Geochemical Constraints on Antimony Mineralization in the Gutaishan Au–Sb Deposit, China: Insights from Trace Elements in Quartz and Sulfur Isotopes in Stibnite
Abstract
1. Introduction
2. Geological Setting
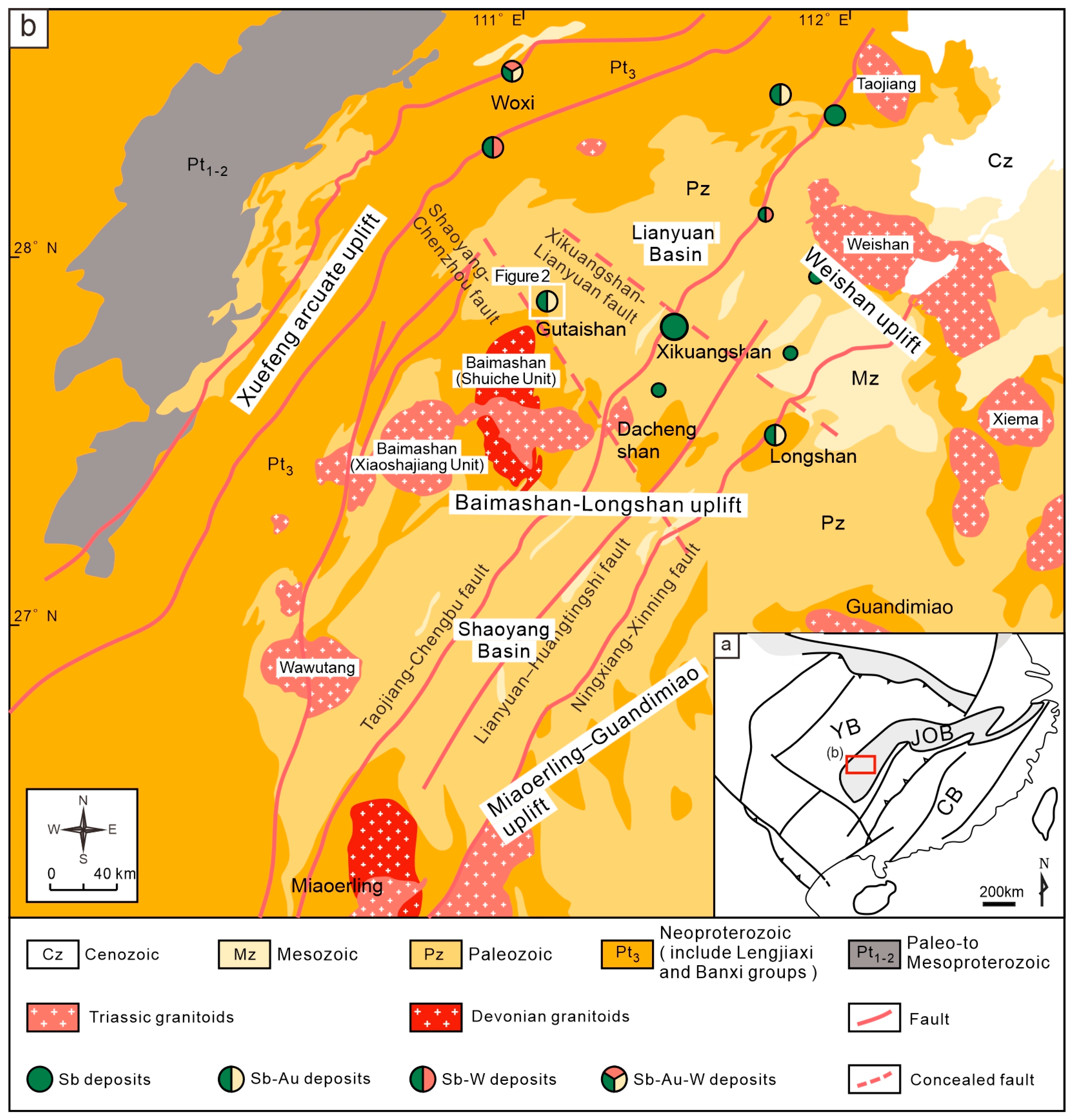
2.1. Regional Geology
2.2. Deposit Geology
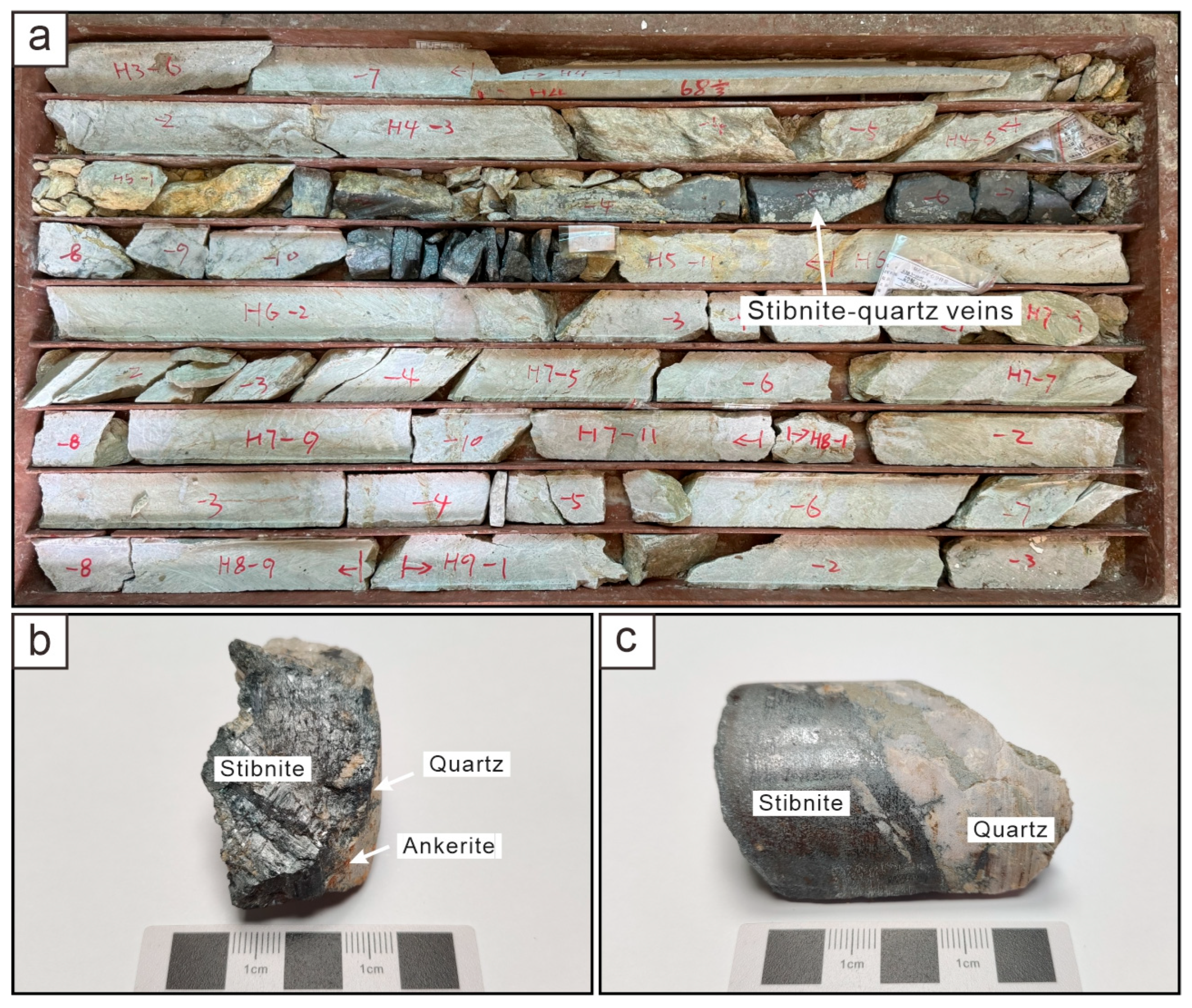
3. Samples and Analytical Methods
4. Results
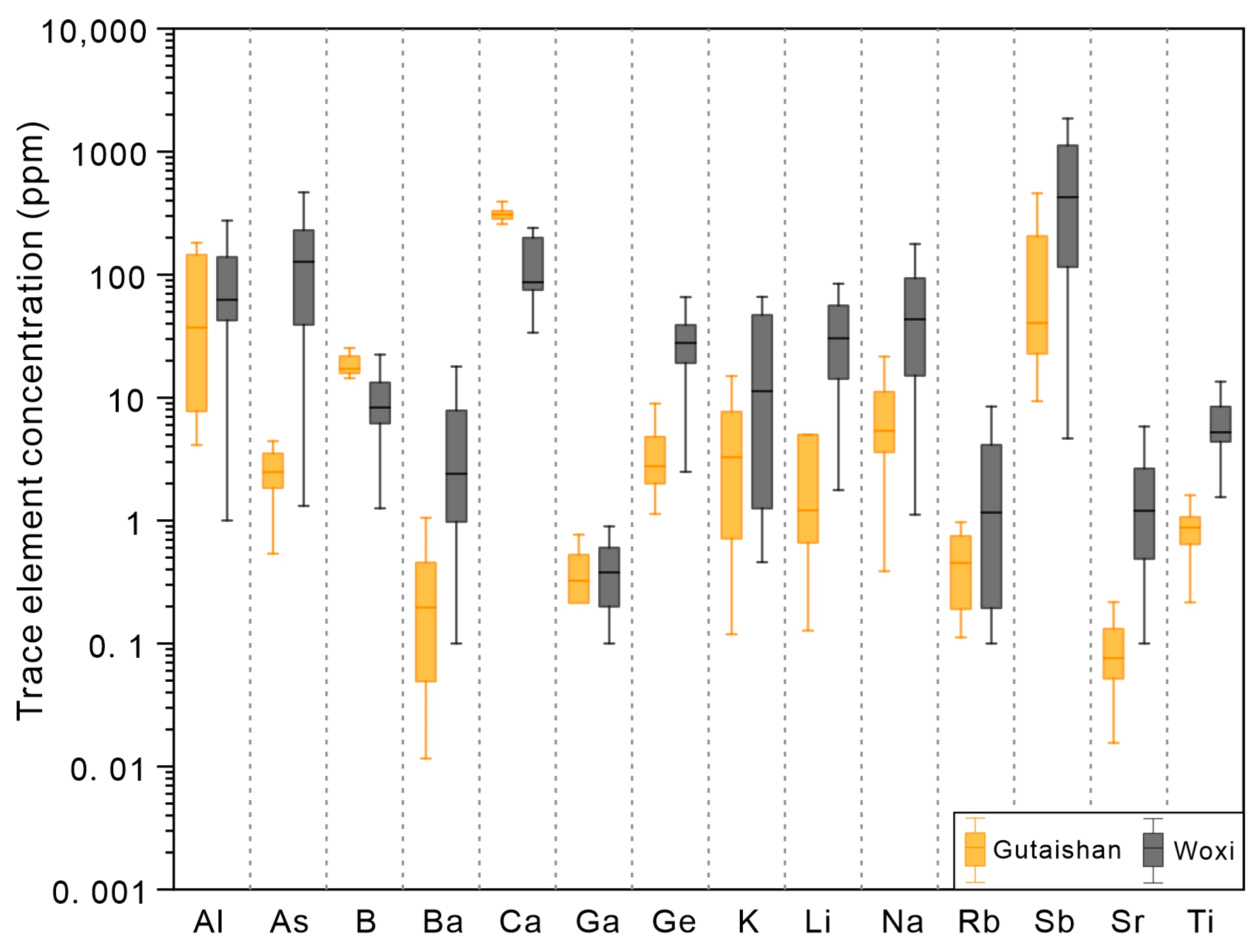
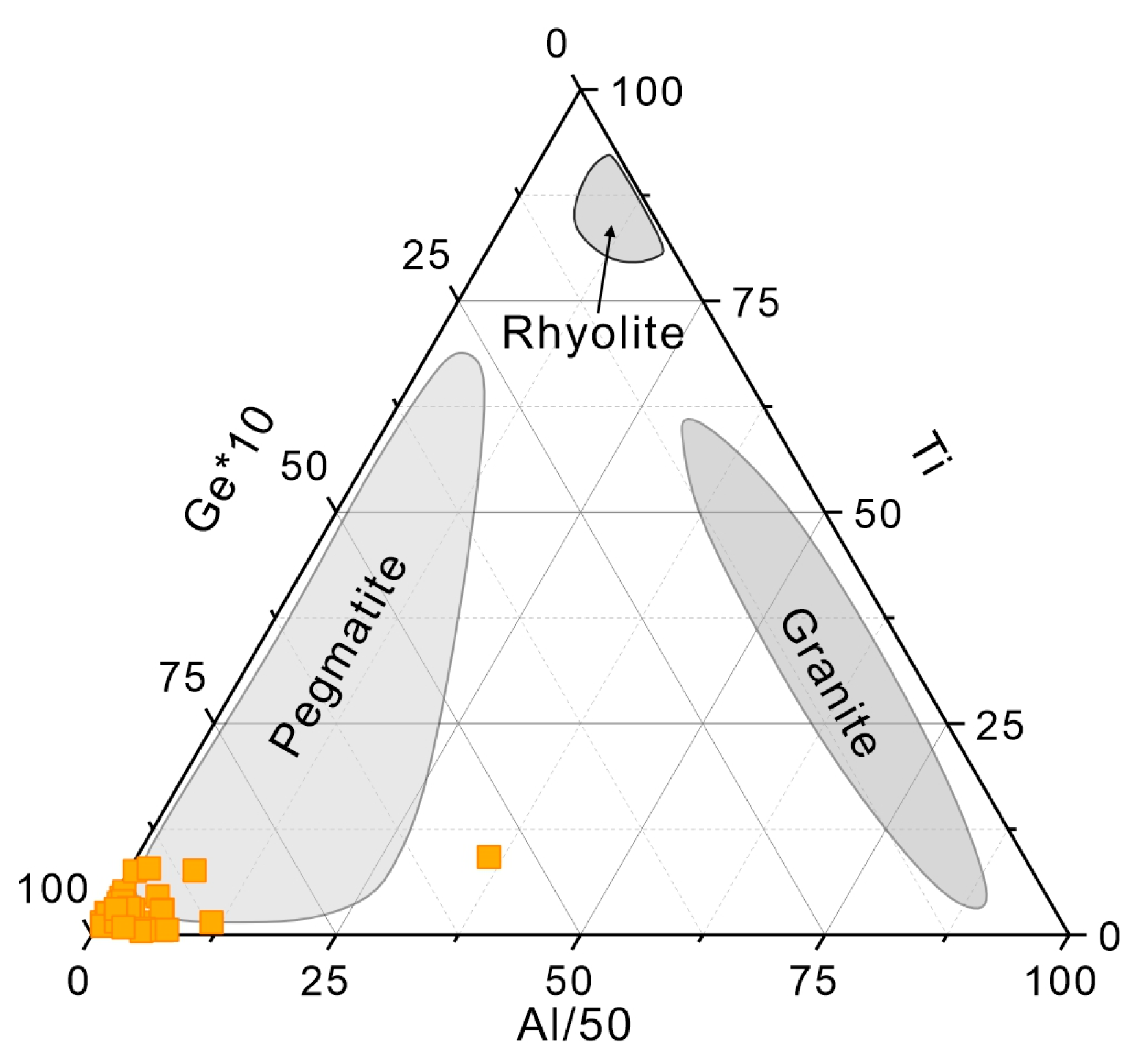
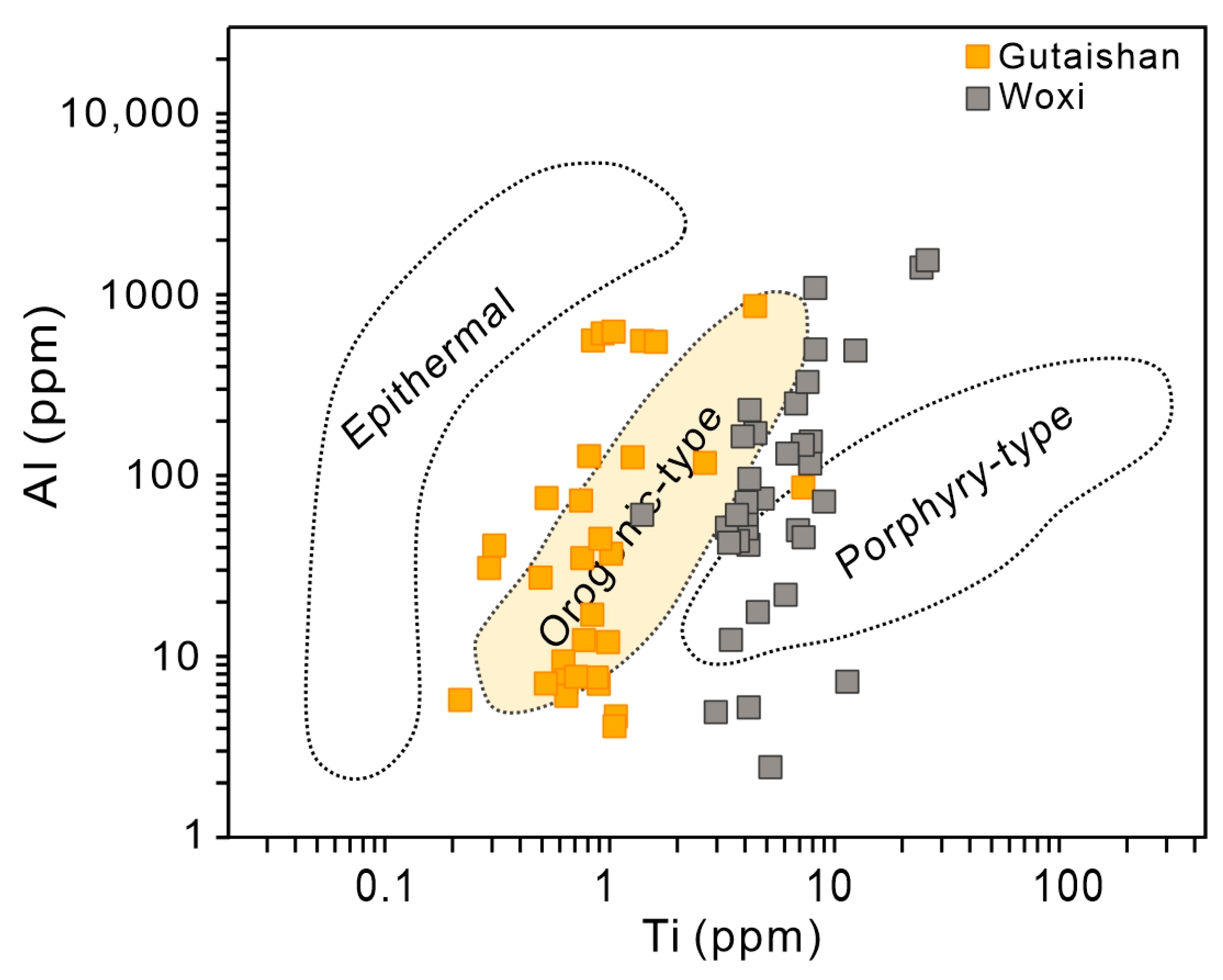
4.1. Trace Element Composition of Quartz
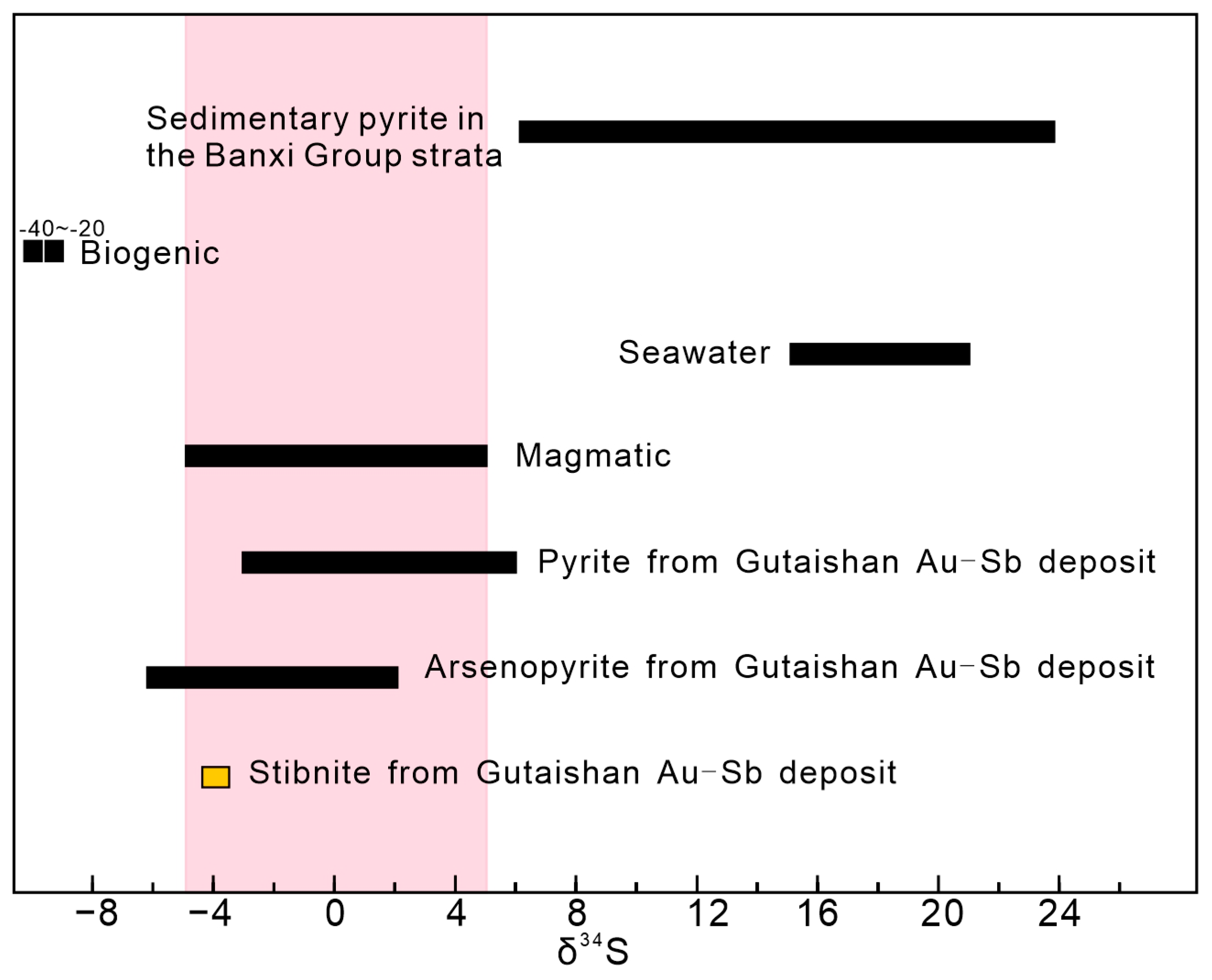
4.2. Sulfur Isotopic Composition of Stibnite
5. Discussion
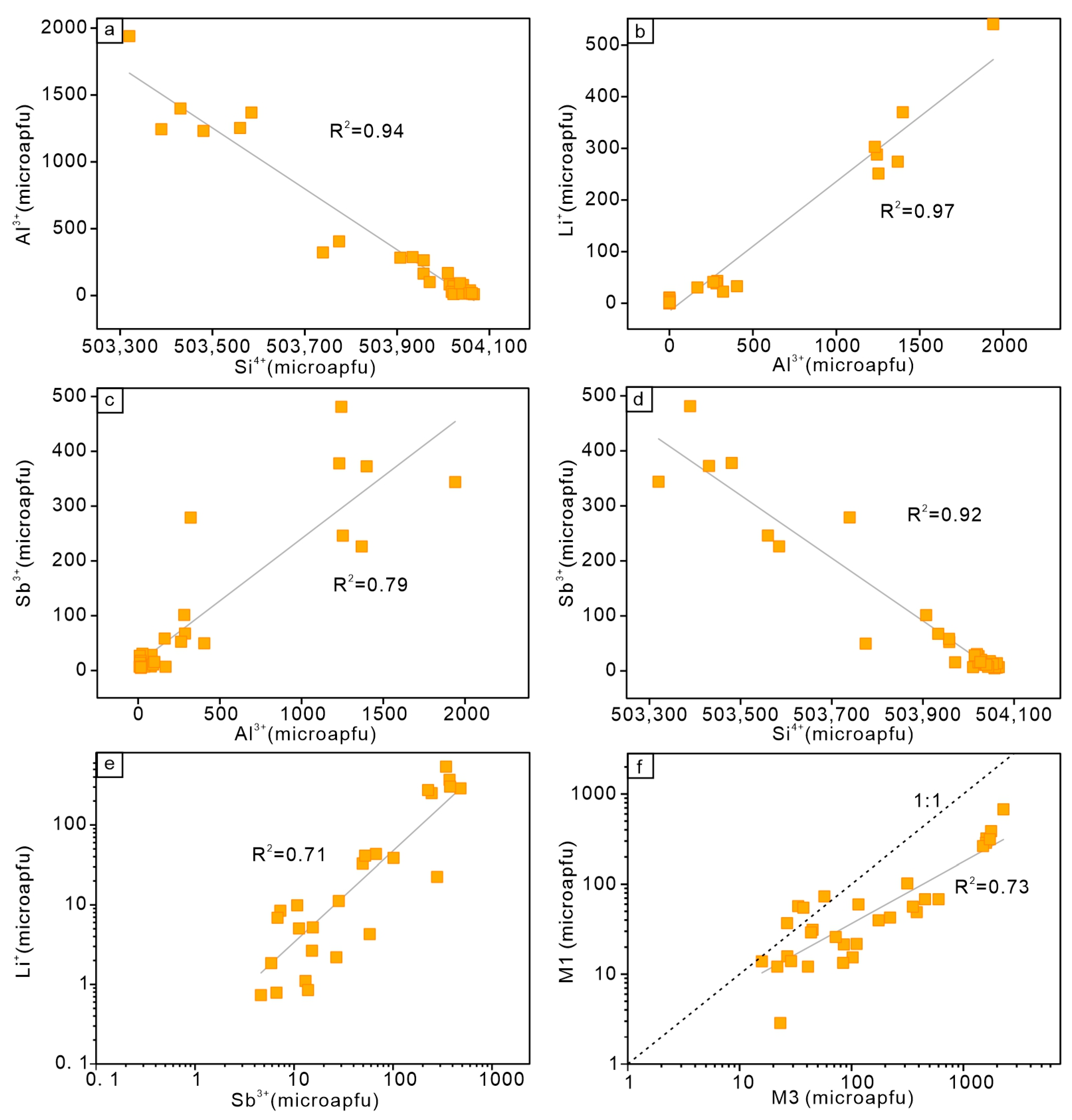
5.1. Substitution Mechanisms of Trace Elements in Quartz
5.2. Genesis of the Gutaishan Au–Sb Deposit
5.3. Magmatic–Hydrothermal Contributions to Sb Mineralization
5.4. Mineralization Model of the Gutaishan Au–Sb Deposit
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, J.; Zhang, Y.; Ma, Y.; Wang, Y.; Zhang, J.; Zhang, T. Metallogenic characteristics and resource potential of antimony in China. J. Geochem. Explor. 2021, 230, 106834. [Google Scholar] [CrossRef]
- Peng, J.T.; Hu, R.Z.; Burnard, P.G. Samarium–neodymium isotope systematics of hydrothermal calcites from the Xikuangshan antimony deposit (Hunan, China): The potential of calcite as a geochronometer. Chem. Geol. 2003, 200, 129–136. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Xie, Y.; Shao, Y.; Lai, C. Pyrite geochemistry and metallogenic implications of Gutaishan Au deposit in Jiangnan Orogen, South China. Ore Geol. Rev. 2020, 117, 103298. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Xie, Y.; Shao, Y.; Tan, H.; Li, H.; Lai, C. Ore-forming mechanism and physicochemical evolution of Gutaishan Au deposit, South China: Perspective from quartz geochemistry and fluid inclusions. Ore Geol. Rev. 2020, 119, 103382. [Google Scholar] [CrossRef]
- Li, W.; Xie, G.Q.; Mao, J.W.; Zhang, Z.Y.; Fu, B.; Lu, S. Muscovite 40Ar/39Ar and in situ sulfur isotope analyses of the slate-hosted Gutaishan Au–Sb deposit, South China: Implications for possible Late Triassic magmatic-hydrothermal mineralization. Ore Geol. Rev. 2018, 101, 839–853. [Google Scholar] [CrossRef]
- Li, W.; Cook, N.J.; Ciobanu, C.L.; Xie, G.; Wade, B.P.; Gilbert, S.E. Trace element distributions in (Cu)-Pb-Sb sulfosalts from the Gutaishan Au–Sb deposit, South China: Implications for formation of high fineness native gold. Am. Mineral. 2019, 104, 425–437. [Google Scholar] [CrossRef]
- Li, W.; Cook, N.J.; Xie, G.Q.; Mao, J.W.; Ciobanu, C.L.; Li, J.W.; Zhang, Z.Y. Textures and trace element signatures of pyrite and arsenopyrite from the Gutaishan Au–Sb deposit, South China. Miner. Depos. 2019, 54, 591–610. [Google Scholar] [CrossRef]
- Yu, J.G. Alteration features and prospecting direction in the Gutaishan gold deposit, Hunan. Hunan Geol. 1998, 3, 155–159, (In Chinese with English abstract). [Google Scholar]
- Zhang, Z.Y.; Zhu, Q.Q.; Li, W.; Han, Y.X. Deep information of W–Au–Sb deposits in the Central Hunan Basin. Miner. Depos. 2014, 33 (Suppl. S1), 491–492. (In Chinese) [Google Scholar]
- Wu, J.C.; Wang, J.R.; Ou, J.; Wang, S.S.; Yang, S.F.; Li, Y.H. Fluid inclusions and isotopic geochemistry of the Baima–Longshan gold belt, Hunan: Implications for ore-forming fluids. Miner. Resour. Geol. 2007, 21, 673–678. (In Chinese) [Google Scholar]
- Li, W.; Xie, G.Q.; Zhang, Z.Y.; Zhang, X.K. Constraint on the genesis of the Gutaishan gold deposit in central Hunan Province: Evidence from fluid inclusions and C–H–O isotopes. Acta Petrol. Sin. 2016, 32, 3489–3506, (In Chinese with English abstract). [Google Scholar]
- Zhou, C.; Sun, J.; Guo, A.M.; Jia, P.Y.; Lu, W.; Wei, H.T.; Guo, D.; Cai, Y. A comparative study of the ore-forming fluids of the typical gold–antimony deposits along the Middle Xuefeng arc structural belt. Geol. China 2020, 47, 1241–1259, (In Chinese with English abstract). [Google Scholar]
- Rusk, B. Cathodoluminescent textures and trace elements in hydrothermal quartz. In Quartz: Deposits, Mineralogy and Analytics; Götze, J., Möckel, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 307–329. [Google Scholar]
- Thomas, J.B.; Watson, E.B.; Spear, F.S.; Shemella, P.T.; Nayak, S.K.; Lanzirotti, A. TitaniQ under pressure: The effect of pressure and temperature on the solubility of Ti in quartz. Contrib. Mineral. Petrol. 2010, 160, 743–759. [Google Scholar] [CrossRef]
- Liu, E.T.; Zhao, J.X.; Pan, S.Q.; Yan, D.T.; Wang, H. In-situ U-Pb Dating of Quartz: A Preliminary Study. J. Earth Sci. 2024, 35(2), 726–728. [Google Scholar] [CrossRef]
- Lubben, J.D. Silicification Across the Betze-Post Carlin-Type Gold Deposit: Clues to Ore Fluid Properties and Sources, Northern Carlin Trend, Nevada. Master’s Thesis, University of Nevada, Las Vegas, NV, USA, 2004. [Google Scholar]
- Wang, X.; Qi, N.; Zhu, X.Y.; He, X.H.; Gu, H.W.; Deng, X.H. Magmatic to Hydrothermal Evolution of Bianjiadayuan Ag-Pb-Zn-Sn Deposit, NE China: A Quartz Texture and Trace Elements Study. J. Earth Sci. 2025, 36, 1493–1504. [Google Scholar] [CrossRef]
- Rusk, B.G.; Lowers, H.A.; Reed, M.H. Trace elements in hydrothermal quartz: Relationships to cathodoluminescent textures and insights into vein formation. Geology 2008, 36, 547–550. [Google Scholar] [CrossRef]
- Monecke, T.; Kempe, U.; Götze, J. Genetic significance of the trace element content in metamorphic and hydrothermal quartz: A reconnaissance study. Earth Planet. Sci. Lett. 2002, 202, 709–724. [Google Scholar] [CrossRef]
- Agangi, A.; McPhie, J.; Kamenetsky, V.S. Magma chamber dynamics in a silicic LIP revealed by quartz: The Mesoproterozoic Gawler Range Volcanics. Lithos 2011, 126, 68–83. [Google Scholar] [CrossRef]
- Götze, J.; Plötze, M.; Habermann, D. Origin, spectral characteristics and practical applications of the cathodoluminescence (CL) of quartz—A review. Mineral. Petrol. 2001, 71, 225–250. [Google Scholar] [CrossRef]
- Landtwing, M.R.; Pettke, T. Relationships between SEM-cathodoluminescence response and trace-element composition of hydrothermal vein quartz. Am. Mineral. 2005, 90, 122–131. [Google Scholar] [CrossRef]
- Mercer, C.N.; Reed, M.H.; Mercer, C.M. Time scales of porphyry Cu deposit formation: Insights from titanium diffusion in quartz. Econ. Geol. 2015, 110, 587–602. [Google Scholar] [CrossRef]
- Wark, D.A.; Watson, E.B. TitaniQ: A titanium-in-quartz geothermometer. Contrib. Mineral. Petrol. 2006, 152, 743–754. [Google Scholar] [CrossRef]
- Monnier, L.; Lach, P.; Salvi, S.; Melleton, J.; Bailly, L.; Béziat, D.; Monnier, Y.; Gouy, S. Quartz trace-element composition by LA-ICP-MS as proxy for granite differentiation, hydrothermal episodes, and related mineralization: The Beauvoir Granite (Echassières district), France. Lithos 2018, 320–321, 355–377. [Google Scholar] [CrossRef]
- Wertich, V.; Leichmann, J.; Dosbaba, M.; Götze, J. Multi-stage evolution of gold-bearing hydrothermal quartz veins at the Mokrsko gold deposit (Czech Republic) based on cathodoluminescence, spectroscopic, and trace elements analyses. Minerals 2018, 8, 335. [Google Scholar] [CrossRef]
- Krupp, R.E. Solubility of stibnite in hydrogen sulfide solutions, speciation, and equilibrium constants, from 25 to 350 °C. Geochim. Cosmochim. Acta 1988, 52, 3005–3015. [Google Scholar] [CrossRef]
- Fu, S.; Hu, R.; Yin, R.; Yan, J.; Mi, X.; Song, Z.; Sullivan, N.A. Mercury and in situ sulfur isotopes as constraints on the metal and sulfur sources for the world’s largest Sb deposit at Xikuangshan, southern China. Miner. Depos. 2020, 55, 1353–1364. [Google Scholar] [CrossRef]
- Canfield, D.E. Isotope fractionation by natural populations of sulfate-reducing bacteria. Geochim. Cosmochim. Acta 2001, 65, 1117–1124. [Google Scholar] [CrossRef]
- Ohmoto, H.; Goldhaber, M.B. Sulfur and carbon isotopes. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Wiley: New York, NY, USA, 1997; pp. 517–611. [Google Scholar]
- Seal, R.R. Sulfur isotope geochemistry of sulfide minerals. Rev. Mineral. Geochem. 2006, 61, 633–677. [Google Scholar] [CrossRef]
- Craddock, P.R.; Rouxel, O.J.; Ball, L.A.; Bach, W. Sulfur isotope measurement of sulfate and sulfide by high-resolution MC-ICP-MS. Chem. Geol. 2008, 253, 102–113. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, X.; Chen, L.; Bao, Z.; Chen, K.; Zong, C.; Li, X.C.; Qiu, J.W. Simultaneous measurement of sulfur and lead isotopes in sulfides using nanosecond laser ablation coupled with two multi-collector inductively coupled plasma mass spectrometers. J. Asian Earth Sci. 2018, 154, 386–396. [Google Scholar] [CrossRef]
- Dai, Z.H.; Fu, S.L.; Liu, Y.F.; Meng, Y.M.; Bao, Z.A.; Hou, K.J.; Lan, T.G. A potential stibnite reference material for sulfur isotope determination by LA-MC-ICP-MS. J. Anal. At. Spectrom. 2024, 39, 216–226. [Google Scholar] [CrossRef]
- Mason, P.R.D.; Košler, J.; de Hoog, J.C.M.; Sylvester, P.J.; Meffan-Main, S.K. In situ determination of sulfur isotopes in sulfur-rich materials by laser ablation multiple-collector inductively coupled plasma mass spectrometry (LA-MC-ICP-MS). J. Anal. At. Spectrom. 2006, 21, 177–186. [Google Scholar] [CrossRef]
- Hou, L.; Peng, H.J.; Ding, J.; Zhang, J.R.; Zhu, S.B.; Wu, S.Y.; Wu, Y.; Ouyang, H.G. Textures and in situ chemical and isotopic analyses of pyrite, Huijiabao Trend, Youjiang basin, China: Implications for paragenesis and source of sulfur. Econ. Geol. 2016, 111, 331–353. [Google Scholar] [CrossRef]
- Zhao, G. Jiangnan Orogen in South China: Developing from divergent double subduction. Gondwana Res. 2015, 27, 1173–1180. [Google Scholar] [CrossRef]
- Qiu, L.; Yan, D.P.; Tang, S.L.; Wang, Q.; Yang, W.X.; Tang, X.; Wang, J. Mesozoic geology of southwestern China: Indosinian foreland overthrusting and subsequent deformation. J. Asian Earth Sci. 2016, 122, 91–105. [Google Scholar] [CrossRef]
- Hu, R.Z.; Zhou, M.F. Multiple Mesozoic mineralization events in South China—An introduction to the thematic issue. Miner. Depos. 2012, 47, 579–588. [Google Scholar] [CrossRef]
- Li, H.; Kong, H.; Zhou, Z.K.; Tindell, T.; Tang, Y.Q.; Wu, Q.H.; Xi, X.S. Genesis of the Banxi Sb deposit, South China: Constraints from wall-rock geochemistry, fluid inclusion microthermometry, Rb–Sr geochronology, and H–O–S isotopes. Ore Geol. Rev. 2019, 115, 103162. [Google Scholar] [CrossRef]
- Ma, D.S.; Pan, J.Y.; Xie, Q.L. Ore sources of Sb (Au) deposits in central Hunan: II. Evidence of isotopic geochemistry. Miner. Depos. 2003, 22, 78–87. (In Chinese) [Google Scholar]
- Peng, J.T. Hunan Xuefeng area gold metallogenic evolution mechanism discussion. Tecton. Metallog. 1999, 23, 144–151. (In Chinese) [Google Scholar]
- Wang, F.R.; Quan, Z.Y.; Hu, N.Y.; Li, Y.P. Metallogenic conditions and distribution-enrichment patterns of gold deposits in Hunan Province. Hunan Geol. 1993, 12, 163–170. (In Chinese) [Google Scholar]
- Wang, G. Geological-Geochemical Characteristics of Caledonian Granitoids and Its Geological Significance: Taking Baimashan, Xuefengshan, Hongxiaqiao Plutons as Examples. Master’s Thesis, Central South University, Changsha, China, 2013; pp. 10–70, (In Chinese with English abstract). [Google Scholar]
- Chen, W.; Chen, P.; Huang, H.; Ding, X.; Sun, T. Chronological and geochemical studies of granite and enclave in Baimashan pluton, Hunan, South China. Sci. China Ser. D Earth Sci. 2007, 50, 1606–1627. [Google Scholar] [CrossRef]
- Luo, Z.G.; Wang, Y.J.; Zhang, F.F.; Zhang, A.M.; Zhang, Y.Z. LA-ICPMS zircon U–Pb dating for Baimashan and Jintan Indosinian granitic plutons and its petrogenetic implications. Geotecton. Metallog. 2010, 34, 282–290. [Google Scholar]
- Liu, J.Q.; Yuan, X.; Zhan, Z.; Lin, J.S.; Feng, W.M.; Huang, X.P. The geochronologic characteristics of Baimashan granite in western Hunan Province and its geotectonic significance. Earth Sci. Front. 2013, 20, 25–35. [Google Scholar]
- Kang, R.H. Analysis of Exploration Perspectives of Gold–Antimony Deposits in the Baimashan–Longshan EW-Striking Structural Zone, Hunan Province. Geol. Miner. Resour. South China 2002, 18, 57–61, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Jiang, C.; Xie, B.W.; Duan, A.J.; Huang, X.J.; Ai, G.; Liu, W. Study on Geological Characteristics and Prospecting Direction of No.3 Vein in Gutaishan Gold-antimony Deposit, Hunan Province. Mod. Min. 2024, 40, 49–53+61. [Google Scholar]
- Jochum, K.P.; Willbold, M.; Raczek, I.; Stoll, B.; Herwig, K. Chemical Characterisation of the USGS Reference Glasses GSA-1G, GSC-1G, GSD-1G, GSE-1G, BCR-2G, BHVO-2G and BIR-1G Using EPMA, ID-TIMS, ID-ICP-MS and LA-ICP-MS. Geostand. Geoanal. Res. 2005, 29, 285–302. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Chen, L.; Chen, K.; Bao, Z.; Liang, P.; Sun, T.; Yuan, H. Preparation of standards for in situ sulfur isotope measurement in sulfides using femtosecond laser ablation MC-ICP-MS. J. Anal. At. Spectrom. 2017, 32, 107–116. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, L.; Zong, C.; Yuan, H.; Chen, K.; Dai, M. Development of pressed sulfide powder tablets for in situ sulfur and lead isotope measurement using LA-MC-ICP-MS. Int. J. Mass Spectrom. 2017, 421, 255–262. [Google Scholar] [CrossRef]
- Fu, S.; Lan, Q.; Yan, J. Trace element chemistry of hydrothermal quartz and its genetic significance: A case study from the Xikuangshan and Woxi giant Sb deposits in southern China. Ore Geol. Rev. 2020, 126, 103732. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Peng, J.T. Infrared microthermometric and noble gas isotope study of fluid inclusions in ore minerals at the Woxi orogenic Au–Sb–W deposit, western Hunan, South China. Ore Geol. Rev. 2015, 65, 55–69. [Google Scholar] [CrossRef]
- Müller, A.; Wiedenbeck, M.; van den Kerkhof, A.M.; Kronz, A.; Simon, K. Trace elements in quartz—A combined electron microprobe, secondary ion mass spectrometry, laser ablation ICP-MS, and cathodoluminescence study. Eur. J. Mineral. 2003, 15(4), 747–763. [Google Scholar] [CrossRef]
- Müller, A.; Koch-Müller, M. Hydrogen speciation and trace element contents of igneous, hydrothermal and metamorphic quartz from Norway. Mineral. Mag. 2009, 73, 569–583. [Google Scholar] [CrossRef]
- Götte, T.; Ramseyer, K. Trace element characteristics, luminescence properties and real structure of quartz. In Quartz: Deposits, Mineralogy and Analytics; Rusk, B.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 265–285. [Google Scholar]
- Griffiths, J.H.E.; Owen, J.; Ward, I.M. Paramagnetic resonance in neutron-irradiated diamond and smoky quartz. Nature 1954, 173, 439–440. [Google Scholar] [CrossRef]
- Walsby, C.J.; Lees, N.S.; Claridge, R.F.; Weil, J.A. The magnetic properties of oxygen-hole aluminum centres in crystalline SiO2. VI: A stable AlO4/Li centre. Can. J. Phys. 2003, 81, 583–598. [Google Scholar] [CrossRef]
- Götze, J. Chemistry, textures and physical properties of quartz—Geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Rusk, B.G.; Reed, M.H.; Dilles, J.H.; Kent, A.J. Intensity of quartz cathodoluminescence and trace-element content in quartz from the porphyry copper deposit at Butte, Montana. Am. Mineral. 2006, 91, 1300–1312. [Google Scholar] [CrossRef]
- Weil, J.A. A review of electron spin spectroscopy and its application to the study of paramagnetic defects in crystalline quartz. Phys. Chem. Miner. 1984, 10, 149–165. [Google Scholar] [CrossRef]
- Miyoshi, N.; Yamaguchi, Y.; Makino, K. Successive zoning of Al and H in hydrothermal vein quartz. Am. Mineral. 2005, 90, 310–315. [Google Scholar] [CrossRef]
- Lehmann, K.; Berger, A.; Götte, T.; Ramseyer, K.; Wiedenbeck, M. Growth related zonations in authigenic and hydrothermal quartz characterized by SIMS-, EPMA-, SEM-CL-, and SEM-CC-imaging. Mineral. Mag. 2009, 73, 633–643. [Google Scholar] [CrossRef]
- Lehmann, K.; Pettke, T.; Ramseyer, K. Significance of trace elements in syntaxial quartz cement, Haushi Group sandstones, Sultanate of Oman. Chem. Geol. 2011, 280, 47–57. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Schott, J. Thermodynamic properties of aqueous Ge (IV) hydroxide complexes from 25 to 350 °C: Implications for the behavior of germanium and the Ge/Si ratio in hydrothermal fluids. Geochim. Cosmochim. Acta 1998, 62, 1631–1642. [Google Scholar] [CrossRef]
- Blankenburg, H.J.; Götze, J.; Schulz, H. Quarzrohstoffe; Deutscher Verlag für Grundstoffindustrie: Leipzig-Stuttgart, Germany, 1994; p. 296. [Google Scholar]
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and mineral chemistry of quartz: A review. Mineral. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]
- Rottier, B.; Casanova, V. Trace element composition of quartz from porphyry systems: A tracer of the mineralizing fluid evolution. Miner. Depos. 2021, 56, 843–862. [Google Scholar] [CrossRef]
- Peterková, T.; Dolejš, D. Magmatic-hydrothermal transition of Mo-W-mineralized granite-pegmatite-greisen system recorded by trace elements in quartz: Krupka district, Eastern Krušné hory/Erzgebirge. Chem. Geol. 2019, 523, 179–202. [Google Scholar] [CrossRef]
- Breiter, K.; Badanina, E.; Ďurišová, J.; Dosbaba, M.; Syritso, L. Chemistry of quartz—A new insight into the origin of the Orlovka Ta-Li deposit, Eastern Transbaikalia, Russia. Lithos 2021, 348–349, 105206. [Google Scholar] [CrossRef]
- Obolensky, A.A.; Gushchina, L.V.; Borisenko, A.S.; Borovikov, A.A.; Pavlova, G.G. Antimony in hydrothermal processes: Solubility, conditions of transfer, and metal-bearing capacity of solutions. Russ. Geol. Geophys. 2007, 48, 992–1001. [Google Scholar] [CrossRef]
- Biver, M.; Shotyk, W. Stibnite (Sb2S3) oxidative dissolution kinetics from pH 1 to 11. Geochim. Cosmochim. Acta 2012, 79, 127–139. [Google Scholar] [CrossRef]
- Ohmoto, H. Systematics of sulfur and carbon isotopes in hydrothermal ore deposits. Econ. Geol. 1972, 67, 551–578. [Google Scholar] [CrossRef]
- Rees, C.E.; Jenkins, W.J.; Monster, J. The sulphur isotopic composition of ocean water sulphate. Geochim. Cosmochim. Acta 1978, 42, 377–381. [Google Scholar] [CrossRef]
- Rao, J.R.; Wang, J.H.; Cao, Y.Z. Deep structure in Hunan. Hunan Geol. 1993, 12, 1–101, (In Chinese with English abstract). [Google Scholar]
- Peng, J.; Hu, R.; Zhao, J.; Fu, Y.; Lin, Y. Scheelite Sm-Nd dating and quartz Ar-Ar dating for Woxi Au–Sb-W deposit, western Hunan. Chin. Sci. Bull. 2003, 48, 2640–2646. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Baker, T.; Dubé, B.; Groves, D.; Hart, C.; Gosselin, P. Distribution, character and genesis of gold deposits in metamorphic terranes. In Economic Geology 100th Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists, Inc.: Littleton, CO, USA, 2005; pp. 407–450. [Google Scholar]
- Phillips, G.N.; Powell, R. Formation of gold deposits: A metamorphic devolatilization model. J. Metamorph. Geol. 2010, 28, 689–718. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Q.; Yang, Y.L.; Zhou, Y.L.; Cui, X.D.; Zou, H. Petrogenesis and geodynamic implications of the Baimashan granitic complex in central Hunan, South China. Geol. J. 2022, 57, 4718–4745. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Norman, C. Controls of mineral parageneses in the system Fe-Sb-SO. Econ. Geol. 1997, 92, 308–324. [Google Scholar] [CrossRef]
- Robert, F.; Poulsen, K.H.; Cassidy, K.F.; Hodgson, C.J. Gold Metallogeny of the Superior and Yilgarn Cratons; Society of Economic Geologists: Littleton, CO, USA, 2005. [Google Scholar]
- Thébaud, N.; Sugiono, D.; LaFlamme, C.; Miller, J.; Fisher, L.; Voute, F.; Tessalina, S.; Sonntag, I.; Fiorentini, M. Protracted and polyphased gold mineralisation in the Agnew district (Yilgarn craton, Western Australia). Precambrian Res. 2018, 310, 291–304. [Google Scholar] [CrossRef]
- Moncada, D.; Rimstidt, J.D.; Bodnar, R.J. How to form a giant epithermal precious metal deposit: Relationships between fluid flow rate, metal concentration of ore-forming fluids, duration of the ore-forming process, and ore grade and tonnage. Ore Geol. Rev. 2019, 113, 103066. [Google Scholar] [CrossRef]
- Sasmaz, A.; Sukach, V.; Bondarenko, S.; Aleksiienko, H.; Erfanian Kaseb, H.; Sasmaz, B.; Kurylo, S.; Hrinchenko, O.; Somka, V.; Voudouris, P. Newly Identified Au-Ag-Bi-Te Mineralization in the Aydindere Skarn Fe and Cu Deposit, Giresun, NE Turkey: Implications of Gold Mineralization during Retrograde Skarn Evolution. J. Earth Sci. 2025, 36(2), 543–561. [Google Scholar] [CrossRef]
- Hou, X.N.; Fu, S.L.; Kong, H.; Liu, B.; Tang, Y.W.; Huang, J.G. Is there superimposed mineralization occurring within the Longshan Sb-Au deposit, South China? A perspective from U-Pb dating of apatite and in-situ S isotopes of pyrite and stibnite. Ore Geol. Rev. 2025, 181, 106631. [Google Scholar] [CrossRef]


| Sample Name | Sample Types | Minerals | 34SV-CDT | 1σ |
|---|---|---|---|---|
| K2B3-1001-1-1 | Stibnite ores | Stibnite | −4.42 | 0.17 |
| K2B3-1001-1-2 | Stibnite ores | Stibnite | −4.19 | 0.18 |
| K2B3-1001-1-3 | Stibnite ores | Stibnite | −4.13 | 0.18 |
| K2B3-1001-1-4 | Stibnite ores | Stibnite | −4.22 | 0.18 |
| K2B3-1001-1-5 | Stibnite ores | Stibnite | −4.43 | 0.20 |
| K2B3-1001-1-6 | Stibnite ores | Stibnite | −4.27 | 0.16 |
| K2B3-1001-1-7 | Stibnite ores | Stibnite | −4.05 | 0.17 |
| K2B3-1001-1-8 | Stibnite ores | Stibnite | −4.36 | 0.19 |
| K2B3-1001-1-9 | Stibnite ores | Stibnite | −4.08 | 0.18 |
| K2B3-1001-1-10 | Stibnite ores | Stibnite | −4.46 | 0.18 |
| K2B3-209-1-1 | Stibnite ores | Stibnite | −3.80 | 0.18 |
| K2B3-209-1-2 | Stibnite ores | Stibnite | −3.77 | 0.21 |
| K2B3-209-1-3 | Stibnite ores | Stibnite | −3.68 | 0.21 |
| K2B3-209-1-4 | Stibnite ores | Stibnite | −3.95 | 0.19 |
| K2B3-209-1-5 | Stibnite ores | Stibnite | −3.51 | 0.22 |
| K2B3-209-1-6 | Stibnite ores | Stibnite | −3.85 | 0.18 |
| K2B3-209-1-7 | Stibnite ores | Stibnite | −3.87 | 0.17 |
| K2B3-209-1-8 | Stibnite ores | Stibnite | −3.61 | 0.17 |
| K2B3-209-1-9 | Stibnite ores | Stibnite | −3.50 | 0.17 |
| K2B3-209-1-10 | Stibnite ores | Stibnite | −3.76 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Kang, L.; Li, B.; Kang, P. Geochemical Constraints on Antimony Mineralization in the Gutaishan Au–Sb Deposit, China: Insights from Trace Elements in Quartz and Sulfur Isotopes in Stibnite. Minerals 2025, 15, 953. https://doi.org/10.3390/min15090953
Feng J, Kang L, Li B, Kang P. Geochemical Constraints on Antimony Mineralization in the Gutaishan Au–Sb Deposit, China: Insights from Trace Elements in Quartz and Sulfur Isotopes in Stibnite. Minerals. 2025; 15(9):953. https://doi.org/10.3390/min15090953
Chicago/Turabian StyleFeng, Jingping, Linyan Kang, Bin Li, and Peixuan Kang. 2025. "Geochemical Constraints on Antimony Mineralization in the Gutaishan Au–Sb Deposit, China: Insights from Trace Elements in Quartz and Sulfur Isotopes in Stibnite" Minerals 15, no. 9: 953. https://doi.org/10.3390/min15090953
APA StyleFeng, J., Kang, L., Li, B., & Kang, P. (2025). Geochemical Constraints on Antimony Mineralization in the Gutaishan Au–Sb Deposit, China: Insights from Trace Elements in Quartz and Sulfur Isotopes in Stibnite. Minerals, 15(9), 953. https://doi.org/10.3390/min15090953





