Abstract
The extraction of lithium from clay-type lithium ores has attracted significant attention, but the leaching behavior of associated elements, such as Rb, K, and Sr, remains less explored. This study quantitatively investigated the leaching behaviors and mechanisms of Li, Rb, K, Sr, and Mg in clay-type lithium ore through water leaching and roasting–water leaching processes. The results show that during direct water leaching, the leaching efficiency of K ranged between 10% and 13%, while Li and Sr exhibited lower extraction rates, requiring prolonged high-temperature leaching. Rb dissolution was minimal, and the leaching efficiency of Mg was significantly affected by temperature. In contrast, roasting–water leaching significantly enhanced the leaching efficiency, achieving extraction rates of 90.65% for Li, 92.91% for Rb, 75.85% for K, and 36.99% for Sr. However, Mg leaching was suppressed to below 1%. Roasting disrupted the original silicate and carbonate lattices, generating new phases that altered the ore’s microstructure into aggregated dense phases and needle-like porous phases upon water leaching, thereby facilitating the release of Li, Rb, K, and Sr. A research finding was that the new phase generated by magnesium inhibited its leaching, which indirectly enhanced subsequent Li, Rb, K, and Sr extraction and separation. These findings provide a quantitative foundation for optimizing multi-element co-extraction from clay-type lithium ores.
1. Introduction
Li, Rb, and K, classified as critical metals, have been gaining increasing strategic significance due to their essential roles in new energy technologies, advanced materials development, and modern agricultural applications. As the core raw material for power batteries and energy storage systems [1,2,3], Li has experienced continuously rapid growth in demand [4,5]. Rb exhibits unique and versatile applications in electronics and aerospace technologies [6,7,8]. K, as an indispensable nutrient for crop growth, plays a vital role in ensuring food security [9,10]. Concurrently, Sr has drawn significant attention as a key additive in electronic ceramics and medical imaging [11,12]. Against this backdrop, clay-type lithium ores have emerged as important targets for multi-element recovery due to their abundant lithium reserves and frequent association with valuable elements, such as potassium, rubidium, and strontium. Notably, the clay deposits in the high-salinity mudflat areas of the Qaidam Basin, China [13,14,15], possess distinctive resource characteristics, making their exploitation crucial for alleviating strategic resource shortages.
The extraction of lithium from clay-type deposits presents complex technical challenges. These minerals exhibit diverse genetic origins and are typically associated with silicate minerals (e.g., quartz, kaolinite, chlorite, and illite) and carbonate minerals (e.g., calcite). Lithium and rubidium primarily occur as isomorphous substitutions within stable crystal lattices [1,16,17], while potassium is often adsorbed in clay interlayers or hosted in aluminosilicates such as feldspar [18]. In contrast, strontium tends to form secondary mineral phases with carbonates or sulfates [11]. The differences in occurrence states make it difficult for a single leaching system to simultaneously break different chemical bonding structures. Meanwhile, during the roasting process, major elements, such as K and Mg, preferentially react with sodium sulfate to form double salts, which not only consume the roasting agent but also may inhibit the release of target elements through a wrapping effect. Secondly, the similarity in chemical properties between elements can lead to competitive reactions during roasting and reduce the efficiency of subsequent element separation, making it difficult to achieve comprehensive recovery of elements. Roasting pretreatment has been proven effective in enhancing the leaching performance of target elements by inducing high-temperature lattice reconstruction and phase transformation [19]. In particular, the sodium sulfate roasting–water leaching process significantly improves lithium extraction efficiency [20,21]. However, existing studies have mostly focused on the extraction efficiency of a single element, Li, and failed to solve the core problem of synergistic leaching of multiple elements. Systematic exploration is still lacking regarding the synergistic leaching laws and potential values of Rb, K, and Sr, which have similar chemical properties to Li.
The comprehensive utilization of clay-type lithium ores requires giving equal attention to the leaching of Rb and Sr as to Li extraction. Furthermore, the potential interactions between high-abundance elements (K and Mg) and these target metals during leaching remain poorly understood. To address these research gaps, this study focuses on clay minerals from the hypersaline mudflat deposits in the Qaidam Basin, Qinghai Province, employing systematic Na2SO4 roasting–water leaching experiments to investigate the migration patterns and occurrence state transformations of multiple elements during the roasting process. The scientific novelty of this work lies not only in lithium extraction but particularly in quantitatively revealing the differentiated leaching behaviors of various elements (Li, Rb, K, Mg, and Sr) resulting from their distinct occurrence states. This study provides clear answers to key questions regarding the co-extraction of lithium with rubidium, potassium, magnesium, and strontium, as well as their mutual influences in clay-type lithium ores, thereby offering fundamental support for the development of low-grade complex clay mineral resources. Compared with the traditional sulfuric acid method, the optimized process parameters achieved multi-element extraction without the involvement of strong acids while significantly reducing magnesium leaching rates, thereby facilitating subsequent element separation.
2. Materials and Methods
The investigated samples consisted of grayish-brown clay collected from a high-salinity mudflat deposit in the Qaidam Basin, Qinghai Province, China. To ensure representativeness, all samples were processed following standard geological protocols, including drying and crushing procedures. XRD and FTIR analyses revealed that the main mineral phases in the samples include silicate minerals (quartz, chlorite, illite), carbonate minerals (calcite), halite, and more, along with organic impurities.
In the water leaching experiments, pretreated ore samples weighing precisely 10.00 g were placed in sealed glass bottles. Ultra-pure water was added at solid-to-liquid ratios of 1:3 for leaching experiments conducted at 15 °C, 25 °C, and 50 °C, with agitation at 200 rpm. Additional tests were performed at 50 °C using different solid-to-liquid ratios (1:2, 1:3, and 1:5) to examine the leaching efficiency, with periodic sampling during the process. For the roasting–water leaching experiments, ore samples weighing exactly 10.00 g were thoroughly mixed with anhydrous sodium sulfate (AR ≥ 99.0%) at different mass ratios (10:1, 10:3, and 10:5), then placed in crucibles and roasted at various temperatures (600–1000 °C) for different durations (0.5–4 h). After cooling to room temperature, the roasted products were subjected to water leaching at 80 °C with a solid-to-liquid ratio of 1:5. Following leaching, the leachate was separated from the residue by vacuum filtration, and the elemental concentrations in the leachate were analyzed using ICP-OES. The key parameter ranges in this study were determined through preliminary experimental validation to ensure effective ore decomposition and optimal leaching efficiencies of target elements, thereby guaranteeing the reliability of the experimental methodology. During preliminary experiments, K and Mg were identified as the predominant components in the leachate. Meanwhile, among high-value elements, Li, Rb, and Sr demonstrated leaching characteristics with industrial recovery potential. The leaching efficiency for each element was calculated using the following formula.
In the above equation, is the leaching efficiency of elements; c1 is the element concentration of the leaching solution (g/L); v1 is the volume of the leached liquid (L); ω1 is the sample element grade (%); and m1 is the mass of the ore sample (g).
The elemental content was quantified using inductively coupled plasma–optical emission spectrometry (ICP-OES, AVIO 220 Max, PerkinElmer Management Co., Ltd., Shanghai, China). The mineral phases and their treatment-induced transformations were characterized using X-ray diffraction (XRD, D2PHASER, Bruker, Billerica, MA, USA). The mineralogical features and microstructural evolution were revealed using scanning electron microscopy with energy-dispersive spectroscopy (SEM-EDS, JSM-IT500HR, Japan Electron Co., Ltd., Tokyo, Japan). The functional group dynamics were monitored using Fourier-transform infrared spectroscopy (FTIR, NVENSIO-S, Bruker Scientific Technology Co., Ltd., Billerica, MA, USA) throughout the processing stages. The specific surface area of the samples was analyzed using a specific surface area and pore size analyzer (Bet, ASAP2460, Micromeritics, Norcross, GA, USA).
3. Results and Discussion
3.1. Water Leaching
As shown in Figure 1, the leaching efficiency of potassium exhibited minimal temperature dependence, maintaining stable values between 10% and 13% across the tested temperature range (15–50 °C), with no significant temporal fluctuations observed during the 0–60 min leaching period. This behavior indicates rapid attainment of leaching equilibrium, suggesting that potassium primarily exists as soluble salts or easily desorbed adsorbed species on clay mineral surfaces or interlayers, with its leaching process governed by dissolution–diffusion mechanisms rather than temperature-dependent chemical reactions. Magnesium demonstrated generally higher leaching efficiencies, particularly at 50 °C, while showing similarly time-independent leaching patterns across all temperatures. In contrast, lithium and strontium exhibited gradual but limited increases in leaching efficiency with both elevated temperature and prolonged duration, reflecting their predominant occurrence within stable silicate lattices (e.g., kaolinite, chlorite) that resist efficient extraction through thermally driven dissolution alone. Rubidium displayed negligible leaching (<1%) throughout all conditions, with minimal response to parameter variations, confirming its isomorphic substitution into highly stable mineral phases that remain essentially unleachable in pure aqueous systems.
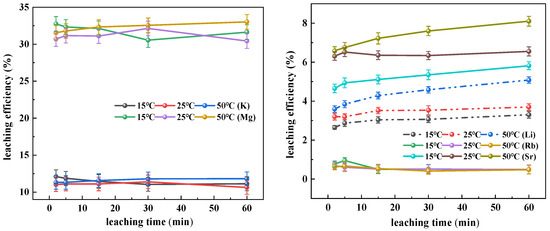
Figure 1.
Influence of temperature on the leaching efficiency.
As shown in Figure 2, at 50 °C, a solid-to-liquid ratio of 1:2 was observed to enhance the initial magnesium leaching, although the final magnesium leaching efficiency showed limited dependence on the solid-to-liquid ratio. Potassium exhibited consistently low leaching efficiencies with minimal variations across different solid-to-liquid ratios and leaching durations, confirming that the occurrence states of elements constitute the predominant factor governing leaching differences. For lithium and strontium, positive correlations were identified between leaching efficiency and both increased solid-to-liquid ratios and prolonged leaching times, though the absolute improvements remained modest. Rubidium leaching efficiencies demonstrated negligible variations with changing solid-to-liquid ratios or leaching durations, providing further evidence that the leaching processes of lithium, strontium, and rubidium are primarily constrained by their host mineral lattices rather than being solution-concentration-dependent.
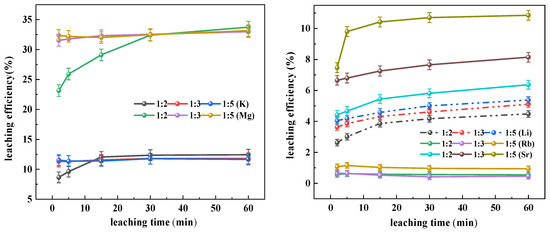
Figure 2.
Influence of solid–liquid ratio on the leaching efficiency.
3.2. Roasting–Water Leaching
The roasting temperature was identified as a critical parameter affecting elemental leaching efficiencies, with Figure 3 presenting the leaching rates of various elements under different temperatures at a fixed ore-to-salt ratio of 10:3 and a roasting duration of 4 h. As demonstrated in Figure 3, K and Rb exhibited similar temperature-dependent leaching patterns, showing increasing efficiencies from 600 °C to 800 °C, followed by declining trends at higher temperatures, with optimal leaching achieved at 800 °C (75.85% for K and 85.51% for Rb). Under identical conditions, Rb consistently demonstrated higher leachability than K, indicating greater mobility of Rb in the Na2SO4 roasting–water leaching system. Distinct leaching behaviors were observed for Li and Sr: Li extraction increased rapidly between 600 and 800 °C, which was corroborated by XRD analysis showing significant reduction in quartz and illite diffraction peaks alongside the emergence of new phases (reyerite and gloriaite), attributable to high-temperature solid-state reactions between Na2SO4 and silicates that decomposed the original mineral structures. Above 800 °C, Li leaching showed gradual improvement from 86.63% to 90.65%. The Sr leaching efficiency was observed to increase within the 600–800 °C temperature range, followed by a significant decrease beyond 800 °C from 36.99% to 20.74%. This temperature-dependent behavior was attributed to the partial decomposition of strontium-bearing mineral lattices at intermediate temperatures, which facilitated the release of Sr ions and their subsequent reaction with sodium sulfate to form soluble Sr compounds. However, when the roasting temperature exceeded 800 °C, the formation of stronalsite (a thermally stable calcium-strontium aluminosilicate phase) was identified through XRD analysis, effectively immobilizing Sr within the crystalline structure and consequently reducing its leachability. Research indicates that stronalsite formation is significantly promoted under high-temperature conditions [22].
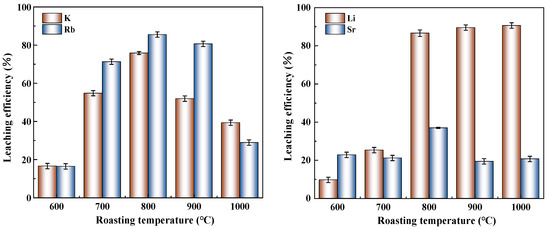
Figure 3.
Influence of roasting temperature on leaching efficiency.
Figure 4 presents the leaching efficiencies of various elements under different ore-to-salt ratios at a fixed roasting temperature of 800 °C for 4 h. As shown in Figure 4, the maximum leaching efficiencies for K (77.67%) and Rb (87.82%) were achieved at an ore-to-salt ratio of 10:1, with both elements exhibiting decreasing extraction trends as the ratio increased (indicating higher Na2SO4 dosage). This inverse correlation suggests that lower salt concentrations enabled the leaching of K and Rb by promoting more complete reaction conditions. In contrast, Li and Sr demonstrated a different pattern, with their leaching efficiencies initially increasing before decreasing, peaking at an intermediate ore-to-salt ratio of 10:3. This phenomenon occurs because an appropriate amount of sodium sulfate reacts effectively with silicate minerals in the raw ore to form more soluble lithium- and strontium-bearing phases, while excessive sodium sulfate leads to excessively high Na+ concentrations in the system, resulting in the formation of insoluble sodium silicate salts that inhibit Li and Sr leaching.
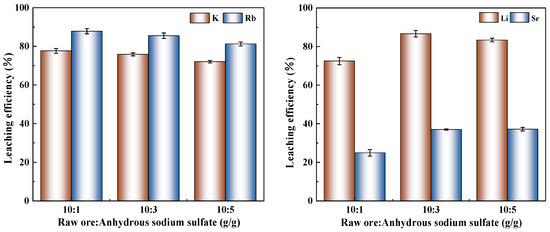
Figure 4.
Influence of ore-to-salt ratio on the leaching efficiency.
As shown in Figure 5, the leaching efficiencies of K and Rb increased with prolonged leaching time from 0.5 to 2 h, as extended roasting time allowed for more complete reactions between sodium sulfate and minerals, generating increased soluble products. The maximum Rb leaching efficiency (92.91%) was achieved after 2 h of roasting, followed by a fluctuating decrease with further extension, possibly due to the recombination of soluble components into insoluble phases during prolonged roasting. However, K leaching efficiency showed renewed increase after 4 h roasting. In contrast, Li leaching efficiency exhibited continuous growth (from 66.75% to 86.64%) throughout the 0.5–4 h period, indicating its prolonged reaction kinetics where extended roasting time promoted further reactions between lithium-bearing minerals and sodium sulfate. Similarly, Sr leaching efficiency demonstrated gradual improvement (from 18.37% to 36.99%) with increasing roasting duration.
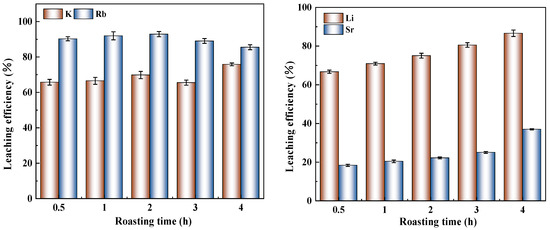
Figure 5.
Influence of roasting time on the leaching efficiency.
Figure 6 presents the leaching behavior of magnesium under varying roasting temperatures and ore-to-salt ratios. As shown in the figure, although magnesium leaching efficiency exhibited an increasing trend with higher salt ratios and elevated temperatures, the overall extraction rates remained extremely low (<1%). SEM-EDS analysis revealed that magnesium was predominantly concentrated in the needle-like porous phase (Structure 2) within the leaching residues. Combined with the XRD patterns in Figure 7, this observation suggests the probable formation of stable forsterite phases [23] during roasting. A study showed that forsterite has an orthorhombic crystal structure, in which Mg2+ is tightly bound to SiO44− tetrahedra. This makes it difficult for Mg2+ to break away from the structure during dissolution, resulting in a low leaching rate of Mg2+ [24].
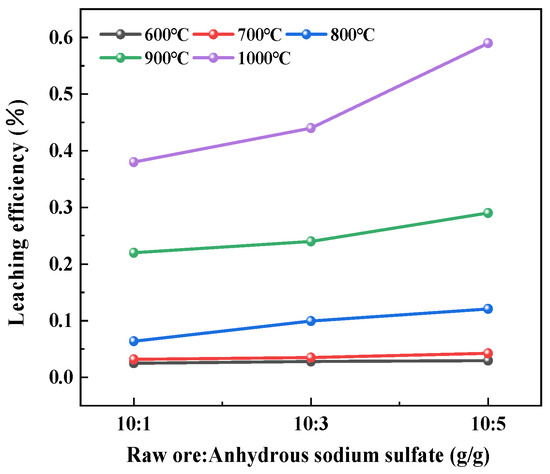
Figure 6.
Changes in Mg leaching efficiency under different roasting temperatures and ore-to-salt ratios.
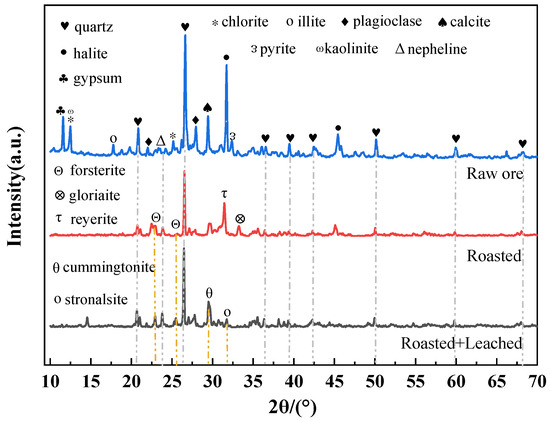
Figure 7.
XRD patterns of samples under different conditions (quartz, SiO2; halite, NaCl; gypsum, CaSO4·2H2O; chlorite, (Mg,Fe)5Al(AlSi3O10)(OH)8; illite, K0.65Al2(Si3.35Al0.65)O10(OH)2; plagioclase, NaAlSi3O8; calcite, CaCO3; pyrite, FeS2; kaolinite, Al2Si2O5(OH)4; nepheline, Na3K(AlSiO4)4; forsterite, Mg2SiO4; gloriaite, Na2Mg(SO4)2·4H2O; reyerite, K2Na6Ca(SO4)3; cummingtonite, (Mg,Fe)7Si8O22(OH)2; stronalsite, SrNa2Al4Si4O16).
3.3. Mineral Phase Transformation During Leaching
The XRD patterns clearly demonstrate the phase transformation sequence of clay-type lithium ore during the “raw ore → roasting → roasting–water leaching” process. As shown in Figure 7, the raw ore was predominantly composed of silicate minerals (quartz, chlorite, illite) and carbonate minerals (calcite), reflecting its natural mineralogical composition. After roasting, the characteristic diffraction peaks of original minerals (e.g., quartz, illite) were significantly weakened or had completely disappeared, while new phases including reyerite, gloriaite, and forsterite were detected, indicating that high-temperature reactions between sodium sulfate and ore minerals destroyed the original silicate/carbonate lattices and promoted new phase formation. Following water leaching of roasted products, the diffraction intensities of roasting-generated phases (reyerite, gloriaite) were further reduced, accompanied by the appearance of post-leaching phases (cummingtonite, stronalsite), demonstrating that the leaching process dissolved soluble roasting products (e.g., sulfates) and induced secondary phase reconstruction. The main chemical reactions occurring during the roasting process include the following:
Na2SO4 + Al2O3·2SiO2 → 2NaAlSiO4 + 2SO3↑
Na2SO4 + K(Li)AlSi3O8 → K(Li)NaSO4 + NaAlSi3O8
The FTIR spectra clearly reveal the evolution of functional groups in clay-type lithium ore from raw material to samples roasted at different temperatures. As shown in Figure 8, the raw ore exhibited characteristic peaks, including hydroxyl stretching vibrations at 3406.66 cm−1, alkyl C-H vibrations at 2922.56 cm−1 and 2851.87 cm−1, and silicate features around 1032.5 cm−1, reflecting its natural composition containing hydroxyl-bearing minerals (e.g., illite, chlorite) and organic impurities. With increasing roasting temperature (600–1000 °C), the hydroxyl peaks near 3600 cm−1 gradually weakened and eventually disappeared, demonstrating thermal dehydration of hydroxyl-containing minerals (e.g., illite), consistent with the XRD observations showing diminished illite diffraction peaks. In the 2000–1500 cm−1 region, the asymmetric stretching vibrations of carbonate (CO32−) disappeared after roasting due to decomposition (e.g., CaCO3 → CaO + CO2). Significant changes were observed in the 1000–400 cm−1 region, where silicate characteristic peaks (e.g., 1026.87 cm−1) shifted and broadened, while new peaks emerged at 690.28 cm−1 and 660.61 cm−1 above 600 °C, coinciding with the formation of new phases (e.g., forsterite) in XRD patterns and indicating high-temperature-induced silicate lattice rearrangement. At 1000 °C, the spectral features became smoother, suggesting further densification and crystallinity improvement of mineral phases. Figure 9 shows that after 800 °C roasting, samples with different ore-to-salt ratios exhibited varying degrees of hydroxyl peak weakening near 3403.3 cm−1, while in the 1000–400 cm−1 region, the shifting and broadening of silicate characteristic peaks (e.g., 1032.5 cm−1) differed among samples, with more pronounced features of new phases (e.g., reyerite, gloriaite) around 1013.4 cm−1 observed at the 10:5 ore-to-salt ratio.
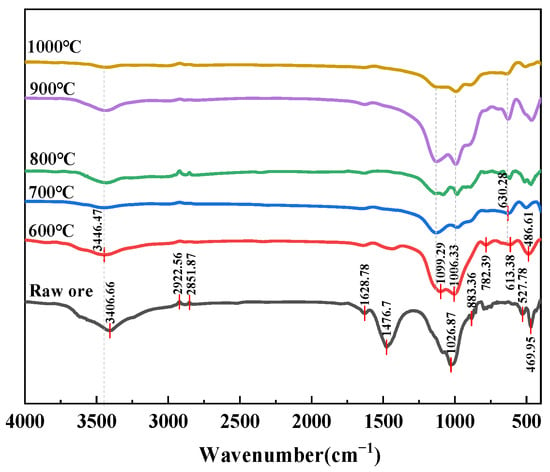
Figure 8.
FTIR spectra of samples at different roasting temperatures.
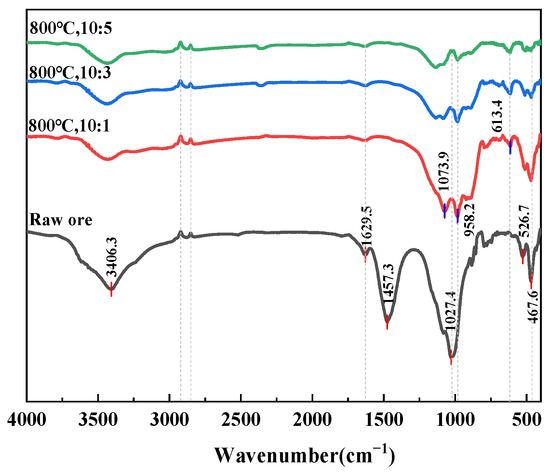
Figure 9.
FTIR spectra of samples at different ore-to-salt ratios.
3.4. Morphological Transformation During Leaching
The SEM images distinctly demonstrate the morphological evolution of clay-type lithium ore during the roasting–leaching process. Figure 10 presents the microstructures of (a) raw ore, (b) samples after roasting at 800 °C with an ore-to-salt ratio of 10:3 for 4 h, and (c) leaching residues obtained after water leaching at 80 °C with a solid-to-liquid ratio of 1:5 for 1 h. In the raw ore stage (a), irregular particle morphology with rough surfaces and good dispersion was observed, though the stable mineral lattices required roasting for structural destruction. After roasting (b), significant particle agglomeration occurred, with small particles sintering into large clusters and surfaces becoming smoother and denser, indicating that high-temperature roasting effectively destroyed the original silicate lattices and liberated elements. During subsequent water leaching (c), partial disintegration of agglomerates was observed, where the dissolution of soluble salts disrupted dense surface layers, exposing new pores and partially restoring particle dispersion.
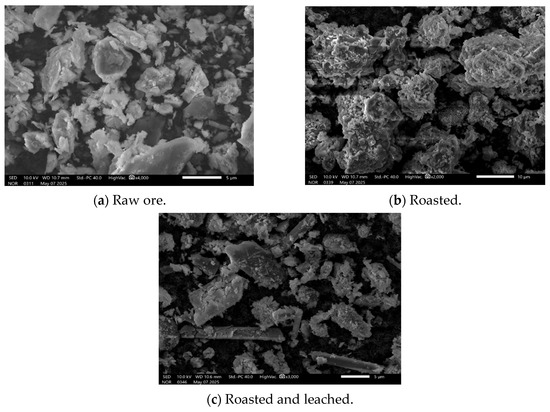
Figure 10.
SEM images of samples under different processing conditions: raw ore (a), roasted (b), and roasted and leached (c).
Figure 11 displays SEM images of the minerals roasted at 800 °C with an ore-to-salt ratio of 10:3 for 4 h, showing distinct structural and compositional differentiation. As revealed in Figure 11, the roasted minerals primarily exhibited two characteristic structures. Structure 1 appeared as dense agglomerates enriched with Na and S (Table 1), which, combined with the added anhydrous sodium sulfate and newly formed phases (reyerite, gloriaite) as identified by XRD, were inferred to be complex salt phases generated through reactions between sodium sulfate and calcium/silicon-bearing minerals. The detection of K and Rb indicates the partial migration and enrichment of these elements during roasting. Structure 2 consisted of loosely aggregated needle-like and rod-shaped assemblages, with high contents of Si, Ca, and Mg, suggesting residual silicate phases from the original ore. This structure showed higher Rb enrichment than Structure 1, along with significant carbon content (12.15%), implying the possible formation of complex carbonates through reactions between CO2 (from carbonate decomposition) and elements such as Si and Mg (Table 2). The standard deviation values of element content measurements can effectively reflect their occurrence state characteristics. As evidenced by the data analysis in Table 1 and Table 2, elements with larger standard deviations (e.g., Rb ± 0.86) demonstrate significant distribution heterogeneity within the minerals, which aligns with Rb’s characteristic occurrence as inclusions within clay mineral interlayers. Conversely, elements with smaller standard deviations (e.g., Na ± 0.32) exhibit homogeneous distribution features in mineral phases.

Figure 11.
SEM images of roasted minerals.

Table 1.
Surface element contents of Structure 1 in the roasted minerals.

Table 2.
Surface element contents of Structure 2 in the roasted minerals.
Figure 12 and Figure 13 present the nitrogen adsorption–desorption isotherms and pore size distributions of samples under different roasting conditions, with the structural parameters summarized in Table 3. As shown in Figure 12, all samples exhibited Type IV isotherms, indicating predominantly mesoporous structures (2–50 nm), though some samples also showed macropore (>50 nm) distributions. The H3-type hysteresis loops suggest irregular pore structures formed through interlayer reactions and sintering during roasting. Figure 13 demonstrates that clay-type lithium ore roasted at 600 °C with an ore-to-salt ratio of 10:3 maintained more open mesoporous/macroporous structures, revealing limited mineral sintering and mild reactions between anhydrous sodium sulfate and minerals at lower temperatures. When the roasting temperature was increased from 600 °C to 800 °C at the same ore-to-salt ratio, macropores were reduced and mesopores were refined, with the average pore diameter decreasing from 38.1939 nm to 24.6321 nm, demonstrating that elevated temperatures accelerated mineral reactions and new phase (e.g., sulfates, silicates) formation, leading to sintering-induced densification and significant pore volume reduction. However, the leaching efficiency of all elements at 600 °C was lower than that in the 800 °C group, which indicates that temperature was the core factor affecting leaching under these conditions. At identical roasting temperatures, decreasing the ore-to-salt ratio (increased sodium sulfate dosage) further reduced the average pore diameter from 24.6321 nm to 11.4159 nm and decreased the BET surface area from 1.1126 m2/g to 0.5738 m2/g, indicating more compact new-phase packing with compressed porosity. As a result, the leaching efficiency of K and Rb decreased continuously, which indicates that smaller pores hindered the diffusion and dissolution of K and Rb, leading to a decrease in leaching efficiency. The leaching efficiency of Li and Sr reached a peak at an ore-to-salt ratio of 10:3 and then decreased. This is because when the ore-to-salt ratio was 10:3, the dosage of Na2SO4 was sufficient for the reaction with silicate minerals, while when the ore-to-salt ratio was 10:5, the high salt content led to sintering densification, which inhibited the leaching efficiency. It can be seen that the leaching of Li and Sr not only depended on the diffusion channels of pores but was also affected by the formation of soluble phases through chemical reactions.
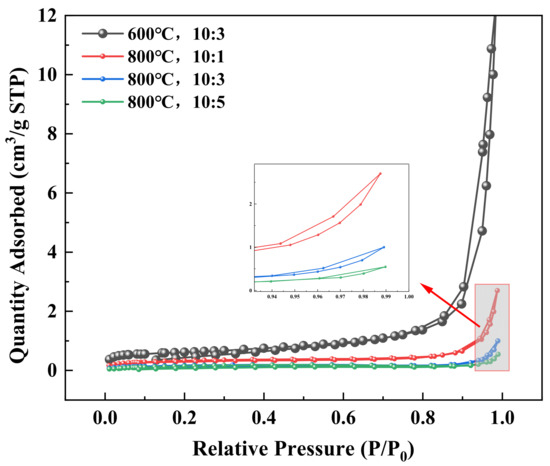
Figure 12.
Nitrogen adsorption–desorption isotherms.
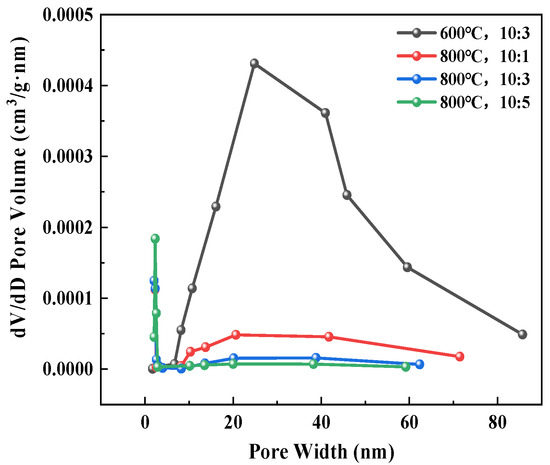
Figure 13.
Pore size distribution maps of the ore treated under different conditions.

Table 3.
Pore structure characteristics of clay-type lithium ores under different roasting conditions.
4. Conclusions
Clay-type lithium ores represent an important source of lithium, and lithium extraction has received considerable attention. However, the leaching behaviors of associated elements, such as Rb, K, Sr, and Mg, remain less studied, with their mutual influences unclear. To provide fundamental support for comprehensive element recovery from clay-type lithium ores, this investigation systematically examined the leaching patterns and mechanisms of Li, Rb, K, Sr, and Mg through water leaching and roasting–water leaching processes using high-salinity mudflat clay-type lithium ore. In direct water leaching, potassium exhibited stable leaching efficiencies (10%–13%) with minimal dependence on temperature, duration, or solid-to-liquid ratio, while magnesium showed higher extractability with optimal performance at 50 °C. Lithium and strontium demonstrated limited but gradually increasing leaching efficiencies with elevated temperatures, extended duration, and higher liquid-to-solid ratios, whereas rubidium extraction remained negligible (<1%). The roasting–water leaching process significantly enhanced extraction efficiencies, achieving 90.65% lithium recovery at 1000 °C, with maximum Rb (92.91%) and K (75.85%) recoveries observed at 800 °C with an ore-to-salt ratio of 10:3 after 2 h of roasting. Strontium reached its peak leaching efficiency (36.99%) under identical conditions (800 °C, 10:3 ratio), while magnesium extraction remained consistently below 1%. Advanced characterization revealed that roasting decomposed original silicate (quartz, chlorite, illite) and carbonate (calcite) lattices, generating new phases, including reyerite, gloriaite, and forsterite. FTIR analysis identified the evolution of functional groups (hydroxyl, carbonate, silicate), reflecting high-temperature dehydration, carbonate decomposition, silicate lattice rearrangement, and new phase formation. SEM-EDS mapping showed differentiated microstructures—dense agglomerates enriched with Na, S, and partially, with K/Rb, versus porous needle-like residues retaining silicate phases with Rb/Sr/Mg enrichment. Porosity analysis demonstrated that lower temperatures and moderate ore-to-salt ratios (10:3) maintained open pore structures, which facilitated reagent diffusion, whereas excessive salt addition promoted sintering densification, which hindered leaching. Another research finding was that the new phase generated by magnesium inhibited its leaching, which indirectly enhanced subsequent Li, Rb, K, and Sr extraction and separation. These results collectively elucidate the leaching characteristics and underlying mechanisms of multiple elements in clay-type lithium ores, providing a scientific basis for optimizing multi-element co-extraction processes.
Author Contributions
Methodology, D.A.; validation, X.Z. and J.Z.; formal analysis, D.A.; investigation, X.Z.; writing—original draft preparation, B.F.; writing—review and editing, B.F.; visualization, J.Z.; supervision, J.Z.; project administration, H.C.; funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Guided Research Project of Qinghai (2024ZY006), the National Natural Science Foundation of China (22478232), and The Open Project of Salt Lake Chemical Engineering Research Complex, Qinghai University (2024-DXSSKF-Z02).
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xie, R.; Tong, X.; Xie, X.; Song, Q.; Ren, S.; Wang, X.; Zhao, Z.; Liu, Y. Study on process mineralogy of clay-type lithium ore in central region of Yunnan province. J. Taiwan Inst. Chem. Eng. 2025, 168, 105900. [Google Scholar] [CrossRef]
- Tang, X.T.; Chen, J.; Zhang, Y.; Yu, J.G.; Lin, S. Echelon extraction of valuable components from salt lake brine substrate. Desalination 2025, 594, 118307. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.Q.; Luo, M.Z.; Li, G.M.; He, J. Separation and utilization of lithium, magnesium, and boron resources from salt lakes through Ionic liquid extraction and CO2 mineralization. Sep. Purif. Technol. 2025, 358, 130444. [Google Scholar] [CrossRef]
- Liu, B.C.; Liu, X. Prediction of metal recovery potential of end-of-life NEV batteries in China based on GRA-BiLSTM. Waste Manag. 2024, 190, 339–349. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Gao, D.; Li, D.; Fan, Y.; Li, W. Economically Utilization of the Rubidium resources in Qarhan Salt Lake: From Fundamental Study to Technology Innovation. J. Salt Lake Res. 2022, 30, 1–11+41. (In Chinese) [Google Scholar]
- Huang, S.Y.; Ma, Y.F.; Liu, X.; Li, K.S.; Zhang, Z.H.; Du, Y.S.; Fu, Z.H.; Li, S.T.; Xie, J.M.; Wu, Y.L.; et al. Study on the enrichment of Li, Rb and Cs in Salt Lake Brine and the mechanism of Li at the mineral interface. J. Water Process Eng. 2024, 65, 105889. [Google Scholar] [CrossRef]
- Chen, X.L.; Hu, W.P. Direct and efficient in situ rubidium extraction from potassium chloride salts. Nat. Sustain. 2024, 7, 1672–1680. [Google Scholar] [CrossRef]
- Ma, F.T.; Zeng, Y.; Yu, X.D.; Chen, K.; Ren, S.Y. The Leaching Behavior of Potassium Extraction from Polyhalite Ore in Water. Acs Omega 2023, 8, 37162–37175. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Mesa, D.; Gowda, V.; Ortega, F.; Bhadani, K.; Ariza-Rodríguez, N.; Asbjörnsson, G.; Brito-Parada, P.R. Strontium minerals as critical raw materials—Market dynamics, processing techniques, and future challenges. Miner. Eng. 2025, 220, 109065. [Google Scholar] [CrossRef]
- Kicinska, A.; Caba, G. Strontium leaching from municipal waste subjected to incineration. Environ. Geochem. Health 2024, 46, 220. [Google Scholar] [CrossRef]
- Yu, Z.F.; Zeng, Y.; Sun, H.B.; Li, L.G.; He, W.H.; Huang, P.; Yu, X.D. Solid-Liquid Phase Equilibria of a Quaternary Sulfate-Type System Containing Lithium, Rubidium, and Magnesium at 298.2 K. Acs Omega 2024, 9, 22196–22202. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.F.; Liu, Y.T.; Sun, L.Q.; Liu, Q.F. Integrated separation of precipitation and three-liquid-phase extraction for boron, lithium and magnesium from salt lake brine with high magnesium-lithium ratio. Sep. Purif. Technol. 2024, 347, 127541. [Google Scholar] [CrossRef]
- Ding, C.W.; Ma, Y.L.; Chen, J.Z.; Ren, W.K.; Du, X.C.; Zhang, T.; Zhao, H.D.; Zhang, F.G. Mineralogy and geochemical characteristics of rare elements in clays from Balun Mahai Salt Lake of Qaidam Basin. J. Salt Lake Res. 2024, 32, 32–39. (In Chinese) [Google Scholar] [CrossRef]
- Huang, R.; Liu, J.; Ru, S.S.; Wang, P.; Hao, J.M.; Gao, H.L. Structure-activity relationships in lithium-hosting montmorillonite: Octahedral lithium locking mechanisms. Particuology 2025, 104, 42–51. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, J.C.; Tan, X.M.; Yin, Y.J.; Zhang, L.Z.; Shi, L.J. Review on extraction of rubidium and cesium from ores. J. Salt Lake Res. 2024, 32, 89–96. (In Chinese) [Google Scholar] [CrossRef]
- López-Hernández, D.; Mahia, M.; Meléndez, W.; López-Contreras, A.Y. Potassium fixation and ammonium competition on a soil with expansive clays. Bioagro 2021, 33, 229–233. [Google Scholar] [CrossRef]
- Xie, R.Q.; Zhou, W.C.; Tong, X.; Liu, Y.; Xie, X.; Wang, X. Study on extraction of lithium from clay-type lithium ore with low roasting temperature and water and its mechanism. Sep. Purif. Technol. 2025, 364, 132450. [Google Scholar] [CrossRef]
- Qiu, H.; Shao, S.; Zhang, B.; Li, B.; Wang, H.; Wei, Y.G. A promising approach for efficient and selective extraction of lithium from clay-type lithium resource: Process optimization and reaction mechanism. Process Saf. Environ. Prot. 2025, 195, 106848. [Google Scholar] [CrossRef]
- Zhong, W.L.; Feng, H.P.; Tong, L.Z.; Li, D.; Yang, L.; Rao, F. Lithium extraction from a Li-rich kaolin resource through Na2SO4 roasting and water leaching. Miner. Eng. 2024, 218, 109004. [Google Scholar] [CrossRef]
- Ahijado, A.; Casillas, R.; Nagy, G.; Fernández, C. Sr-rich minerals in a carbonatite skarn, Fuerteventura, Canary Islands (Spain). Mineral. Petrol. 2005, 84, 107–127. [Google Scholar] [CrossRef]
- Bai, C.; Zhong, G.S.; Li, D.D.; Wei, Y.W. Influence of SiO2 Particle Size on Formation of Forsterite in MgO-SiO2 System. Bull. Chin. Ceram. Soc. 2013, 32, 1048–1051. (In Chinese) [Google Scholar] [CrossRef]
- Furstoss, J.; Hirel, P.; Carrez, P.; Gouriet, K.; Meko-Fotso, V.; Cordier, P. Structures and energies of twist grain boundaries in Mg2SiO4 forsterite. Comput. Mater. Sci. 2024, 233, 112768. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).