Formation Conditions of Early Cambrian Witherite (BaCO3) Deposit in Chongqing: Implications for Differential Oceanic Changes
Abstract
1. Introduction
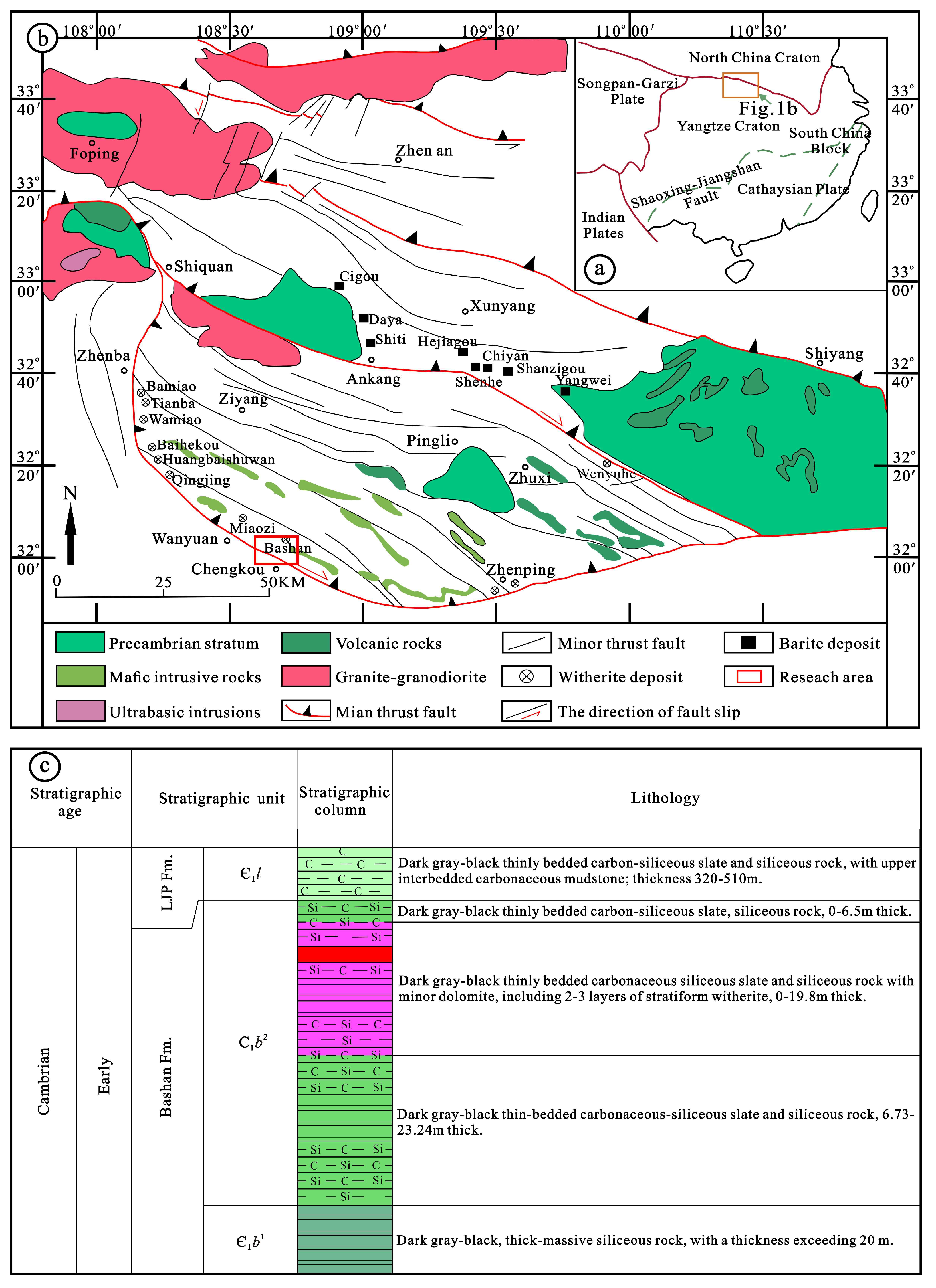
2. Regional and Deposit Geology
3. Methods
3.1. Samples
3.2. Fluid Inclusions
4. Results
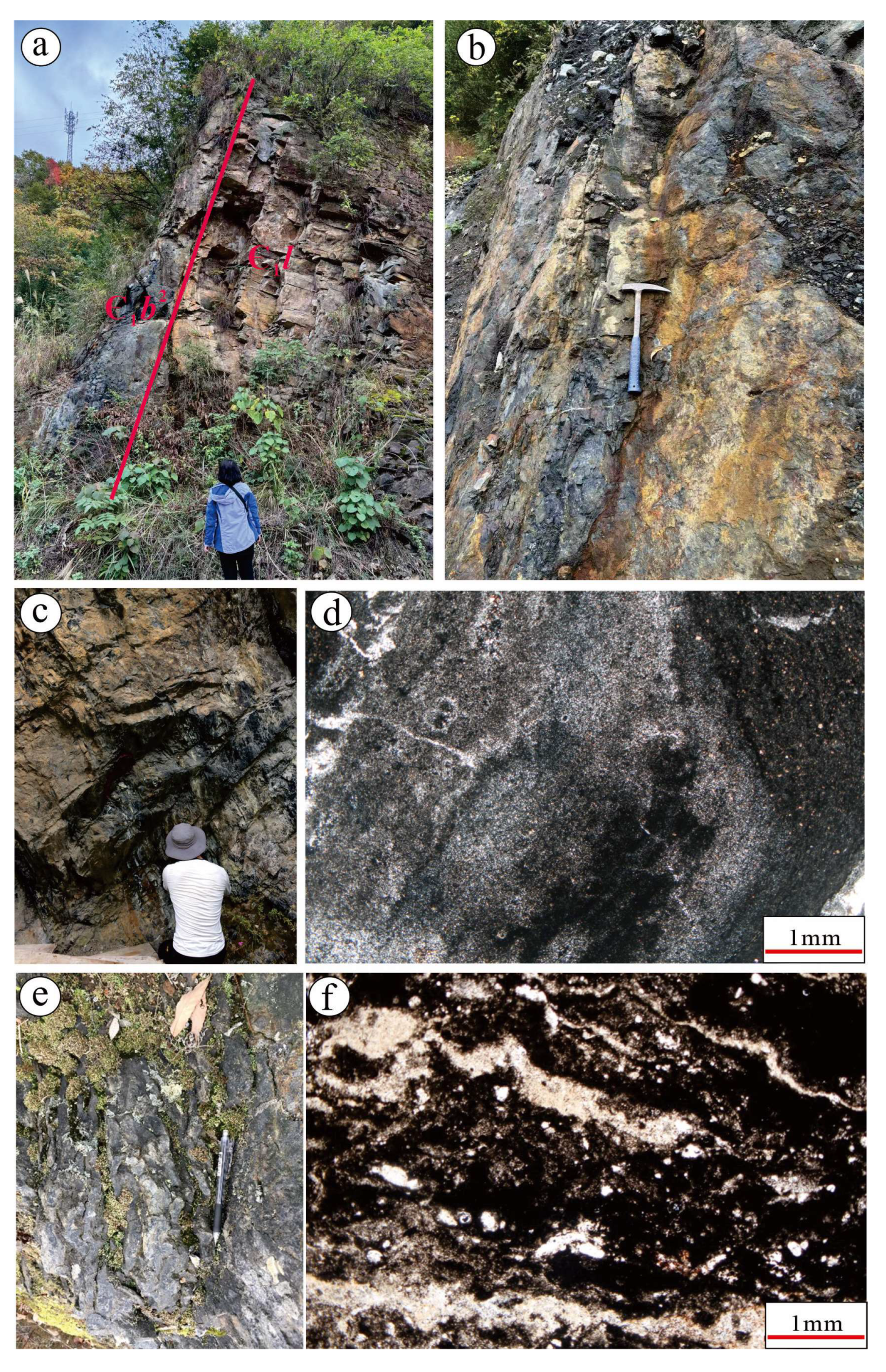
4.1. Petrologic and Petrographic Characteristics
4.2. Petrography of Fluid Inclusions
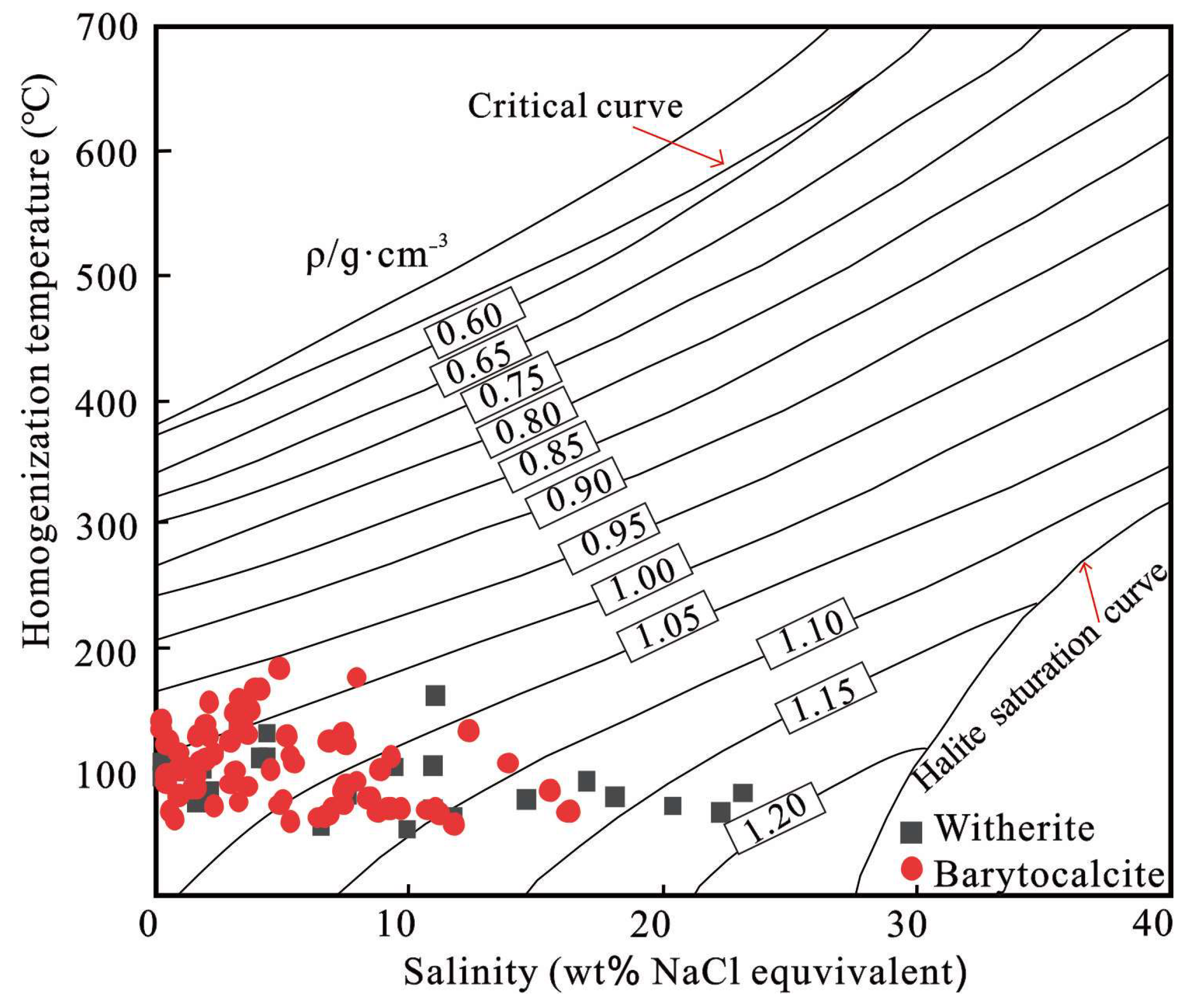
4.3. Microthermometric Results
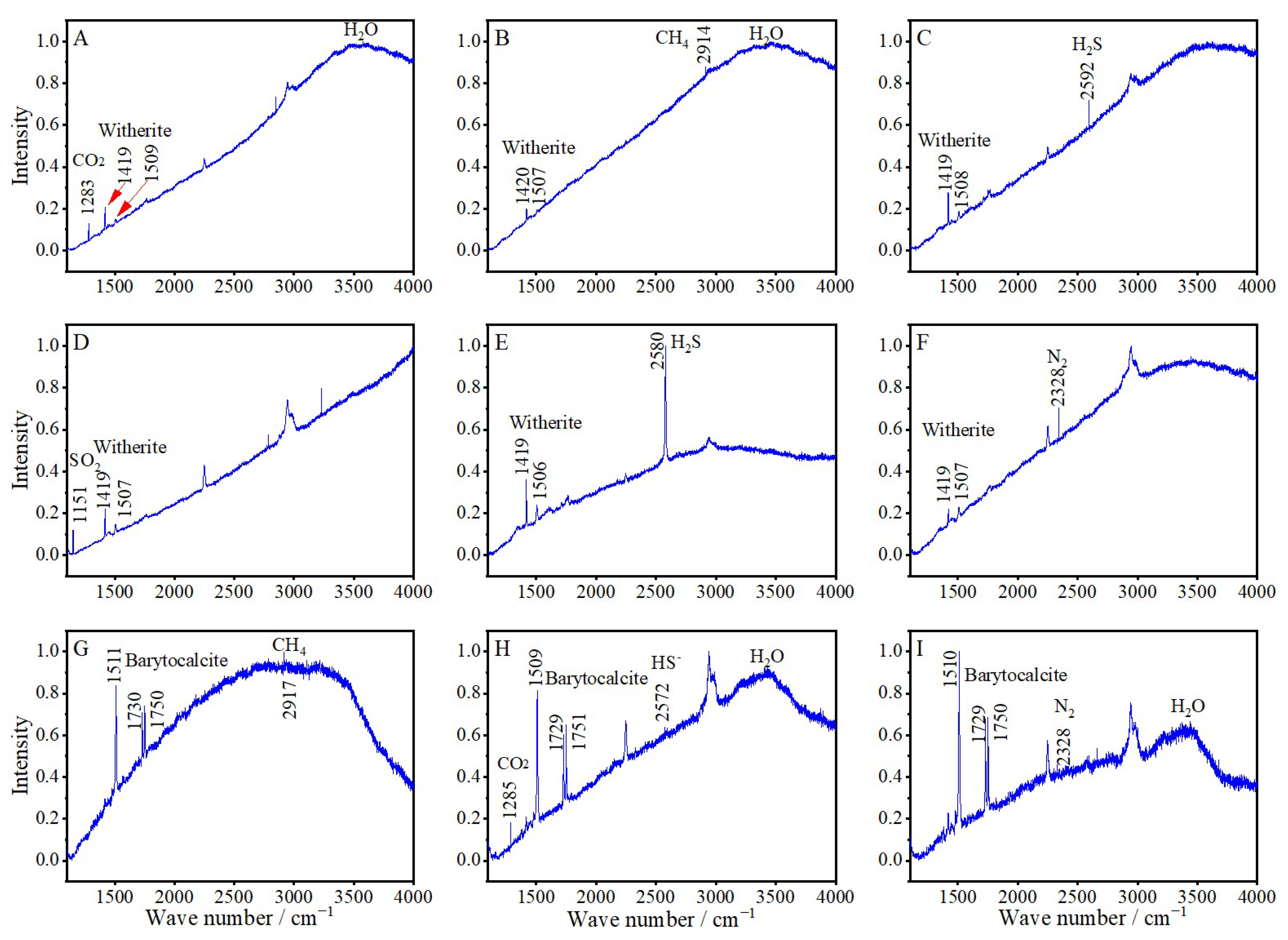
4.4. Laser Raman Spectroscopy
5. Discussion
5.1. Nature of Ore-Forming Fluids
5.2. Sources of Ore-Forming Materials
5.3. Implications for the Marine Environment
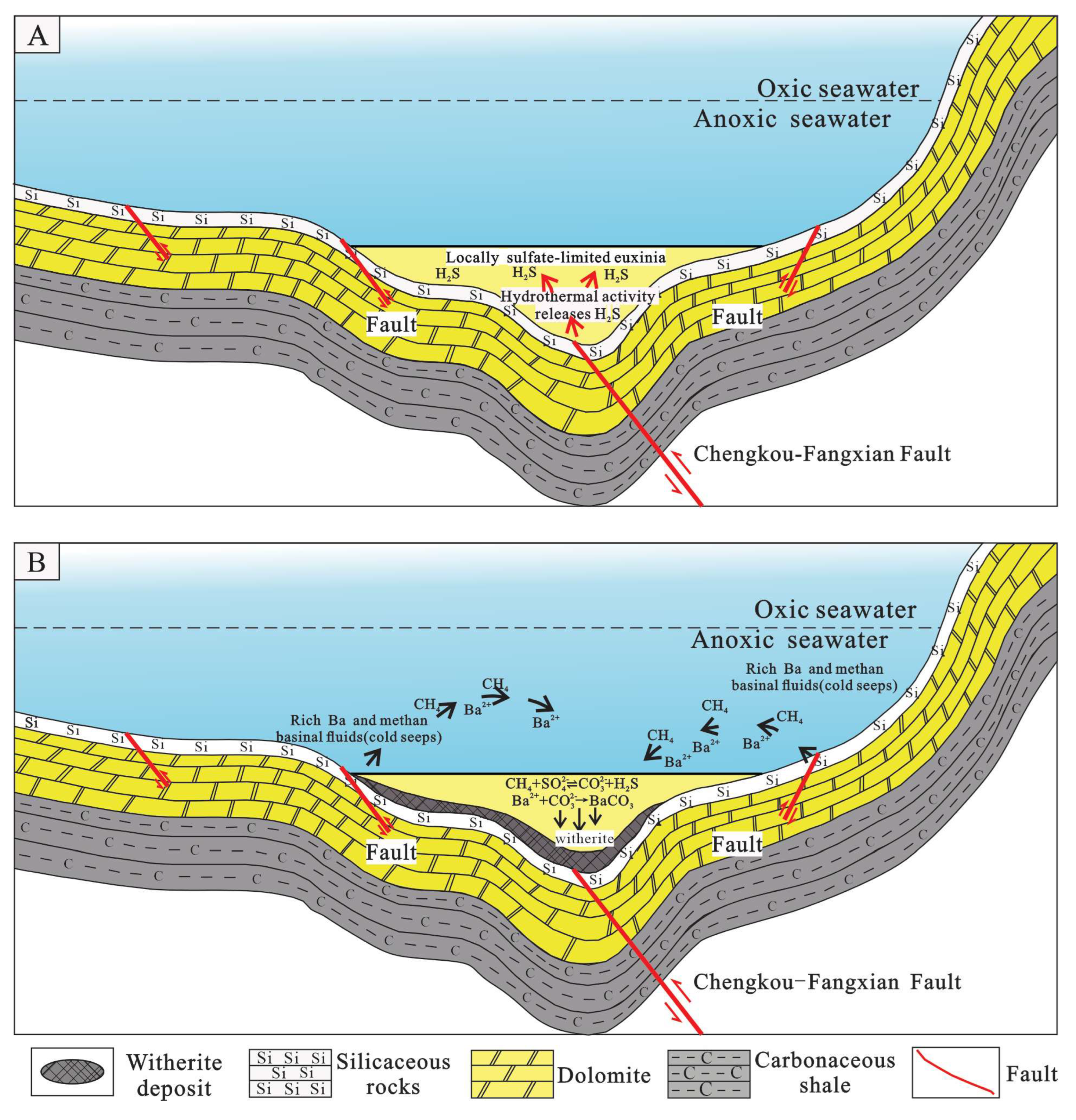
5.4. The Mechanism of Ore-Forming
6. Conclusions
- Strontium isotope data indicate that the Chengkou witherite deposit shares a common seawater origin with other deposits in the Qinling-Dabashan region. The δ13C values of witherite fall between marine carbonate and organic matter, suggesting a specific contribution of organic matter to the formation of witherite. The wide range of homogenization temperatures (54.2 to 162.7 °C) does not reflect the original trapping temperatures of the ore-forming fluids but rather results from post-entrapment thermal re-equilibration of the inclusions. Fluids contain H2S, CH4, HS−, etc., indicating the formation of witherite in a sulfur-rich and oxygen-deficient stratified water environment, revealing the complexity of the marine environment in the study area during the Early Cambrian.
- The large-scale precipitation of witherite deposits in South China during the Early Cambrian was controlled by unique paleo-marine sedimentary environments. Although atmospheric and oceanic oxygen concentration had risen substantially during this period, the restricted marginal basins of the Yangtze Platform evolved a distinct stratified water column characterized by oxic surface waters overlying euxinic (anoxic and sulfidic) deep waters. This persistent physicochemical stratification created a stable, low-energy sedimentary environment, which is conducive to witherite formation.
- During the Early Cambrian, Chengkou was situated within a restricted marginal basin, where the low sulfate content in the ocean favored the enrichment of Ba+. The extensive proliferation and subsequent death of Cambrian organisms led to the accumulation and degradation of a large amount of organic matter in the Chengkou region, creating a limited marine environment rich in . This unique restricted marine environment ultimately became a crucial control factor for forming the largest witherite deposit in the world.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Ling, H.F.; Struck, U.; Zhu, X.K.; Zhu, M.; He, T.; Yang, B.; Gamper, A.; Shields, G.A. Coupling of ocean redox and animal evolution during the Ediacaran-Cambrian transition. Nat. Commun. 2018, 9, 2575. [Google Scholar] [CrossRef]
- He, T.; Zhu, M.; Mills, B.J.; Wynn, P.M.; Zhuravlev, A.Y.; Tostevin, R.; Pogge von Strandmann, P.A.; Yang, A.; Poulton, S.W.; Shields, G.A. Possible links between extreme oxygen perturbations and the Cambrian radiation of animals. Nat. Geosci. 2019, 12, 468–474. [Google Scholar] [CrossRef]
- Wei, G.Y.; Planavsky, N.J.; Tarhan, L.G.; He, T.; Wang, D.; Shields, G.A.; Wei, W.; Ling, H.F. Highly dynamic marine redox state through the Cambrian explosion highlighted by authigenic δ238U records. Earth Planet. Sci. Lett. 2020, 544, 116361. [Google Scholar] [CrossRef]
- Chen, X.; Ling, H.F.; Vance, D.; Shields-Zhou, G.A.; Zhu, M.; Poulton, S.W.; Och, L.M.; Jiang, S.Y.; Li, D.; Cremonese, L.; et al. Rise to modern levels of ocean oxygenation coincided with the Cambrian radiation of animals. Nat. Commun. 2015, 6, 7142. [Google Scholar] [CrossRef]
- Yang, L.W.; Yang, T.; Li, J.; Lin, Y.; Ling, H.F. The marine redox evolution and the formation model for the early Cambrian Gongxi-Tianzhu barite deposits in the South China Block. Ore Geo. Rev. 2023, 158, 105502. [Google Scholar] [CrossRef]
- Pi, D.H.; Jiang, S.Y.; Luo, L.; Yang, J.H.; Ling, H.F. Depositional environments for stratiform witherite deposits in the Lower Cambrian black shale sequence of the Yangtze Platform, southern Qinling region, SW China: Evidence from redox-sensitive trace element geochemistry. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 398, 125–131. [Google Scholar] [CrossRef]
- Xu, L.G.; Lehmann, B.; Mao, J.W.; Zheng, W.; Ye, H.S.; Li, H.Y. Strontium, sulfur, carbon, and oxygen isotope geochemistry of the Early Cambrian strata-bound barite and witherite deposits of the Qinling-Daba Region, Northern Margin of the Yangtze Craton, China. Econ. Geol. 2016, 111, 695–718. [Google Scholar] [CrossRef]
- Bates, S.L.; Hendry, K.R.; Pryer, H.V.; Kinsley, C.W.; Pyle, K.M.; Woodward, E.M.S.; Horner, T.J. Barium isotopes reveal role of ocean circulation on barium cycling in the Atlantic. Geochim. Cosmochim. Acta 2017, 204, 286–299. [Google Scholar] [CrossRef]
- Charbonnier, Q.; Moynier, F.; Bouchez, J. Barium isotope cosmochemistry and geochemistry. Sci. Bull. 2018, 63, 385–394. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Henderson, G.M. Barium stable isotopes in the global ocean: Tracer of Ba inputs and utilization. Earth Planet. Sci. Lett. 2017, 473, 269–278. [Google Scholar] [CrossRef]
- Wei, W.; Zeng, Z.; Shen, J.; Tian, L.L.; Wei, G.Y.; Ling, H.F.; Huang, F. Dramatic changes in the carbonate-hosted barium isotopic compositions in the Ediacaran Yangtze Platform. Geochim. Cosmochim. Acta 2021, 299, 113–129. [Google Scholar] [CrossRef]
- Crockford, P.W.; Wing, B.A.; Paytan, A.; Hodgskiss, M.S.; Mayfield, K.K.; Hayles, J.A.; Middleton, J.E.; Ahm, A.S.C.; Johnston, D.T.; Caxito, F.; et al. Barium-isotopic constraints on the origin of post-Marinoan barites. Earth Planet. Sci. Lett. 2019, 519, 234–244. [Google Scholar] [CrossRef]
- Alaminia, Z.; Sharifi, M. Geological, geochemical and fluid inclusion studies on the evolution of barite mineralization in the Badroud area of Iran. Ore Geo. Rev. 2018, 92, 613–626. [Google Scholar] [CrossRef]
- Keveshk, H.H.; Ehya, F.; Paydar, G.R.; Kheymehsari, S.M. Rare earth elements geochemistry, O and S isotopic compositions, and microthermometric data of barite from the Kuh–Ghalagheh deposit, Markazi Province, Iran. Appl. Geochem. 2021, 135, 105128. [Google Scholar] [CrossRef]
- Griffith, E.M.; Paytan, A. Barite in the ocean–occurrence, geochemistry and palaeoceanographic applications. Sedimentology 2012, 59, 1817–1835. [Google Scholar] [CrossRef]
- Mißbach, H.; Duda, J.P.; Van Den Kerkhof, A.M.; Lüders, V.; Pack, A.; Reitner, J.; Thiel, V. Ingredients for microbial life preserved in 35 billion-year-old fluid inclusions. Nat. Commun. 2021, 12, 1101. [Google Scholar] [CrossRef]
- Green, D.I.; Young, B. Hydromagnesite and dypingite from the Northern Pennine Orefield, northern England. P. Yorks. Geol. Soc. 2006, 56, 151–154. [Google Scholar] [CrossRef]
- Fitch, A.A. Barite and witherite from near el portal, mariposa county, california. Am. Mineral. J. Earth Planet. Mat. 1931, 16, 461–468. [Google Scholar]
- Tu, H.K. Mineralizing conditions and mechanism of the barite deposits in the meeting area of Shaanxi, Gansu and Sichuan provinces. Geol. Chem. Miner. 1998, 20, 295–300. (In Chinese) [Google Scholar]
- Tang, J.X.; Lin, W.D.; Gao, D.R.; Mu, J.L. The genesis of the Miaozi witherite-barytocalcite-barytodolomite deposit in Wanyuan city, Sichuan province. Miner. Depos. 1998, 17, 264–276. (In Chinese) [Google Scholar]
- Lv, Z.C.; Liu, C.Q.; Liu, J.J.; Wu, F.C. Geochemical studies on the Lower Cambrian witherite-bearing cherts in the northern Daba Mountains. Acta Geol. Sin. 2004, 78, 390–406. (In Chinese) [Google Scholar]
- Luo, L. The Depositional Environment and C-O-Sr Isotopic Characters of Witherite Deposits in Qinling-Daba Mountain Areas, SW China. Master’s Thesis, Nanjing University, Nanjing, China, 2011. (In Chinese). [Google Scholar]
- Chamberlain, S.C.; Dossert, W.P.; Siegel, D.I. A new paragenesis and new localities for witherite. Can. Mineral. 1986, 24, 79–90. [Google Scholar]
- Lv, Z.C.; Liu, C.Q.; Liu, J.J.; Zhao, Z.Q.; Wu, F.C.; Li, J. Carbon, oxygen and strontium isotopic studies of the Huangbashuwan witherite deposit at Ziyang and Wenyuhe witherite deposit at Zhushan, China. Geochimica 2005, 34, 557–573. (In Chinese) [Google Scholar]
- Liu, J.J.; Wu, S.H.; Liu, Z.J.; Su, W.C.; Wang, J.P. A discussion on the origin of witherite deposits in large-scale barium metallogenic belt, southern Qinling Mountains, China: Evidence from individual fluid inclusion. Earth Sci. Front. 2010, 17, 222–238. (In Chinese) [Google Scholar]
- Wang, Z.C.; Li, G.Z. Barite and witherite deposits in Lower Cambrian shales of South China: Stratigraphic distribution and geochemical characterization. Econ. Geol. 1991, 86, 354–363. [Google Scholar] [CrossRef]
- Cheng, Z.; Qi, S.; Jiang, X.; Chen, X.; Zhang, M.; Tang, L.; Liu, L.; Zhu, Y. Source and genesis of Chapai carbonate-hosted Zn-Pb deposit, South China: Constraints from in-situ S and Pb isotopes of sulfide and sulfate minerals. Ore Geol. Rev. 2023, 164, 105808. [Google Scholar] [CrossRef]
- Gao, H.Z. The biochemical sedimentary metallogenic model of baritic and witheritic deposits in Lower Cambrian in China. J. Miner. Petrol. 1998, 2, 70–77. [Google Scholar]
- Roedder, E. Volume 12: Fluid inclusions. In Reviews in Mineralogy; Ribbe, P.H., Ed.; Mineralogical Society of America: Blacksburg, WV, USA, 1984; Volume 12, p. 644. [Google Scholar]
- Kesler, S.E. Ore-forming fluids. Elements 2005, 1, 13–18. [Google Scholar] [CrossRef]
- Fall, A.; Bodnar, R.J. How precisely can the temperature of a fluid event be constrained using fluid inclusions? Econ. Geol. 2018, 113, 1817–1843. [Google Scholar] [CrossRef]
- Chi, G.; Diamond, L.W.; Lu, H.; Lai, J.; Chu, H. Common problems and pitfalls in fluid inclusion study: A review and discussion. Minerals 2020, 11, 7. [Google Scholar] [CrossRef]
- Goldstein, R.H. Fluid inclusions in sedimentary and diagenetic systems. Lithos 2001, 55, 159–193. [Google Scholar] [CrossRef]
- Zaw, K.; Gemmell, J.B.; Large, R.R.; Mernagh, T.P.; Ryan, C.G. Evolution and source of ore fluids in the stringer system, Hellyer VHMS deposit, Tasmania, Australia: Evidence from fluid inclusion microthermometry and geochemistry. Ore Geol. Rev. 1996, 10, 251–278. [Google Scholar] [CrossRef]
- Kerrich, R.; Goldfarb, R.; Groves, D.; Garwin, S.; Jia, Y. The characteristics, origins, and geodynamic settings of supergiant gold metallogenic provinces. Sci. China Series D Earth Sci. 2000, 43, 1–68. [Google Scholar] [CrossRef]
- Ronde, C.E.D.; Faure, K.; Bray, C.J.; Chappell, D.A.; Wright, I.C. Hydrothermal fluids associated with seafloor mineralization at two southern Kermadec arc volcanoes, offshore New Zealand. Miner. Deposita 2003, 38, 217–233. [Google Scholar] [CrossRef]
- Safina, N.P.; Melekestseva, I.Y.; Nimis, P.; Ankusheva, N.N.; Yuminov, A.M.; Kotlyarov, V.A.; Sadykov, S.A. Barite from the Saf’yanovka VMS deposit (Central Urals) and Semenov-1 and Semenov-3 hydrothermal sulfide fields (Mid-Atlantic Ridge): A comparative analysis of formation conditions. Miner. Deposita 2016, 51, 491–507. [Google Scholar] [CrossRef]
- Bodnar, R.J. Hydrothermal solutions. In Encyclopedia of Geochemistry; Marshall, C.P., Fairbridge, R.W., Eds.; Kluwer Academic Publishers: Lancaster, PA, USA, 1999; pp. 333–337. [Google Scholar]
- Bischoff, J.L.; Rosenbauer, R.J. The critical point and two-phase boundary of seawater, 200–500 °C. Earth Planet Sci. Lett. 1984, 68, 172–180. [Google Scholar] [CrossRef]
- Hu, Y.X. Research of Metallogenic Geological Background and Metallogenic Model of “Bashan Type” Witherite Deposits in Chongqing. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2021. [Google Scholar]
- Yang, X.Q.; Zhang, Z.H.; Duan, S.G. Origin of the Mesoproterozoic Jingtieshan bedded barite deposit, north Qilian Mountains, NW China: Geochemical and isotope (O, S, Sr) evidence. Geol. J. 2018, 53, 21–32. [Google Scholar] [CrossRef]
- McArthur, J.M.; Howarth, R.J.; Shields, G.A.; Zhou, Y. Strontium isotope stratigraphy. In Geologic Time Scale 2020; Gradstein, F.M., Ogg, J.G., Schmitz, M.D., Ogg, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 211–238. [Google Scholar]
- Chen, X.; Zhou, Y.; Shields, G.A. Progress towards an improved Precambrian seawater 87Sr/86Sr curve. Earth Sci. Rev. 2022, 224, 103869. [Google Scholar] [CrossRef]
- Palmer, M.R.; Edmond, J.M. The strontium isotope budget of the modern ocean. Earth Planet. Sci. Let. 1989, 92, 11–26. [Google Scholar] [CrossRef]
- Present, T.M.; Adkins, J.F.; Fischer, W.W. Variability in Sulfur Isotope Records of Phanerozoic Seawater Sulfate. Geophy. Res. Lett. 2020, 47, e2020GL088766. [Google Scholar] [CrossRef]
- Absar, N.; Kalam, T.D.A.; Raza, M.Q.; Ashok, M.; Islam, R. Redox conditions of Early Cambrian Ocean as deciphered from multi-proxy geochemical and isotopic studies of Proto-Tethys carbonaceous sediments from Outer Lesser Himalaya, India. J. Earth Syst. Sci. 2024, 13326, 26. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhou, X.; Hu, D.P. High-resolution paired carbon isotopic records from the Meishucun section in South China: Implications for carbon cycling and environmental changes during the Ediacaran-Cambrian transition. Precambrian Res. 2020, 337, 105561. [Google Scholar] [CrossRef]
- Shields, G.; Veizer, J. Precambrian marine carbonate isotope database: Version 1.1. Geochem. Geophy. Geosy. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Goddéris, Y.; Le Hir, G.; Macouin, M.; Donnadieu, Y.; Hubert-Théou, L.; Dera, G.; Aretz, M.; Fluteau, F.; Li, Z.X.; Halverson, G.P. Paleogeographic forcing of the strontium isotopic cycle in the Neoproterozoic. Gondwana Res. 2017, 42, 151–162. [Google Scholar] [CrossRef]
- Cui, H.; Kaufman, A.J.; Zou, H.; Kattan, F.H.; Trusler, P.; Smith, J.; Ivantsov, A.Y.; Rich, T.H.; Al Qubsani, A.; Yazedi, A.; et al. Primary or secondary? A dichotomy of the strontium isotope anomalies in the Ediacaran carbonates of Saudi Arabia. Precambrian Res. 2020, 343, 105720. [Google Scholar] [CrossRef]
- Derry, L.A.; Brasier, M.D.; Corfield, R.E.A.; Rozanov, A.Y.; Zhuravlev, A.Y. Sr and C isotopes in Lower Cambrian carbonates from the Siberian craton: A paleoenvironmental record during the ‘Cambrian explosion’. Earth Planet. Sci. Lett. 1994, 128, 671–681. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Wang, Y.L.; Zhang, X.J.; Wei, Z.F.; Wang, G.; Zhang, T.; Ma, H.; Wei, J.Y.; He, W.; Ma, X.Y.; et al. Carbon, oxygen and strontium isotopic and elemental characteristics of the Cambrian Longwangmiao Formation in South China: Paleoenvironmental significance and implications for carbon isotope excursions. Gondwana Res. 2022, 106, 174–190. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, D.; Huang, L.L.; Zhu, G.Y.; Zhang, T.F.; Liu, J.J.; Zhai, X.F.; Xiong, R.; Wang, S.; Zhang, Y.Y. Characteristics and genesis of dolomite in the lower Cambrian Xiaoerbulake Formation of the western Tarim Basin, China. Front. Earth Sci. 2023, 10, 1075941. [Google Scholar] [CrossRef]
- Nicolaides, S. Origin and modification of Cambrian dolomites (Red Heart Dolomite and Arthur Creek Formation), Georgina Basin, central Australia. Sedimentology 1995, 42, 249–266. [Google Scholar] [CrossRef]
- Huang, H.X.; Wen, H.G.; Wen, L.; Zhang, B.J.; Zhou, G.; He, Y.; Wen, L.B.; Zhao, Y.; Jiang, H.C. Multistage dolomitization of deeply buried dolomite in the Lower Cambrian Canglangpu Formation, central and northern Sichuan Basin. Mar. Petrol. Geol. 2023, 152, 106261. [Google Scholar] [CrossRef]
- Zhou, C.M.; Zhang, J.M.; Li, G.X.; Yu, Z.Y. Early Cambrian Carbon and Oxygen Isotope Records in Xiaotan, Yongshan, Yunnan. Geol. Sci. 1997, 32, 201–211. (In Chinese) [Google Scholar]
- Zhang, W.; Deng, X.; Peng, L.; Zhang, Y.; Xu, D.; Liu, H.; Jin, X.; Sun, J.; Lai, C. Rare earth elements and carbon-oxygen isotopes of calcite from the Tongjiachong Cu deposit, South China: Implications for fluid source and mineral precipitation. Ore Geol. Rev. 2020, 116, 103236. [Google Scholar] [CrossRef]
- Amthor, J.E.; Grotzinger, J.P.; Schröder, S.; Bowring, S.A.; Ramezani, J.; Martin, M.W.; Matter, A. Extinction of Cloudina and Namacalathus at the Precambrian-Cambrian boundary in Oman. Geology 2003, 31, 431–434. [Google Scholar] [CrossRef]
- Gaucher, C.; Sial, A.N.; Ferreira, V.P.; Pimentel, M.M.; Chiglino, L.; Sprechmann, P. Chemostratigraphy of the Cerro Victoria Formation (Lower Cambrian, Uruguay): Evidence for progressive climate stabilization across the Precambrian–Cambrian boundary. Chem. Geol. 2007, 237, 28–46. [Google Scholar] [CrossRef]
- Gregg, J.M.; Shelton, K.L. Dolomitization and dolomite neomorphism in the back reef facies of the Bonneterre and Davis formations (Cambrian), southeastern Missouri. J. Sed. Res. 1990, 60, 549–562. [Google Scholar]
- Mehrabi, B.; Karimi Shahraki, B.; Bazargani Guilani, K.; Masoudi, F. Early Cambrian high-temperature dolomite of the Rizu Series in the Jalal-Abad iron ore deposit, Central Iran. Arab. J. Geosci. 2015, 8, 7163–7176. [Google Scholar] [CrossRef]
- Yan, Z.B.; Guo, F.S.; Pan, J.Y.; Guo, G.L.; Zhang, Y.J. Application of C, O and Sr isotope composition of carbonates in the research of paleoclimate and paleooceanic environment. Contrib. Geol. Miner. Reso. Res. 2005, 20, 53–56. (In Chinese) [Google Scholar]
- He, X.Y.; Shou, J.F.; Shen, A.J.; Wu, X.N.; Wang, Y.S.; Hu, Y.Y.; Zhu, Y.; Wei, X.D. Geochemical Characteristics and Genesis of Dolomite. Petrol. Explor. Dev. 2014, 41, 375. (In Chinese) [Google Scholar]
- Ackerman, L.; Žák, J.; Žák, K.; Pašava, J.; Kachlik, V.; Hora, J.; Veselovský, F.; Hajná, J. Carbon, oxygen, and strontium isotopic fingerprint in Neoproterozoic to early Cambrian limestones in an active margin setting: A record of local environment or global changes? Precambrian Res. 2022, 370, 106538. [Google Scholar] [CrossRef]
- Kim, S.T.; O’Neil, J.R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Li, C.; Planavsky, N.J.; Shi, W.; Zhang, Z.H.; Zhou, C.M.; Cheng, M.; Tarhan, L.G.; Luo, G.M.; Xie, S.C. Ediacaran marine redox heterogeneity and early animal ecosystems. Sci. Rep. 2015, 5, 17097. [Google Scholar] [CrossRef]
- Han, T.; Peng, Y.B.; Bao, H.M. Sulfate-limited euxinic seawater facilitated Paleozoic massively bedded barite deposition. Earth Planet. Sci. Lett. 2022, 582, 117419. [Google Scholar] [CrossRef]
- Sperling, E.A.; Melchin, M.J.; Fraser, T.; Stockey, R.G.; Farrell, U.C.; Bhajan, L.; Brunoir, T.N.; Cole, D.B.; Gill, B.C.; Lenz, A.; et al. A long-term record of early to mid-Paleozoic marine redox change. Sci. Adv. 2021, 7, 4382. [Google Scholar] [CrossRef]
- Chang, C.; Hu, W.; Huang, K.J.; Wang, Z.; Zhang, X. Mass extinction coincided with expanded continental margin euxinia during the Cambrian Age 4. Geophy. Res. Lett. 2023, 50, e2023GL105560. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, D.; Zhou, X.; Guo, C.; Huang, T.; Zhang, G. Tectono-depositional pattern and evolution of the middle Yangtze Platform (South China) during the late Ediacaran. Precambrian Res. 2019, 333, 105426. [Google Scholar] [CrossRef]
- Gwiazda, R.H.; Paull, C.K.; Caress, D.W.; Preston, C.M.; Johnson, S.B.; Lundsten, E.M.; Anderson, K. The Extent of Fault-Associated Modern Authigenic Barite Deposits Offshore Northern Baja California Revealed by High-Resolution Mapping. Front. Mar. Sci. 2019, 6, 460. [Google Scholar] [CrossRef]
- Craig, H. Geochemical implications of the isotopic composition of carbon in the ancient rocks. Geochim. Cosmochim. Acta 1954, 6, 186–196. [Google Scholar] [CrossRef]
- Emrich, K.; Ehhalt, D.H.; Vogel, J.C. Carbon isotope fractionation during the precipitation of calcium carbonate. Earth Planet. Sci. Lett. 1970, 8, 363–371. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Stolper, D.A.; Pu, X.; Lloyd, M.K.; Christensen, N.I.; Bucholz, C.E.; Lange, R.A. Constraints on Early Paleozoic deep-ocean oxygen concentrations from the iron geochemistry of the bay of islands ophiolite. Geochem. Geophy. Geosy. 2022, 23, e2021GC010196. [Google Scholar] [CrossRef]
- Shi, L. Discussion on Forming Conditions and Process of the Barite-Witherite Deposits in Lower Cambrian, Northern Daba Mountains, China. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2007. (In Chinese). [Google Scholar]
- Wang, Z.C.; Fan, D.L.; Chen, J.S. origin of witherite ore deposits in early Cambrian Dabashan black shale series. Chin. J. Geol. 1992, 27, 237–248. [Google Scholar]






| Sample No. | Lithology | Formation |
|---|---|---|
| LH-001B1 | Laminated chert | Baziping Fm. |
| LH-002B1 | Chert, C-rich | Baziping Fm. |
| LH-003B1 | Barytocalcite | Bashan Fm. |
| LH-003B2 | Barytocalcite | Bashan Fm. |
| LH-004B1 | Barytocalcite | Bashan Fm. |
| LH-004B3 | Shale | Bashan Fm. |
| LH-004B4 | Witherite | Bashan Fm. |
| LH-004B5 | Barytocalcite | Bashan Fm. |
| LH-006B2 | Witherite | Bashan Fm. |
| LH-007B1 | Barytocalcite | Bashan Fm. |
| LH-007B2 | Barytocalcite | Bashan Fm. |
| LH-008B1 | Dolostone | Lujiaping Fm. |
| Sample Number | Mineral | N | Long Axis Size (um) | Tm, ice (°C) | Th (°C) | Salinity (wt.% NaCl eq.) | Pressure (MPa) | Density (g/cm3) |
|---|---|---|---|---|---|---|---|---|
| Range (Mean) | Range (Mean) | Range (Mean) | Range (Mean) | Range (Mean) | ||||
| 004b5 | barytocalcite | 27 | 1.4~7.5 | −0.1~−5.0 (−1.7) | 62.3~167.7 (109.5) | 0.2~7.9 (2.67) | 3.89~13.24 (7.84) | 0.932~1.027 (0.973) |
| 007b1 | barytocalcite | 6 | 1.1~3.1 | −0.9~−9.9 (−5.5) | 89.4~126.3 (111.9) | 1.6~13.8 (5.99) | 6.73~12.53 (9.61) | 0.952~1.049 (0.994) |
| 007b2 | barytocalcite | 12 | 1.2~5.3 | −0.2~−4.6 (−1.7) | 64.1~140.7 (95.6) | 0.4~7.3 (2.85) | 4.24~9.07 (6.91) | 0.954~1.026 (0.984) |
| 003b1 | barytocalcite | 10 | 1.0~2.9 | −0.2~−5.0 (−2.3) | 102.3~185.7 (142.3) | 0.4~7.9 (3.78) | 7.51~16.89 (11.07) | 0.918~0.992 (0.953) |
| 003b2 | barytocalcite | 27 | 1.3~8.5 | −1.3~−12.3 (−5.7) | 66.1~136.7 (85) | 3.1~16.2 (8.48) | 5.12~14.87 (8.17) | 0.957~1.093 (1.027) |
| 004b4 | witherite | 14 | 1.0~2.9 | −0.1~−21.0 (−8.4) | 68.3~138.3 (88.7) | 0.2~23.0 (9.51) | 5.08~11.78 (8.18) | 0.955~1.141 (1.036) |
| 006b2 | witherite | 15 | 1.0~5.0 | −0.1~−10.6 (−3.2) | 54.2~162.7 (100.8) | 0.2~14.6 (6) | 5.13~17.35 (8.64) | 0.967~1.055 (1.001) |
| Total | 111 | 1.0~7.5 | −0.1~−21.0 (−4.1) | 54.2~185.7 (101.3) | 0.2~23.0 (5.69) | 3.89~17.35 (8.63) | 0.918~1.141 (0.995) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Liang, F.; Wang, C.; Tian, Y.; Hu, Y.; Liu, H.; Xia, Z.; Yuan, C.; Han, K.; Zhou, S. Formation Conditions of Early Cambrian Witherite (BaCO3) Deposit in Chongqing: Implications for Differential Oceanic Changes. Minerals 2025, 15, 978. https://doi.org/10.3390/min15090978
Jiang J, Liang F, Wang C, Tian Y, Hu Y, Liu H, Xia Z, Yuan C, Han K, Zhou S. Formation Conditions of Early Cambrian Witherite (BaCO3) Deposit in Chongqing: Implications for Differential Oceanic Changes. Minerals. 2025; 15(9):978. https://doi.org/10.3390/min15090978
Chicago/Turabian StyleJiang, Jie, Feng Liang, Chan Wang, Yaming Tian, Yunxi Hu, Hao Liu, Zhipeng Xia, Changjian Yuan, Kaibin Han, and Susu Zhou. 2025. "Formation Conditions of Early Cambrian Witherite (BaCO3) Deposit in Chongqing: Implications for Differential Oceanic Changes" Minerals 15, no. 9: 978. https://doi.org/10.3390/min15090978
APA StyleJiang, J., Liang, F., Wang, C., Tian, Y., Hu, Y., Liu, H., Xia, Z., Yuan, C., Han, K., & Zhou, S. (2025). Formation Conditions of Early Cambrian Witherite (BaCO3) Deposit in Chongqing: Implications for Differential Oceanic Changes. Minerals, 15(9), 978. https://doi.org/10.3390/min15090978






