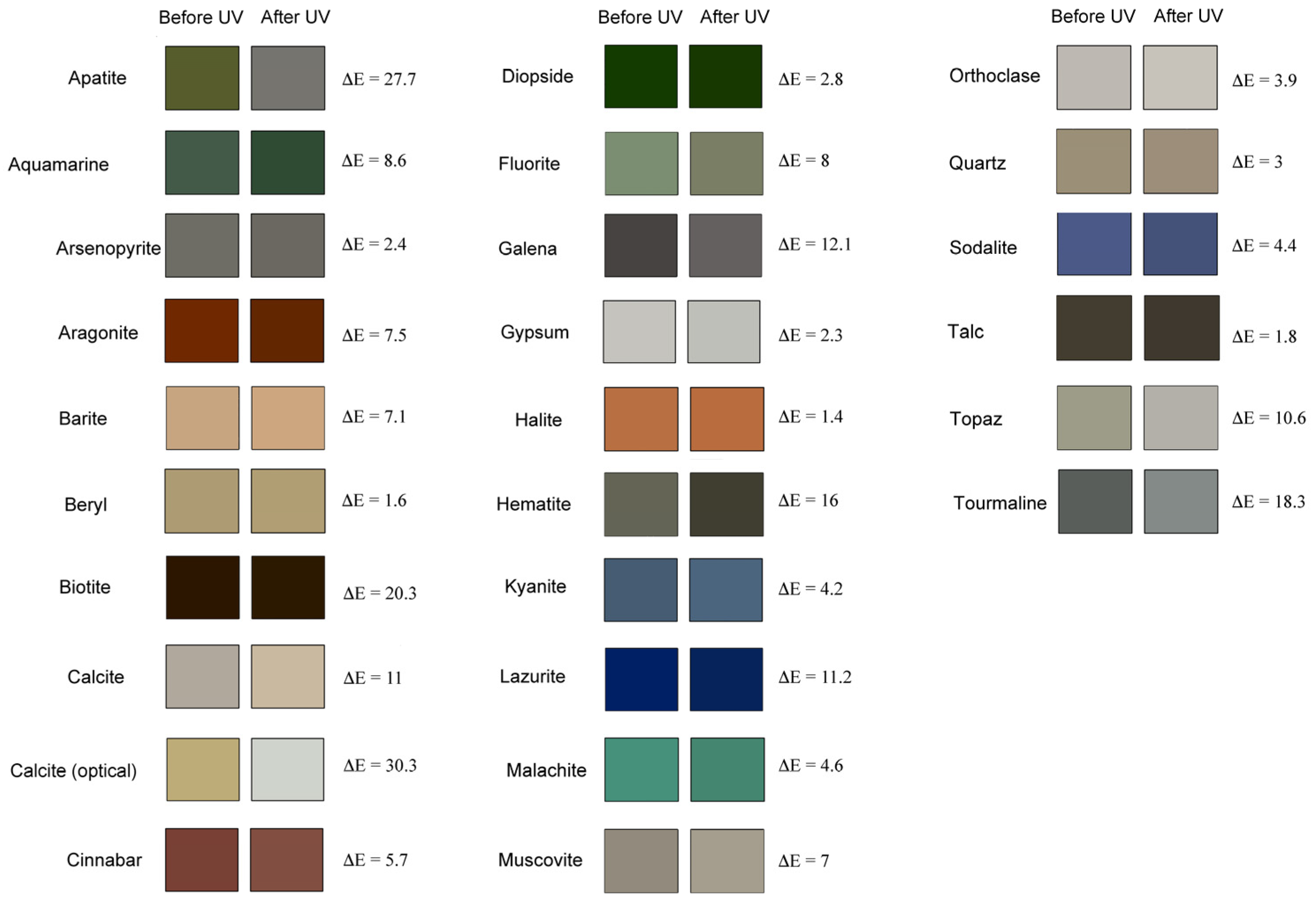
2.2.3. Colorimetric Analyses
2.2.4. UV Photoaging Tests
3. Results and Discussion
- (1)
- The particle size alters light scattering and absorption [34].Normally, when a mineral is ground into smaller particles, thus reducing its grain size distribution, its colour changes compared to the cohesive mineral, usually accompanied by an increase in lightness (L).
- (2)
- The crystal structure, which affects electronic transitions and interference patterns [35]. In some minerals, the colour variations could be due to the oxidation of organic matter, iron (from Fe+2 to Fe+3) and manganese (from Mn+2 to Mn+3).
- (3)
- The surface properties (roughness) influence reflectance and adsorption [36]
- (4)
- Anisotropy/orientation changes polarisation and rotation of reflected light [39].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISO 21348:2007; Space Environment (Natural and Artificial)-Process for Determining Solar Irradiances. ISO: Geneva, Switzerland, 2007.
- Kopp, G.; Lean, J.L. A New, Lower Value of Total Solar Irradiance: Evidence and Climate Significance. Geophys. Res. Lett. 2011, 38, 714. [Google Scholar] [CrossRef]
- El-Nouby Adam, M. Effect of The Atmosphere on UVB Radiation Reaching the Earth’s Surface: Dependence on Solar Zenith Angle. Atmos. Ocean. Sci. Lett. 2011, 4, 139–145. [Google Scholar] [CrossRef]
- Bennson, G. Handbook on Synchrotron Radiation. Opt. Acta Int. J. Opt. 1984, 31, 979–980. [Google Scholar] [CrossRef]
- Cogulet, A.; Blanchet, P.; Landry, V. Wood Degradation under UV Irradiation: A Lignin Characterization. J. Photochem. Photobiol. B Biol. 2016, 158, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, N.; Khanna, A.S. Effect of Methyltrimethoxy Silane Modification on Yellowing of Epoxy Coating on UV (B) Exposure. J. Coatings 2014, 2014, 22. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-Change and Yellowing of Polyurethane as a Result of UV Irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Careddu, N.; Marras, G. The Effects of Solar UV Radiation on the Gloss Values of Polished Stone Surfaces. Constr. Build. Mater. 2013, 49, 828–834. [Google Scholar] [CrossRef]
- Navarro, R.; Catarino, L.; Pereira, D.; Paulo, F.; Campos, D.S. Effect of UV Radiation on Chromatic Parameters in Serpentinites Used as Dimension Stones. Bull. Eng. Geol. Environ. 2019, 78, 5345–5355. [Google Scholar] [CrossRef]
- Sitzia, F.; Lisci, C.; Mirão, J. Accelerate Ageing on Building Stone Materials by Simulating Daily, Seasonal Thermo-Hygrometric Conditions and Solar Radiation of Csa Mediterranean Climate. Constr. Build. Mater. 2021, 266, 121009. [Google Scholar] [CrossRef]
- Sitzia, F.; Lisci, C.; Mirão, J. Building Pathology and Environment: Weathering and Decay of Stone Construction Materials Subjected to a Csa Mediterranean Climate Laboratory Simulation. Constr. Build. Mater. 2021, 300, 124311. [Google Scholar] [CrossRef]
- Virgil Lueth Light Sensitive Minerals. In Proceedings of the 39th Annual New Mexico Mineral Symposium, Socorro, New Mexico, 10–11 November 2018; pp. 34–35.
- Bringley, J.F.; Rajeswaran, M.; Olson, L.P.; Liebert, N.M. Silver-Halide/Organic-Composite Structures: Toward Materials with Multiple Photographic Functionalities. J. Solid State Chem. 2005, 178, 3074–3089. [Google Scholar] [CrossRef]
- Kirk, R.D. The Luminescence and Tenebrescence of Natural and Synthetic Sodalite. Am Min. 1955, 40, 22–31. [Google Scholar]
- Foord, E.E.; Berendsen, P.; Storey, L.O. Corderoite, First Natural Occurrence of Alpha -Hg//3s//2cl//2, From The Cordero Mercury Deposit, Humboldt County, Nevada; American Mineralogist: Chantilly, VA, USA, 1985; ISBN 0895204444. [Google Scholar]
- Nassau, K. Conserving Light-Sensitive Minerals and Gems. In The Care and Conservation of Geological Material: Minerals, Rocks, Meteorites and Lunar Finds; Routledge: Abingdon, Oxfordshire, UK, 2013; ISBN 9781135385149. [Google Scholar]
- Horak., J.M. Environmental Effects on Geological Material: Light Induced Changes of Minerals. In Conservation of Geological Collections; Child, R.E., Ed.; Archetype Publications: London, UK, 1994; pp. 23–30. ISBN 1873132603. [Google Scholar]
- Rossman, G.R. Colored Varieties of the Silica Minerals. In Silica: Physical Behavior, Geochemistry, and Materials Applications; de Gruyter: Berlin, Germany, 2019; ISBN 9781501509698. [Google Scholar]
- Howie, F.M. The Care and Conservation of Geological Material: Minerals, Rocks, Meteorites and Lunar Finds; Routledge: Abingdon, Oxfordshire, UK, 1992. [Google Scholar] [CrossRef]
- Ballirano, P.; Botticelli, M.; Maras, A. Thermal Behaviour of Cinnabar, α-HgS, and the Kinetics of the β-HgS (Metacinnabar)—α-HgS Conversion at Room Temperature. Eur. J. Mineral. 2014, 25, 957–965. [Google Scholar] [CrossRef]
- Douglass, D.L.; Shing, C.; Wang, G. The Light-Induced Alteration of Realgar to Pararealgar. Am. Mineral. 1992, 77, 1266–1274. [Google Scholar]
- Brunton, C.H.C.; Besterman, T.P.; Cooper, J.A. Guidelines for the Curation of Geological Material; The Geological Society: London, UK, 1985. [Google Scholar]
- Howie, F.M.P. Museum Climatology and the Conservation of Palaeontological Material. In Curation of Palaeontological Collections: Special Papers in Palaeontology; The Palaeontological Association: Durham, UK, 1979. [Google Scholar]
- Lisci, C.; Sitzia, F.; Pires, V.; Mirão, J. Building Stones Durability by UVA Radiation, Moisture and Spray Accelerated Weathering. J. Build. Pathol. Rehabil. 2022, 7, 60. [Google Scholar] [CrossRef]
- Dang, R.; Yuan, Y.; Luo, C.; Liu, J. Chromaticity Shifts Due to Light Exposure of Inorganic Pigments Used in Traditional Chinese Painting. Light. Res. Technol. 2017, 49, 818–828. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.; Dong, X.; Huang, L.; Zeng, H.; Lin, Z.; Zou, G. Corrugated 1D Hybrid Metal Halide [C6H7ClN]CdCl3Exhibiting Broadband White-Light Emission. Inorg. Chem. 2022, 61, 4752–4759. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Li, T.; Huang, F.; Li, Z.; Zhou, Y.; Lin, T.; Ouyang, Y.; Tao, X.; Pan, C. Highly-Efficient All-Inorganic Lead-Free 1D CsCu2I3 Single Crystal for White-Light Emitting Diodes and UV Photodetection. Nano Energy 2021, 81, 105570. [Google Scholar] [CrossRef]
- Worku, M.; Tian, Y.; Zhou, C.; Lee, S.; Meisner, Q.; Zhou, Y.; Ma, B. Sunlike White-Light-Emitting Diodes Based on Zero-Dimensional Organic Metal Halide Hybrids. ACS Appl. Mater. Interfaces 2018, 10, 30051–30057. [Google Scholar] [CrossRef] [PubMed]
- Dang, R.; Yuan, Y.; Liu, G.; Liu, J. Chromaticity Changes of Inorganic Pigments in Chinese Traditional Paintings Due to the Illumination of Frequently-Used Light Sources in Museum. Color Res. Appl. 2018, 43, 596–605. [Google Scholar] [CrossRef]
- Vitorino, T.; Picollo, M.; del Hoyo-Meléndez, J.M. An Investigation of the Photostability of 19th Century Artists’ Cochineal Lake Pigments Using MFT and Conventional Accelerated Ageing Tests. Nondestruct. Test. Eval. 2024, 39, 2530–2548. [Google Scholar] [CrossRef]
- Grandjean, F.; Samain, L.; Long, G.J. Characterization and Utilization of Prussian Blue and Its Pigments. Dalt. Trans. 2016, 45, 18018–18044. [Google Scholar] [CrossRef] [PubMed]
- Sitzia, F.; Moita, P.; Bottura-Scardina, S.; Lisci, C. Colour Fading and Changing in Light-Sensitive Minerals Exposed to UV Rays. In Proceedings of the Global Stone Congress 2023; Lopes, L., Peres, M., Marques, P., Eds.; University of Evora: Batalha, Portugal, 2023. [Google Scholar]
- ASTM E308-22: 2022; Standard Practice for Computing The Colors of Objects by Using the CIE System. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- Yang, X.L.; Wan, X.X. Analysis of the Spectral Reflectance and Color of Mineral Pigments Affected by Their Particle Size. Color Res. Appl. 2020, 45, 246–261. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, B.; Wang, X.; Wen, L.; Wang, A. Formation and Coloring Mechanism of Typical Aluminosilicate Clay Minerals for CoAl2O4 Hybrid Pigment Preparation. Front. Chem. 2018, 6, 125. [Google Scholar] [CrossRef]
- Lu, L.; Xu, B.; Zhang, Q.; Lu, T.; Farooq, U.; Chen, W.; Zhou, Q.; Qi, Z. Adsorption Features of Pigments onto Iron Oxides: Co-Effect of the Molecular Structure of Adsorbates and the Varied Surface Properties of Minerals. J. Mol. Liq. 2023, 388, 122766. [Google Scholar] [CrossRef]
- Sitzia, F.; Lisci, C.; Mirão, J. The Interaction between Rainwater and Polished Building Stones for Flooring and Cladding—Implications in Architecture. J. Build. Eng. 2022, 52, 104495. [Google Scholar] [CrossRef]
- Sitzia, F.; Lisci, C.; Pires, V.; Alves, T.; Mirão, J. Laboratorial Simulation for Assessing the Performance of Slates as Construction Materials in Cold Climates. Appl. Sci. 2023, 13, 2761. [Google Scholar] [CrossRef]
- Green, L.H. The Relationship between Polarization Colors and Rotation Properties of Anisotropic Minerals. Econ. Geol. 1952, 47, 451–458. [Google Scholar] [CrossRef]
- Witzel, R.F.; Burnham, R.W.; Onley, J.W. Threshold and Suprathreshold Perceptual Color Differences. J. Opt. Soc. Amer. 1973, 63, 615–625. [Google Scholar] [CrossRef]
- Moazed, C.; Overbey, R.; Spector, R.M. Alpha-Induced Coloration Reversal in Biotite. Nature 1977, 267, 818–819. [Google Scholar] [CrossRef]
- Waychunas, G.A. Apatite Luminescence. Rev. Mineral. Geochemistry 2002, 48, 701–742. [Google Scholar] [CrossRef]
- Jayaprakash, R.; Ratnam, V.V.; Durrani, S.A. Optical Bleaching of Radiation-Induced Colour Centres in Fluorapatite. Nucl. Tracks Radiat. Meas. 1985, 10, 601–604. [Google Scholar] [CrossRef]
- Liu, H.; Liao, L.; Pan, X.; Su, K.; Shuai, P.; Yan, Z.; Guo, Q.; Mei, L. Recent Research Progress of Luminescent Materials with Apatite Structure: A Review. Open Ceram. 2022, 10, 100251. [Google Scholar] [CrossRef]
- Anufrieva, A.V.; Andrienko, O.S.; Buynovskiy, A.S.; Makaseev, Y.N.; Mazov, I.N.; Nefedov, R.A.; Sachkov, V.I.; Stepanova, O.B.; Valkov, A.V. Rare Earth Elements Materials Production from Apatite Ores. IOP Conf. Ser. Mater. Sci. Eng. 2016, 112, 012002. [Google Scholar]
- Knutson, C.; Peacor, D.R.; Kelly, W.C. Luminescence, Color and Fission Track Zoning in Apatite Crystals of the Panasqueira Tin-Tungsten Deposit, Beira-Baixa, Portugal. Am. Mineral. 1985, 70, 829–837. [Google Scholar]
- Bačík, P.; Fridrichová, J.; Štubňa, J.; Bancík, T.; Illášová, L.; Pálková, H.; Škoda, R.; Mikuš, T.; Milovská, S.; Vaculovič, T.; et al. The Ree-Induced Absorption and Luminescence in Yellow Gem-Quality Durango-Type Hydroxylapatite from Muránska Dlhá Lúka, Slovakia. Minerals 2020, 10, 1001. [Google Scholar] [CrossRef]
- Clavé, E.; Beyssac, O.; Bernard, S.; Royer, C.; Lopez-Reyes, G.; Schröder, S.; Rammelkamp, K.; Forni, O.; Fau, A.; Cousin, A.; et al. Radiation-Induced Alteration of Apatite on the Surface of Mars: First in Situ Observations with SuperCam Raman Onboard Perseverance. Sci. Rep. 2024, 14, 11284. [Google Scholar] [CrossRef]
- Silva, T.P.; de Oliveira, D.; Veiga, J.P.; Lisboa, V.; Carvalho, J.; Barreiros, M.A.; Coutinho, M.L.; Salas-Colera, E.; Vigário, R. Contribution to the Understanding of the Colour Change in Bluish-Grey Limestones. Heritage 2022, 5, 1479–1503. [Google Scholar] [CrossRef]
- Kabacińska, Z.; Yate, L.; Wencka, M.; Krzyminiewski, R.; Tadyszak, K.; Coy, E. Nanoscale Effects of Radiation (UV, X-Ray, and γ) on Calcite Surfaces: Implications for Its Mechanical and Physico-Chemical Properties. J. Phys. Chem. C 2017, 121, 13357–13369. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, B.; Xu, T.; Tan, L.; Xin, W.; Du, J.; Bao, J. Efficient Synergistic Photocatalytic Degradation by Sunlight-Driven Natural Tourmaline Modified g-C3N4 Interface Electric Field. Sep. Purif. Technol. 2025, 361, 131496. [Google Scholar] [CrossRef]
- Tzeng, J.H.; Weng, C.H.; Lin, Y.H.; Huang, S.M.; Yen, L.T.; Anotai, J.; Lin, Y.T. Synthesis, Characterization, and Visible Light Induced Photoactivity of Tourmaline-N-TiO2 Composite for Photooxidation of Ethylene. J. Ind. Eng. Chem. 2019, 80, 376–384. [Google Scholar] [CrossRef]
- Vuori, S.; Colinet, P.; Norrbo, I.; Steininger, R.; Saarinen, T.; Palonen, H.; Paturi, P.; Rodrigues, L.C.V.; Göttlicher, J.; Le Bahers, T.; et al. Detection of X-Ray Doses with Color-Changing Hackmanites: Mechanism and Application. Adv. Opt. Mater. 2021, 9, 2100762. [Google Scholar] [CrossRef]
- Song, C.; Guo, Q.; Liu, Y.; Rao, Y.; Liao, L. Photochromism, UV-Vis, Vibrational and Fluorescence Spectroscopy of Differently Colored Hackmanite. Crystals 2023, 11, 1607. [Google Scholar] [CrossRef]
- Norrbo, I.; Curutchet, A.; Kuusisto, A.; Mäkelä, J.; Laukkanen, P.; Paturi, P.; Laihinen, T.; Sinkkonen, J.; Wetterskog, E.; Mamedov, F.; et al. Solar UV Index and UV Dose Determination with Photochromic Hackmanites: From the Assessment of the Fundamental Properties to the Device. Mater. Horizons 2018, 5, 569–576. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.C. The Kinetics of Carrier Trap Parameters in Na8Al6Si6O24(Cl,S)2 Hackmanite. Materials 2023, 16, 6776. [Google Scholar] [CrossRef]
- Rogers, J.J.W. Geochemistry of Solids. Geochim. Cosmochim. Acta 1964, 28, 10–11. [Google Scholar] [CrossRef]
- Dickson, F.W.; Tunell, G. The Stability Relations of Cinnabar and Metacinnabar. Am. Mineral. 1959, 44, 471–487. [Google Scholar]
- Atalaya, M. Preventive Conservation Applied to the Mineralogical Collection of the National Museum of Natural History and Science of the University of Lisbon; Universidade Nova de Lisboa: Lisbon, Portugal, 2020. [Google Scholar]
- Homem, P.M. Ferramentas Inovadoras Para Monitorização Ambiental e Avaliação de Danos Para Objectos Em Museus, Palácios, Arquivos e Bibliotecas: A Exposição Luminosa e Os Dosímetros LightCheck. Ciências E Técnicas Do Património 2006, V–VI, 225–240. [Google Scholar]

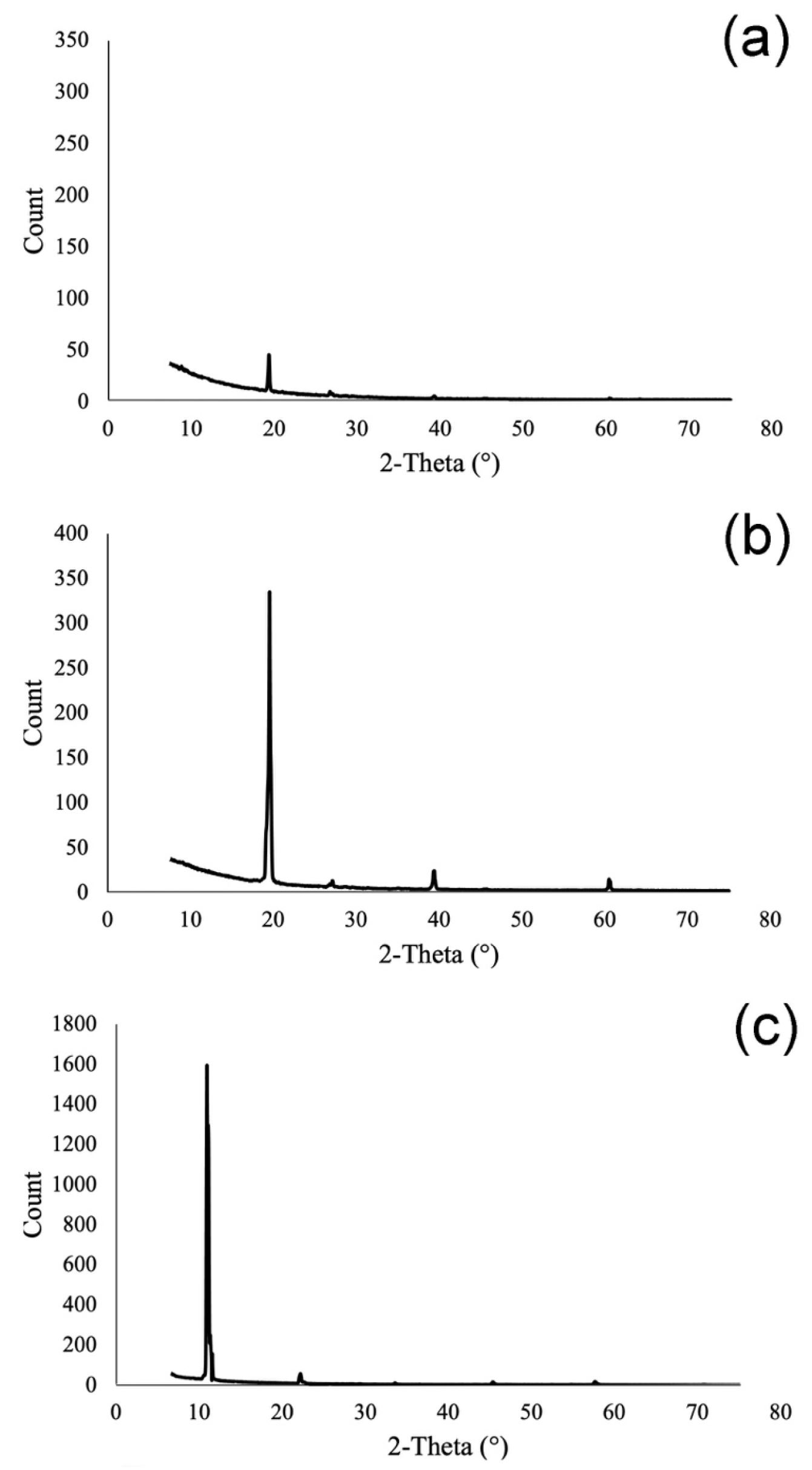
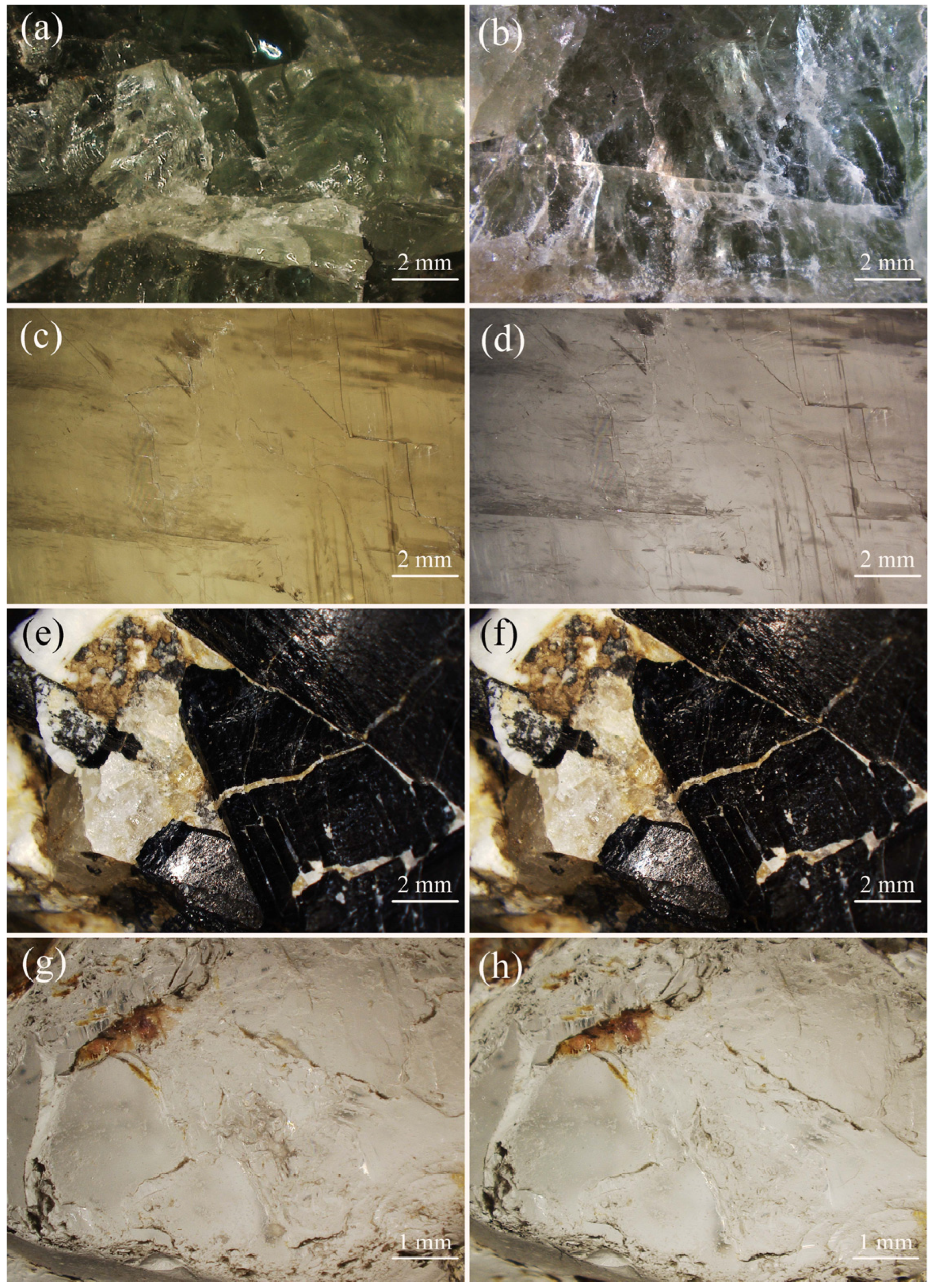

| Mineral | Chemical Formula | Nickel–Strunz Classification | Crystal System | Provenance |
|---|---|---|---|---|
| Apatite | Ca5(PO4)3(F,Cl,OH) | Phosphate (08.BN.05) | Hexagonal | Portugal |
| Aquamarine | Be3Al2Si6O18 | Cyclosilicate (9.CJ.05) | Hexagonal | Brazil |
| Arsenopyrite | (FeAs)S | Sulphide (02.EB.20) | Monoclinic | Portugal |
| Aragonite | CaCO3 | Carbonate (5.AB.15) | Orthorhombic | Morocco |
| Barite | BaSO4 | Sulphate (7.AD.35) | Orthorhombic | Morocco |
| Beryl | Be3Al2Si6O18 | Cyclosilicate (9.CJ.05) | Hexagonal | Portugal |
| Biotite | K(Mg, Fe)3 AlSi3O10 (F,OH)2 | Phyllosilicate (9.EC.20 09) | Monoclinic | Portugal |
| Calcite | CaCO3 | Carbonate (5.AB.05) | Trigonal | Italy |
| Calcite (Optical) | CaCO3 | Carbonate (5.AB.05) | Trigonal | China |
| Cinnabar | HgS | Sulphide (2.CD.15a) | Trigonal | Spain |
| Diopside | MgCaSi2O6 | Inosilicate (09.DA.15) | Monoclinic | Portugal |
| Fluorite | CaF2 | Fluoride (3.AB.25) | Cubic | Italy |
| Galena | PbS | Sulphide (2.CD.10) | Cubic | Italy |
| Gypsum | CaSO4·2H2O | Sulphate (07.CD.40) | Monoclinic | Portugal |
| Halite | NaCl | Chloride (3.AA.20) | Cubic | Portugal |
| Hematite | Fe2O3 | Oxide (4.CB.05) | Trigonal | Portugal |
| Kyanite | Al2SiO5 | Nesosilicate (9.AF.15) | Triclinic | Italy |
| Lazurite | Na3Ca(Al3Si3O12)S | Tectosilicate (9.FB.10) | Cubic | Afghanistan |
| Malachite | Cu2CO3(OH)2 | Carbonate (5.BA.10) | Monoclinic | Spain |
| Muscovite | KAl2(AlSi3O10)(F,OH)2 | Phyllosilicate (9.EC.15) | Monoclinic | Portugal |
| Orthoclase | KAlSi3O8 | Tectosilicate (09.FA.30) | Monoclinic | Portugal |
| Quartz | SiO2 | Tectosilicate (4.DA.05) | Trigonal | Morocco |
| Sodalite | Na8(Al6Si6O24)Cl2 | Tectosilicate (09.FB.10) | Cubic | Brazil |
| Talc | Mg3Si4O10(OH)2 | Phyllosilicate (9.EC.05) | Monoclinic | Italy |
| Topaz | Al2SiO4(F,OH)2 | Nesosilicate (9.AF.35) | Orthorhombic | Portugal |
| Tourmaline | NaMg3(Al,Mg)6B3Si6O27(OH) | Cyclosilicate (8/E.19X) | Trigonal | Portugal |
| Mineral | Before UV | After UV | ΔE | ΔC* | ΔH* | ||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | ||||
| Apatite | 37.79 | −8.82 | 27.08 | 48.84 | −0.01 | 3.24 | 27.7 | −25.23 | 2.97 |
| Aquamarine | 36.12 | −11.80 | 6.52 | 29.07 | −15.13 | 10.18 | 8.6 | 4.76 | 1.37 |
| Arsenopyrite | 45.80 | −0.68 | 5.26 | 43.83 | −0.33 | 3.88 | 2.4 | −1.42 | 0.20 |
| Aragonite | 27.17 | 30.51 | 77.99 | 24.41 | 26.20 | 72.49 | 7.5 | −6.67 | 2.09 |
| Barite | 68.85 | 8.75 | 23.86 | 71.68 | 12.30 | 29.34 | 7.1 | 6.40 | 1.29 |
| Beryl | 65.09 | 2.11 | 24.08 | 66.36 | 2.07 | 25.07 | 1.6 | 0.99 | 0.13 |
| Biotite | 9.68 | 9.51 | 26.59 | 11.07 | 7.49 | 46.77 | 20.3 | 19.13 | 6.74 |
| Calcite | 68.70 | 1.57 | 6.78 | 75.62 | 3.04 | 15.21 | 11.0 | 8.55 | 0.31 |
| Calcite (optical) | 70.76 | 1.07 | 30.62 | 83.67 | −1.73 | 3.38 | 30.3 | −26.74 | 5.33 |
| Cinnabar | 34.25 | 24.15 | 18.45 | 38.84 | 20.99 | 17.23 | 5.7 | −3.24 | 1.00 |
| Diopside | 20.76 | −24.79 | 65.35 | 20.02 | −22.18 | 64.70 | 2.8 | −1.50 | 2.23 |
| Fluorite | 56.56 | −11.23 | 13.13 | 52.03 | −4.69 | 13.28 | 8.0 | −3.19 | 5.71 |
| Galena | 28.68 | 0.16 | 3.03 | 40.66 | 0.53 | 1.28 | 12.1 | −8.61 | 1.65 |
| Gypsum | 78.72 | −0.27 | 3.48 | 76.62 | −0.90 | 2.90 | 2.3 | −0.452 | 0.73 |
| Halite | 22.74 | 64.81 | 72.13 | 23.10 | 66.13 | 71.88 | 1.4 | 0.70 | 1.14 |
| Hematite | 41.67 | −2.25 | 7.94 | 25.72 | −0.99 | 7.88 | 16.0 | −0.31 | 1.22 |
| Kyanite | 37.67 | −3.95 | −16.04 | 41.33 | −3.60 | −18.04 | 4.2 | 1.88 | 0.78 |
| Lazurite | 13.06 | 10.30 | −48.11 | 14.96 | 9.38 | −37.10 | 11.2 | −10.94 | 1.60 |
| Malachite | 54.68 | −28.67 | 3.69 | 51.32 | −25.93 | 5.11 | 4.6 | −2.47 | 1.84 |
| Muscovite | 57.97 | 0.45 | 9.27 | 64.97 | 1.13 | 8.83 | 7.0 | −0.38 | 0.72 |
| Orthoclase | 75.33 | 0.50 | 4.24 | 79.09 | −0.25 | 4.78 | 3.9 | 0.51 | 0.76 |
| Quartz | 59.91 | 1.19 | 15.35 | 60.40 | 2.69 | 12.83 | 3 | −2.59 | 0.08 |
| Sodalite | 37.83 | 3.84 | −28.18 | 34.85 | 3.32 | −24.95 | 4.4 | −3.27 | 0.08 |
| Talc | 25.91 | 1.48 | 9.11 | 24.25 | 1.14 | 8.61 | 1.8 | −0.54 | 0.26 |
| Topaz | 63.55 | −1.94 | 10.24 | 72.32 | −0.41 | 4.42 | 10.6 | −5.98 | 0.64 |
| Tourmaline | 39.34 | −1.77 | 0.95 | 57.61 | −2.50 | 1.43 | 18.3 | 0.87 | 4.80 |
| Mineral | Weight Norm. % | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Be | Ca | B | C | O | F | Na | Mg | Al | Si | P | S | Cl | K | Fe | Cu | As | Sr | Hg | Pb | Ba | TOT | |
| Apatite | - | 36.26 | - | - | 42.42 | 4.45 | 0.21 | - | - | - | 16.66 | - | - | - | - | - | - | - | - | - | - | 100 |
| Aquamarine | N.M. | - | - | - | 54.26 | - | - | 0.33 | 11.87 | 33.54 | - | - | - | - | - | - | - | - | - | - | - | 100 |
| Arsenopyrite | - | - | - | - | - | - | - | - | - | - | - | 17.22 | - | - | 35.05 | - | 47.73 | - | - | - | - | 100 |
| Aragonite | - | 40.14 | - | 11.89 | 47.31 | - | - | - | - | - | - | - | - | - | - | - | - | 0.66 | - | - | - | 100 |
| Barite | - | 0.26 | - | - | 35.48 | - | - | 0.58 | - | - | - | 13.51 | - | - | - | - | - | - | - | - | 50.17 | 100 |
| Beryl | N.M. | - | - | - | 56.02 | - | 0.42 | 0.28 | 10.76 | 32.52 | - | - | - | - | - | - | - | - | - | - | - | 100 |
| Biotite | - | - | - | - | 42.61 | 1.44 | - | 2.68 | 10.55 | 16.77 | - | - | - | 7.15 | 18.8 | - | - | - | - | - | - | 100 |
| Calcite | - | 38.57 | - | 11.35 | 50.08 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 100 |
| Calcite (Optical) | - | 36.06 | - | 11.69 | 52.25 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 100 |
| Cinnabar | - | - | - | - | - | - | - | - | - | - | - | 9.97 | - | - | - | - | - | - | 90.03 | - | - | 100 |
| Diopside | - | 17.27 | - | - | 47.83 | - | - | 9.22 | 0.26 | 22.57 | - | - | - | - | 2.85 | - | - | - | - | - | - | 100 |
| Fluorite | - | 45.7 | - | - | - | 54.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 100 |
| Galena | - | - | - | - | - | - | - | - | - | - | - | 13.06 | - | - | - | - | - | - | - | 86.94 | - | 100 |
| Gypsum | - | 23.64 | - | - | 59.75 | - | - | - | - | - | - | 16.61 | - | - | - | - | - | - | - | - | - | 100 |
| Halite | N.A. | |||||||||||||||||||||
| Hematite | - | - | - | - | 28.88 | - | - | - | - | - | - | - | - | - | 71.12 | - | - | - | - | - | - | 100 |
| Kyanite | N.A. | |||||||||||||||||||||
| Lazurite | - | 8.55 | - | - | 36.86 | - | 12.54 | 0.97 | 16.2 | 16.45 | - | 7.02 | - | 0.57 | 0.84 | - | - | - | - | - | - | 100 |
| Malachite | - | - | - | 5.89 | 36.02 | - | - | 0.17 | - | 0.45 | - | - | - | - | 1.23 | 56.24 | - | - | - | - | - | 100 |
| Muscovite | - | - | - | - | 51.67 | 0.7 | - | 0.42 | 18.19 | 20.04 | - | - | - | 6.95 | 2.03 | - | - | - | - | - | - | 100 |
| Orthoclase | - | - | - | - | 48.83 | - | 1.1 | - | 9.67 | 29.49 | - | - | - | 10.91 | - | - | - | - | - | - | - | 100 |
| Quartz | - | - | - | - | 54.97 | - | - | - | - | 45.03 | - | - | - | - | - | - | - | - | - | - | - | 100 |
| Sodalite | - | - | - | - | 46.85 | - | 17.87 | - | 13.87 | 14.44 | - | - | 6.97 | - | - | - | - | - | - | - | - | 100 |
| Talc | - | - | - | - | 51.31 | - | - | 19.64 | 0.13 | 28.02 | - | - | - | - | 0.9 | - | - | - | - | - | - | 100 |
| Topaz | - | - | - | - | 45.07 | 12.31 | - | - | 28.78 | 13.84 | - | - | - | - | - | - | - | - | - | - | - | 100 |
| Tourmaline | - | - | N.M. | - | 54.34 | - | 1.73 | 1.34 | 18.22 | 15.57 | - | - | - | - | 8.8 | - | - | - | - | - | - | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitzia, F.; Moita, P.; Bottura-Scardina, S.; Lisci, C. Colour Stability of Light-Sensitive Minerals Under UVA340nm Irradiation: Implications for the Conservation of Cultural Heritage and Museum Display Conditions. Minerals 2025, 15, 999. https://doi.org/10.3390/min15090999
Sitzia F, Moita P, Bottura-Scardina S, Lisci C. Colour Stability of Light-Sensitive Minerals Under UVA340nm Irradiation: Implications for the Conservation of Cultural Heritage and Museum Display Conditions. Minerals. 2025; 15(9):999. https://doi.org/10.3390/min15090999
Chicago/Turabian StyleSitzia, Fabio, Patricia Moita, Silvia Bottura-Scardina, and Carla Lisci. 2025. "Colour Stability of Light-Sensitive Minerals Under UVA340nm Irradiation: Implications for the Conservation of Cultural Heritage and Museum Display Conditions" Minerals 15, no. 9: 999. https://doi.org/10.3390/min15090999
APA StyleSitzia, F., Moita, P., Bottura-Scardina, S., & Lisci, C. (2025). Colour Stability of Light-Sensitive Minerals Under UVA340nm Irradiation: Implications for the Conservation of Cultural Heritage and Museum Display Conditions. Minerals, 15(9), 999. https://doi.org/10.3390/min15090999









