Process Mineralogy Study and Flotation Testwork of a Complex Lead–Gold Rougher Concentrate
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.1.1. Sample
2.1.2. Reagent
2.2. Methods
2.2.1. Process Mineralogy Study
2.2.2. Flotation
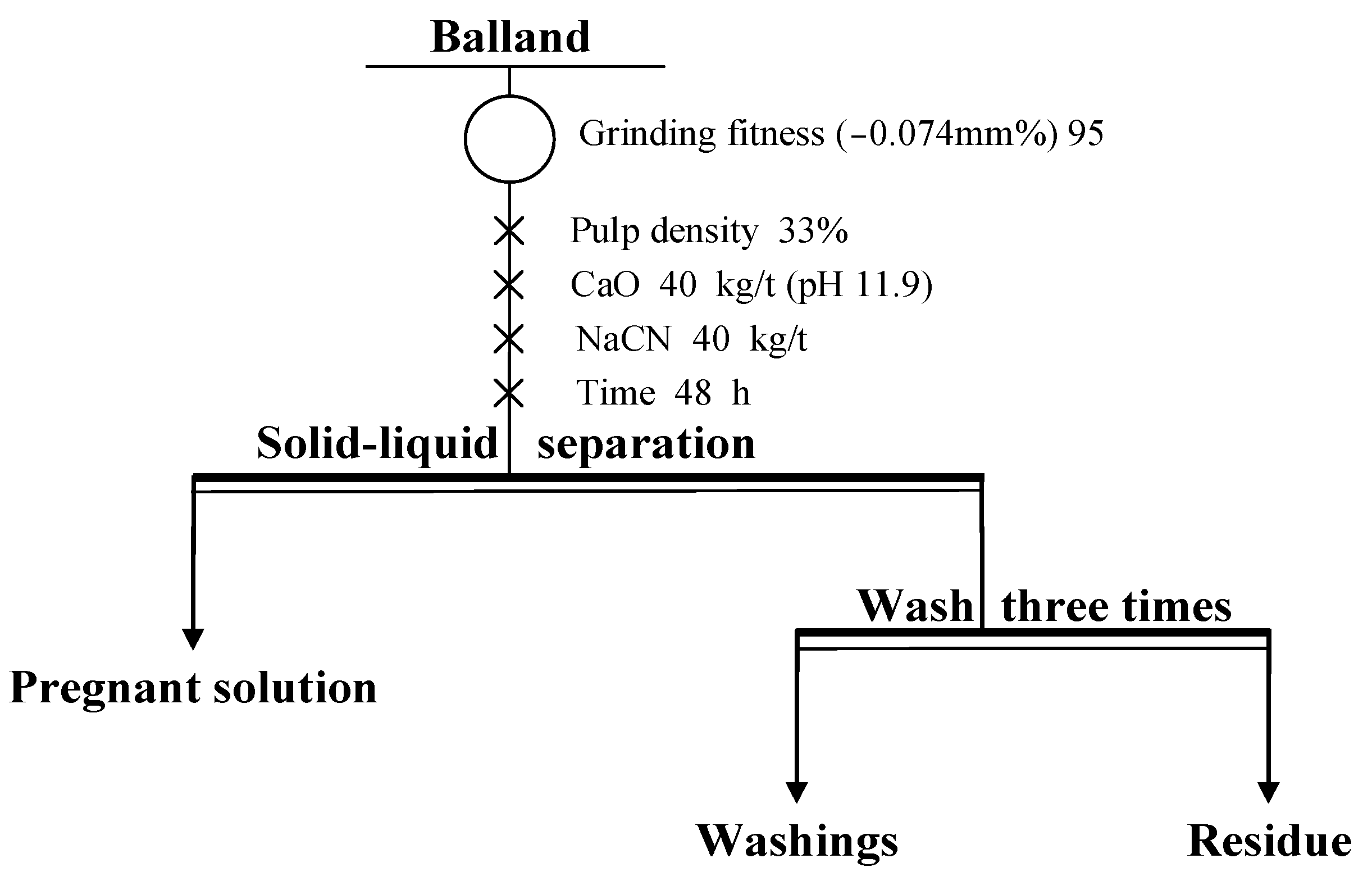
2.2.3. Leaching
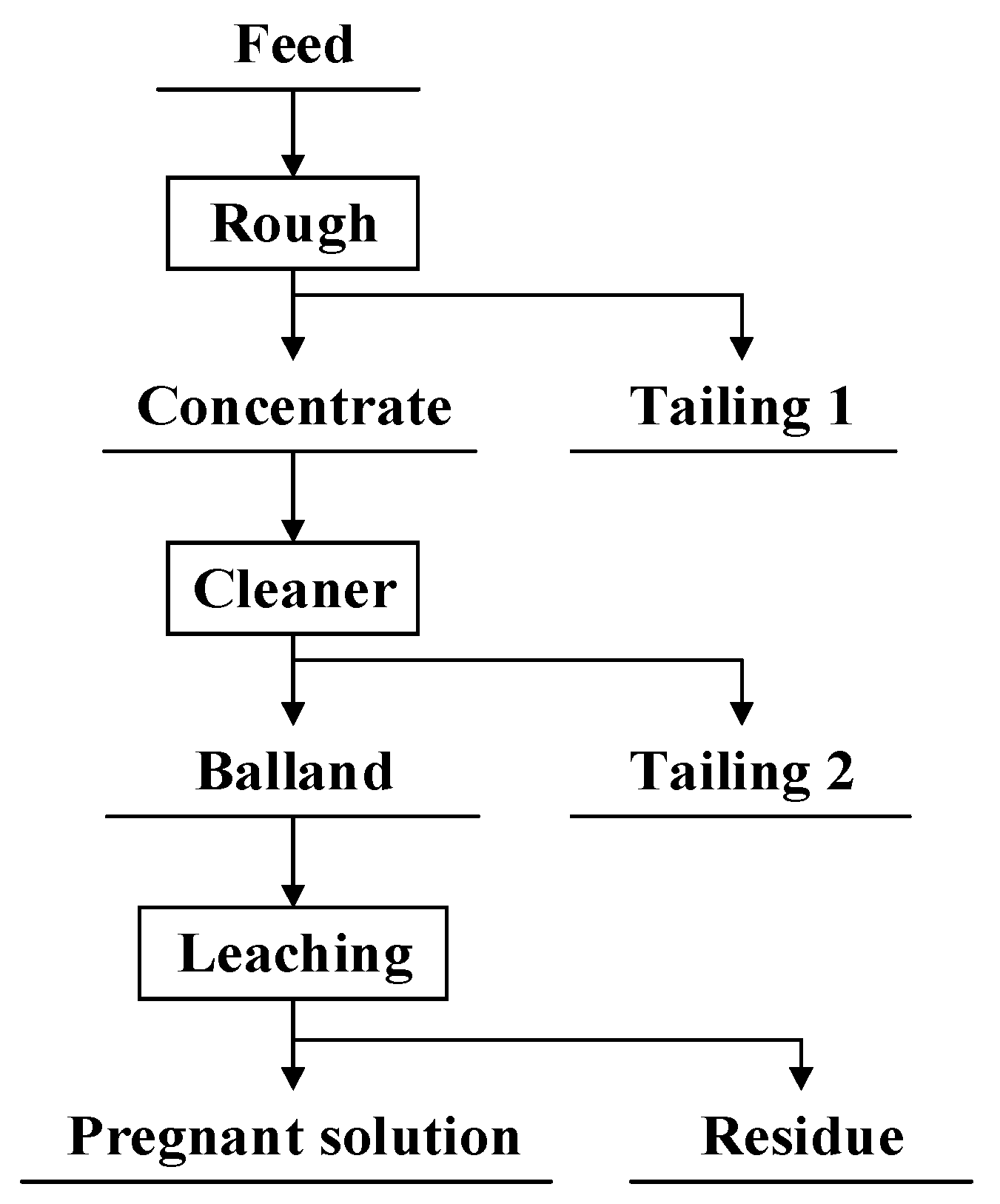
2.3. Process Flow Chart
3. Results and Discussion
3.1. Process Mineralogy Analysis
3.1.1. Chemical Composition
3.1.2. Composition and Mineralogical Characteristics
Composition and Content
Particle Size Distribution of Major Metal Sulfides
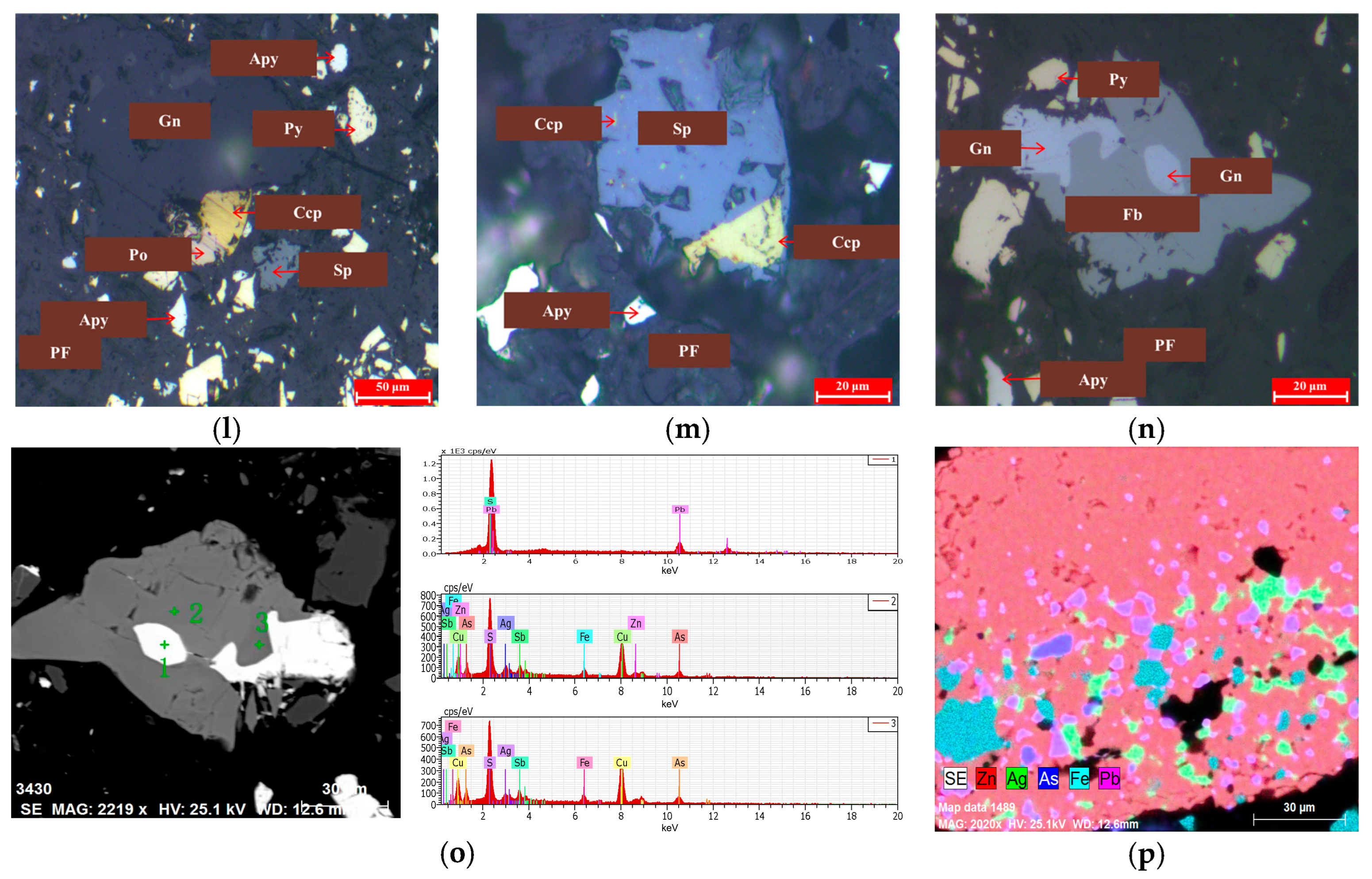
Mineral Locking Characteristics
3.1.3. Process Mineralogy Characteristics of Gold Minerals
Analysis of Minerals and Fineness
Size Distribution of Gold Minerals
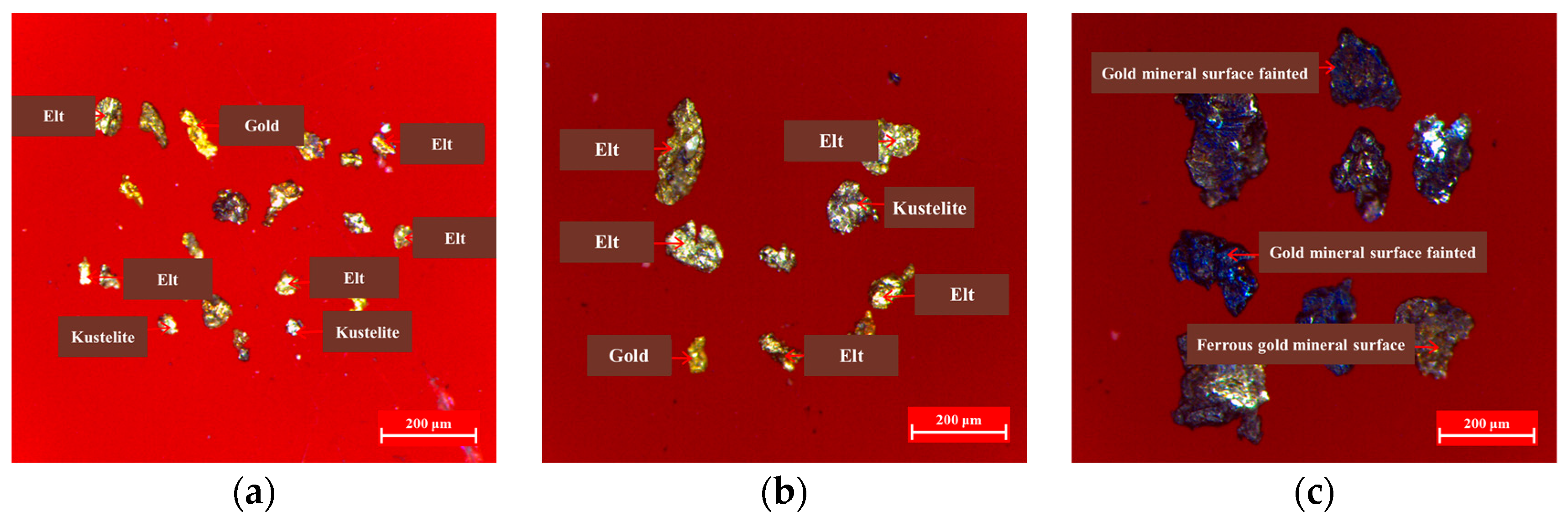
The Morphology of Gold Minerals
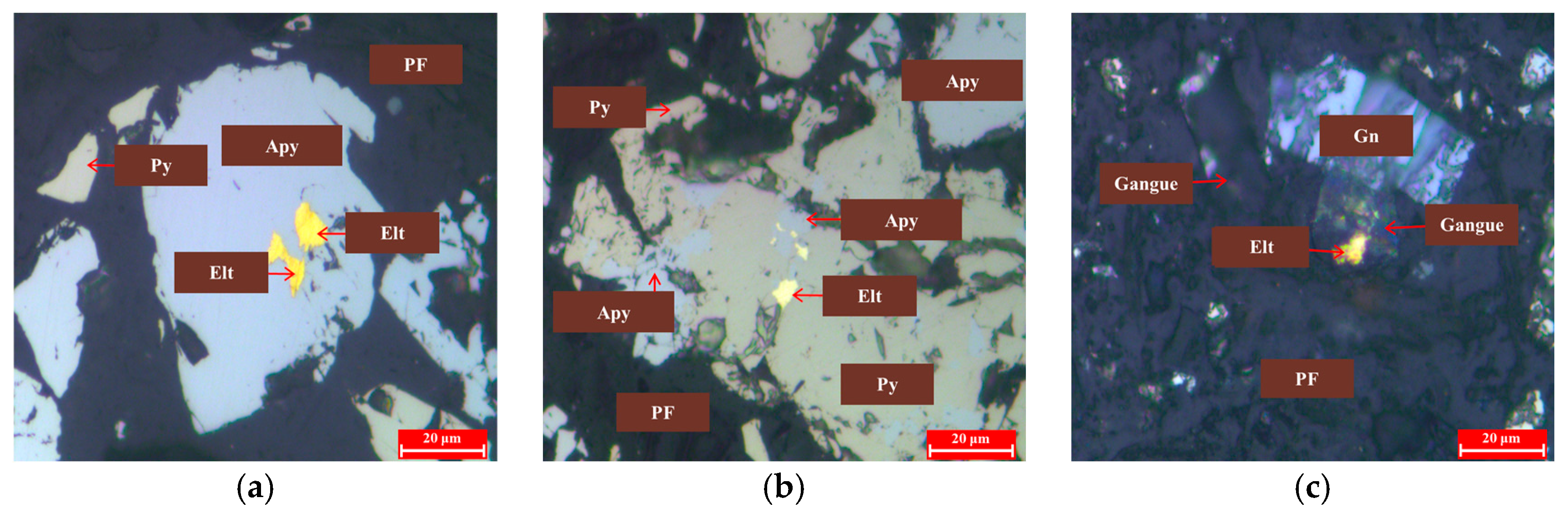
Association of Gold Minerals
3.1.4. Process Mineralogy Characteristics of Silver Minerals
Content and Distribution
Association Characteristics
3.2. Flotation Tests
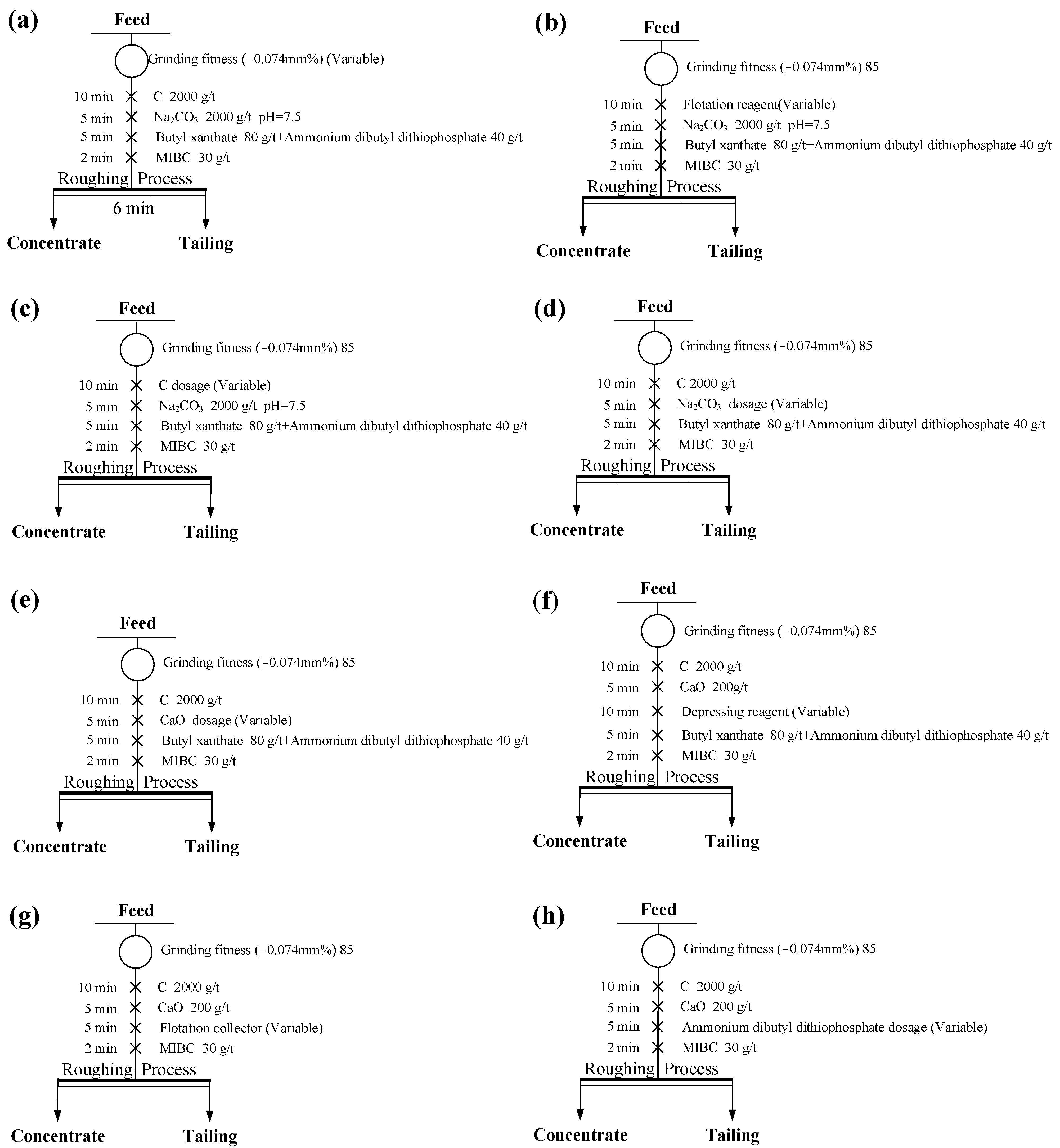
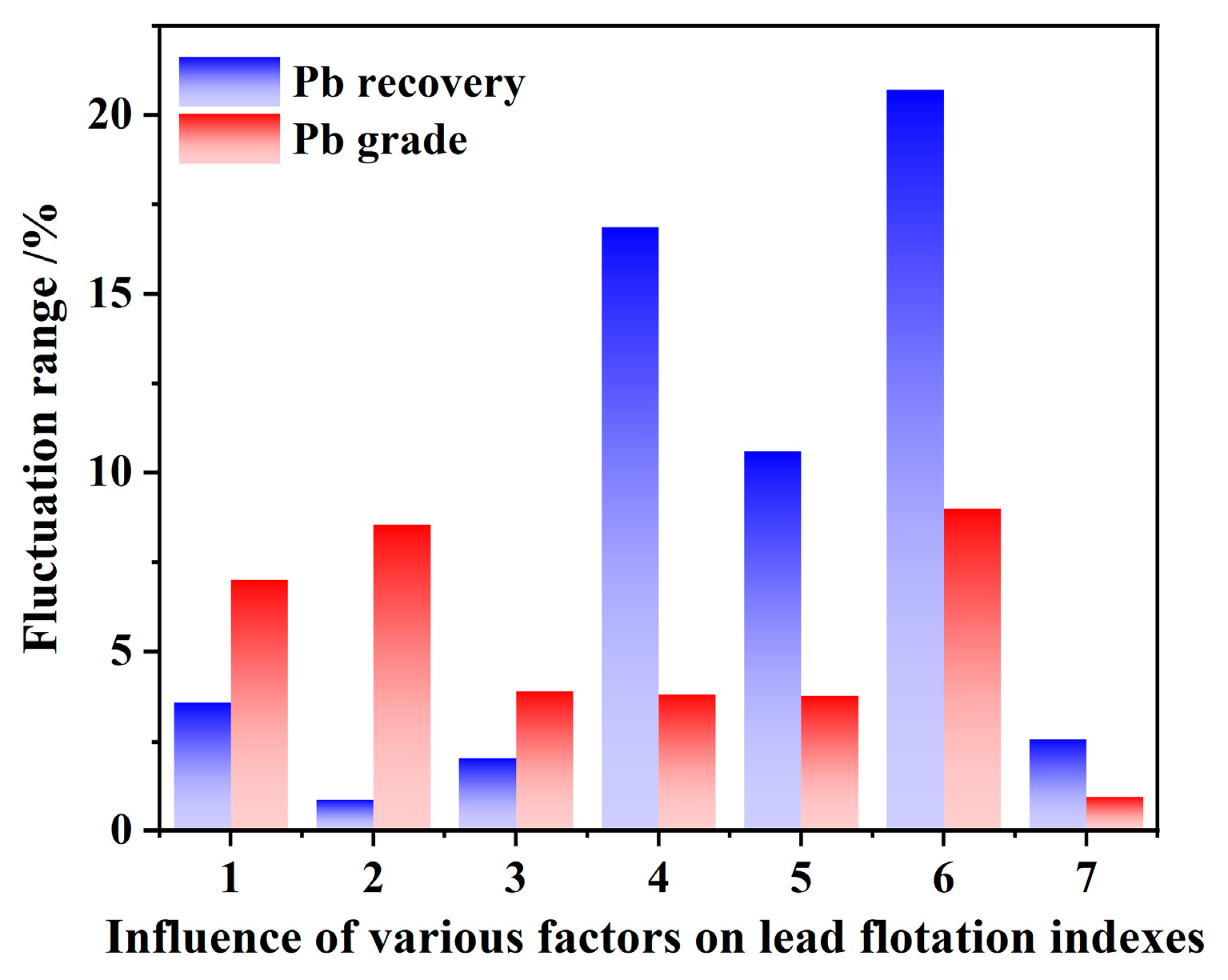
3.2.1. Flotation Conditions Tests
3.2.2. Comprehensive Conditioning Test
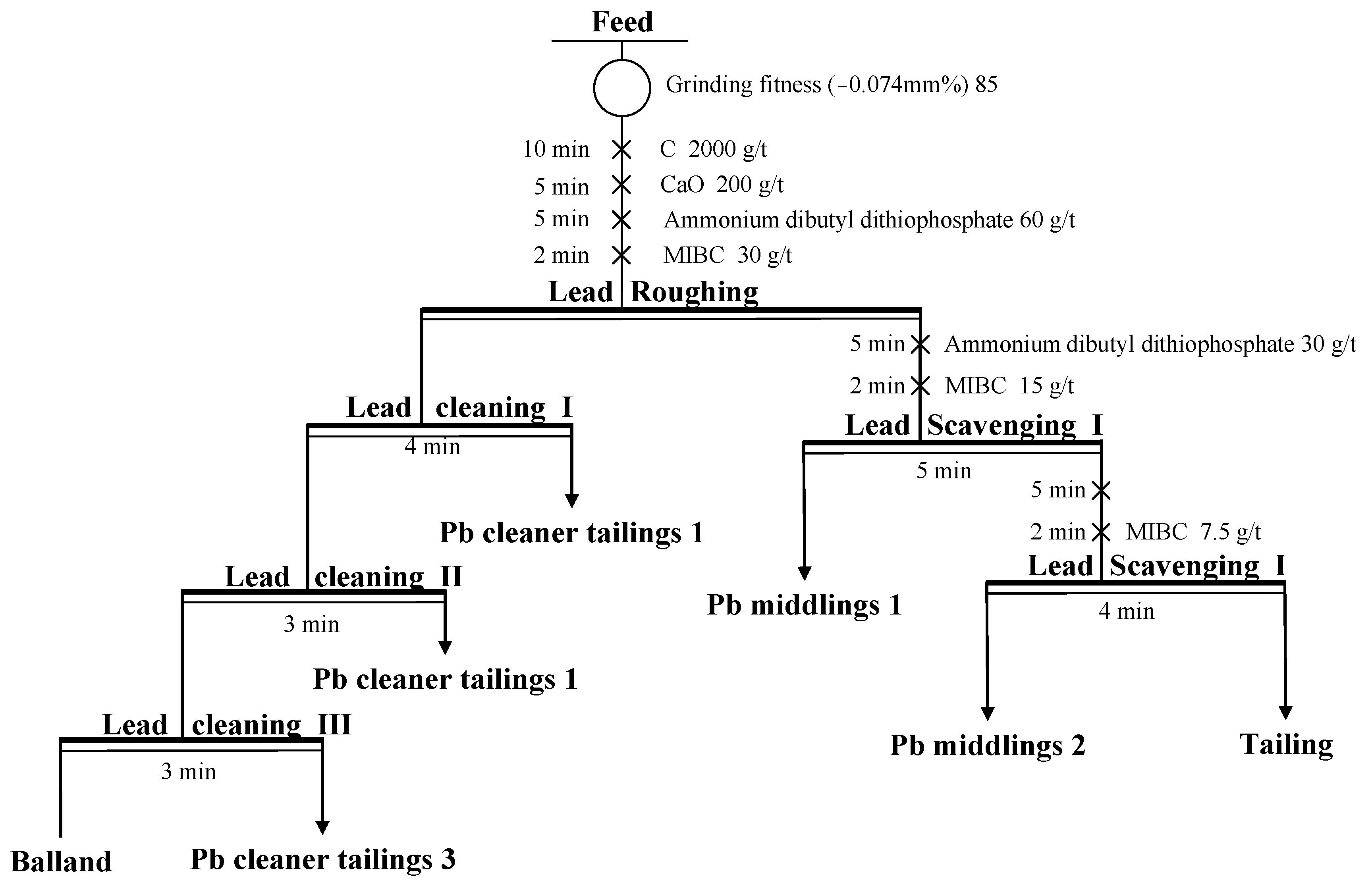
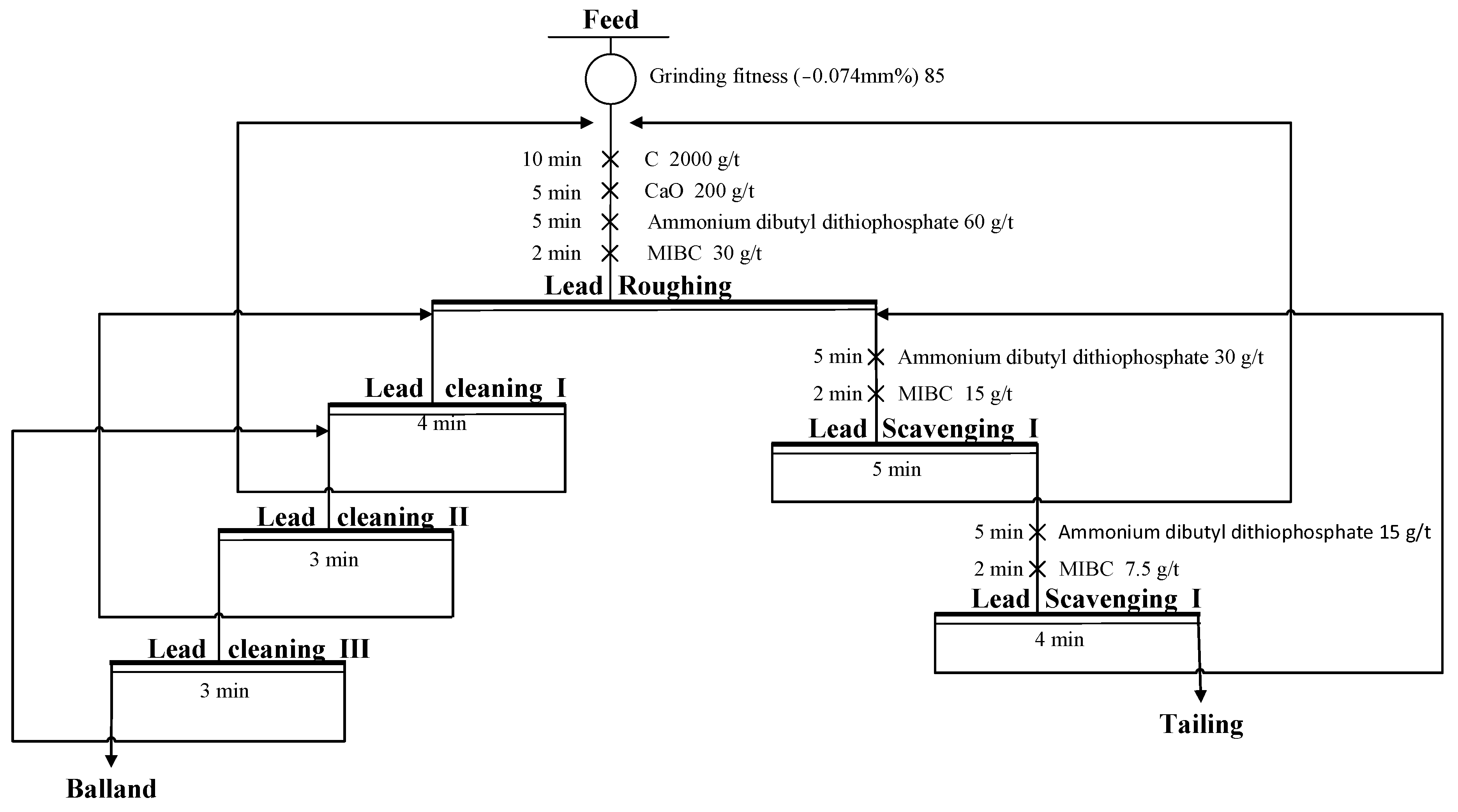
3.2.3. Flotation Flowchart Development
3.3. Leaching Test
3.4. Product Analysis
3.4.1. Association of Balland
3.4.2. Flotation Tailings Analysis
3.4.3. Multi-Element Analysis of Products
4. Conclusions
- (1)
- Process mineralogical analysis indicated a diverse and complex mineral composition in the rougher concentrate sample. Metal sulfides constituted 82.14% of the mineral content, with pyrite being predominant at 54.09%. Gangue minerals made up 16.49%, mainly quartz at 10.67%. Average grades for gold, silver, and lead were 50.50 g/t, 154.80 g/t, and 3.74%, respectively.
- (2)
- Lead minerals were primarily galena, constituting 3.43% of the mineral content. Most galena particles were smaller than 0.037 mm, accounting for 94.72%. Lead phase analysis showed a lead oxidation rate of 10.16%.
- (3)
- Gold minerals were primarily composed of electrum (76.18%). The particle size was mainly fine-grained (49.34%) and micronized gold (47.36%). Gold minerals were primarily liberated gold (44.38%), followed by gold encapsulated in metal sulfides (mainly arsenopyrite and pyrite) at 35.69%. Congenial gold constituted 18.62%, primarily associated with arsenopyrite at 12.52%, with a small amount in other morphologies. Gold encapsulated in gangue accounted for 1.31%.
- (4)
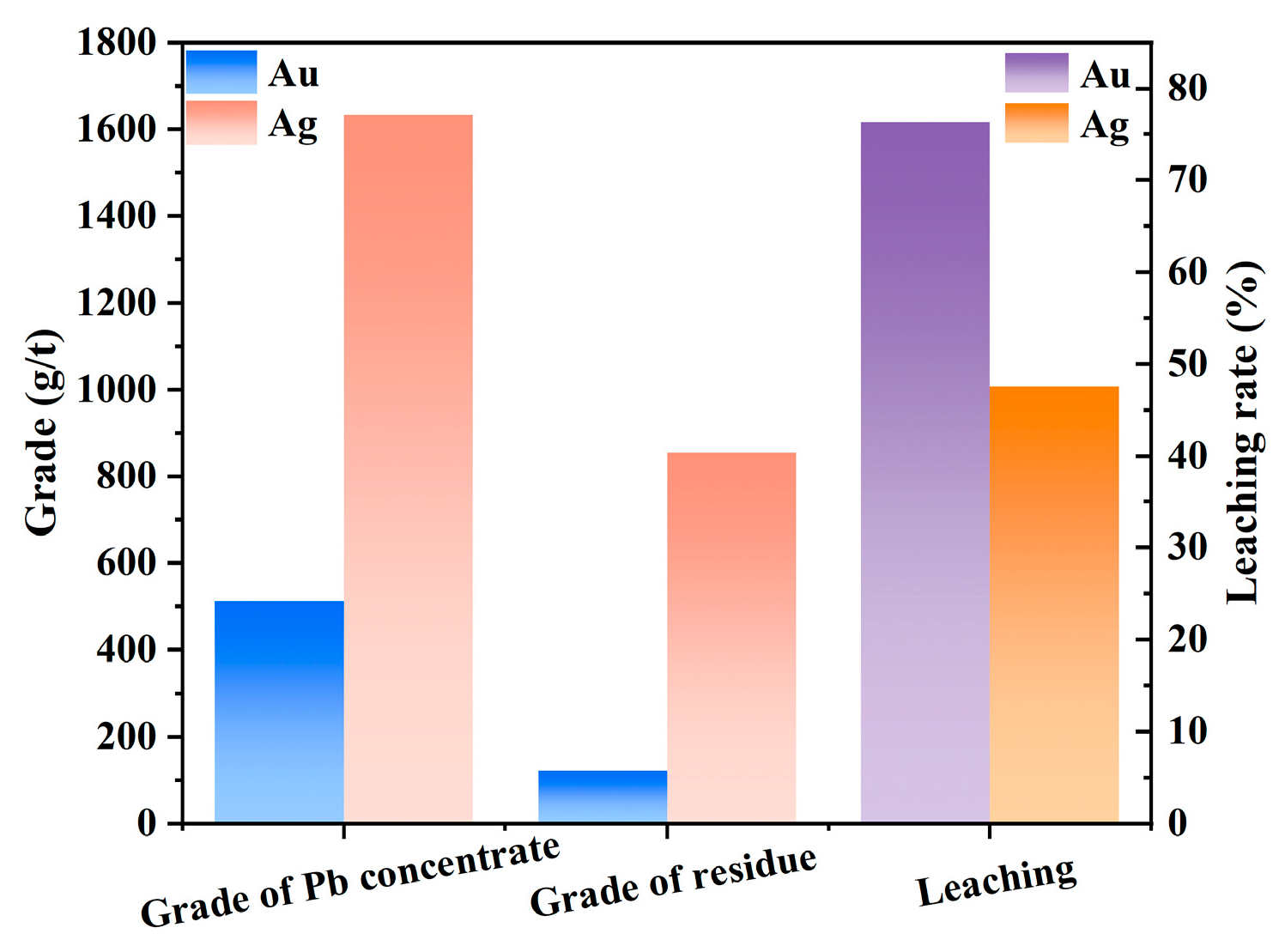
- Flotation closed-circuit testing showed that with three-stage cleaning and two-stage scavenging, the grades of gold, silver, and lead in the balland were 512.10 g/t, 1632.80 g/t, and 40.38%, with recoveries of 70.65%, 73.86%, and 75.37%, respectively.
- (5)
- Cyanide leaching tests on balland, with a regrind fineness of 95% at −0.045 mm, a pulp density of 33%, calcium oxide at 40 kg/t, sodium cyanide at 40 kg/t, and a 48-h leaching time, yielded a gold leaching rate of 76.27%.
- (6)
- An examination of gold and lead loss in flotation tailings revealed that gold minerals were primarily encapsulated in metal sulfides (55.67%), followed by congenial gold (30.87%), liberated gold (9.24%), and a smaller portion encapsulated by gangue and metal oxides (4.22%). Lead minerals were predominantly associated with gangue and metal oxides (62.72%), with 51.47% at attachment and 11.25% as inclusions. Losses of lead associated with sulfides were 20.29%, and liberated lead loss was minimal at 6.10%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, H.Y.; Sun, R.; Wu, J.Z.; Feng, D.X.; Gao, L.K. A Case Study of Enhanced Sulfidization Flotation of Lead Oxide Ore: Influence of Depressants. Minerals 2020, 10, 95. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H.; Sun, W.; Lin, S.; Zhang, C. Beneficiation of silver and silver-bearing lead-zinc ores: A review. Miner. Eng. 2024, 208, 108608. [Google Scholar] [CrossRef]
- Lazic, P.; Vucinic, D.; Stanojev, J.; Micovic, B. Direct selective lead, copper and zinc minerals flotation from polymetallic ore “Podvirovi”. J. Min. Sci. 2010, 46, 690–694. [Google Scholar] [CrossRef]
- Yang, H.M.; Hu, Y.H.; Ao, W.Q.; Qiu, G.Z.; Wang, D.Z. Ultrafine milling for the processing of gold-bearing sulphides. Rare Met. 2002, 21, 133–136. [Google Scholar]
- Arellano-Pina, R.; Sanchez-Ramirez, E.A.; Vazquez-Sanchez, E.E.; Perez-Garibay, R.; Rojas-Montes, J.C. Gold recovery improvements in grinding and flash flotation circuit. Miner. Eng. 2023, 199, 108130. [Google Scholar] [CrossRef]
- Fedotov, P.K.; Senchenko, A.E.; Fedotov, K.V.; Burdonov, A.E. Hydrometallurgical Processing of Gold-Containing Ore and its Enrichment Products. Metallurgist 2021, 65, 214–227. [Google Scholar] [CrossRef]
- Acarkan, N.; Bulut, G.; Gul, A.; Kangal, O.; Karakas, F.; Kokkilic, O.; Onal, G. The Effect of Collector’s Type on Gold and Silver Flotation in a Complex Ore. Sep. Sci. Technol. 2011, 46, 283–289. [Google Scholar] [CrossRef]
- Bas, A.D.; Koc, E.; Yazici, E.Y.; Deveci, H. Treatment of copper-rich gold ore by cyanide leaching, ammonia pretreatment and ammoniacal cyanide leaching. Trans. Nonferrous Met. Soc. China 2015, 25, 597–607. [Google Scholar] [CrossRef]
- Yu, A.M.; Ding, Z.; Yuan, J.Q.; Feng, Q.C.; Wen, S.M.; Bai, S.J. Process Mineralogy Characteristics and Flotation Optimization of a Low-Grade Oxidized Lead and Zinc Ore from Lanping Mine. Minerals 2023, 13, 1167. [Google Scholar] [CrossRef]
- Li, W.-J.; Liu, S.; Song, Y.-S.; Wen, J.-K.; Zhou, G.-Y.; Chen, Y. Comprehensive recovery of gold and base-metal sulfide minerals from a low-grade refractory ore. Int. J. Miner. Metall. Mater. 2016, 23, 1377–1386. [Google Scholar] [CrossRef]
- Monte, M.B.M.; Dutra, A.J.B.; Albuquerque, C.R.F.; Tondo, L.A.; Lins, F.F. The influence of the oxidation state of pyrite and arsenopyrite on the flotation of an auriferous sulphide ore. Miner. Eng. 2002, 15, 1113–1120. [Google Scholar] [CrossRef]
- Chen, D.X.; Xiao, J. Improving gold recovery of Pb-Zn sulfide ore by selective activation with organic acid. Rare Met. 2016, 35, 198–203. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Wei, Q.; Jiao, F.; Yang, C.; Li, W.; Qin, W. Study on flotation recovery of typical carbon-bearing lead-zinc sulphide ore in Guizhou with pre-decarbonization. Geochemistry 2024, 84, 126096. [Google Scholar] [CrossRef]
- Bocharov, V.A.; Ignatkina, V.A.; Kayumov, A.A.; Makavetskas, A.R.; Fishchenko, Y.Y. Influence of Structural Features and Nature of Interaction between Minerals on the Selection of Methods for Lead-Bearing Ore Separation. J. Min. Sci. 2018, 54, 821–830. [Google Scholar] [CrossRef]
- Raji, F.; Kappes, R.; Peng, Y. Deleterious impacts of triethylenetetramine and sodium metabisulphite on gold flotation during pyrrhotite depression. Miner. Eng. 2024, 216, 108884. [Google Scholar] [CrossRef]
- Liu, G.Q.; Yen, W.T. Effects of sulfide minerals and dissolved-oxygen on the gold and silver dissolution in cyanide solution. Miner. Eng. 1995, 8, 111–123. [Google Scholar] [CrossRef]
- Swinkels, L.J.; Burisch, M.; Rossberg, C.M.; Oelze, M.; Gutzmer, J.; Frenzel, M. Gold and silver deportment in sulfide ores—A case study of the Freiberg epithermal Ag-Pb-Zn district, Germany. Miner. Eng. 2021, 174, 107235. [Google Scholar] [CrossRef]
- Chandra; Mubarok, M.Z. On the use of lignin-based biopolymer in improving gold and silver recoveries during cyanidation leaching. Miner. Eng. 2016, 89, 1–9. [Google Scholar] [CrossRef]
- Klein, B.; Altun, N.E.; Ghaffari, H.; McLeavy, M. A hybrid flotation-gravity circuit for improved metal recovery. Int. J. Miner. Process. 2010, 94, 159–165. [Google Scholar] [CrossRef]
- Azizi, A.; Olsen, C.; Larachi, F. Efficient strategies to enhance gold leaching during cyanidation of multi-sulfidic ores. Can. J. Chem. Eng. 2014, 92, 1687–1692. [Google Scholar] [CrossRef]
- Chang, C.; Xiao, K.Y.; Feng, G.H.; Sun, L.; Yang, J.S. Reactive transport numerical modeling of intermediate sulfidation epithermal deposit: A case study of Haopinggou Ag-Au-Pb-Zn deposit, Henan province, China. J. Geochem. Explor. 2024, 263, 107500. [Google Scholar] [CrossRef]
- Song, B.X.; Dong, X.R.; Qiu, X.Y.; Hu, Z.; Wang, Y. Electronic structure and flotation behavior of Ag-bearing galena. J. Alloys Compd. 2021, 868, 159105. [Google Scholar] [CrossRef]
- Hu, Y.H.; Wu, M.R.; Liu, R.Q.; Sun, W. A review on the electrochemistry of galena flotation. Miner. Eng. 2020, 150, 106272. [Google Scholar] [CrossRef]
- Aydin, S.B.; Gül, A. Kinetic modelling and optimization of flotation process of electrum. Physicochem. Probl. Miner. Process. 2021, 57, 80–94. [Google Scholar] [CrossRef]
- Liu, X.Q.; Fang, Y.; Jiang, H.; Xiao, Y.; Li, L.G. Study on the Integrative Utilization of Cyanide Tailings of Polymetallic Sulfide Ore of gold and silver. In Proceedings of the International Conference on Materials for Environmental Protection and Energy Application (MEPEA 2011), Kuala Lumpur, Malaysia, 27–28 September 2011; pp. 43–55. [Google Scholar]
- Cui, W.Y.; Chen, J.H. Insight into mineral flotation fundamentals through the DFT method. Int. J. Min. Sci. Technol. 2021, 31, 983–994. [Google Scholar] [CrossRef]
- Klimpel, R.R. Industrial experiences in the evaluation of various flotation reagent schemes for the recovery of gold. Miner. Metall. Process. 1999, 16, 1–11. [Google Scholar]
- Luo, X.; Feng, B.; Wong, C.; Miao, J.; Ma, B.; Zhou, H. The critical importance of pulp concentration on the flotation of galena from a low grade lead-zinc ore. J. Mater. Res. Technol. 2016, 5, 131–135. [Google Scholar] [CrossRef]
- Özçelik, S.; Ekmekçi, Z. Surface Chemistry and Flotation of Gold-Bearing Pyrite. Minerals 2024, 14, 914. [Google Scholar] [CrossRef]
- Bidari, E.; Aghazadeh, V. Pyrite from Zarshuran Carlin-type gold deposit: Characterization, alkaline oxidation pretreatment, and cyanidation. Hydrometallurgy 2018, 179, 222–231. [Google Scholar] [CrossRef]
- Chen, T.T.; Cabri, L.J.; Dutrizac, J.E. Characterizing gold in refractory sulfide gold ores and residues. Jom J. Miner. Met. Mater. Soc. 2002, 54, 20–22. [Google Scholar] [CrossRef]
- Ran, J.; Qiu, X.; Hu, Z.; Liu, Q.; Song, B.; Yao, Y. Effects of particle size on flotation performance in the separation of copper, gold and lead. Powder Technol. 2019, 344, 654–664. [Google Scholar] [CrossRef]
- Sen, S.; Ipekoglu, U.; Cilingir, Y. Flotation of Fine Gold Particles by the Assistance of Coal-Oil Agglomerates. Sep. Sci. Technol. 2010, 45, 610–618. [Google Scholar] [CrossRef]
- Lan, Z.Y.; Lai, Z.N.; Zheng, Y.X.; Lv, J.F.; Pang, J.; Ning, J.L. Recovery of Zn, Pb, Fe and Si from a low-grade mining ore by sulfidation roasting-beneficiation-leaching processes. J. Cent. South Univ. 2020, 27, 37–51. [Google Scholar] [CrossRef]
- Mütevellioglu, N.A.; Yekeler, M. Beneficiation of Oxidized Lead-Zinc Ores by Flotation Using Different Chemicals and Test Conditions. J. Min. Sci. 2019, 55, 327–332. [Google Scholar] [CrossRef]
- Seksenova, N.; Bykov, R.; Mamyachenkov, S.; Daumova, G.; Kozhakanova, M. Optimization of Conditions for Processing of Lead-Zinc Ores Enrichment Tailings of East Kazakhstan. Metals 2021, 11, 1802. [Google Scholar] [CrossRef]
- Lv, C.C.; Ding, J.; Qian, P.; Li, Q.C.; Ye, S.F.; Chen, Y.F. Comprehensive recovery of metals from cyanidation tailing. Miner. Eng. 2015, 70, 141–147. [Google Scholar] [CrossRef]



| Elements | Au (g/t *) | Ag (g/t) | S (%) | Fe (%) | As (%) | SiO2 (%) | Pb (%) | Zn (%) |
| Concentration | 50.50 | 154.80 | 35.89 | 34.19 | 10.61 | 11.06 | 3.74 | 1.59 |
| Elements | Al2O3 (%) | C (%) | CaO (%) | MgO (%) | Cu (%) | Sb (%) | Cr (%) | Cd (%) |
| Concentration | 1.02 | 0.48 | 0.47 | 0.31 | 0.11 | 0.054 | 0.043 | 0.035 |
| Phase | Pb/Sulfide | Pb/Oxide | Pb/Total |
|---|---|---|---|
| Concentration (%) | 3.36 | 0.38 | 3.74 |
| Relative content (%) | 89.84 | 10.16 | 100.00 |
| Mineral | Content (%) | Mineral | Content (%) |
|---|---|---|---|
| Pyrite | 54.09 | Quartz | 10.67 |
| Arsenopyrite | 21.21 | Feldspar | 2.12 |
| Galena, Boulangerite | 4.03 | Pyroxenes | 1.57 |
| Sphalerite | 2.39 | Calcite, Carbonates | 1.38 |
| Chalcopyrite, Chalcocite, covellite | 0.41 | Micas | 0.51 |
| Tetrahedrite, Freibergite | 0.01 | Wollastonite, Kaolinite, graphite | 0.24 |
| Lead oxide | 0.43 | — | — |
| Limonite, Hematite, Magnetite | 0.94 | — | — |
| Total | 83.51 | Total | 16.49 |
| Size (mm) | +0.100 | −0.100~ +0.074 | −0.074~ +0.053 | −0.053~ +0.037 | −0.037~ +0.010 | −0.010 | Total | |
|---|---|---|---|---|---|---|---|---|
| Minerals | ||||||||
| Pyrite | 10.24 | 18.47 | 25.74 | 25.47 | 14.11 | 5.97 | 100.00 | |
| Arsenopyrite | 7.04 | 20.53 | 23.07 | 25.29 | 16.59 | 7.48 | 100.00 | |
| Chalcopyrite | — | — | 1.78 | 11.52 | 72.62 | 14.08 | 100.00 | |
| Galena | — | — | — | 5.28 | 74.01 | 20.71 | 100.00 | |
| Locked | Liberated | Sulfide-linked * | Sulfide-Coated * | Associated with Chalcopyrite | Locked in Chalcopyrite | Total | |
|---|---|---|---|---|---|---|---|
| Minerals | |||||||
| Pyrite | 79.76 | 10.72 | 4.38 | 3.06 | 2.08 | 100.00 | |
| Arsenopyrite | 74.20 | 13.02 | 6.05 | 4.75 | 1.98 | 100.00 | |
| Galena | 64.02 | 16.83 | 8.36 | 8.21 | 2.58 | 100.00 | |
| Gold Minerals | Native Gold | Electrum | Total (%) |
|---|---|---|---|
| Relative content (%) | 23.82 | 76.18 | 100.00 |
| Fineness (‰) | 938.9 | 634.9 | — |
| Gold content (%) | 48.37 | 22.37 | 70.74 |
| Distribution rate (%) | 31.62 | 68.38 | 100.00 |
| Size (mm) | +0.074 | −0.074~ +0.053 | −0.053~ +0.037 | −0.037~ +0.01 | −0.010 | Total |
|---|---|---|---|---|---|---|
| Content (%) | 0.36 | 0.81 | 2.13 | 49.34 | 47.36 | 100.00 |
| Morphology | Slab-Flaky | Elongated Angular Granular | Angular Granular | Shape Angular Granular | Total |
|---|---|---|---|---|---|
| Content (%) | 47.38 | 21.67 | 19.82 | 11.13 | 100.00 |
| Locked Association | Gold | Locked | Enclosed in Metallic Sulfides | Enclosed in Gangue | Total | ||
|---|---|---|---|---|---|---|---|
| Arsenopyrite | Pyrite | Gangue | |||||
| Relative content (%) | 44.38 | 12.52 | 4.95 | 1.15 | 35.69 | 1.31 | 100.00 |
| Silver Minerals | Relative Content (%) | Ag (%) | Metal Volume | Distribution (%) |
|---|---|---|---|---|
| Tetrahedrite containing silver | 0.0013 | 4.88 | 63.44 | 40.98 |
| Freibergite | 0.0004 | 16.79 | 67.16 | 43.38 |
| Silver gold | 0.0000505 | 29.27 | 14.78 | 9.55 |
| Others | 99.9982495 | 9.42 × 10−6 | 9.42 | 6.09 |
| Total | 100.00 | — | 154.80 | 100.00 |
| Locked Association | Leachable | Enclosed in Metallic Sulfides | Enclosed in Metallic Oxide and Gangue | Total |
|---|---|---|---|---|
| Content (%) | 60.03 | 21.23 | 18.74 | 100.00 |
| Minerals | Yield/% | Pb Grade /% | Pb Recovery/% |
|---|---|---|---|
| Balland | 3.52 | 55.51 | 52.18 |
| Cleaner tailings 3 | 1.53 | 20.93 | 8.55 |
| Cleaner tailings 2 | 3.25 | 9.85 | 8.55 |
| Cleaner tailings 1 | 6.87 | 6.52 | 11.96 |
| Middlings 1 | 8.75 | 2.71 | 6.33 |
| Middlings 2 | 3.36 | 2.15 | 1.93 |
| Tailing | 72.72 | 0.54 | 10.49 |
| Rougher concentrate | 100.00 | 3.74 | 100.00 |
| Minerals | Yield (%) | Grade | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|
| Au (g/t) | Ag (g/t) | Pb (%) | Au | Ag | Pb | ||
| Balland | 6.98 | 512.10 | 1632.80 | 40.38 | 70.65 | 73.86 | 75.37 |
| Tailing | 93.02 | 15.96 | 43.37 | 0.99 | 29.35 | 26.14 | 24.63 |
| Feed | 100.00 | 50.59 | 154.31 | 3.74 | 100.00 | 100.00 | 100.00 |
| Minerals | Yield (%) | Grade | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|
| Au (g/t) | Ag (g/t) | Pb (%) | Au | Ag | Pb | ||
| Balland | 7.31 | 468.80 | 1543.70 | 37.47 | 67.77 | 73.03 | 73.05 |
| Tailing | 92.69 | 17.58 | 44.96 | 1.09 | 32.23 | 26.97 | 26.95 |
| Feed | 100.00 | 50.56 | 154.52 | 3.75 | 100.00 | 100.00 | 100.00 |
| Locked Association | Liberated | Sulfide-Linked | Sulfide-Coated | Hyphenated with Gangues and Metal Oxides | Coated by Gangues and Metal Oxides | Total |
|---|---|---|---|---|---|---|
| Content (%) | 80.66 | 7.85 | 3.84 | 3.90 | 3.75 | 100.00 |
| Locked Association | Liberated | Arsenopyrite | Sulfide-Coated | Coated by Gangues and Metal Oxides | Total |
|---|---|---|---|---|---|
| Content (%) | 61.88 | 10.37 | 26.52 | 1.23 | 100.00 |
| Morphology | Liberated | Sulfide-Linked | Sulfide-Coated | Hyphenated with Gangues and Metal Oxides | Coated by Gangues and Metal Oxides | Total |
|---|---|---|---|---|---|---|
| Content (%) | 6.10 | 20.29 | 10.89 | 51.47 | 11.25 | 100.00 |
| Morphology | Liberated | Intergrowth | Enveloped by Metal Sulfides | Coated by Gangues and Metal Oxides | Total |
|---|---|---|---|---|---|
| Content (%) | 9.24 | 30.87 | 55.67 | 4.22 | 100.00 |
| Element | Au (g/t) | Ag (g/t) | Pb | S | Fe | Zn | SiO2 | As |
| Content (%) | 512.10 | 1632.80 | 40.38 | 25.69 | 13.74 | 7.26 | 3.40 | 2.31 |
| Element | C | Cu | Al2O3 | MgO | CaO | Sb | Cd | Cr |
| Content (%) | 1.41 | 0.92 | 0.62 | 0.31 | 0.30 | 0.26 | 0.14 | 0.019 |
| Element | Au (g/t) | Ag (g/t) | Cu | Pb | Zn | Fe | S | Cr |
| Content (%) | 15.69 | 43.37 | 0.08 | 0.99 | 0.87 | 36.38 | 38.15 | 0.042 |
| Element | As | C | Al2O3 | MgO | SiO2 | CaO | Sb | Cd |
| Content (%) | 11.46 | 0.41 | 0.97 | 0.27 | 8.80 | 0.49 | 0.033 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Zhao, H.; Zhou, J.; Liu, Z.; Yang, H. Process Mineralogy Study and Flotation Testwork of a Complex Lead–Gold Rougher Concentrate. Minerals 2025, 15, 967. https://doi.org/10.3390/min15090967
Chen G, Zhao H, Zhou J, Liu Z, Yang H. Process Mineralogy Study and Flotation Testwork of a Complex Lead–Gold Rougher Concentrate. Minerals. 2025; 15(9):967. https://doi.org/10.3390/min15090967
Chicago/Turabian StyleChen, Guomin, Han Zhao, Joe Zhou, Zilong Liu, and Hongying Yang. 2025. "Process Mineralogy Study and Flotation Testwork of a Complex Lead–Gold Rougher Concentrate" Minerals 15, no. 9: 967. https://doi.org/10.3390/min15090967
APA StyleChen, G., Zhao, H., Zhou, J., Liu, Z., & Yang, H. (2025). Process Mineralogy Study and Flotation Testwork of a Complex Lead–Gold Rougher Concentrate. Minerals, 15(9), 967. https://doi.org/10.3390/min15090967






