The Effect of Particle Size and Dodecylamine Concentration on the Flotation of Lepidolite in Alkaline Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Mineral, Reagents and Equipment
2.2. Experimental Procedure
3. Results and Discussion
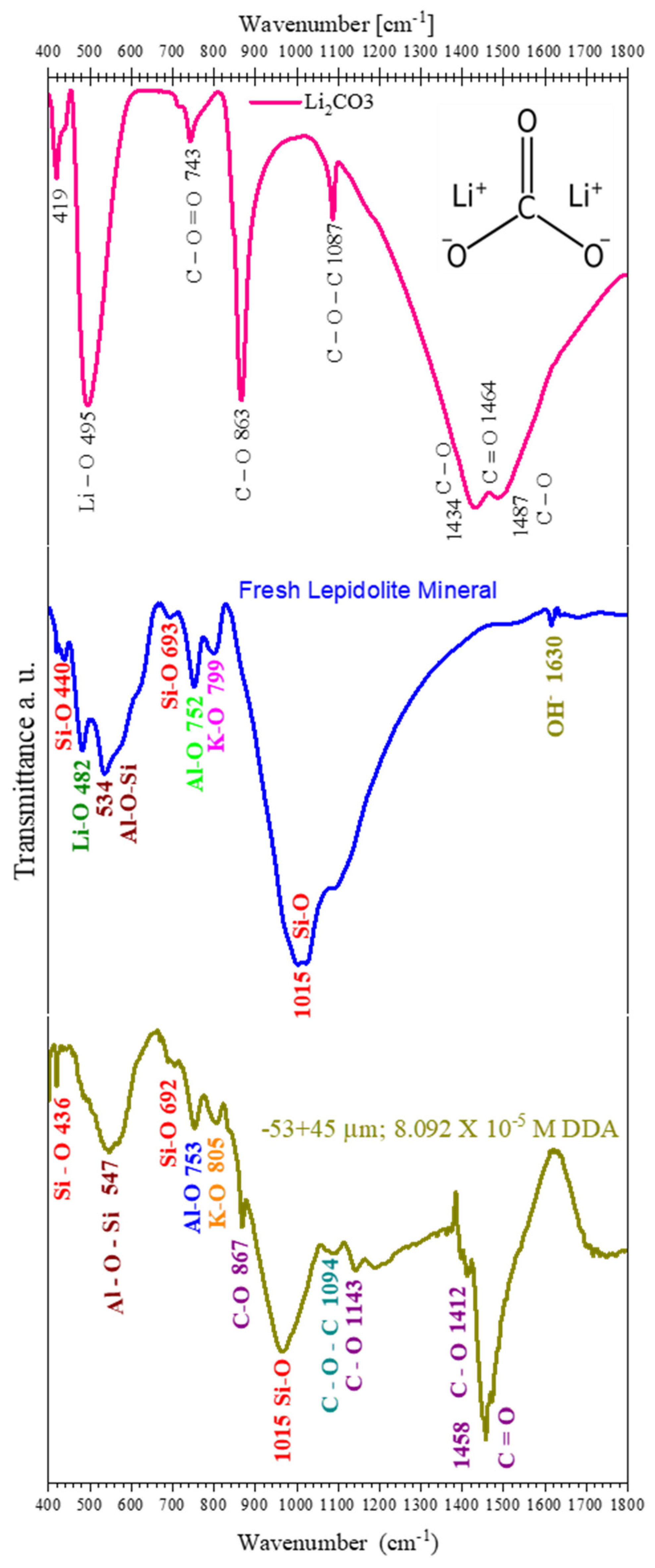
3.1. Mineral Characterization
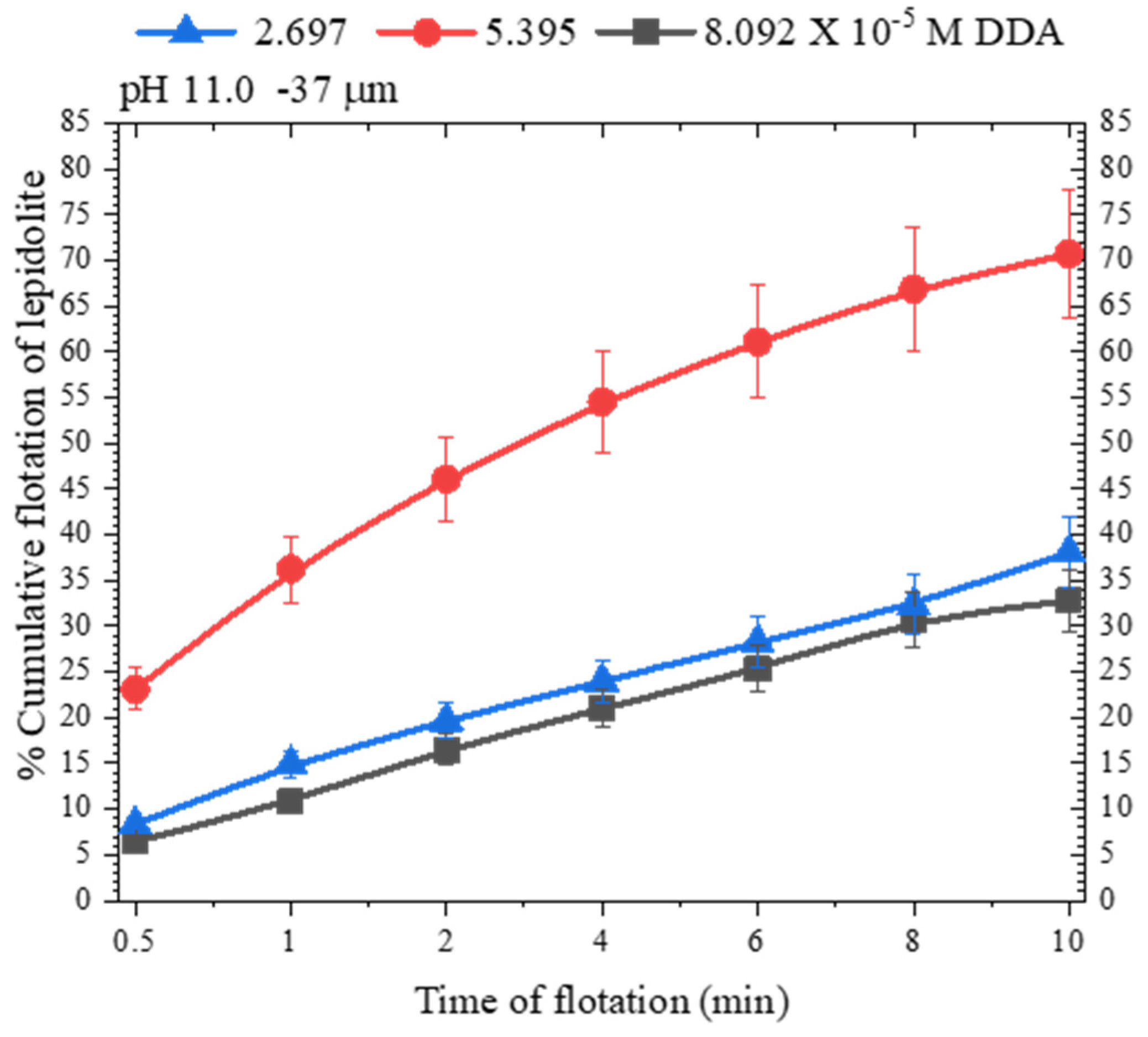
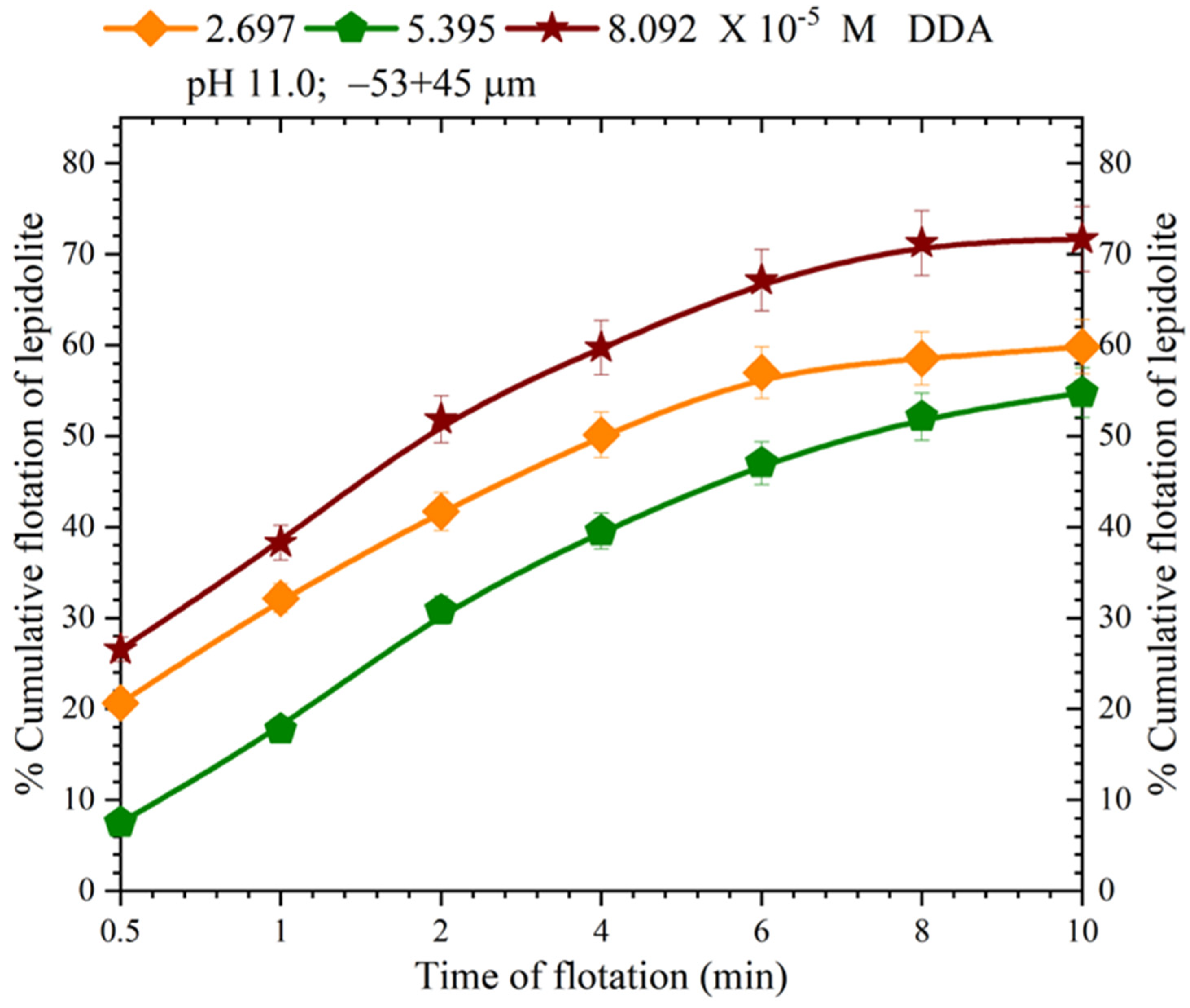
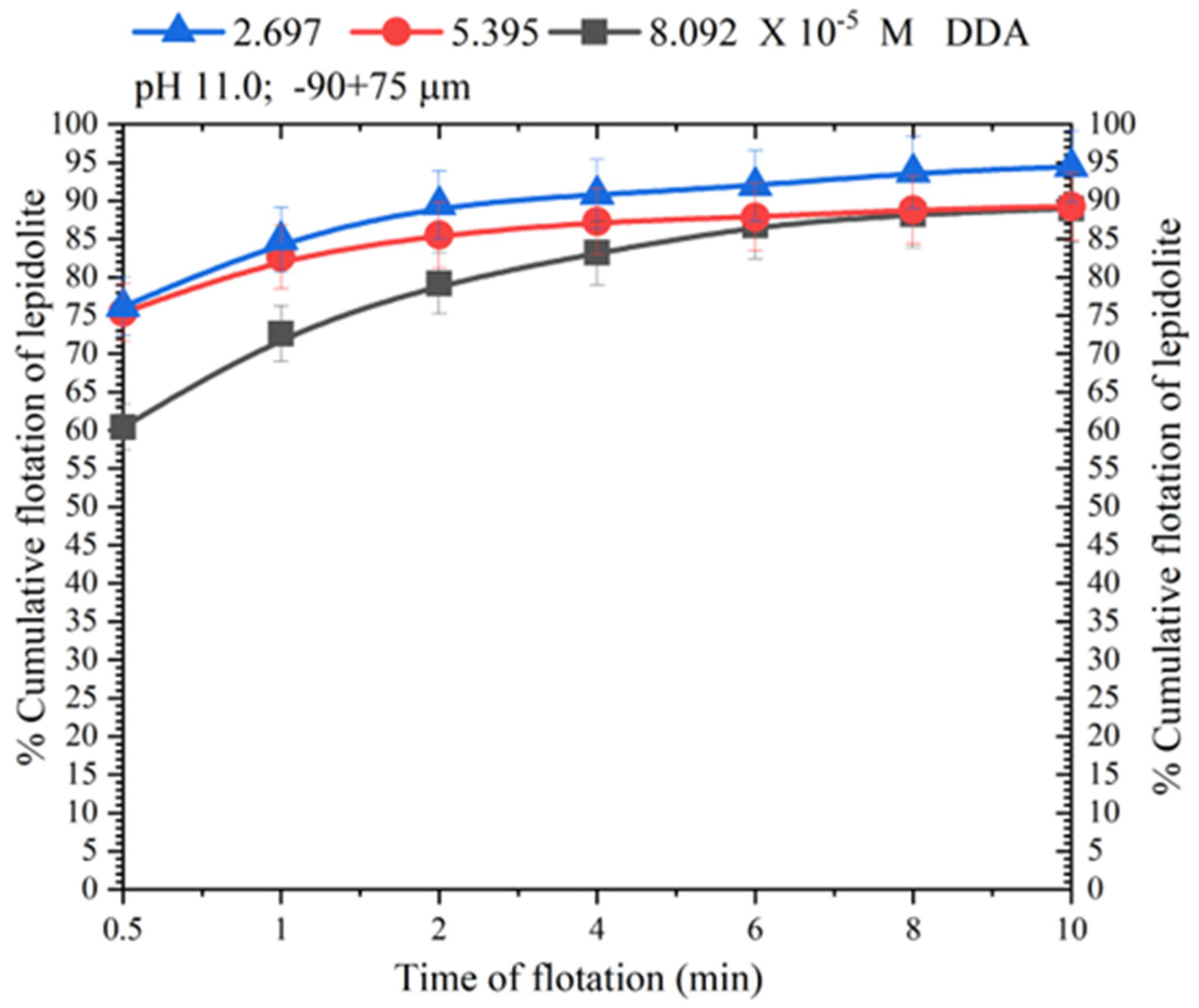
3.2. Lepidolite Flotation with DDA: Effect of Particle Size
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Tang, X.; Qing, D.; Li, J.; Wang, H. Research progress of technology of lithium extraction. Sep. Purif. Technol. 2025, 359, 130561. [Google Scholar] [CrossRef]
- Mojid, M.R.; Lee, K.J.; You, J. A review on advances in direct lithium extraction from continental brines: Ion-sieve adsorption and electrochemical methods for varied Mg/Li ratios. Sustain. Mater. Technol. 2024, 40, e00923. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, Z.; Wei, Q.; Qin, W. Key technologies and development trends for efficient flotation recovery of lepidolite. Green Smart Min. Eng. 2024, 1, 273–288. [Google Scholar] [CrossRef]
- Calisaya Azpilcueta, D.; Herrera-Leon, S.; Cisternas, L.A. Current and future global lithium production till 2025. Open Chem. Eng. J. 2020, 14, 36–51. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2017; U.S. Geological Survey: Reston, VA, USA, 2017; 110p. [Google Scholar]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Grosjean, C.; Miranda, P.H.; Perrin, M.; Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energy Rev. 2012, 16, 1735–1744. [Google Scholar] [CrossRef]
- Petrakis, E.; Alexopoulos, I.; Pantelaki, O.; Karmali, V.; Komnitsas, K. Advances in Mineral Processing of Hard-Rock Lithium Ores: A Comprehensive Review. Min. Metall. Explor. 2025, 42, 1251–1283. [Google Scholar] [CrossRef]
- Filippov, L.; Farrokhpay, S.; Lyo, L.; Filippova, I. Spodumene flotation mechanism. Minerals 2019, 9, 372. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Li, Y.; Han, Y. Flotation behavior and mechanism of a new mixed collector on separation of spodumene from feldspar. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124932. [Google Scholar] [CrossRef]
- Liu, K. Research progress in flotation collectors for lepidolite mineral: An overview. Miner. Process. Extr. Metall. Rev. 2024, 45, 728–742. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Wang, X.; Wu, L.; Wang, Y.; Xu, T. Electrodialytic concentrating lithium salt from primary resource. Desalination 2018, 425, 30–36. [Google Scholar] [CrossRef]
- Choi, J.; Kim, W.; Chae, W.; Kim, S.B.; Kim, H. Electrostatically controlled enrichment of lepidolite via flotation. Mater. Trans. 2012, 53, 2191–2194. [Google Scholar] [CrossRef]
- Vieceli, N.; Durão, F.O.; Guimarães, C.; Nogueira, C.A.; Pereira, M.F.; Margarido, F. Kinetic approach to the study of froth flotation applied to a lepidolite ore. Int. J. Miner. Metall. Mater. 2016, 23, 731–742. [Google Scholar] [CrossRef]
- Vieceli, N.; Durão, F.O.; Guimarães, C.; Nogueira, C.A.; Pereira, M.F.; Margarido, F. Grade-recovery modelling and optimization of the froth flotation process of a lepidolite ore. Int. J. Miner. Process. 2016, 157, 184–194. [Google Scholar] [CrossRef]
- He, G.C.; Feng, J.N.; Mao, M.X.; Wu, Y.P. Application of combined collectors in flotation of lepidolite. Adv. Mater. Res. 2013, 734, 921–924. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Beneficiation of barite ores. In Handbook of Flotation Reagents: Chemistry, Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2015; pp. 129–141. [Google Scholar]
- Korbel, C.; Filippova, I.V.; Filippov, L.O. Froth flotation of lithium micas—A review. Miner. Eng. 2023, 192, 107986. [Google Scholar] [CrossRef]
- Cook, B.K.; Aghamirian, M.; Gibson, C.E. Optimization of spodumene flotation with a fatty acid collector. Miner. Eng. 2023, 204, 108412. [Google Scholar] [CrossRef]
- Rao, S.R. Electrical Characteristics of Interfaces. Electrical Double Layer and Zeta Potential. In Surface Chemistry of Froth Flotation: Volume 1: Fundamentals; Springer: Boston, MA, USA, 2004; pp. 209–255. [Google Scholar]
- Ku, J.; Shi, X.; Wang, Q.; Lin, H.; Shang, H.; Shen, Z. Efficient exploitation of lepidolite resources: A review on beneficiation techniques, extraction methods, and synergistic optimization. Separations 2025, 12, 130. [Google Scholar] [CrossRef]
- Feng, F.; Wen, S.; Han, G.; Feng, Q. Effect of CuSO4 activation on lepidolite flotation performance using sodium oleate as a collector. Appl. Surf. Sci. 2025, 707, 163635. [Google Scholar] [CrossRef]
- He, J.; Xu, H.; Yang, B.; Gong, W.; Zhang, Z.; Shan, Y.; Song, T. Adsorption properties of an efficient combined collector and its enhanced separation of lepidolite from feldspar. J. Ind. Eng. Chem. 2025, 148, 797–808. [Google Scholar] [CrossRef]
- Fei, H.; Likun, G.; Bing, R.; Hairong, S.; Kebo, P.; Guangyan, G.; Ming, Z. Research on lithium extraction and comprehensive utilization from lepidolite. Multipurp. Util. Miner. Resour. 2022, 43, 83–90. [Google Scholar]
- Yang, Z.; Xu, H.; Tang, X.; Zhou, H.; Xie, T.; Shen, L.; Luo, X. Application of a novel mixed anionic/cationic collector in the selective flotation separation of lepidolite and quartz. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134919. [Google Scholar] [CrossRef]
- Shen, L.; Gong, J.; Liu, Y.; Qiao, E. Substituent Effects in Kaolinite Flotation Using Dodecylamine: Experiment and DFT Study. Processes 2023, 11, 703. [Google Scholar] [CrossRef]
- Yang, X.; Feng, B.; Jiang, L. Froth Flotation of Lepidolite—A Review. Minerals 2025, 15, 750. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Fu, W. Froth flotation separation of lepidolite ore using a new Gemini surfactant as the flotation collector. Sep. Purif. Technol. 2022, 282, 119122. [Google Scholar] [CrossRef]
- Isse, A.A.; Gennaro, A. Absolute potential of the standard hydrogen electrode and the problem of interconversion of potentials in different solvents. J. Phys. Chem. B 2010, 114, 7894–7899. [Google Scholar] [CrossRef]
- Pasierb, P.; Komornicki, S.; Rokita, M.; Rȩkas, M. Structural properties of Li2CO3–BaCO3 system derived from IR and Raman spectroscopy. J. Mol. Struct. 2001, 596, 151–156. [Google Scholar] [CrossRef]
- Saikia, N.J.; Bharali, D.J.; Sengupta, P.; Bordoloi, D.; Goswamee, R.L.; Saikia, P.C.; Borthakur, P.C. Characterization, beneficiation and utilization of a kaolinite clay from Assam, India. Appl. Clay Sci. 2003, 24, 93–103. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, X.; Wu, W. Application of fourier transform infrared (FTIR) spectroscopy in sample preparation: Material characterization and mechanism investigation. Adv. Sample Prep. 2024, 11, 100122. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, Y.; Tian, C.; Zhao, B.; Sheng, Q. Agglomeration and floatability characteristics of Ar plasma-modified siderite. Int. J. Miner. Metall. Materials. 2025. [Google Scholar] [CrossRef]
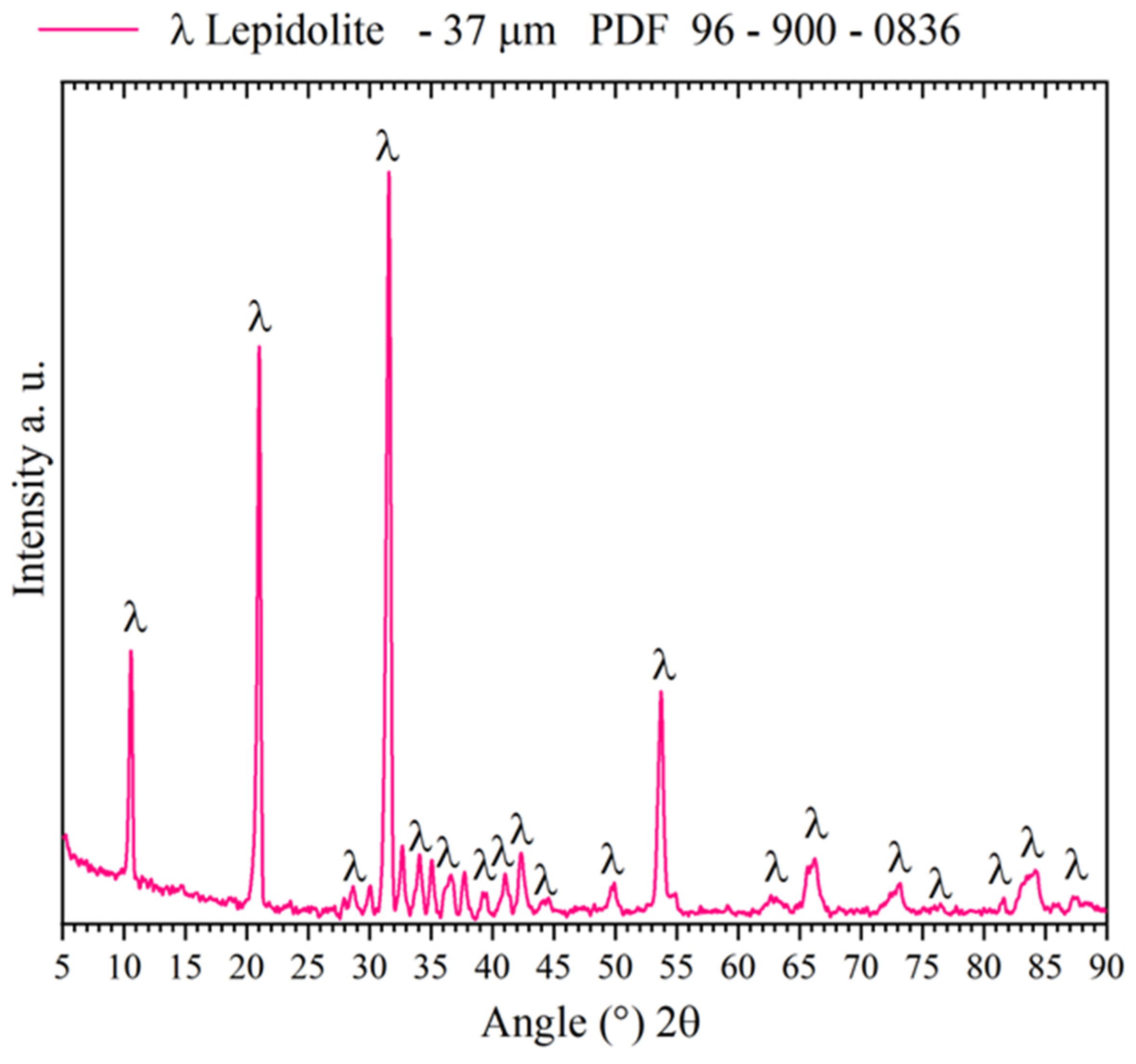

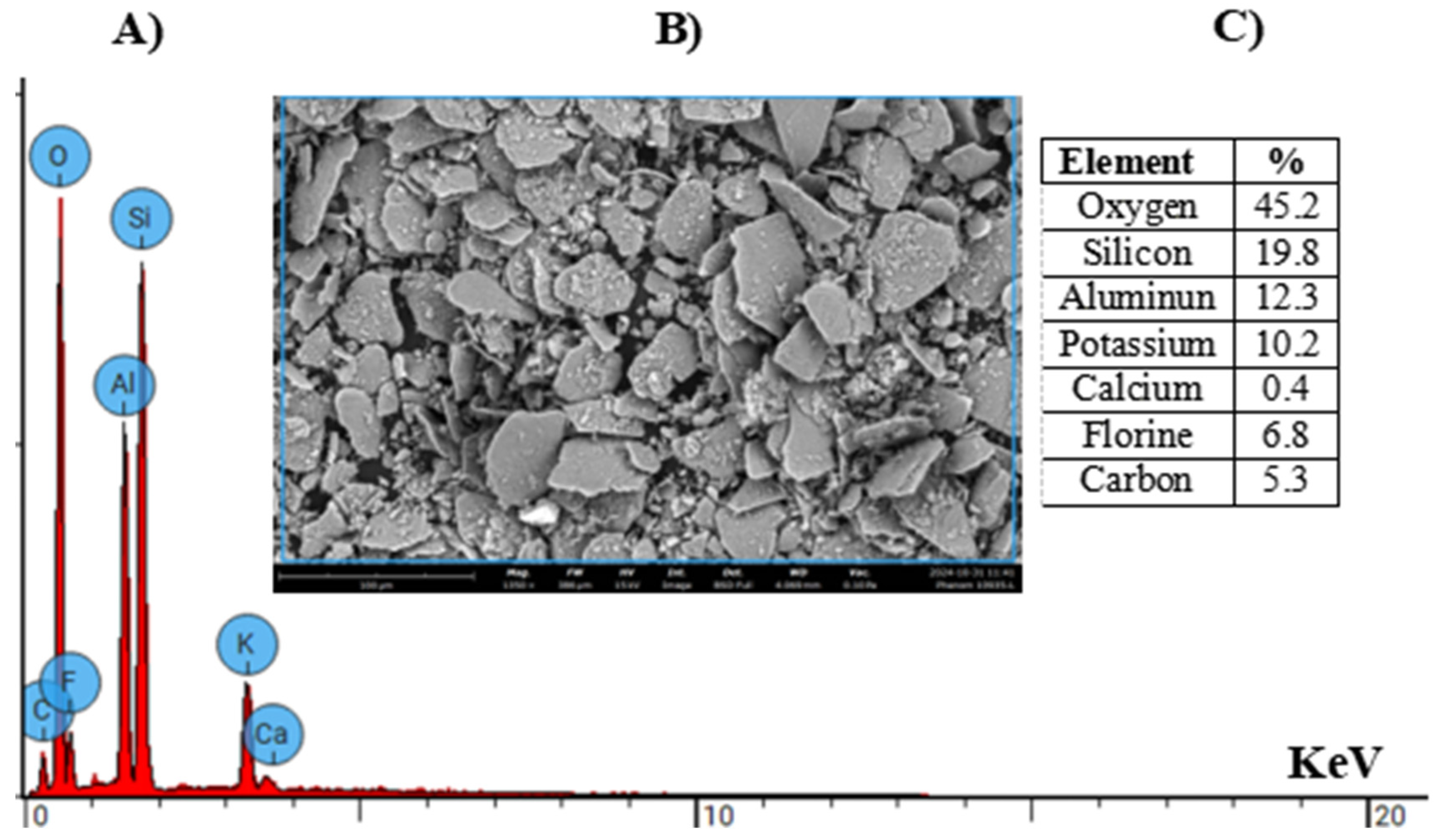


| Mineral | Elemental Composition (%) | |||||
|---|---|---|---|---|---|---|
| O | Si | F | Al | K | Li | |
| Lepidolite | 54.5 | 16.0 | 9.8 | 9.8 | 8.3 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes Pérez, M.; Patiño Cardona, F.; Islas Vázquez, H.; Reyes Domínguez, I.A.; Flores Guerrero, M.U.; Pérez Labra, M.; Juárez Tapia, J.C.; Tolentino Mendoza, D.Y.; Sánchez Acosta, M.M. The Effect of Particle Size and Dodecylamine Concentration on the Flotation of Lepidolite in Alkaline Medium. Minerals 2025, 15, 954. https://doi.org/10.3390/min15090954
Reyes Pérez M, Patiño Cardona F, Islas Vázquez H, Reyes Domínguez IA, Flores Guerrero MU, Pérez Labra M, Juárez Tapia JC, Tolentino Mendoza DY, Sánchez Acosta MM. The Effect of Particle Size and Dodecylamine Concentration on the Flotation of Lepidolite in Alkaline Medium. Minerals. 2025; 15(9):954. https://doi.org/10.3390/min15090954
Chicago/Turabian StyleReyes Pérez, Martín, Francisco Patiño Cardona, Hernan Islas Vázquez, Iván Alejandro Reyes Domínguez, Mizraim Uriel Flores Guerrero, Miguel Pérez Labra, Julio Cesar Juárez Tapia, Dayli Yamileth Tolentino Mendoza, and Miroslava Mishelle Sánchez Acosta. 2025. "The Effect of Particle Size and Dodecylamine Concentration on the Flotation of Lepidolite in Alkaline Medium" Minerals 15, no. 9: 954. https://doi.org/10.3390/min15090954
APA StyleReyes Pérez, M., Patiño Cardona, F., Islas Vázquez, H., Reyes Domínguez, I. A., Flores Guerrero, M. U., Pérez Labra, M., Juárez Tapia, J. C., Tolentino Mendoza, D. Y., & Sánchez Acosta, M. M. (2025). The Effect of Particle Size and Dodecylamine Concentration on the Flotation of Lepidolite in Alkaline Medium. Minerals, 15(9), 954. https://doi.org/10.3390/min15090954







