The Effect of Ore Pre-Heating on the Operation of a 300 kVA Submerged Arc Furnace for High Carbon Ferromanganese Alloy Production—Pilot Study Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.2.1. Chemical Composition
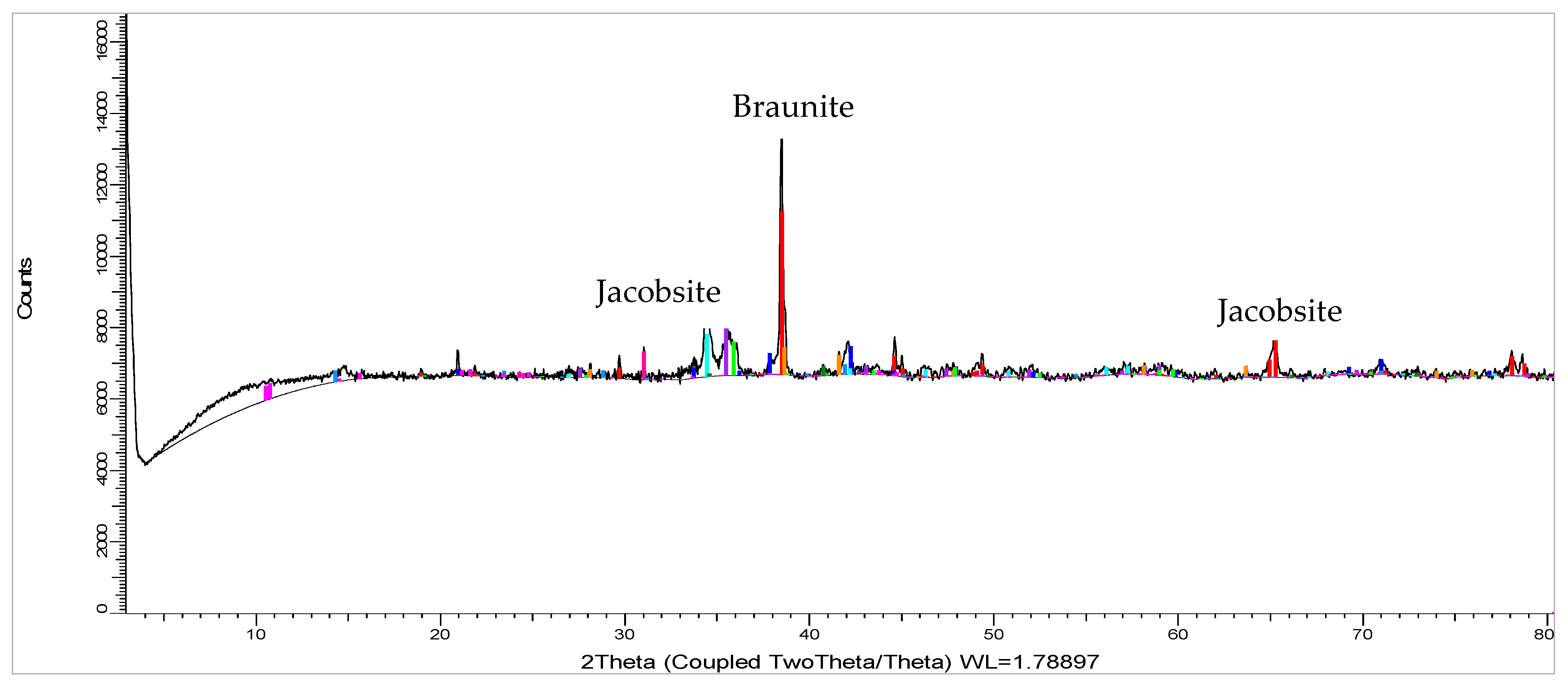
2.2.2. Phase Chemical Composition
2.3. Experimental Equipment
2.4. Experimental Procedure
3. Results and Discussion
3.1. Heat Losses Estimation
3.2. Specific Energy Requirement (SER)
3.3. CO/CO2 Emissions
3.4. Overall Mass Balance
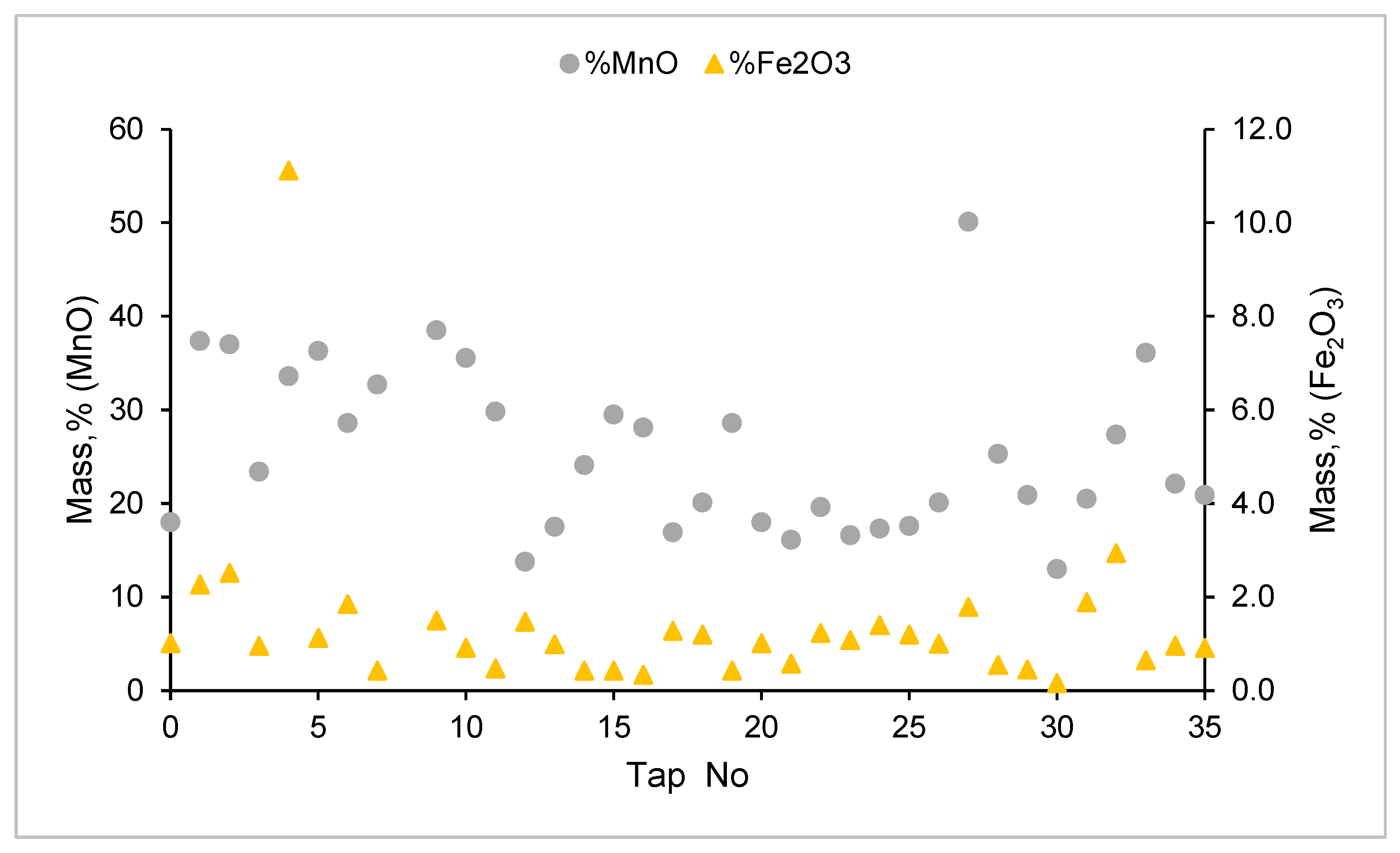
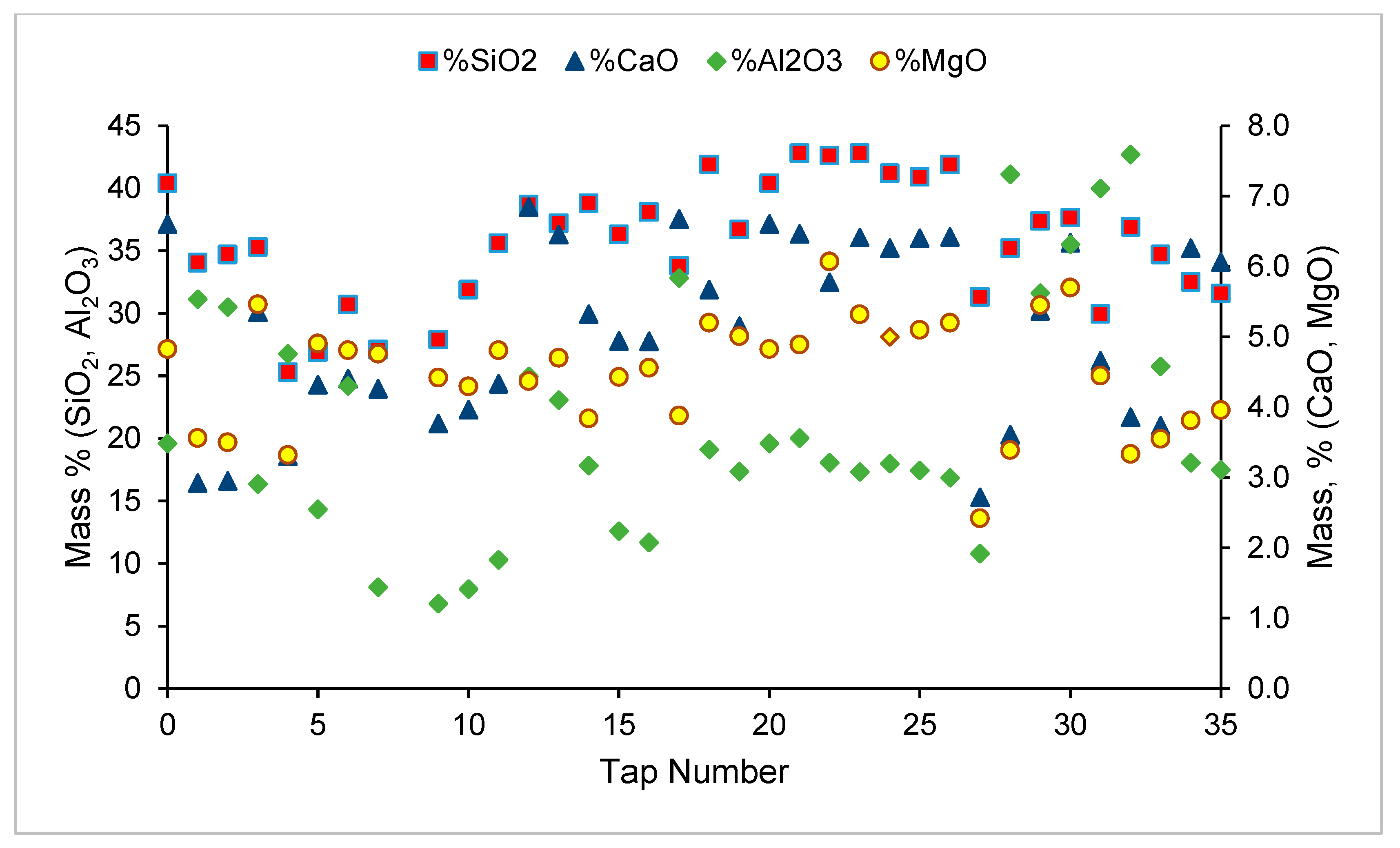
3.5. Elemental Mass Balance
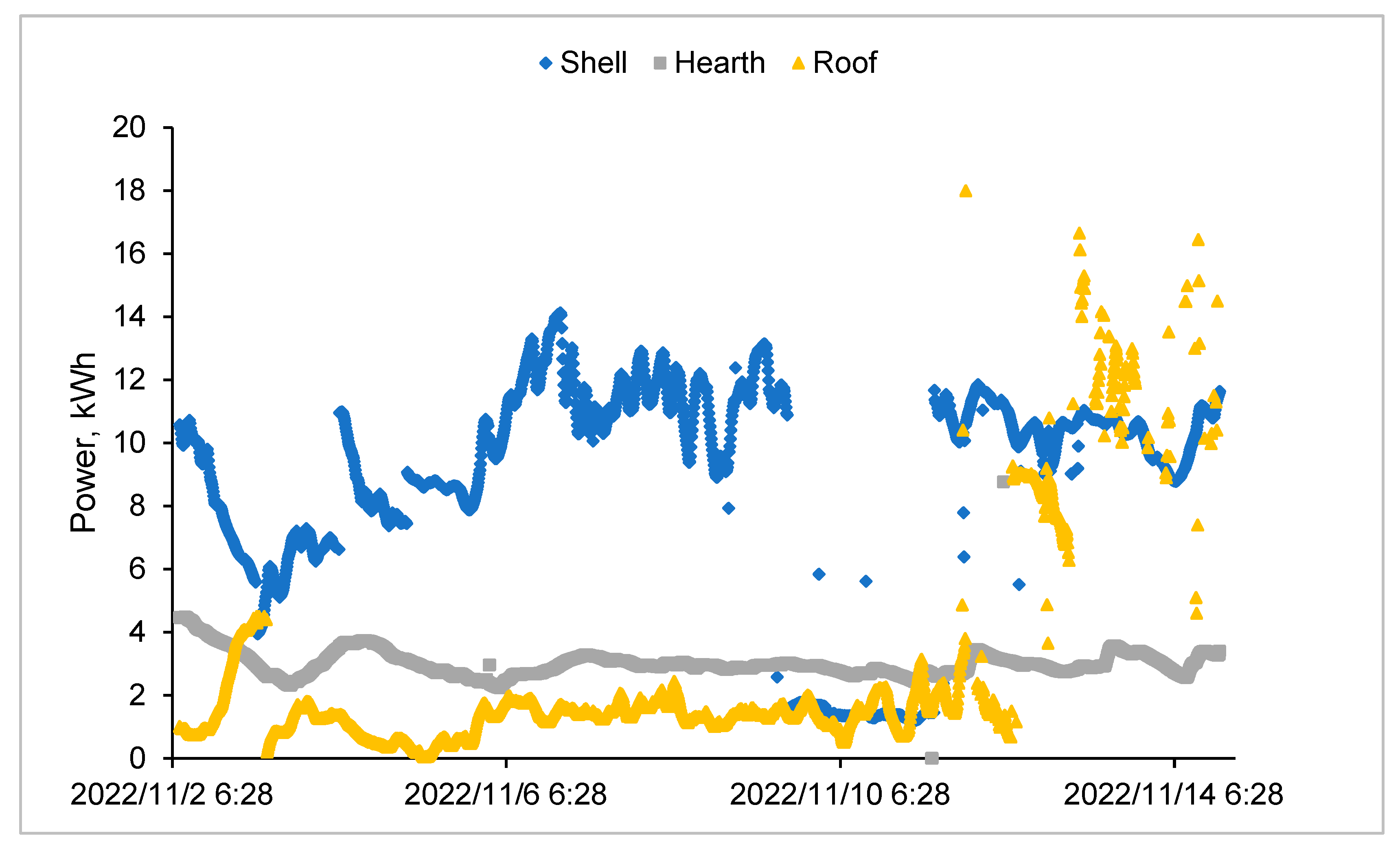
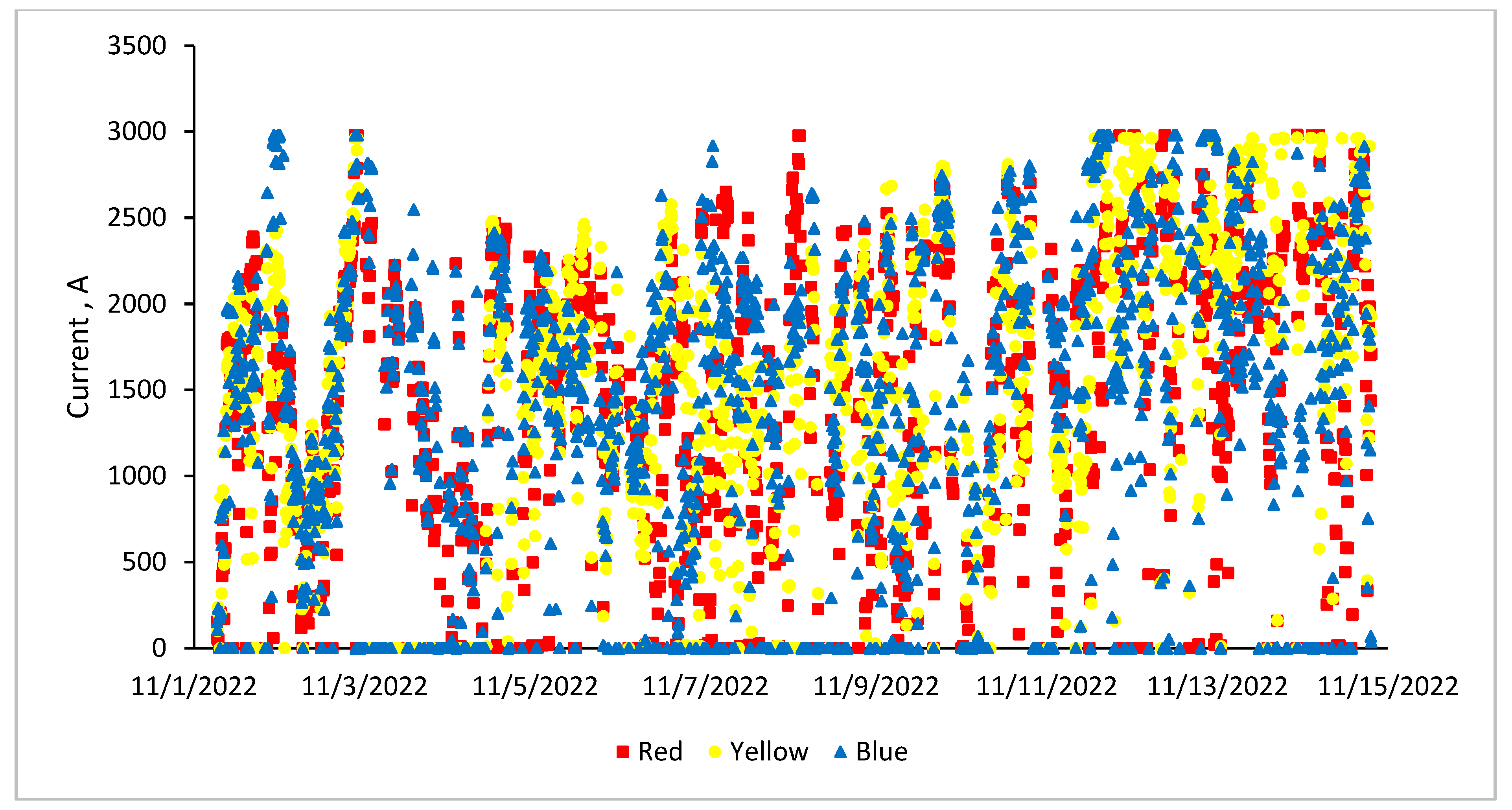
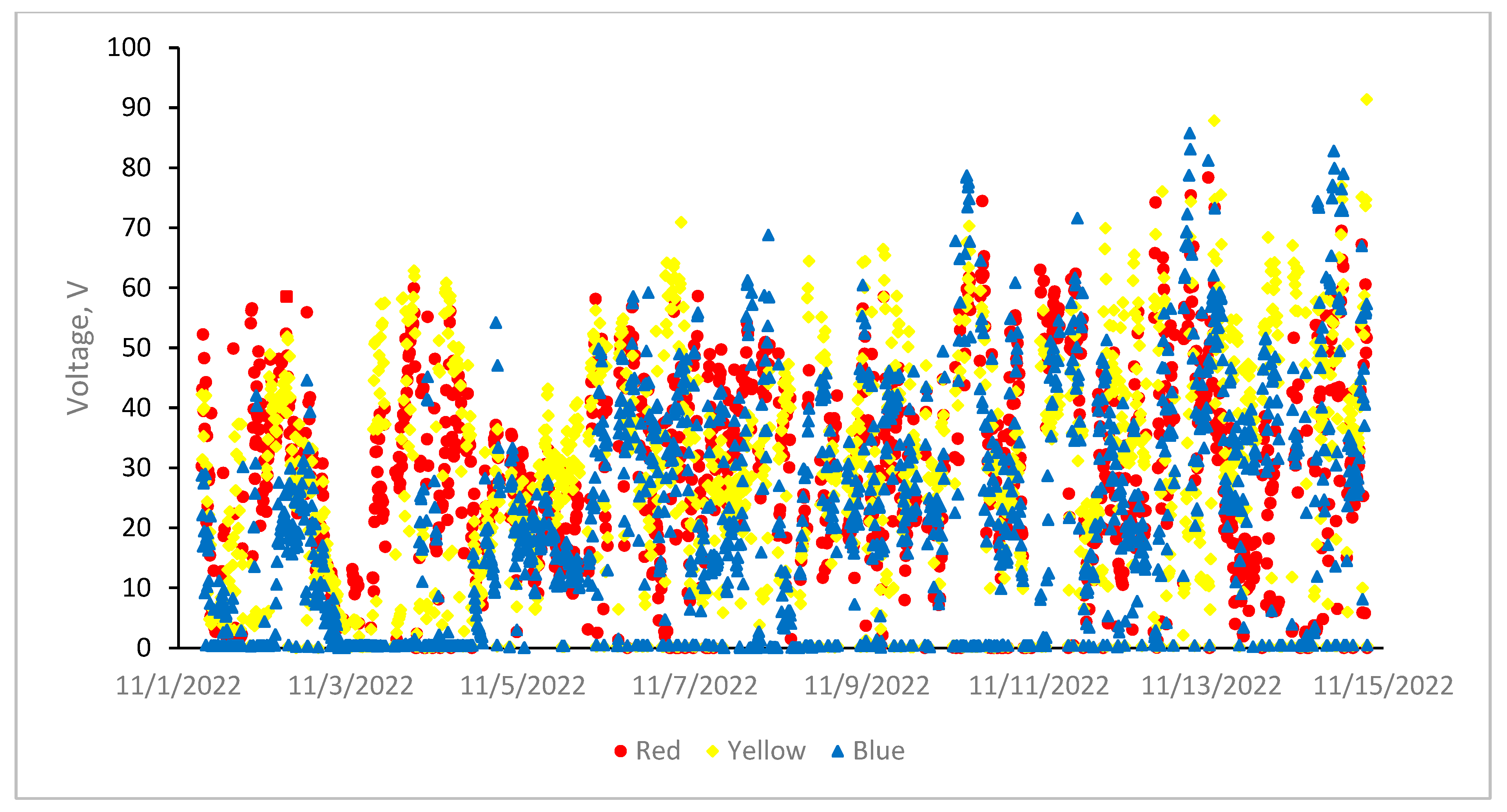
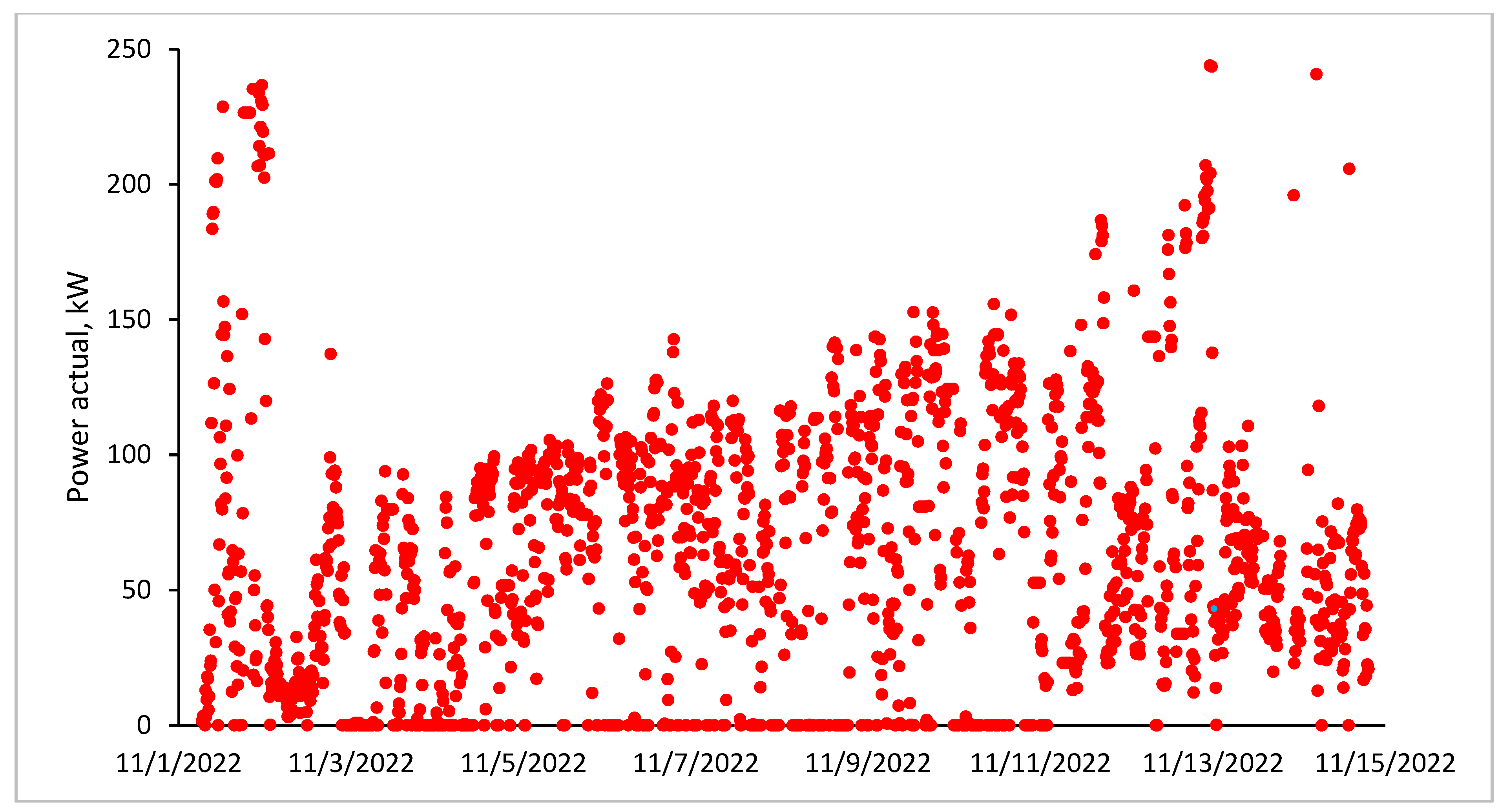
3.6. Furnace Operating Parameters (Current, Voltage and Power)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, Z.; Shao, G.; Ge, Y.; Wang, S.; Xie, Z.; Zhu, Q.; Li, H. From laboratory research to industrial application: A green technology of fluidized mineral processing for manganese dioxide ore reduction. Green Chem. Eng. 2020, 1, 40–47. [Google Scholar] [CrossRef]
- Mchabe, D.; Tsebe, S.; Matinde, E. Evaluation of the Effects of Fluidization Conditions on Hydrogen Reduction in Manganese Ore Fines. Minerals 2025, 15, 368. [Google Scholar] [CrossRef]
- Banda, W.K.; Steenkamp, J.D.; Matinde, E. An investigation into the wear mechanisms of carbon-and silicon carbide-based refractory materials by silicomanganese alloy. J. S. Afr. Inst. Min. Metall. 2020, 120, 333–344. [Google Scholar] [CrossRef]
- Gordon, Y.; Nell, J.; Yaroshenko, Y. Manganese ore thermal treatment prior to smelting. KnE Eng. 2018, 3, 71–86. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, L.; Wang, G.; Luo, C.; Shun, Y.; Yan, K. Novel reduction roasting and leaching method for manganese dioxide ore using FeP slag as the reductant. Hydrometallurgy 2019, 189, 105113. [Google Scholar] [CrossRef]
- Julia, N.; Hecquet, A.; Nussbaum, G.; Blancher, S.; Amalric, A. Pre-heating manganese ore in a pilot-scale rotary kiln. In Proceedings of the 16th International Ferroalloys Congress (INFACON XVI), Trondheim, Norway, 27–29 September 2021. [Google Scholar]
- PREMA. Available online: https://www.aspire2050.eu/prema (accessed on 24 July 2024).
- Olsen, S.E.; Olsen, S.; Tangstad, M.; Lindstad, T. Production of Manganese Ferroalloys; Tapir Academic Press: Trondheim, Norway, 2007. [Google Scholar]
- Ringdalen, E.; Gjøvik, J.E.; Larssen, T.A.; Tangstad, M. Pretreatment of manganese ores in different gas-atmospheres-a method to reduce energy consumption and CO2 emissions in Mn-alloy production. In Proceedings of the 16th International Ferro-Alloys Congress (INFACON XVI), Trondheim, Norway, 27–29 September 2021. [Google Scholar]
- Pegels, A. Renewable energy in South Africa: Potentials, barriers and options for support. Energy Policy 2010, 38, 4945–4954. [Google Scholar] [CrossRef]
- Matinde, E.; Steenkamp, J.D. Metallurgical Overview and Production of Slags; Royal Society of Chemistry: Cambridge, UK, 2021. [Google Scholar]
- Lubkoll, M.; Hockaday, S.A.C.; Harms, T.M.; von Backström, T.W.; Amsbeck, L.; Buck, R. Integrating solar process heat into manganese ore pre-heating. In Proceedings of the 5th Southern African Solar Energy Conference, SASEC 2018, Durban, South Africa, 25–27 June 2018. [Google Scholar]
- Sambo, S.N.; Hockaday, L.; Seodigeng, T.; Reynolds, Q.G. Numerical and Experimental Study of Packed Bed Heat Transfer on the Preheating of Manganese Ore with Air up to 600 °C. Metals 2025, 15, 269. [Google Scholar] [CrossRef]
- Kazdal, T.; Haas-Wittmuess, R.; Richter, S.; Lang, S.; Binder, C.; Reuter, M. Process design for the pre-treatment of manganese ores. In Proceedings of the 16th International Ferroalloys Congress (INFACON XVI), Trondheim, Norway, 27–29 September 2021. [Google Scholar]
- Singh, V.; Reddy, K.V.K.; Tripathy, S.K.; Kumari, P.; Dubey, A.K.; Mohanty, R.; Satpathy, R.R.; Mukherjee, S. A sustainable reduction roasting technology to upgrade the ferruginous manga-nese ores. J. Clean. Prod. 2021, 284, 124784. [Google Scholar] [CrossRef]
- Moholwa, M.S.; Tsebe, S.P.; Hayman, D.A.; Bezuidenhout, P.J.A.; Sitefane, M.B.; Steenkamp, J.D. Effect of ore pre-heating on furnace operation in high carbon ferromanganese production—lessons learnt from pilot-scale test work. In TMS Annual Meeting & Exhibition; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 237–252. [Google Scholar]
- Hockaday, L.; Dinter, F.; Reynolds, Q.G.; McGregor, C. Evaluation of solar thermal pretreatment of car-bonate-rich manganese ores in high-carbon ferromanganese production through dynamic process modelling. J. S. Afr. Inst. Min. Metall. 2024, 124, 763–775. [Google Scholar] [CrossRef]






| Mn | Fe | MgO | Al2O3 | SiO2 | CaO | C | S | Fe2O3 | Vol. | Ash | MC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ore #1 | 36.7 | 3.8 | 3.3 | 0.5 | 5.2 | 15.4 | ||||||
| Ore #2 | 37.4 | 4.5 | 3.1 | 0.6 | 6.2 | 15.2 | ||||||
| Reductant | 0.2 | 4.1 | 5.9 | 0.5 | 84.4 | 1.12 | 0.93 | 2.5 | 12.4 | 0.2 | ||
| Quartz | 99.7 |
| Mineral | Ideal Chemical Formula | Ore #1 | Ore #2 |
|---|---|---|---|
| Quartz | SiO2 | 1.2 | 1.3 |
| Braunite | Mn6SiO12 | 32.2 | 34.3 |
| Hausmannite | Mn2O4 | 14.5 | 20.8 |
| Kutnohorite | Ca(Mn,Mg,Fe)(CO3)2 | 18.4 | 12.0 |
| Jacobsite | (Mn,Fe,Mg)(Fe,Mn)2O4 | 1.2 | 3.8 |
| Calcite | CaCO3 | 14.4 | 14.5 |
| Dolomite | CaMg(CO3)2 | 12.4 | 10.2 |
| Hematite | Fe2O3 | 3.1 | 3.2 |
| Lizardite | Mg3Si2O5(OH)4 | <1 | <1 |
| Todorokite | (Na,Ca,K)2(Mn)6O12·3–4.5(H2O) | 2.5 | - |
| Reading | CO | CO2 | H2 | O2 | N2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cold | Hot | Cold | Hot | Cold | Hot | Cold | Hot | Cold | Hot | |
| 1 | 20.2 | 25.4 | 15.6 | 9.96 | 6.54 | 6.99 | 2.41 | 2.23 | 12.6 | 11.6 |
| 2 | 19.5 | 24.2 | 14.2 | 9.09 | 5.96 | 7.03 | 2.59 | 2.44 | 13.2 | 12.4 |
| 3 | 21.5 | 25.0 | 15.7 | 9.53 | 6.12 | 7.11 | 2.96 | 2.35 | 12.0 | 12.2 |
| 4 | 19.7 | 23.3 | 15.8 | 10 | 5.99 | 7.56 | 3.02 | 2.63 | 12.0 | 12.0 |
| Standard dev | 0.9 | 0.9 | 0.8 | 0.4 | 0.3 | 0.3 | 0.3 | 0.2 | 0.6 | 0.3 |
| Average | 20.2 | 24.5 | 15.3 | 9.65 | 6.15 | 7.17 | 2.75 | 2.41 | 12.5 | 12.1 |
| Feed | IN, kg | Products | OUT, kg |
|---|---|---|---|
| UMK ore | 4651 | Slag | 4087 |
| Kudumane ore | 3026 | Metal | 1733 |
| Quartz | 1081 | Off-gas Dust | 510 |
| Coke | 1667 | Dig-out Metal | 80 |
| Start-up Heel | 125 | Dig-out (unprocessed material) | 498 |
| Electrodes | 566 | Electrodes | 96.3 |
| Total | 11,115 | Total | 7004 |
| Recovery, mass % | ||||||
| Product stream | Mn | Fe | Si | C | Ca | Mg |
| Metal | 48 | 86 | 5 | 4 | 0 | 0 |
| Slag | 31 | 15 | 75 | 0 | 81 | 49 |
| Dust | 7 | 1 | 5 | 0 | 3 | 13 |
| Total | 86 | 103 | 85 | 4 | 85 | 62 |
| Distribution, mass % | ||||||
| Product stream | Mn | Fe | Si | C | Ca | Mg |
| Metal | 55 | 84 | 6 | 98 | 0 | 0 |
| Slag | 37 | 15 | 88 | 2 | 96 | 79 |
| Dust | 8 | 1 | 6 | 0 | 4 | 21 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moholwa, M.S.; Tsebe, S.P.; Hayman, D.; Moloane, S.; Steenkamp, J.; Sitefane, M.; Bezuidenhout, D. The Effect of Ore Pre-Heating on the Operation of a 300 kVA Submerged Arc Furnace for High Carbon Ferromanganese Alloy Production—Pilot Study Results. Minerals 2025, 15, 968. https://doi.org/10.3390/min15090968
Moholwa MS, Tsebe SP, Hayman D, Moloane S, Steenkamp J, Sitefane M, Bezuidenhout D. The Effect of Ore Pre-Heating on the Operation of a 300 kVA Submerged Arc Furnace for High Carbon Ferromanganese Alloy Production—Pilot Study Results. Minerals. 2025; 15(9):968. https://doi.org/10.3390/min15090968
Chicago/Turabian StyleMoholwa, Matale Samuel, Sello Peter Tsebe, Derek Hayman, Sanda Moloane, Joalet Steenkamp, Martin Sitefane, and Driaan Bezuidenhout. 2025. "The Effect of Ore Pre-Heating on the Operation of a 300 kVA Submerged Arc Furnace for High Carbon Ferromanganese Alloy Production—Pilot Study Results" Minerals 15, no. 9: 968. https://doi.org/10.3390/min15090968
APA StyleMoholwa, M. S., Tsebe, S. P., Hayman, D., Moloane, S., Steenkamp, J., Sitefane, M., & Bezuidenhout, D. (2025). The Effect of Ore Pre-Heating on the Operation of a 300 kVA Submerged Arc Furnace for High Carbon Ferromanganese Alloy Production—Pilot Study Results. Minerals, 15(9), 968. https://doi.org/10.3390/min15090968





