Engineered Organo-Clay Nanocomposites for Dual Cationic/Anionic Dye Removal: Role of Polyethylene Glycol Chain Length
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedure for Clay Pretreatment
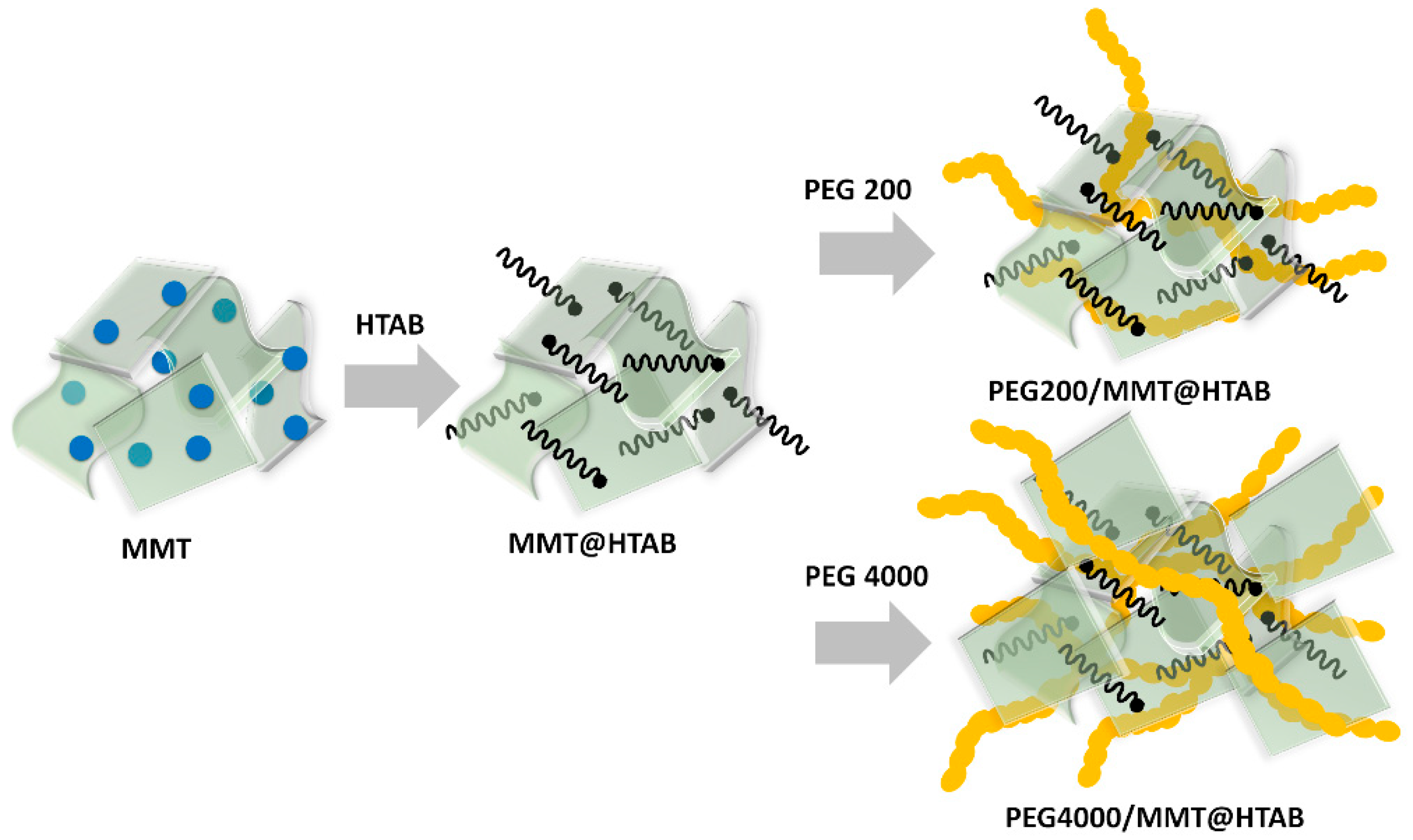
2.3. Preparation of Adsorbents
2.4. Characterization
2.5. Batch Adsorption
3. Results and Discussion
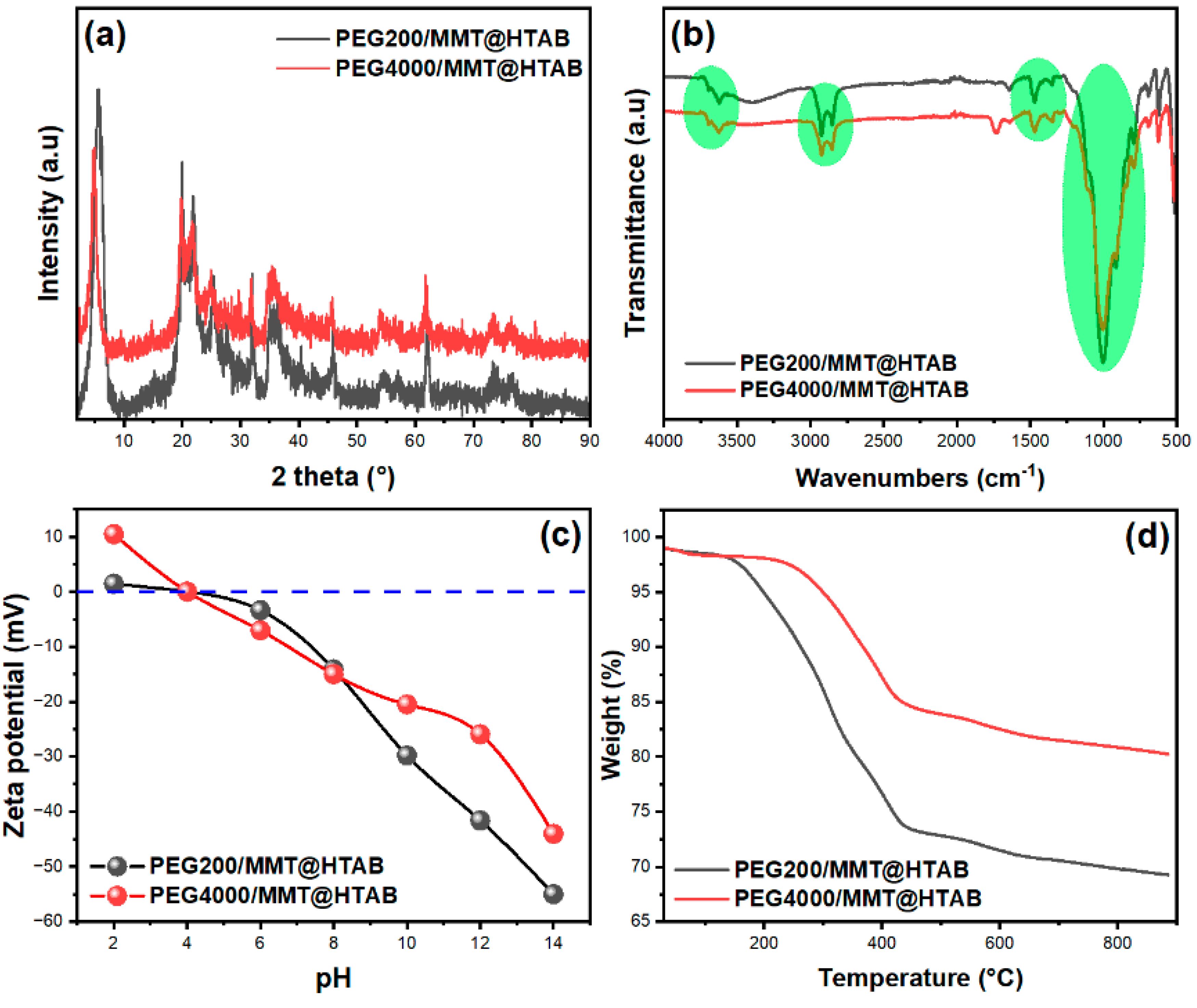
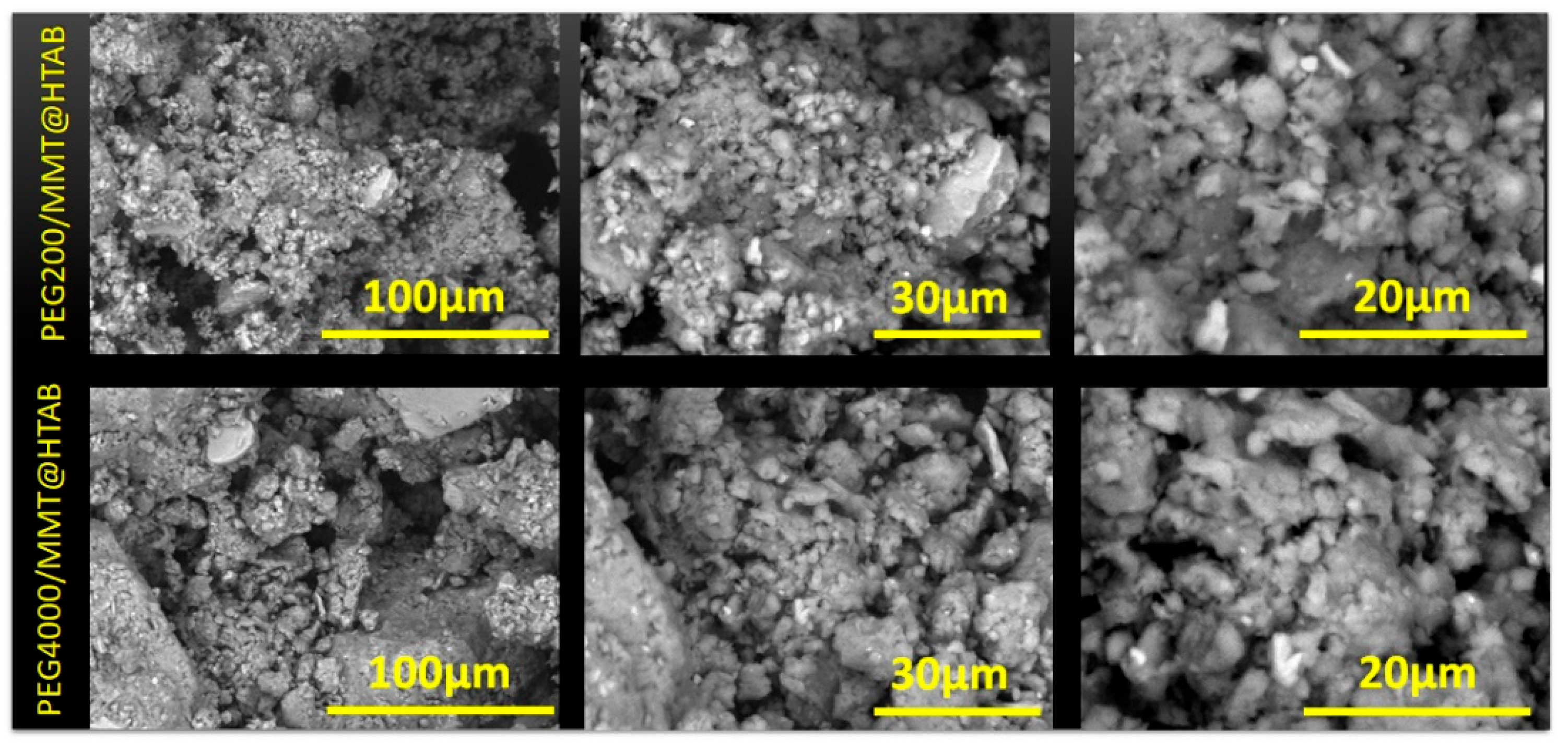
3.1. Characterization of the Adsorbents
3.2. Adsorption Study
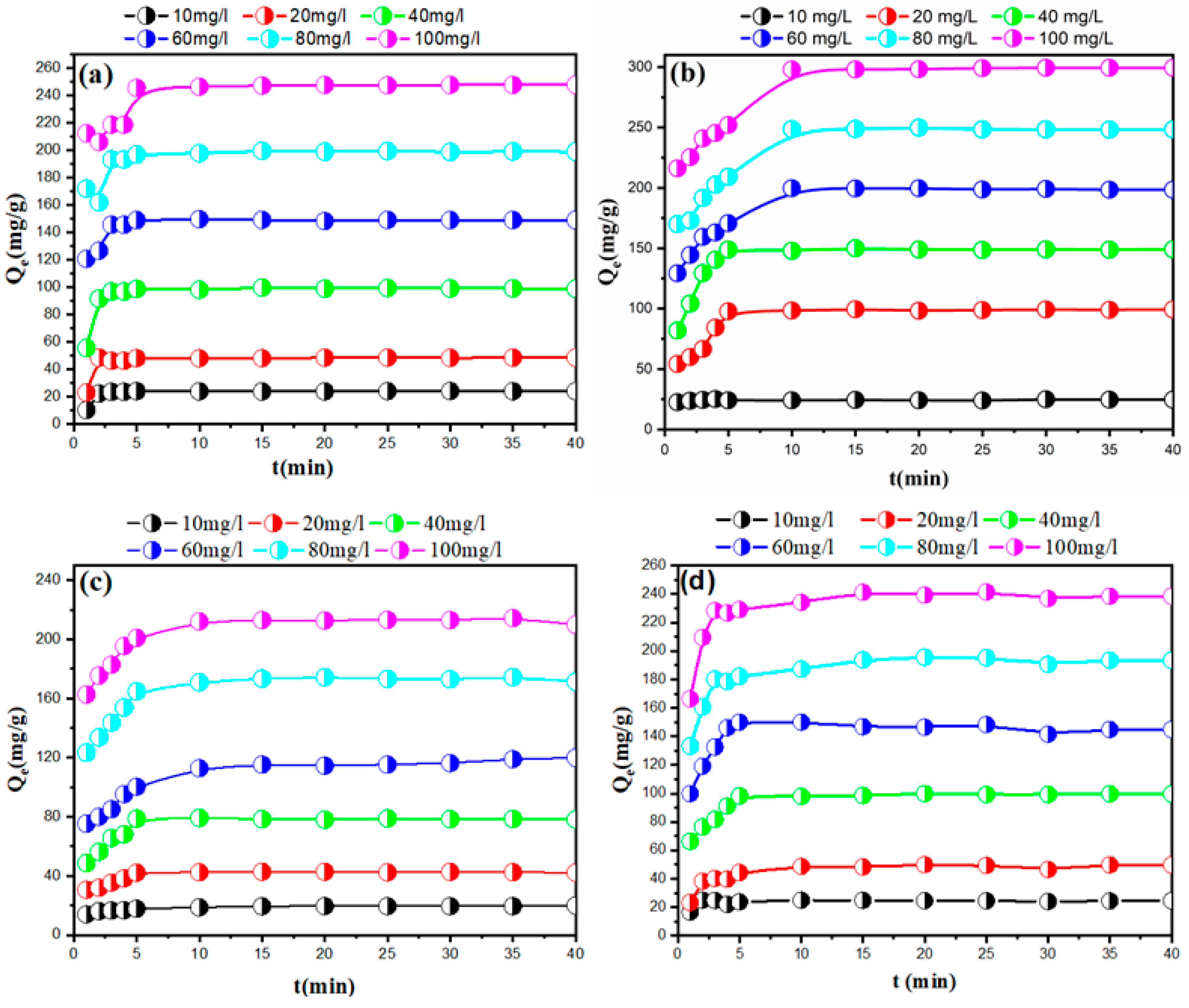
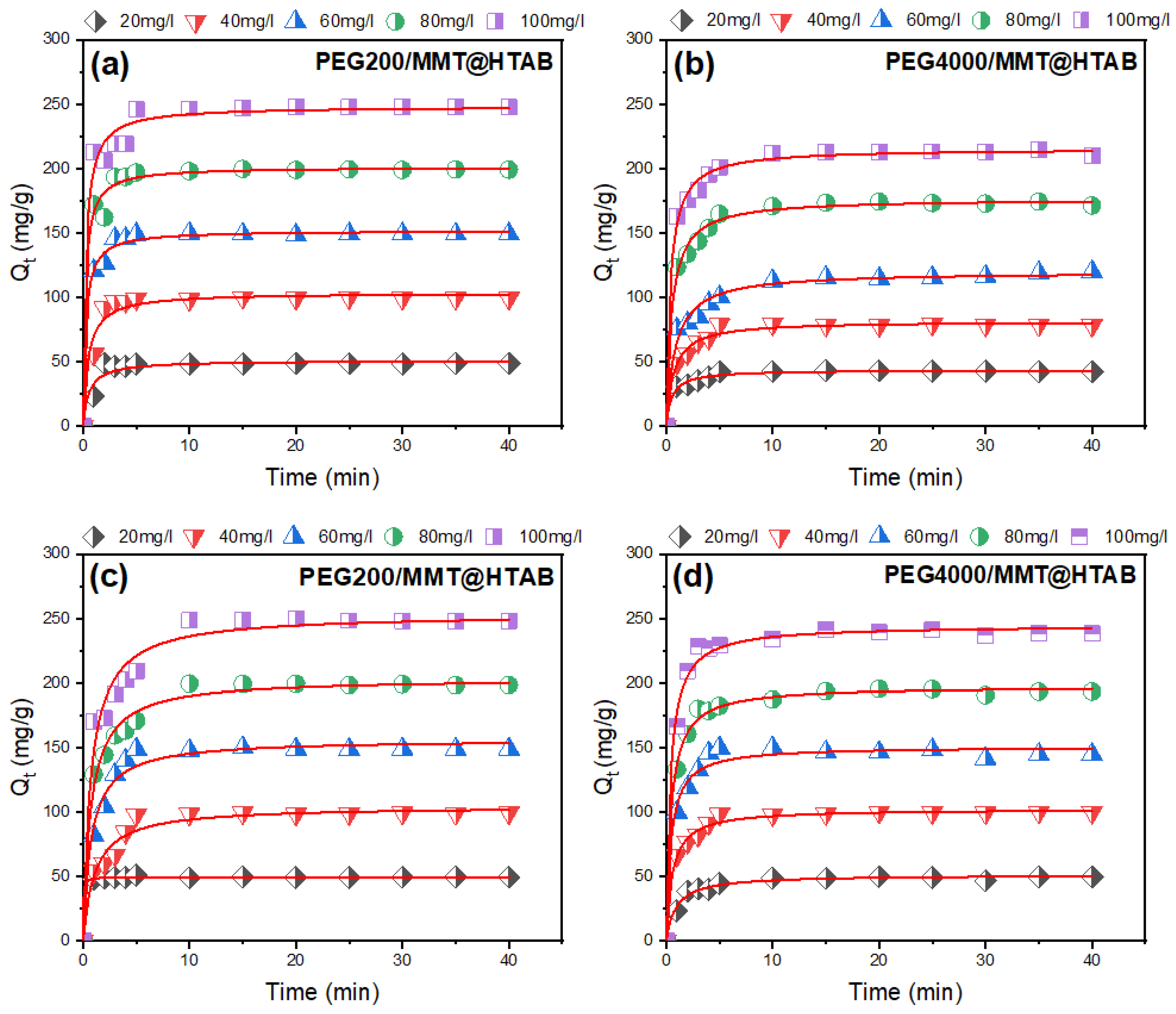
3.2.1. Impact of Contact Duration on Adsorption at Varied Dye Concentrations
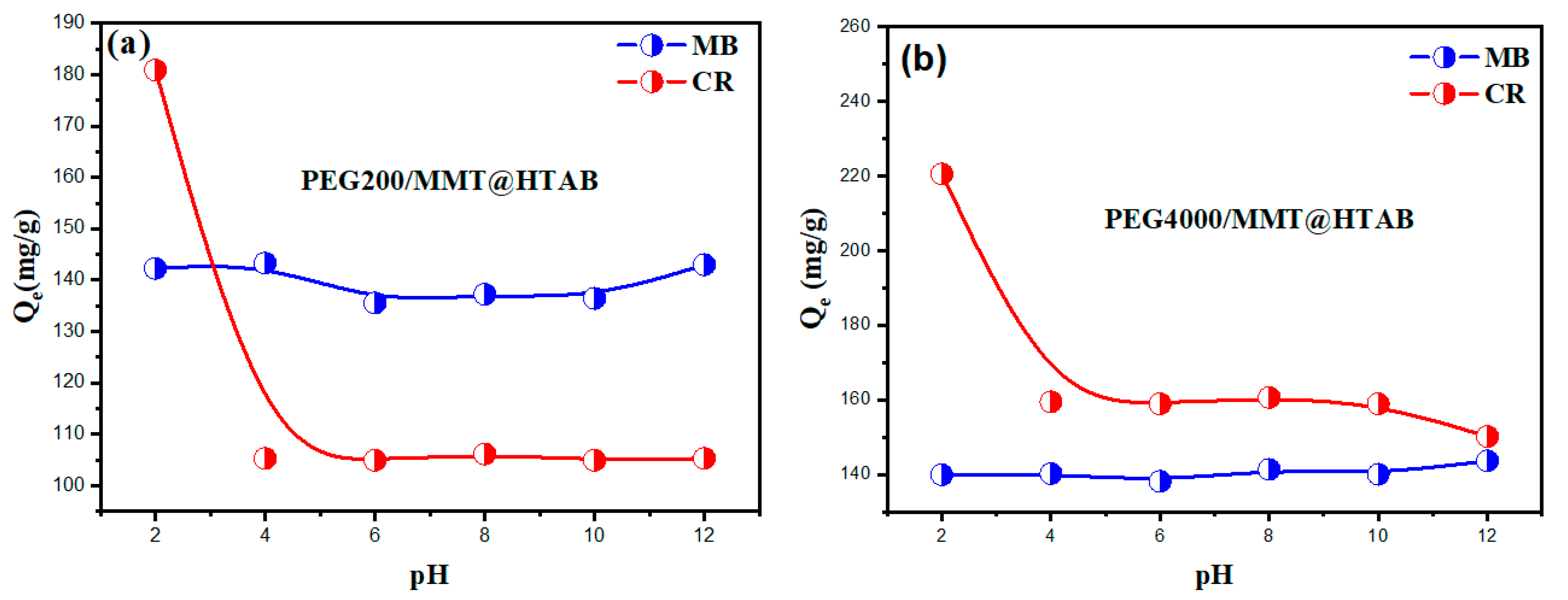
3.2.2. Influence of pH on the Adsorption of MB and CR
3.2.3. Impact of Adsorbent Quantity on Dye Elimination Efficacy
3.2.4. Adsorption Kinetic Models
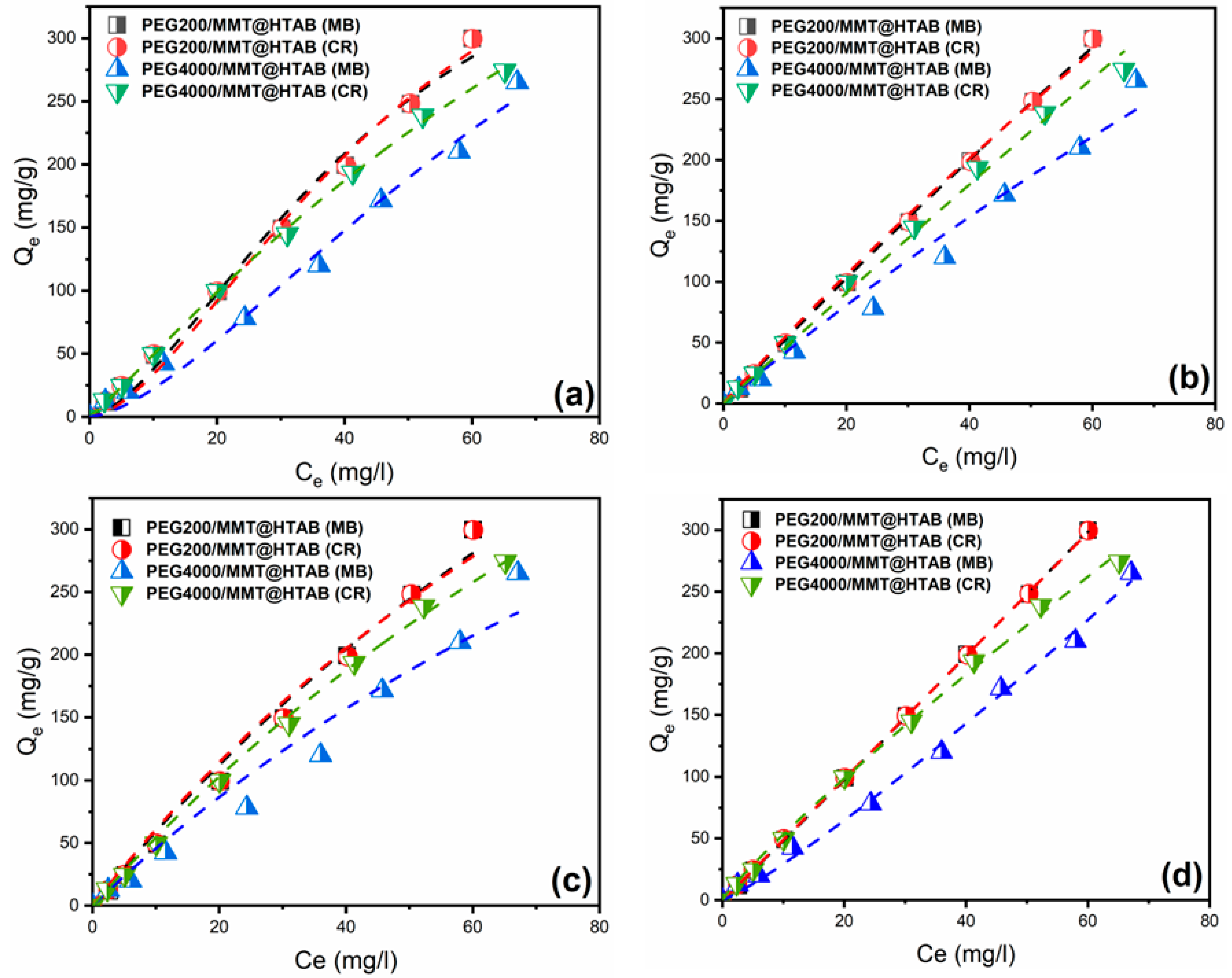
3.2.5. Adsorption Isotherm Models
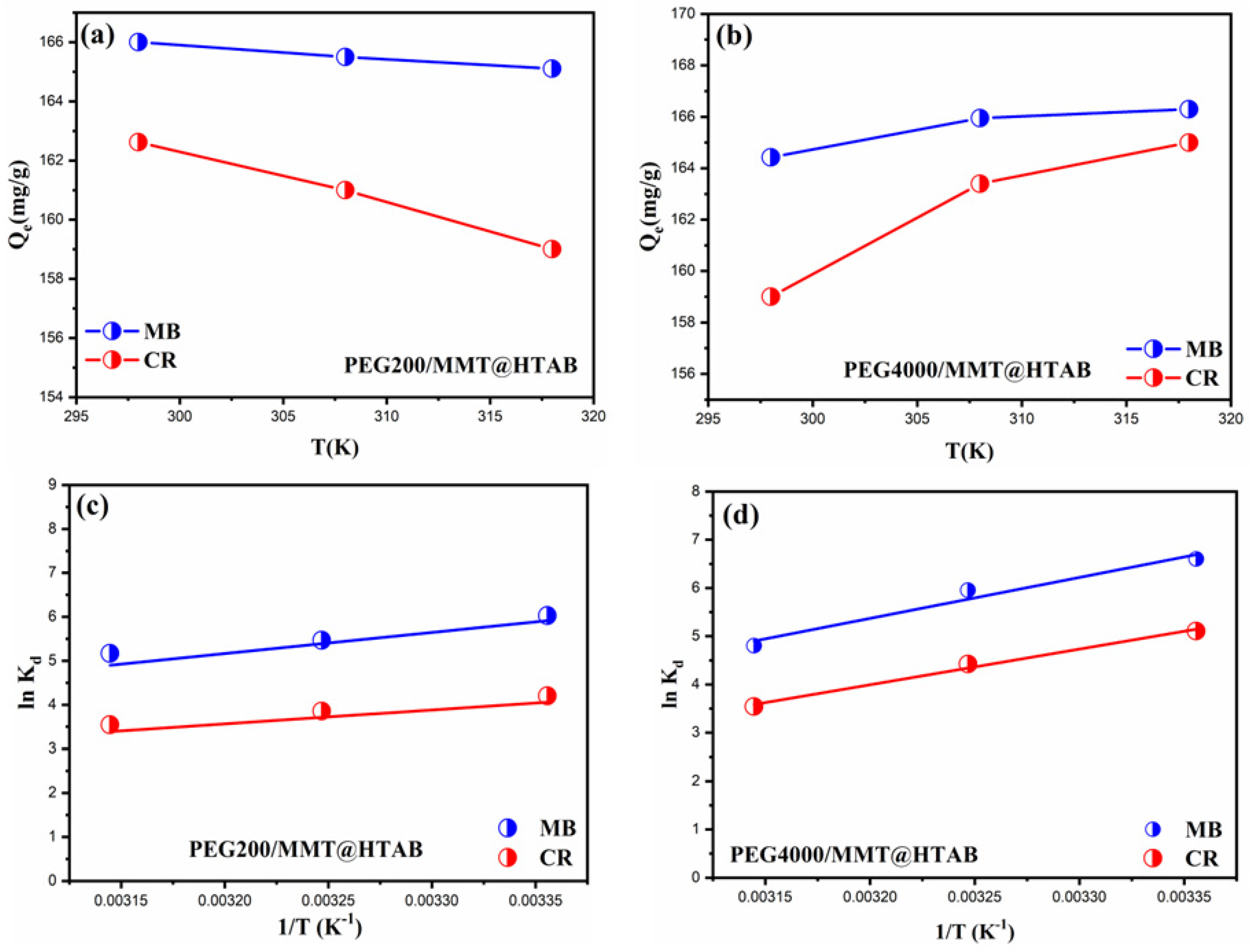
3.2.6. Thermodynamic Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.; Chen, S.; Duan, H.; He, J.; Luo, Y. Removal of Anionic and Cationic Dyes Using Porous Chitosan/Carboxymethyl Cellulose-PEG Hydrogels: Optimization, Adsorption Kinetics, Isotherm and Thermodynamics Studies. Int. J. Biol. Macromol. 2023, 231, 123213. [Google Scholar] [CrossRef]
- Mokhtar, A.; Abdelkrim, S.; Sardi, A.; Hachemaoui, M.; Chaibi, W.; Chergui, F.; Boukoussa, B.; Djelad, A.; Sassi, M.; Abboud, M. A Strategy for the Efficient Removal of Acidic and Basic Dyes in Wastewater by Organophilic Magadiite@alginate Beads: Box-Behnken Design Optimization. Int. J. Biol. Macromol. 2024, 277, 134348. [Google Scholar] [CrossRef]
- Mokhtar, A.; Asli, B.; Abdelkrim, S.; Hachemaoui, M.; Boukoussa, B.; Sassi, M.; Viscusi, G.; Abboud, M. Polymer/Clay Nanocomposites as Advanced Adsorbents for Textile Wastewater Treatment. Minerals 2024, 14, 1216. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Shunmugam, R. Unraveling the Effect of PEG Chain Length on the Physical Properties and Toxicant Removal Capacities of Cross-Linked Network Synthesized by Thiol–Norbornene Photoclick Chemistry. ACS Omega 2020, 5, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of Methylene Blue from Aqueous Solution with Magnetite Loaded Multi-Wall Carbon Nanotube: Kinetic, Isotherm and Mechanism Analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Salama, I.E.; Slavchov, R.I.; Filip, S.V.; Clarke, S.M. Chemisorption and Physisorption of Alcohols on Iron(III) Oxide-Terminated Surfaces from Nonpolar Solvents. J. Colloid Interface Sci. 2025, 685, 15–28. [Google Scholar] [CrossRef]
- Sharma, K.; Dalai, A.K.; Vyas, R.K. Removal of Synthetic Dyes from Multicomponent Industrial Wastewaters. Rev. Chem. Eng. 2018, 34, 107–134. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.-M. Application of Chitosan, a Natural Aminopolysaccharide, for Dye Removal from Aqueous Solutions by Adsorption Processes Using Batch Studies: A Review of Recent Literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of Low-Cost Adsorbents for Dye Removal—A Review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Zare, K.; Sadegh, H.; Shahryari-ghoshekandi, R.; Maazinejad, B.; Ali, V.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Enhanced Removal of Toxic Congo Red Dye Using Multi Walled Carbon Nanotubes: Kinetic, Equilibrium Studies and Its Comparison with Other Adsorbents. J. Mol. Liq. 2015, 212, 266–271. [Google Scholar] [CrossRef]
- Zhang, F.; Song, W.; Lan, J. Effective Removal of Methyl Blue by Fine-Structured Strontium and Barium Phosphate Nanorods. Appl. Surf. Sci. 2015, 326, 195–203. [Google Scholar] [CrossRef]
- Ghorai, S.; Sarkar, A.; Raoufi, M.; Panda, A.B.; Schönherr, H.; Pal, S. Enhanced Removal of Methylene Blue and Methyl Violet Dyes from Aqueous Solution Using a Nanocomposite of Hydrolyzed Polyacrylamide Grafted Xanthan Gum and Incorporated Nanosilica. ACS Appl. Mater. Interfaces 2014, 6, 4766–4777. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Schadeck Netto, M.; Oliveira, M.L.S.; Seliem, M.K.; Luiz Dotto, G.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of Congo Red and Methylene Blue Dyes on an Ashitaba Waste and a Walnut Shell-Based Activated Carbon from Aqueous Solutions: Experiments, Characterization and Physical Interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Chinoune, K.; Mekki, A.; Boukoussa, B.; Mokhtar, A.; Sardi, A.; Hachemaoui, M.; Iqbal, J.; Ismail, I.; Abboud, M.; Aboneama, W.A. Adsorption Behavior of MB Dye on Alginate-Sepiolite Biocomposite Beads: Adsorption, Kinetics, and Modeling. Inorg. Chem. Commun. 2024, 165, 112558. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, W.; He, S.; Yu, S.; Chen, Y.; Lu, L.; Shu, Z.; Cui, H.; Zhang, Y.; Jin, H. Rapid Adsorption of Cationic Dye-Methylene Blue on the Modified Montmorillonite/Graphene Oxide Composites. Appl. Clay Sci. 2019, 168, 304–311. [Google Scholar] [CrossRef]
- Roufegari-Nejhad, E.; Sirousazar, M.; Abbasi-Chiyaneh, V.; Kheiri, F. Removal of Methylene Blue from Aqueous Solutions Using Poly(Vinyl Alcohol)/Montmorillonite Nanocomposite Hydrogels: Taguchi Optimization. J. Polym. Environ. 2019, 27, 2239–2249. [Google Scholar] [CrossRef]
- Lackovičová, M.; Baranyaiová, T.; Bujdák, J. The Chemical Stabilization of Methylene Blue in Colloidal Dispersions of Smectites. Appl. Clay Sci. 2019, 181, 105222. [Google Scholar] [CrossRef]
- Crini, G. Non-Conventional Low-Cost Adsorbents for Dye Removal: A Review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Ramalingam, G.; Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Hoang, T.K.A. Biomass and Waste Derived Silica, Activated Carbon and Ammonia-Based Materials for Energy-Related Applications—A Review. Fuel 2024, 355, 129490. [Google Scholar] [CrossRef]
- Bouabbaci, S.; Chougui, A.; Asli, B.; Mokhtar, A.; Belouatek, A.; Boukoussa, B.; Abboud, M. Preparation and Characterization of Activated Carbon from Pine Bark Biomass for Malachite Green Removal. Int. J. Environ. Anal. Chem. 2025, 105, 1542–1565. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, N. Removal of Dyes from Waste Water Using Low-Cost Adsorbents. Macromol. Symp. 2024, 413, 2400156. [Google Scholar] [CrossRef]
- Mao, S.; Gao, M. Functional Organoclays for Removal of Heavy Metal Ions from Water: A Review. J. Mol. Liq. 2021, 334, 116143. [Google Scholar] [CrossRef]
- Raninga, M.; Mudgal, A.; Patel, V.K.; Patel, J.; Kumar Sinha, M. Modification of Activated Carbon-Based Adsorbent for Removal of Industrial Dyes and Heavy Metals: A Review. Mater. Today Proc. 2023, 77, 286–294. [Google Scholar] [CrossRef]
- Boldog, I.; Dušek, M.; Jelínek, T.; Švec, P.; Ramos, F.S.d.O.; Růžička, A.; Bulánek, R. Porous 10- and 12-Vertex (Bi)-p-Dicarba-Closo-Boranedicarboxylates of Cobalt and Their Gas Adsorptive Properties. Microporous Mesoporous Mater. 2018, 271, 284–294. [Google Scholar] [CrossRef]
- Li, G.L.; Zhou, C.H.; Fiore, S.; Yu, W.H. Interactions between Microorganisms and Clay Minerals: New Insights and Broader Applications. Appl. Clay Sci. 2019, 177, 91–113. [Google Scholar] [CrossRef]
- Natarajan, S.; Anitha, V.; Gajula, G.P.; Thiagarajan, V. Synthesis and Characterization of Magnetic Superadsorbent Fe3O4-PEG-Mg-Al-LDH Nanocomposites for Ultrahigh Removal of Organic Dyes. ACS Omega 2020, 5, 3181–3193. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Sahoo, G.; Swain, S.K. Graphene Quantum Dot Decorated Magnetic Graphene Oxide Filled Polyvinyl Alcohol Hybrid Hydrogel for Removal of Dye Pollutants. J. Mol. Liq. 2020, 302, 112591. [Google Scholar] [CrossRef]
- Edathil, A.A.; Pal, P.; Banat, F. Alginate Clay Hybrid Composite Adsorbents for the Reclamation of Industrial Lean Methyldiethanolamine Solutions. Appl. Clay Sci. 2018, 156, 213–223. [Google Scholar] [CrossRef]
- Sardi, A.; Bounaceur, B.; Mokhtar, A.; Boukoussa, B.; Abbes, M.T.; Chaibi, W.; Nacer, A.; Khadidja, K.B.; Issam, I.; Iqbal, J.; et al. Kinetics and Thermodynamic Studies for Removal of Trypan Blue and Methylene Blue from Water Using Nano Clay Filled Composite of HTAB and PEG and Its Antibacterial Activity. J. Polym. Environ. 2023, 31, 5065–5088. [Google Scholar] [CrossRef]
- dos Santos, A.; Viante, M.F.; Pochapski, D.J.; Downs, A.J.; Almeida, C.A.P. Enhanced Removal of P-Nitrophenol from Aqueous Media by Montmorillonite Clay Modified with a Cationic Surfactant. J. Hazard. Mater. 2018, 355, 136–144. [Google Scholar] [CrossRef]
- Oueslati, W.; Ben Rhaiem, H.; Lanson, B.; Ben Haj Amara, A. Selectivity of Na–Montmorillonite in Relation with the Concentration of Bivalent Cation (Cu2+, Ca2+, Ni2+) by Quantitative Analysis of XRD Patterns. Appl. Clay Sci. 2009, 43, 224–227. [Google Scholar] [CrossRef]
- Abdelkrim, S.; Mokhtar, A.; Djelad, A.; Bennabi, F.; Souna, A.; Bengueddach, A.; Sassi, M. Chitosan/Ag-Bentonite Nanocomposites: Preparation, Characterization, Swelling and Biological Properties. J. Inorg. Organomet. Polym. 2020, 30, 831–840. [Google Scholar] [CrossRef]
- McLauchlin, A.R.; Thomas, N.L. Preparation and Characterization of Organoclays Based on an Amphoteric Surfactant. J. Colloid Interface Sci. 2008, 321, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, M.; Dubois, P. Polymer-Layered Silicate Nanocomposites: Preparation, Properties and Uses of a New Class of Materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Mittal, V. Polymer Layered Silicate Nanocomposites: A Review. Materials 2009, 2, 992–1057. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/Layered Silicate Nanocomposites: A Review from Preparation to Processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Yang, W.; Xia, X.; Liu, X.; Zhang, S. Interlayer Structure and Dynamic Properties of CTMAB–Montmorillonite: Experiment and Molecular Dynamics. RSC Adv. 2023, 13, 13324–13336. [Google Scholar] [CrossRef] [PubMed]
- Lertsutthiwong, P.; Noomun, K.; Khunthon, S.; Limpanart, S. Influence of Chitosan Characteristics on the Properties of Biopolymeric Chitosan–Montmorillonite. Prog. Nat. Sci. Mater. Int. 2012, 22, 502–508. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer Chitosan/Montmorillonite Nanocomposites: Preparation and Characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Baa, N.B.; Mokhnachi, N.B.; Haddadine, N. System Based on Clay/Polymer for Biomedical Application. J. Fundam. Appl. Sci. 2020, 12, 108–117. [Google Scholar]
- Sahu, M.; Reddy, V.R.M.; Kim, B.; Patro, B.; Park, C.; Kim, W.K.; Sharma, P. Fabrication of Cu2ZnSnS4 Light Absorber Using a Cost-Effective Mechanochemical Method for Photovoltaic Applications. Materials 2022, 15, 1708. [Google Scholar] [CrossRef]
- Mokhtar, A.; Bennabi, F.; Abdelkrim, S.; Sardi, A.; Boukoussa, B.; Souna, A.; Bengueddach, A.; Sassi, M. Evaluation of Intercalated Layered Materials as an Antimicrobial and Drug Delivery System: A Comparative Study. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 353–364. [Google Scholar] [CrossRef]
- Zaichik, S.; Steinbring, C.; Jelkmann, M.; Bernkop-Schnürch, A. Zeta Potential Changing Nanoemulsions: Impact of PEG-Corona on Phosphate Cleavage. Int. J. Pharm. 2020, 581, 119299. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Char, K.; Lee, S.W.; Park, Y.W. Structural Changes of Polyaniline/Montmorillonite Nanocomposites and Their Effects on Physical Properties. J. Mater. Chem. 2003, 13, 2942–2947. [Google Scholar] [CrossRef]
- Moslemizadeh, A.; Khezerloo-ye Aghdam, S.; Shahbazi, K.; Khezerloo-ye Aghdam, H.; Alboghobeish, F. Assessment of Swelling Inhibitive Effect of CTAB Adsorption on Montmorillonite in Aqueous Phase. Appl. Clay Sci. 2016, 127–128, 111–122. [Google Scholar] [CrossRef]
- Nie, J.; Ke, Y.; Zheng, H.; Yi, Y.; Qin, Q.; Pan, F.; Dong, P. Preparation and Characterization of Organo Montmorillonite Modified by a Novel Gemini Surfactant. Integr. Ferroelectr. 2012, 137, 67–76. [Google Scholar] [CrossRef]
- Șerban, M.V.; Nazarie (Ignat), S.-R.; Dinescu, S.; Radu, I.-C.; Zaharia, C.; Istrătoiu, E.-A.; Tănasă, E.; Herman, H.; Gharbia, S.; Baltă, C.; et al. Silk ProteinsEnriched Nanocomposite Hydrogels Based on Modified MMT Clay and Poly(2-Hydroxyethyl Methacrylate-Co-2-Acrylamido-2-Methylpropane Sulfonic Acid) Display Favorable Properties for Soft Tissue Engineering. Nanomaterials 2022, 12, 503. [Google Scholar] [CrossRef]
- Abid, L.H.; Mussa, Z.H.; Al-Qaim, F.F.; Kamyab, H.; Al-Saedi, H.F.S.; Kadhim, N.J.; Deyab, I.F.; Imran, A.F.; Al-Asadi, S.T. Application of ZnCl2-Modified Biowaste to the Removal of Highly Polluted Dye: A Case Study of Investigating the Kinetics and Adsorption Isotherms. Energy Nexus 2025, 19, 100481. [Google Scholar] [CrossRef]
- Zeghoud, L.; Gouamid, M.; Ben Mya, O.; Rebiai, A.; Saidi, M. Adsorption of Methylene Blue Dye from Aqueous Solutions Using Two Different Parts of Palm Tree: Palm Frond Base and Palm Leaflets. Water Air Soil Pollut. 2019, 230, 195. [Google Scholar] [CrossRef]
- Hua, Z.; Pan, Y.; Hong, Q. Adsorption of Congo Red Dye in Water by Orange Peel Biochar Modified with CTAB. RSC Adv. 2023, 13, 12502–12508. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, R.; Ray, S.K. Removal of Congo Red and Methyl Violet from Water Using Nano Clay Filled Composite Hydrogels of Poly Acrylic Acid and Polyethylene Glycol. Chem. Eng. J. 2015, 260, 269–283. [Google Scholar] [CrossRef]
- Ozola-Davidane, R.; Burlakovs, J.; Tamm, T.; Zeltkalne, S.; Krauklis, A.E.; Klavins, M. Bentonite-Ionic Liquid Composites for Congo Red Removal from Aqueous Solutions. J. Mol. Liq. 2021, 337, 116373. [Google Scholar] [CrossRef]
- Hidayat, A.R.P.; Sulistiono, D.O.; Murwani, I.K.; Endrawati, B.F.; Fansuri, H.; Zulfa, L.L.; Ediati, R. Linear and Nonlinear Isotherm, Kinetic and Thermodynamic Behavior of Methyl Orange Adsorption Using Modulated Al2O3@UiO-66 via Acetic Acid. J. Environ. Chem. Eng. 2021, 9, 106675. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- El-Khiyami, S.S.; Ali, H.; Ismail, A.M.; Hafez, R.S. Tunable Physical Properties and Dye Removal Application of Novel Chitosan Polyethylene Glycol and Polypyrrole/Carbon Black Films. Sci. Rep. 2025, 15, 20124. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Hassan, S.; Imran, Z.; Mazhar, D.; Afzal, S.; Ullah, S.A. Green Approach to Water Purification: Investigating Methyl Orange Dye Adsorption Using Chitosan/Polyethylene Glycol Composite Membrane. J. Polym. Environ. 2024, 32, 194–212. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ovejero, G.; Mestanza, M.; García, J. Removal of Dyes from Wastewaters by Adsorption on Sepiolite and Pansil. Ind. Eng. Chem. Res. 2010, 49, 3207–3216. [Google Scholar] [CrossRef]
- Farghali, A.A.; Bahgat, M.; El Rouby, W.M.A.; Khedr, M.H. Preparation, Decoration and Characterization of Graphene Sheets for Methyl Green Adsorption. J. Alloys Compd. 2013, 555, 193–200. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Lee, J.; Nandi, D.; Parameswaranpillai, J.; Pant, B.; Siengchin, S. Efficient Removal of Organic Dye from Aqueous Solution Using Hierarchical Zeolite-Based Biomembrane: Isotherm, Kinetics, Thermodynamics and Recycling Studies. Catalysts 2022, 12, 886. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Gorrasi, G. Design of Sodium Alginate/Soybean Extract Beads Loaded with Hemp Hurd and Halloysite as Novel and Sustainable Systems for Methylene Blue Adsorption. Polym. Eng. Sci. 2022, 62, 129–144. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K. Adsorption of Methylene Blue from Aqueous Solution by Graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Kumar, V. Adsorption Kinetics and Isotherms for the Removal of Rhodamine B Dye and Pb+ 2 Ions from Aqueous Solutions by a Hybrid Ion-Exchanger. Arab. J. Chem. 2019, 12, 316–329. [Google Scholar]
- Viscusi, G.; Mottola, S.; Boumezough, Y.; Arris, S.; De Marco, I.; Gorrasi, G. A Novel Porous Adsorbentbased on Cactus Powder/Ionic Liquid for the Removal of Nimesulide from Wastewater. Chemosphere 2025, 376, 144293. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Du, Q.; Pi, X.; Wang, Y.; Sun, Y.; Wang, M.; Zhang, Y.; Chen, K.; Zhu, J. Effective Removal of Tetracycline from Water Using Copper Alginate @ Graphene Oxide with In-Situ Grown MOF-525 Composite: Synthesis, Characterization and Adsorption Mechanisms. Nanomaterials 2022, 12, 2897. [Google Scholar] [CrossRef] [PubMed]
- Joudi, M.; Nasserlah, H.; Hafdi, H.; Mouldar, J.; Hatimi, B.; Mhammedi, M.A.E.; Bakasse, M. Synthesis of an Efficient Hydroxyapatite–Chitosan–Montmorillonite Thin Film for the Adsorption of Anionic and Cationic Dyes: Adsorption Isotherm, Kinetic and Thermodynamic Study. SN Appl. Sci. 2020, 2, 1078. [Google Scholar] [CrossRef]
- Kypritidou, Z.; Argyraki, A. A Multi-Site Mechanism Model for Studying Pb and Cu Retention from Aqueous Solutions by Fe-Mg-Rich Clays. Clay Miner. 2018, 53, 175–192. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Alene, A.N. A Comparative Study of Acidic, Basic, and Reactive Dyes Adsorption from Aqueous Solution onto Kaolin Adsorbent: Effect of Operating Parameters, Isotherms, Kinetics, and Thermodynamics. Emerg. Contam. 2022, 8, 59–74. [Google Scholar] [CrossRef]
- Karadag, D.; Turan, M.; Akgul, E.; Tok, S.; Faki, A. Adsorption Equilibrium and Kinetics of Reactive Black 5 and Reactive Red 239 in Aqueous Solution onto Surfactant-Modified Zeolite. J. Chem. Eng. Data 2007, 52, 1615–1620. [Google Scholar] [CrossRef]
- Lima, E.C.; Gomes, A.A.; Tran, H.N. Comparison of the Nonlinear and Linear Forms of the van’t Hoff Equation for Calculation of Adsorption Thermodynamic Parameters (∆S° and ∆H°). J. Mol. Liq. 2020, 311, 113315. [Google Scholar] [CrossRef]
- Li, R.; Chen, J.; Zhang, H.; Rehman, F.; Siddique, J.; Shahab, A.; Mo, Z.; Luo, L. Facile Synthesis of Magnetic-Activated Nanocomposites for Effective Removal of Cationic and Anionic Dyes in an Aqueous Environment: An Equilibrium Isotherm, Kinetics and Thermodynamic Studies. Chem. Eng. Res. Des. 2023, 189, 319–332. [Google Scholar] [CrossRef]
- Sevim, F.; Laçin, Ö.; Demir, F.; Erkiliç, Ö.F. Adsorption Capacity, Isotherm, Kinetics, and Thermodynamics Examinations on the Removal of a Textile Azo Dye by Local Natural Adsorbent. Glob. Chall. 2025, 9, 2500024. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, G.; Lamberti, E.; Gorrasi, G. Hemp Fibers Modified with Graphite Oxide as Green and Efficient Solution for Water Remediation: Application to Methylene Blue. Chemosphere 2022, 288, 132614. [Google Scholar] [CrossRef] [PubMed]




| Adsorbent | Dye | C0 (mg/L) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|---|
| Qe (exp) (mg/g) | K1 (min−1) | R2 | Qe (exp) (mg/g) | K2 (g/mg·min−1) | R2 | |||
| PEG200/MMT@HTAB | MB | 20 | 48.869 | 0.914 | 0.961 | 50.975 | 0.362 | 0.922 |
| 40 | 99.740 | 0.966 | 0.991 | 103.589 | 0.019 | 0.969 | ||
| 60 | 147.921 | 1.489 | 0.988 | 151.917 | 0.025 | 0.993 | ||
| 80 | 196.034 | 1.763 | 0.973 | 201.322 | 0.022 | 0.986 | ||
| 100 | 293.985 | 1.810 | 0.960 | 248.386 | 0.016 | 0.984 | ||
| CR | 20 | 49.394 | 3.612 | 0.998 | 49.652 | 0.765 | 0.998 | |
| 40 | 98.796 | 0.517 | 0.962 | 104.521 | 0.008 | 0.965 | ||
| 60 | 149.222 | 0.694 | 0.993 | 156.533 | 0.009 | 0.978 | ||
| 80 | 193.505 | 0.746 | 0.947 | 203.776 | 0.006 | 0.988 | ||
| 100 | 240.932 | 0.730 | 0.923 | 253.663 | 0.005 | 0.976 | ||
| Adsorbent | Dye | C0 (mg/L) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|---|
| Qe (exp) (mg/g) | K1 (min−1) | R2 | Qe (exp) (mg/g) | K2 (g/mg·min−1) | R2 | |||
| PEG4000/MMT@HTAB | MB | 20 | 49.827 | 0.946 | 0.962 | 43.615 | 0.045 | 0.986 |
| 40 | 77.940 | 0.716 | 0.977 | 81.599 | 0.017 | 0.985 | ||
| 60 | 113.571 | 0.647 | 0.933 | 119.850 | 0.009 | 0.982 | ||
| 80 | 169.029 | 0.974 | 0.963 | 176.674 | 0.011 | 0.993 | ||
| 100 | 207.211 | 1.257 | 0.971 | 215.591 | 0.012 | 0.995 | ||
| CR | 20 | 48.383 | 0.636 | 0.979 | 51.241 | 0.022 | 0.981 | |
| 40 | 98.050 | 0.872 | 0.976 | 102.408 | 0.017 | 0.992 | ||
| 60 | 146.958 | 1.011 | 0.989 | 151.017 | 0.015 | 0.981 | ||
| 80 | 190.085 | 1.079 | 0.989 | 197.755 | 0.011 | 0.996 | ||
| 100 | 236.450 | 1.163 | 0.996 | 244.880 | 0.011 | 0.994 | ||
| Adsorbents | Dye | Langmuir–Freundlich (Sips) | Redlich–Peterson | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qm (mg/g) | B | ns | R2 | RMSE | A | B | Β | R2 | RMSE | ||
| PEG200/MMT@HTAB | MB | 483.24 | 0.021 | 0.63 | 0.985 | 13.95 | 5.29 | 0.0016 | 0.98 | 0.998 | 3.89 |
| CR | 504.61 | 0.020 | 0.61 | 0.982 | 12.38 | 5.68 | 0.0063 | 0.82 | 0.997 | 5.67 | |
| PEG4000/MMT@HTAB | MB | 604.64 | 0.012 | 0.65 | 0.989 | 10.46 | 4.31 | 0.0049 | 0.88 | 0.981 | 13.03 |
| CR | 944.62 | 0.008 | 0.90 | 0.990 | 3.01 | 4.16 | 0.0008 | 0.92 | 0.994 | 7.28 | |
| Langmuir | Freundlich | ||||||||||
| Qm (mg/g) | aL (L/g) | R2 | RMSE | nF | KF(L/g) | R2 | RMSE | ||||
| PEG200/MMT@HTAB | MB | 1132 | 0.0055 | 0.990 | 10.75 | 0.98 | 4.63 | 0.999 | 1.16 | ||
| CR | 981.3 | 0.0067 | 0.998 | 9.12 | 0.99 | 4.79 | 0.999 | 0.68 | |||
| PEG4000/MMT@HTAB | MB | 833.8 | 0.0058 | 0.968 | 17.22 | 0.87 | 2.11 | 0.995 | 6.61 | ||
| CR | 1039.4 | 0.0055 | 0.998 | 4.15 | 1.12 | 6.87 | 0.998 | 4.99 | |||
| Adsorbents | Dubinin-Radushkevich | |||||
|---|---|---|---|---|---|---|
| Dye | Qm (mg/g) | β (104 mol2·J−2) | E (kJ·mol−1) | R2 | RMSE | |
| PEG200/MMT@HTAB | MB | 310.74 | 0.91 | 0.07 | 0.943 | 26.23 |
| CR | 304.05 | 1.01 | 0.07 | 0.944 | 25.76 | |
| PEG4000/MMT@HTAB | MB | 286.76 | 1.51 | 0.06 | 0.914 | 23.12 |
| CR | 285.67 | 0.86 | 0.08 | 0.944 | 26.10 | |
| Adsorbents | Dyes | ΔH° (kJ/mol) | ΔS° (J/mol·K) | ΔG° (kJ/mol) | ||
|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | ||||
| MMT@HTAB@PEG 4000 | CR | −61.5 | −163.5 | −12.7 | −11.1 | −9.5 |
| MB | −70.5 | −181.1 | −16.6 | −14.8 | −12.9 | |
| MMT@HTAB@PEG 200 | CR | −26.1 | −52.5 | −10.4 | −9.8 | −9.4 |
| MB | −33.9 | −64.2 | −14.8 | −14.2 | −13.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardi, A.; Abdelkrim, S.; Mokhtar, A.; Zaiter, K.; Hachemaoui, M.; Boukoussa, B.; Viscusi, G.; Aloui, Z.; Abboud, M. Engineered Organo-Clay Nanocomposites for Dual Cationic/Anionic Dye Removal: Role of Polyethylene Glycol Chain Length. Minerals 2025, 15, 935. https://doi.org/10.3390/min15090935
Sardi A, Abdelkrim S, Mokhtar A, Zaiter K, Hachemaoui M, Boukoussa B, Viscusi G, Aloui Z, Abboud M. Engineered Organo-Clay Nanocomposites for Dual Cationic/Anionic Dye Removal: Role of Polyethylene Glycol Chain Length. Minerals. 2025; 15(9):935. https://doi.org/10.3390/min15090935
Chicago/Turabian StyleSardi, Amina, Soumia Abdelkrim, Adel Mokhtar, Khaled Zaiter, Mohammed Hachemaoui, Bouhadjar Boukoussa, Gianluca Viscusi, Zouhaier Aloui, and Mohamed Abboud. 2025. "Engineered Organo-Clay Nanocomposites for Dual Cationic/Anionic Dye Removal: Role of Polyethylene Glycol Chain Length" Minerals 15, no. 9: 935. https://doi.org/10.3390/min15090935
APA StyleSardi, A., Abdelkrim, S., Mokhtar, A., Zaiter, K., Hachemaoui, M., Boukoussa, B., Viscusi, G., Aloui, Z., & Abboud, M. (2025). Engineered Organo-Clay Nanocomposites for Dual Cationic/Anionic Dye Removal: Role of Polyethylene Glycol Chain Length. Minerals, 15(9), 935. https://doi.org/10.3390/min15090935













