Composition, Properties, and Flotation Reagent Regimes of Carbonaceous Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents of Flotation
2.3. Research Methods
2.3.1. IR Spectroscopy
2.3.2. X-Ray Photoelectron Spectroscopy Analysis
2.3.3. Acid–Base Centres
2.3.4. Froth Flotation
3. Results and Discussion
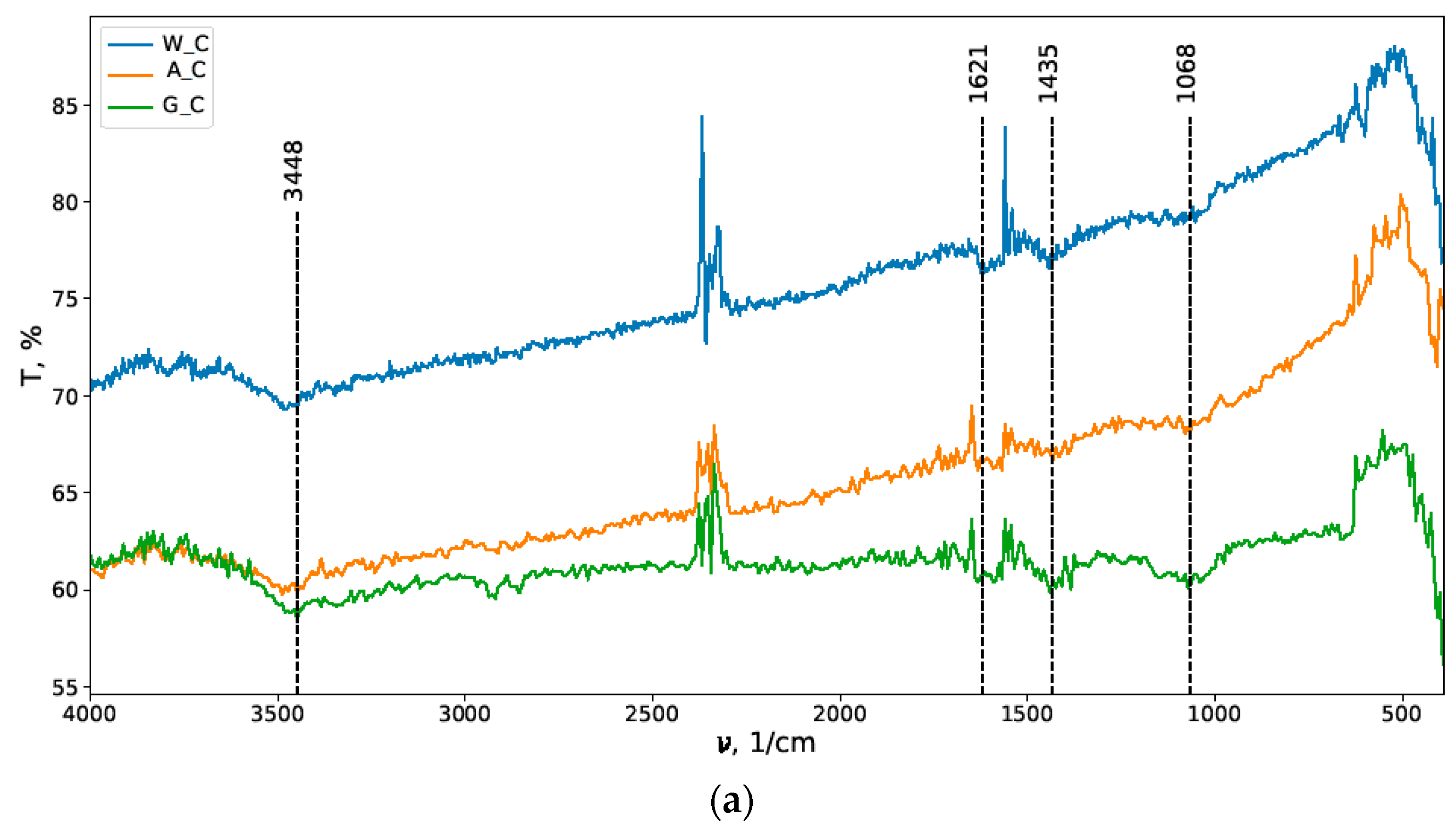
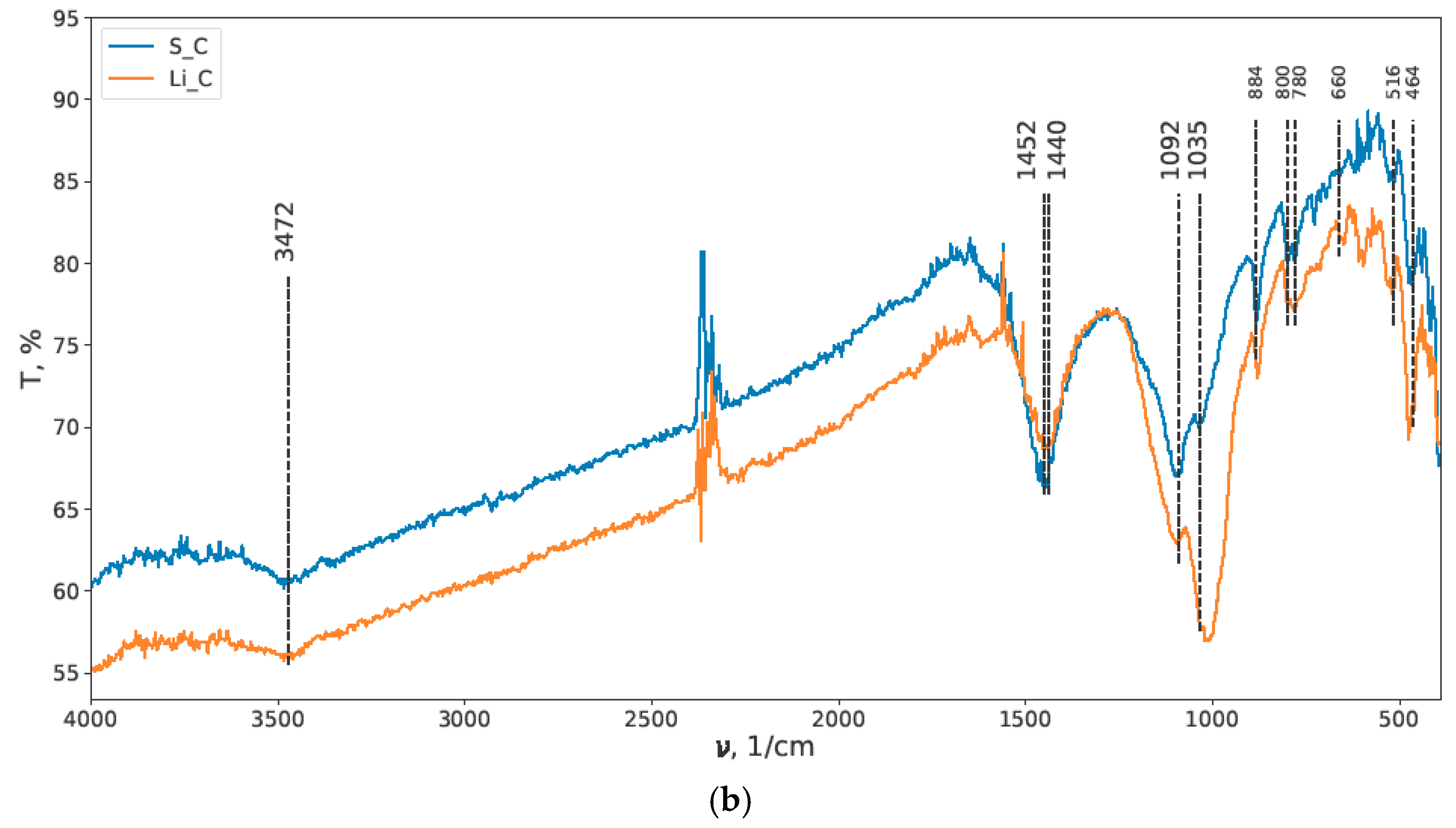
3.1. IR Spectroscopy
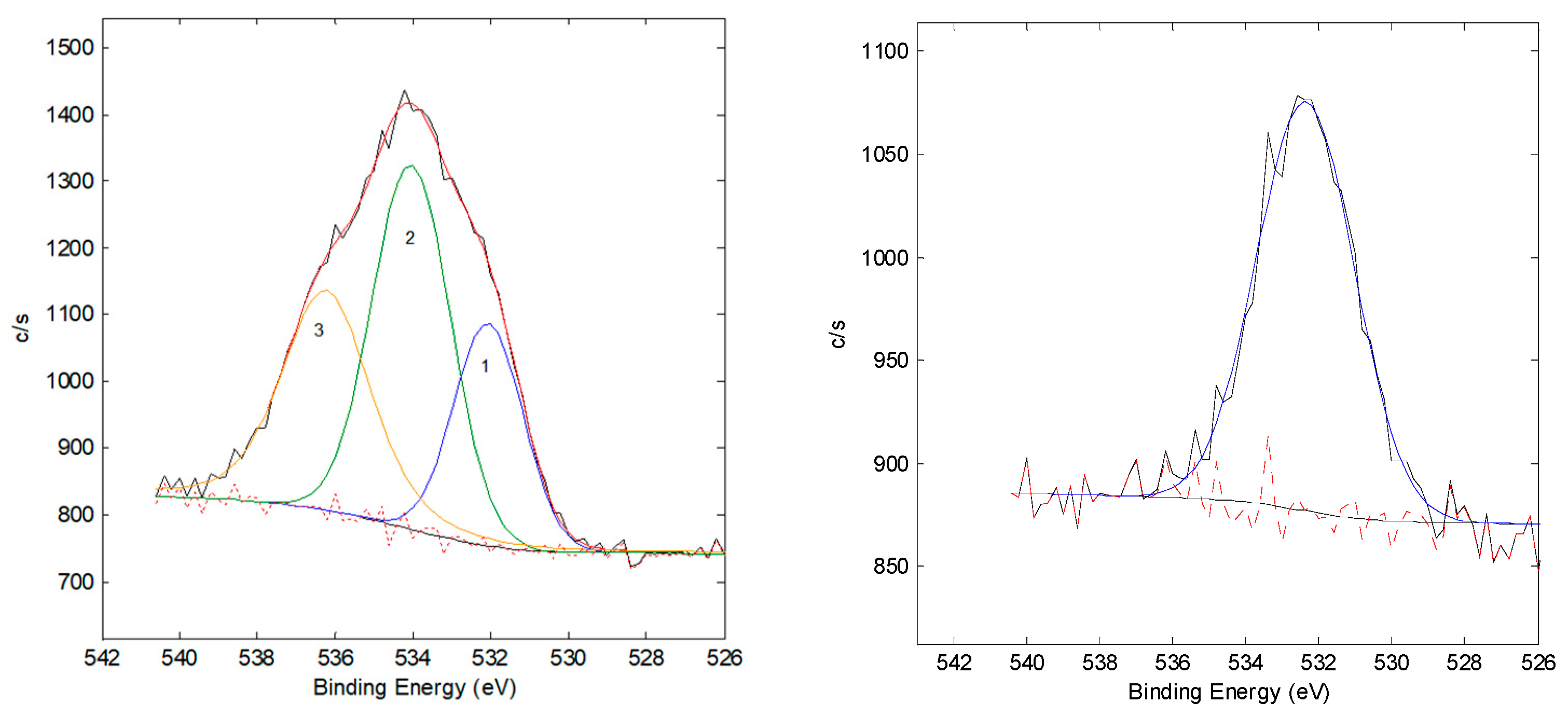
3.2. XPS
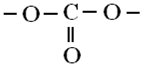
3.3. Acid–Base Centres
3.4. Flotation Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sousa, R.; Futuro, A.; Pires, C.S.; Leite, M.M. Froth flotation of Aljustrel sulphide complex ore. Physicochem. Probl. Miner. Process. 2017, 53, 758. [Google Scholar] [CrossRef]
- Adams, M.D.; Burger, A.M. Characterization and blinding of carbonaceous preg-robbers in gold ores. Miner. Eng. 1998, 11, 919. [Google Scholar] [CrossRef]
- Lee, S.; Gibson, C.; Borschneck, A.; Ghahreman, A. Transformer oil vs. kerosene: Selective collectors for C-matter flotation from a double refractory gold ore. Miner. Eng. 2023, 191, 107951. [Google Scholar] [CrossRef]
- Tabatabaei, R.H.; Nagaraj, D.R.; Vianna, S.M.; Napier-Munn, T.J.; Gorain, B. The effect of non-sulphide gangue minerals on the flotation of sulphide minerals from Carlin-type gold ores. Miner. Eng. 2014, 60, 26. [Google Scholar] [CrossRef]
- Wieniewski, A.; Skorupska, B. Technology of Polish copper ore beneficiation—Perspectives from the past experience. E3S Web Conf. 2016, 8, 01064. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Zaleska, E.; Danczak, O. Flotation of carbonaceous copper shale–quartz mixture with poly(ethylene glycol) alkyl ethers. Trans. Nonferr. Met. Soc. China 2015, 25, 314. [Google Scholar] [CrossRef]
- Magwaneng, R.S.; Haga, K.; Batnasan, A.; Shibayama, A.; Kosugi, M.; Kawarabuki, R.; Kawata, M. Investigation for removal of organic carbon from carbonaceous copper sulphide ore and improving the recovery of copper through flotation. In Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2018; p. 343. [Google Scholar] [CrossRef]
- Miller, J.D.; Wan, R.Y.; Diaz, X. Preg-robbing gold ores. In Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2016; p. 885. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Preg-robbing phenomena in the thiosulphate leaching of gold ores. Miner. Eng. 2001, 14, 1387. [Google Scholar] [CrossRef]
- Pan, Z.; Xiong, J.; Cui, Y.; Wei, Q.; Jia, W.; Zhang, Z.; Qin, W. Effect mechanism of CMs on the flotation separation of lead–zinc ore. Sep. Purif. Technol. 2022, 294, 121101. [Google Scholar] [CrossRef]
- Niu, H.; Yang, H.; Tong, L.; Zhong, S.; Liu, Y. Spectral study of humic substance extract from pressurized oxidizing slag of Carlin-typed gold deposit. J. Phys. Conf. Ser. 2019, 1347, 012027. [Google Scholar] [CrossRef]
- Moroz, T.N.; Ponomarchuk, V.A.; Goryainov, S.V.; Palchik, N.A.; Edwards, H.G.; Zhmodik, S.M. Raman spectra of natural CMs from a black shale formation. J. Raman Spectrosc. 2015, 46, 959. [Google Scholar] [CrossRef]
- Heckmann, A.; Fromm, O.; Rodehorst, U.; Münster, P.; Winter, M.; Placke, T. New insights into electrochemical anion intercalation into CMs for dual-ion batteries: Impact of the graphitization degree. Carbon 2018, 131, 201. [Google Scholar] [CrossRef]
- Morcos, I. On contact angle and dispersion energy of the cleavage graphite/water system. J. Colloid Interface Sci. 1970, 34, 469. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Depci, T.; Assemi, S.; Prisbrey, K.; Miller, J.D. The nature of graphene surfaces as determined from the wettability studies of basal and edge planes. ECS Trans. 2015, 66, 45. [Google Scholar] [CrossRef]
- Sime, F.M.; Jin, J.; Wang, X.; Wick, C.D.; Miller, J.D. Characterization and simulation of graphite edge surfaces for the analysis of CM separation from sulphide ores by flotation. Miner. Eng. 2022, 182, 107590. [Google Scholar] [CrossRef]
- Spieth, V. Zechstein Kupferschiefer at Spremberg and Related Sites: Hot Hydrothermal Origin of the Polymetallic Cu–Ag–Au Deposit. Ph.D. Thesis, Universität Stuttgart, Stuttgart, Germany, 2019. [Google Scholar] [CrossRef]
- Smith, T.; Lin, D.; Lacouture, B.; Anderson, G. Removal of organic carbon with a Jameson Cell at Red Dog Mine. In Proceedings of the 40th Annual Canadian Mineral Processors Conference, Ottawa, ON, Canada, 22–24 January 2008; Available online: https://www.glencoretechnology.com/.rest/api/v1/documents/ed2ef91249f47c139df4a66f1f12ec0e/Removal-of-Organic-Carbon-with-a-Jameson-Cell-at-R.pdf (accessed on 17 August 2025).
- Konieczny, A.; Pawlos, W.; Krzeminska, M.; Kaleta, R.; Kurzydlo, P. Evaluation of organic carbon separation from copper ore by pre-flotation. Physicochem. Probl. Miner. Process. 2013, 49, 189. [Google Scholar] [CrossRef]
- Gredelj, S.; Zanin, M.; Grano, S.R. Selective flotation of carbon in the Pb–Zn carbonaceous sulphide ores of Century Mine, Zinifex. Miner. Eng. 2009, 22, 279. [Google Scholar] [CrossRef]
- Brooke, K.; Bullock, N.; Harvey, R.; O’Sullivan, R.; Phan, C.; Tan, P.; Telford, P.; Edgar, M. Mount Isa and Townsville operations. In Australasian Mining and Metallurgical Operating Practices, 3rd ed.; Rankin, W.J., Ed.; AusIMM: Carlton, Australia, 2013; Volume 1, p. 931. [Google Scholar]
- Martinez-Esparza, G. Productive Versus Non-Productive Porphyry Systems Surrounding the Peñasquito Diatreme-Porphyry System. Ph.D. Thesis, University of Nevada, Reno, NV, USA, 2020; p. 288. Available online: https://search.proquest.com/openview/203d4bda5c89adb9da0e377d657fb9f4/1?pq-origsite=gscholar&cbl=51922&diss=y (accessed on 17 August 2025).
- Polat, M.; Polat, H.; Chander, S. Physical and chemical interactions in coal flotation. Int. J. Miner. Process. 2003, 72, 199–213. [Google Scholar] [CrossRef]
- Xu, M.; Li, C.; Wang, Y.; Zhang, H. Investigation on mechanism of intensifying coal fly ash froth flotation by pretreatment of non-ionic surfactant. Fuel 2019, 254, 115601. [Google Scholar] [CrossRef]
- Kadagala, M.R.; Nikkam, S.; Tripathy, S.K. A review on flotation of coal using mixed reagent systems. Miner. Eng. 2021, 173, 107217. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Beattie, D.A. Adsorption of tailored carboxymethyl cellulose polymers on talc and chalcopyrite: Correlation between coverage, wettability, and flotation. Miner. Eng. 2010, 23, 985–993. [Google Scholar] [CrossRef]
- Chimonyo, W.; Fletcher, B.; Peng, Y. The differential depression of an oxidised starch on the flotation of chalcopyrite and graphite. Miner. Eng. 2020, 146, 106114. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Peng, Y. A new approach to selectively reject naturally hydrophobic gangue in the flotation of base metal sulphide minerals. Min. Metall. Explor. 2021, 38, 713–720. [Google Scholar] [CrossRef]
- Glembozkii, V.A.; Dmitrieva, G.M.; Sorokin, M.M. Nonpolar Reagents and Their Effect during Flotation; Nauka: Moscow, Russia, 1968. (In Russian) [Google Scholar]
- State Standard 2408.3-90; Solid Fuel. Methods for Determination of Oxygen; Standartinform: Moscow, Russia, 1990; 16p. (In Russian)
- State Standard 11022-95; Solid Mineral Fuels. Methods for Determination of Ash; Standartinform: Moscow, Russia, 2006; 8p. (In Russian)
- Fuerstenau, M.C.; Clifford, K.L.; Kuhn, M.C. The role of zinc–xanthate precipitation in sphalerite flotation. Int. J. Miner. Process. 1974, 1, 307–318. [Google Scholar] [CrossRef]
- Pugh, R.J. Macromolecular organic depressants in sulphide flotation—A review, 1. Principles, types and applications. Int. J. Miner. Process. 1989, 25, 101–130. [Google Scholar] [CrossRef]
- Sosipatorov, A.I.; Panchenko, G.M.; Vysotin, V.V.; Vinokurova, M.A.; Chikin, A.Y. Application prospects of domestic depressor reagent under carbonaceous gold-bearing ore flotation. Proc. Irkutsk. State Tech. Univ. 2018, 22, 184–193. (In Russian) [Google Scholar]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part I. Adsorption studies. Miner. Eng. 2007, 20, 600–608. [Google Scholar] [CrossRef]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Uribe, L.; Hernández, V.; Vidal, C.; Mendonça, R.T. Assessment of the use of lignosulfonates to separate chalcopyrite and molybdenite by flotation. Powder Technol. 2020, 359, 216–225. [Google Scholar] [CrossRef]
- Kelebek, S.; Yoruk, S.; Smith, G.W. Wetting behaviour of molybdenite and talc in lignosulphonate/MIBC solutions and their separation by flotation. Sep. Sci. Technol. 2001, 36, 145–157. [Google Scholar] [CrossRef]
- Nechiporenko, A.P. Acid-Base Properties of the Surface of Solid Substances: Methodical Instructions; LTI im. Lensoveta: Leningrad, Russia, 1989. (In Russian) [Google Scholar]
- Nechiporenko, A.P. Donor-Acceptor Properties of the Surface of Solid-Phase Systems. In Indicator Method: Tutorial; Lan: St. Petersburg, Russia, 2017. (In Russian) [Google Scholar]
- Atchabarova, A.A.; Abdimomyn, S.K.; Abduakhytova, D.A.; Zhigalenok, Y.R.; Tokpayev, R.R.; Kishibayev, K.K.; Khavaza, T.N.; Kurbatov, A.P.; Zlobina, Y.V.; Djenizian, T.J. Role of carbon material surface functional groups on their interactions with aqueous solutions. J. Electroanal. Chem. 2022, 922, 116707. [Google Scholar] [CrossRef]
- Tanabe, K. Solid Acids and Bases: Their Catalytic Properties; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Mao, L.; Yoon, R.-H. Predicting flotation rates using a rate equation derived from first principles. Int. J. Miner. Process. 1997, 51, 171–181. [Google Scholar] [CrossRef]
- Kijewski, P.; Leszczyński, R. Węgiel organiczny w rudach miedzi—Znaczenie i problemy. Zesz. Nauk. Inst. Gospod. Surowcami Miner. Energią PAN 2010, 79, 131–146. (In Polish) [Google Scholar]
- Chimonyo, W.; Peng, Y. Review of the Influence of Native and Modified Biopolymers on Carbonaceous Gangue Depression in Selective Flotation. Miner. Process. Extr. Metall. Rev. 2025, 46, 593–610. [Google Scholar] [CrossRef]
- Yergeshev, A.; Tokpayev, R.; Karmeeva, M.; Khavaza, T.; Yergesheva, N.; Atchabarova, A.; Nauryzbayev, M.; Ignatkina, V. Technological Properties Contrast of Galena, Sphalerite, CM and Choice of Flotation Technology. Minerals 2025, 15, 883. [Google Scholar] [CrossRef]



| Sample | C ±2.0 | H ±3.1 | N ±0.2 | O ±0.5 | S ±0.2 | Ash Content A, %, ±0.5 | Specific Surface Area, m2/g, ±2.00 | |
|---|---|---|---|---|---|---|---|---|
| 1 | Wood charcoal (W_C) | 81.1 | 4.1 | 0.3 | 9.9 | 0.2 | 4.4 | 10.34 |
| 2 | Carbon (A_C) | 84.0 | 5.0 | 1.5 | 7.0 | 0.3 | 2.2 | 318.10 |
| 3 | Graphite (G_C) | 98.0 | - | - | 1.0 | - | 1.0 | 9.98 |
| 4 | Li mica (Li_C) | 2.5 | - | 1.0 | 6.0 | 0.1 | 90.5 | 23.36 |
| 5 | Pb-Zn-S ore (S_C) | 10.2 | - | - | 4.7 | 0.7 | 84.4 | 12.93 |
| CM | O ±2.0 | Si ±1.0 | Mg ±0.6 | Al ±0.2 | Ca ±3.0 | Zn ±2.3 | Fe ±2.6 | K ±0.6 | Na ±0.6 | S ±1.7 |
|---|---|---|---|---|---|---|---|---|---|---|
| Li_C | 65.1 | 10.1 | 0.8 | 5.8 | 6.7 | - | 5.2 | 1.7 | 0.9 | 3.7 |
| S_C | 56.9 | 5.1 | 13.3 | 0.3 | 5.0 | 5.2 | 4.6 | - | 3.6 | 6.0 |
| Indicator | Formula | Molecular Mass | λ, nm | pKa | Active Centre Type |
|---|---|---|---|---|---|
| Dinitroaniline | C6H5N3O4 | 183.12 | 340 | −4.4 | Lewis base |
| Brilliant green | C27H34N2O4S | 482.64 | 610 | 1.3 | Brønsted acid |
| Bromphenol blue | C19H10Br4O5S | 669.97 | 590 | 4.1 | |
| Bromocresol purple | C21H16Br2O5S | 540.22 | 590 | 6.4 | |
| Bromthymol blue | C27H28Br2O5S | 624.39 | 430 | 7.3 | Brønsted base |
| Indigo carmine | C16H8N2Na2O8S2 | 466.36 | 610 | 12.8 | |
| M-Dinitrobenzene | C6H4N2O4 | 168.11 | 240 | 16.8 | Lewis acid |
| Flotation Agent | Frother–Pine Oil | Diesel Oil and Frother | Butyl Xanthate and Frother | Depressor (PL) |
|---|---|---|---|---|
| Concentration, mg/L | 10 | 20 and 10 | 20 and 10 | 50 and 500 |
| C ±1.0 | O ±1.0 | Si ±0.5 | Mg ±0.5 | Al ±0.5 | Ca ±0.5 | P ±0.1 | N ±0.3 | F ±0.5 | Zn ±0.5 | Fe ±0.3 | K ±0.1 | Na ±0.1 | S ±0.2 | Pb ±0.1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W_C | 87.8 | 10.2 | - | - | - | 0.7 | 0.1 | 0.9 | - | - | - | 0.3 | - | - | - |
| A_C | 85.1 | 12.0 | 1.4 | - | 0.8 | 0.7 | - | - | - | - | - | - | - | - | - |
| G_C | 98.5 | 1.5 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Li_C | 18.9 | 49.3 | 12.9 | 5.9 | 4.2 | 2.3 | - | 1.1 | 3.6 | - | 0.7 | 1.0 | 0.1 | - | - |
| S_C | 49.2 | 34.5 | 5.1 | 2.5 | 1.9 | 2.5 | - | - | - | 2.8 | 0.4 | - | - | 0.7 | 0.4 |
| Indicator | Active Centre Type | pKa | gpKa, μmol/m2, ±0.25% | ||||
|---|---|---|---|---|---|---|---|
| W_C | A_C | G_C | Li_C | S_C | |||
| Dinitroaniline | Lewis base | −4.4 | 2.45 | 0.77 | 0.08 | 0.24 | 3.84 |
| Brilliant green | Brønsted acid | 1.3 | 0.68 | 0.06 | 0.33 | 0.04 | 0.21 |
| Bromphenol blue | Brønsted acid | 4.1 | 0.06 | 0.17 | 0.02 | 0.13 | 0.37 |
| Bromocresol purple | Brønsted acid | 6.4 | 0.97 | 3.87 | 0.70 | 0.54 | 0.27 |
| Bromthymol blue | Brønsted base | 7.3 | 0.31 | 1.30 | 0.36 | 0.68 | 4.40 |
| Indigo carmine | Brønsted base | 12.8 | 1.95 | 0.41 | 0.53 | 3.63 | 31.34 |
| M-Dinitrobenzene | Lewis acid | 16.8 | 1.61 | 0.33 | 0.21 | 3.86 | 4.27 |
| Sample | Flotation Parameters k, min−1/R2/εmax | |||||
|---|---|---|---|---|---|---|
| Pine Oil | Diesel Oil + Pine Oil | ButX + Pineoil | PL + Diesel Oil + Pine Oil | |||
| 50 mg/L | 500 mg/L | |||||
| 1 | W_C | 0.53/0.90/93.51 | 0.44/0.98/92.37 | 0.46/0.99/90.57 | 0.69/0.95/98.00 | 0.50/0.87/95.7 |
| 2 | A_C | 0.23/0.94/68.43 | 0.27/0.90/77.67 | 0.29/0.99/83.92 | 0.26/1.00/72.83 | 0.24/1.00/69.50 |
| 3 | G_C | 1.08/0.94/99.39 | 1.95/0.93/99.98 | 1.21/0.88/99.40 | 0.94/0.92/99.39 | 0.84/0.94/98.98 |
| 4 | Li_C | 0.18/0.96/61.10 | 0.30/1.00/78.57 | 0.32/0.97/79.50 | 0.23/0.99/68.29 | 0.17/0.99/56.53 |
| 5 | S_C | 0.15/0.87/81.15 | 0.33/0.96/83.02 | 0.36/0.98/84.07 | 0.26/1.00/72.00 | 0.14/0.99/52.54 |
| Reagent Scheme | pHinitial | pHfinal | ||||
|---|---|---|---|---|---|---|
| W_C | A_C | G_C | Li_C | S_C | ||
| pine oil | 5.6 ± 0.4 | 6.20 | 7.30 | 5.40 | 8.56 | 5.53 |
| diesel oil + pine oil | 5.6 ± 0.4 | 6.20 | 7.25 | 5.70 | 8.61 | 5.66 |
| ButX + pineoil | 5.6 ± 0.4 | 5.96 | 7.40 | 5.76 | 8.56 | 5.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yergeshev, A.; Tokpayev, R.; Karmeeva, M.; Khavaza, T.; Nauryzbayev, M.; Ignatkina, V. Composition, Properties, and Flotation Reagent Regimes of Carbonaceous Material. Minerals 2025, 15, 974. https://doi.org/10.3390/min15090974
Yergeshev A, Tokpayev R, Karmeeva M, Khavaza T, Nauryzbayev M, Ignatkina V. Composition, Properties, and Flotation Reagent Regimes of Carbonaceous Material. Minerals. 2025; 15(9):974. https://doi.org/10.3390/min15090974
Chicago/Turabian StyleYergeshev, Akim, Rustam Tokpayev, Marina Karmeeva, Tamina Khavaza, Mikhail Nauryzbayev, and Vladislava Ignatkina. 2025. "Composition, Properties, and Flotation Reagent Regimes of Carbonaceous Material" Minerals 15, no. 9: 974. https://doi.org/10.3390/min15090974
APA StyleYergeshev, A., Tokpayev, R., Karmeeva, M., Khavaza, T., Nauryzbayev, M., & Ignatkina, V. (2025). Composition, Properties, and Flotation Reagent Regimes of Carbonaceous Material. Minerals, 15(9), 974. https://doi.org/10.3390/min15090974









