Abstract
In this study, natural kaolin was modified with hexadecyltrimethylammonium bromide (HDTMA-Br) at two levels corresponding to 50% and 90% of its cation exchange capacity. The resulting materials, designated as HKR-50 and HKR-90, were used as adsorbents for the mycotoxins ochratoxin A (OCHRA) and zearalenone (ZEN). The characterization of the HKRs with several methods (X-ray diffraction, DRIFT spectroscopy, thermal analysis (DTA/TG), SEM, zeta potential measurements, and the determination of the point of zero charge and textural properties) confirmed the presence of surfactant ions on the organokaolinites’ surfaces. The adsorption of ZEN and OCHRA by HKRs followed nonlinear adsorption isotherms, suggesting a complex adsorption mechanism. The adsorption capacities of ZEN and OCHRA were similar for HKR-50 and HKR-90 at pH 3, with higher adsorption observed for ZEN (~13.0 mg/g for HKR-50 and HKR-90 for ZEN and ~8.0 mg/g for HKR-50 and HKR-90 for OCHRA). At pH 7, the adsorption of ZEN and OCHRA was lower than at pH 3, especially for OCHRA, but slightly increased with increased amounts of surfactant on the kaolinite surface (8.5 mg/g for HKR-50 and 10.8 mg/g for HKR-90 for ZEN and 2.6 mg/g for HKR-50 and 4.1 mg/g for HKR-90 for OCHRA). Special attention was paid to the safety assessment of the natural kaolin and HKR-90, and toxicological tests confirmed the safety of both materials, as no adverse effects were observed in rats.
1. Introduction
Feed is an essential component of the food chain, supporting the growth, productivity, and welfare of farm animals [1,2,3]. However, one of the greatest challenges faced by the feed industry and farmers is the contamination of feed ingredients with mycotoxins. These toxic compounds, produced by various species of fungi such as Aspergillus, Fusarium, and Penicillium in feed, represent a substantial risk to the health and well-being of animals, as well as to the safety of foods derived from animal sources [4,5,6,7]. Common mycotoxins found in cereals and grains in the Balkan region are OCHRA and ZEN. OCHRA exhibits nephrotoxic, immunosuppressive, teratogenic, and potentially carcinogenic effects, while ZEN has estrogenic activity due to its ability to bind estrogen receptors and mimic endogenous estrogens [5,6,8]. Various biological, chemical, and physical methods have been used to eliminate, reduce or inactivate all mycotoxins. Among the most studied decontamination methods is adsorption using various types of minerals, such as clays and zeolites. The addition of adsorbents to livestock and poultry feed aims to mitigate the harmful effects of mycotoxins [9]. Zeolites and clays (montmorillonite, kaolinite, halloysite, illite, etc.) adsorb various mycotoxins with different degrees of efficiency. Although kaolin has been widely studied for the removal of heavy metals [10,11], volatile organic compounds [12,13], dyes [14,15], and pharmaceutical contaminants from aqueous solutions [16,17,18], this raw material remains one of the least studied mycotoxin adsorbents, especially for OCHRA and ZEN. In one study, kaolinite together with illite were investigated only as adsorbents for aflatoxin B1 [19]. Kaolin is primarily composed of the mineral kaolinite, while accompanying minerals such as quartz and mica may also be present. Kaolinite is a 1:1 layer aluminosilicate (Figure 1), where each layer of the mineral consists of a silica tetrahedral sheet (SiO4) and an alumina octahedral sheet (AlO4(OH)2) that is bound together by hydrogen bonds between the hydroxyl group in the octahedral sheet and the oxygen of the tetrahedral sheet [20,21,22,23,24]. There are two types of hydroxyl groups in the kaolinite structure. The first type is an outer hydroxyl group attached to aluminium atoms from the octahedral sheet and covered by one of the layer surfaces. The second type of hydroxyl group is an inner hydroxyl group found within the octahedral and tetrahedral sheets but bound to the octahedral sheet [25]. The distance between these sheets is small, so some larger ions, surfactant or water molecules cannot enter the space between sheets, rendering it unable to swell [21,26]. Due to the hydration of inorganic cations in kaolinite, this mineral is hydrophilic and thus less effective in binding relatively nonpolar organic molecules, such as most of the mycotoxins. The substitution of these inorganic cations with long-chain organic cations—surfactants—transforms kaolinite into an organomineral with heightened hydrophobicity. Surfactants used for modification may contain short, medium, or long hydrocarbon chains, as well as double-chain or aromatic groups, which may consequently influence mycotoxin adsorption [27].
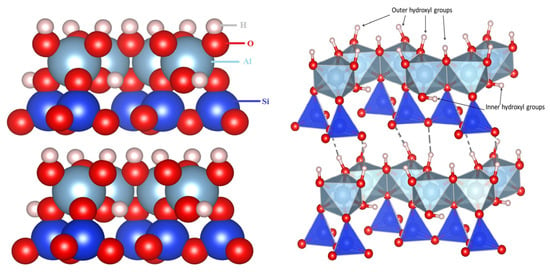
Figure 1.
The structure of kaolinite using structure parameters by D. L. Bish [28]. Structure were generated in the VESTA program [29].
Surfactant-modified kaolinite has been investigated as an adsorbent of different organic pollutants from aqueous solutions. For example, Amine Zenasni et al. [30], studied kaolin modified with hexadecyltrimethylammonium (HDTMA) bromide for the removal of an anionic dye Congo red. They reported a ~4 times higher adsorption capacity of HDTMA-modified kaolin (24.46 mg/g) than of starting natural kaolin (5.94 mg/g). Shamsudin et al. [31] tested the adsorption of the anti-inflammatory drug diclofenac sodium (DCF) onto modified kaolin with hexadecylpyridinium chloride. They reported that DCF adsorption was enhanced by the surface modification of kaolin with hexadecylpyridinium chloride and was dependent on the initial drug concentration, solution pH, adsorbent amount and temperature. In our previous research [32], kaolin was modified with three levels of octadecyldimethylbenzylammonium (ODMBA) ions (Figure 2b) at 25, 50 and 90% of kaolin’s cation exchange capacity (CEC). The prepared organokaolinites (OKR-25, OKR-50 and OKR-90) were tested for OCHRA and ZEN adsorption under various conditions, including different initial toxin concentrations, solid-to-liquid ratios, and pH values (pH 3, 7, and 9). The study demonstrated that increasing the surfactant loading and solid phase concentration enhanced mycotoxin adsorption.
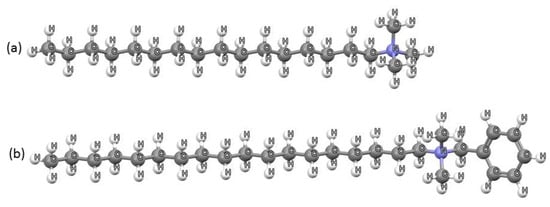
Figure 2.
Example of surfactants used for kaolin modification: (a) HDTMA and (b) ODMBA ions.
Unlike the previous study, which focused on ODMBA as a modifying agent, the present research investigates how the modification of the raw kaolin with HDTMA bromide (Figure 2a) affects the adsorption of OCHRA and ZEN. HDTMA is a widely used surfactant in the pharmaceutical industry, and its adsorption behavior on kaolin has yet to be fully explored in this context. Also, HDTMA used for the preparation of organokaolinites is known as a bactericide in aqueous solution, even at low concentrations [33]. Schulze-Makuch et al. [33] reported that the toxicity of HDTMA to organisms is greatly reduced if the surfactant is tightly bound to the surface of the HDTMA-modified zeolite—clinoptilolite. In this study, kaolin was modified with two levels of HDTMA (50% and 90% of CEC), producing organokaolinites with distinct surface properties. The structural and physicochemical characteristics of the kaolin and organokaolinites were analyzed using X-ray powder diffraction (XRPD), diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy, differential thermal analysis (DTA)/thermogravimetry (TG), scanning electron microscopy (SEM), and the determination of the point of zero charge and zeta potential. The adsorption of OCHRA and ZEN on organokaolinites was followed under different conditions—solution pH values, initial OCHRA and ZEN concentrations, and amount of each adsorbent—in order to determine the best operating parameters for mycotoxins adsorption. Finally, for the first time, toxicological tests of kaolin and organokaolinite with the best capacity for ZEN and OCHRA were conducted, as the evaluation of the safety of mineral-based adsorbents is crucial for their potential application in the decontamination of animal feed contaminated with mycotoxins.
2. Materials and Methods
2.1. Starting Material and Preparation of Organokaolinites
Clay mineral (kaolin) from Rgotina, Southeastern Serbia, was used as the starting material. The raw material was wet sieved to a particle size of <45 μm. The CEC of the starting material had value of 6 meq/100g, determined with the methylene blue adsorption method [34,35]. The chemical composition of kaolin was as follows: 56.98% SiO2, 31.06% Al2O3, 0.77% Fe2O3, 0.51% TiO2, 0.55% CaO,0.07% MgO, 0.06% Na2O, 0.87% K2O and 9.11% ignition loss.
Two organokaolinites (HKR-50 and HKR-90) were prepared by modifying kaolin with HDTMA at 50% and 90% of its CEC. Both concentrations of HDTMA were above its critical micelle concentration—CMC (>9 × 10−4 mol/L at 25 °C) [36]. The procedure for the preparation of organokaolinites was described in a previous study [32]. The surfactant, mycotoxins (OCHRA and ZEN), and all inorganic reagents used in the experiments (H3PO4, NaOH, KH2PO4, K2HPO4, HNO3, and CH3COOH, as well as HPLC-grade acetonitrile and methanol) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All solutions were prepared using distilled water.
2.2. Characterization of Adsorbents
The starting material kaolin and the organokaolinites (HKR-50 and HKR-90) were characterised with several methods.
XRPD data were collected using a X-ray diffractometer (PW 1710, Philips Instrument, Eindhoven, The Netherlands), with a curved graphite monochromator and scintillation counter. The intensities of diffracted CuKα X-ray radiation (λ = 1.54178 Å) were measured at room temperature at intervals of 0.02° 2 θ and a time of 1 s and in a 2 θ range from 4° to 65 °. The X-ray tube was loaded with a voltage of 40 kV and a current of 30 mA, while the slots for directing the primary and diffracted beam were 1° and 0.1 mm.
The textural properties of natural kaolin [32] and organokaolinite (HKR-90) were determined with nitrogen adsorption–desorption isotherms at 77 K using a Thermo Finnigan Sorptomatic 1990 analyser, Thermo Scientific, Wathman, MA, USA. The specific surface area (SBET) was calculated with the Brunauer, Emmet, Teller (BET) method [37].
Thermal analyses (DTA/TG) were performed using a STA 449 F5 Jupiter Simultaneous Thermal Analyser (Netzsch, Selb, Germany) equipped with Proteus® 8.0.0 software. The samples were heated from 25 to 1100 °C at a constant rate of 10 °C/min. Measurements were conducted using platinum crucibles (Pt90Ir10) with lids. An empty lidded crucible served as the DTA reference. Protective gas (20 mL/min synthetic air) and purge gas (50 mL/min synthetic air) were continuously used throughout the analysis. Before analysis, the samples were dried for 2 h at 60 °C, then conditioned for 24 h in a desiccator over a saturated solution of ammonium chloride (NH4Cl) to maintain a relative humidity of 75%.
DRIFT spectroscopy was performed using a Thermo Scientific iS50 spectrometer (Waltham, MA, USA) with the Smart Diffuse Reflectance accessory in a range from 4000 to 400 cm−1 with a resolution of 2 cm−1 and 64 scans. For sample preparation, 20 mg of kaolin or organokaolinites were mixed with 500 mg KBr, while for the background, clear KBr was used.
The morphological and chemical characteristics of kaolin and organokaolinite (HKR-90) were determined using a scanning electron microscope (SEM), model JEOL JSM-7001F (JEOL Ltd., Tokyo, Japan), equipped with an energy-dispersive spectrometer (EDS). The samples were coated with gold before the SEM analysis, thus producing a 15 nm thick coating to improve contrast and make them electrically conductive for analysis. The analyses were performed under high vacuum, with an acceleration voltage of 30 kV. The semi-quantitative chemical composition of the elements was obtained, shown in percentages, and since the analyses were performed on uneven surfaces, the results were normalised to 100%. The detection limit for most elements was around 0.1%.
The determination of the point of zero charge (pHpzc) was performed with the batch equilibration method using the water solutions of KNO3 in concentrations of 0.001–0.1 mol/dm3 as a background electrolyte. The initial pH values (pHi) of each solution were adjusted in the range between 2 and 12 by the small addition of 0.1 mol/dm3 KOH or HNO3 and measured using a pH meter (pH/Ion meter 781, Metrohm, Herisau, Switzerland). In each initial solution (50 mL) where the pH was adjusted, 0.1000 g of kaolin or organokaolinites were added. Erlenmeyer flasks were shaken for 24 h at room temperature and filtered, and the pH of each supernatant was measured (pHf). The pHpzc of the materials was determined as the plateau of the curve pHf = f (pHi) [38].
Zeta potential measurements were performed on a 0.5 mg/mL water solution (pH = 5.7) of starting kaolin and organokaolinites using a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK). The latex dispersion, supplied by the instrument manufacturer, was used as the calibration standard.
2.3. Adsorption of Mycotoxins by Organokaolinites
For in vitro adsorption experiments, 10 mL test solutions containing either OCHRA or ZEN were prepared by diluting an appropriate volume of a 1000 mg/L stock solution (prepared in acetonitrile) with a 0.1 M phosphate buffer at pH 3 or 7. To evaluate the influence of adsorbent mass on mycotoxin adsorption, the organokaolinite samples (50, 30, 15, 10, 5, or 3 mg) were dispersed in 10 mL of buffer containing 2.0 mg/L of OCHRA or ZEN in 15 mL polypropylene tubes. The pH of the buffer solution was adjusted by adding small amounts of diluted H3PO4. The suspensions were shaken for 30 min at room temperature and centrifuged at 13,000 rpm for 2 min, and the OCHRA or ZEN concentrations were determined with high-performance liquid chromatography (HPLC).
The mycotoxin adsorbed amount (%), , was calculated with the following Equation (1):
where and (mg/L) are the initial and equilibrium solution concentrations of OCHRA and ZEN. To investigate mycotoxin adsorption isotherms for two organokaolinites (HKR-50 and HKR-90), 10 mL of OCHRA or ZEN solutions with initial concentrations of 1, 1.5, 2, 2.5, 3, 3.5, 4, and 4.5 mg/L in a phosphate buffer (pH 3 or 7) were added to 15 mL polypropylene tubes containing 3 mg of each organokaolinite for ZEN adsorption and 2.5 mg for OCHRA adsorption. These initial concentrations of both mycotoxins were used in order to avoid their precipitation due to the low solubility of ZEN and OCHRA in water and buffer solutions at different pH values [32]. Also, in order to keep homogenous suspension and prevent the agglomeration of particles, the suspensions were prepared by weighting certain amounts of each adsorbent and their addition to the appropriate volume of the mycotoxin buffer solutions (10 mL). Each sample was prepared in duplicate, and the tubes were placed on a shaker at room temperature for 30 min. Then, mixtures were centrifuged at 13,000 rpm for 2 min and analyzed for the residual OCHRA and ZEN concentration with HPLC. HPLC analyses were performed on a Hitachi L-7100 pump with Hitachi L-7200 autosampler and fluorescence detection with a Hitachi L-7480 fluorescence spectrophotometer (OCHRA—λex = 365 nm, λem = 450 nm; ZEN—λex = 274 nm, λem = 465 nm). The mobile phase for OCHRA and ZEN was acetonitrile: water: acetic acid = 60:40:1, pumped at a flow rate of 1 mL/minute. Freundlich, Langmuir and Sips models were used to fit the experimental data for OCHRA and ZEN adsorption by HKR-50 and HKR-90.
2.4. Acute Oral Toxicity Study in Rats
This study we performed on adult Wistar rats, 6–8 weeks old, weighing 200–220 g, raised at the Veterinary Service Centre, Military Health Care Department, Ministry of Defence, Belgrade, Serbia. A typical macrolon plastic cage (Bioscape, Castrop-Rauxel, Germany) filled with clear sawdust (Versele-Laga, Deinze, Belgium) was used for housing the experimental animals. The animals were housed in centrally regulated ambient conditions, with a temperature of 22 ± 2 °C, humidity of 55 ± 15%, air changes/h of 15–20, and a light/dark cycle of 12/12 h. A commercial diet mixture for rats (Veterinary Institute Subotica, Subotica, Serbia) and tap water from municipal mains, filtered through a 1.0 μm filter (Skala Green, Serbia), were applied ad libitum.
This acute toxicity study was performed at the Department for Experimental Toxicology and Pharmacology, National Poison Control Centre, Military Medical Academy, Belgrade, Serbia, following the OECD Guidelines in a GLP-compliant way as previously described [39,40].
Before the start of the study, experimental procedures and animal care were approved by the Ethical Commission for the Protection of Animal Welfare, Faculty of Veterinary Medicine, University of Belgrade, Serbia (decision No.: 21/2020, 18 June 2020), which was confirmed by the Ethical Committee of the Veterinary Directorate, Ministry of Agriculture, Forestry and Water Management, Belgrade, Serbia (decision No. 323-07-11720/2020-05, 23 October 2020).
After randomization, the acute toxicity of kaolin and HKR-90 was recorded by using six groups of rats, each of them consisting of 5 animals. Increasing doses of kaolin (700, 1400, and 2100 mg/kg) and HKR-90 (800, 1600, and 2400 mg/kg) were administered by the p.o. route in a separate group of animals, respectively. Kaolin and HKR-90 were prepared for administration by dissolving each substance separately in a sterilized and apyrogenic 0.9% NaCl solution ex tempore.
After the 24 h survival registration, the median lethal dose (LD50) for kaolin and HKR-90 was calculated according to the method of Litchfield and Wilcoxon [41]. Then, the general condition of each animal was observed daily throughout the whole study, lasting 7 days.
3. Results and Discussion
3.1. Characterization of Organokaolinites
3.1.1. XRPD Analysis
XRPD analysis was used to determine the crystalline phase composition and structural properties of kaolin and organokaolinites. Mineralogical analysis confirmed that kaolinite is the dominant mineral in kaolin, with smaller amounts of quartz and mica (Figure 3) [34,35]. The semiquantitative distribution of crystalline phases was estimated as clays ≥ 60% and quartz ≤ 40%. The values of the main basal reflection (d001) of kaolinite were 7.090 Å for kaolin, 7.191 Å for HKR-50, and 7.128 Å for HKR-90. These results indicate that modification with the cationic surfactant HDTMA did not significantly change the interlayer spacing of kaolinite, confirming that the surfactant is primarily located on the external surface of the clay mineral. The lack of substantial changes in XRPD patterns indicated that the surfactant remained on the kaolinite surface rather than penetrating the interlayer space. Although Xi et al. [42] used significantly higher amounts of HDTMA (200% and 400% CEC) for the modification of kaolin, they also observed no increase in the basal spacing (d001) for organokaolinites, which remained at 7.1 Å, the same as in unmodified kaolin. This further confirms that HDTMA was adsorbed exclusively on the kaolinite surface without intercalation into the crystal structure.
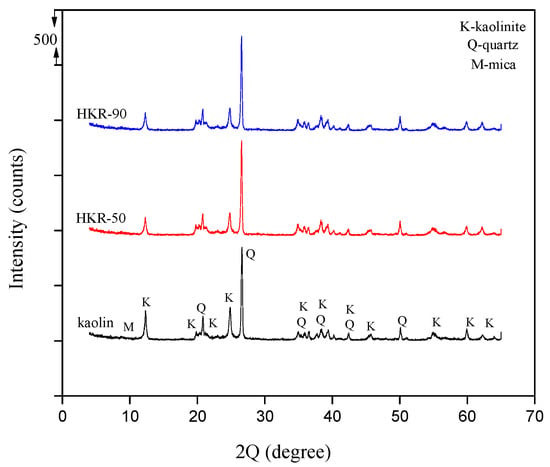
Figure 3.
XRPD patterns of samples kaolin and organokaolinites (HKR-50 and HKR-90).
3.1.2. Textural Properties of Kaolin and Organokaolinites
The specific surface area of kaolin and HKR-90 was determined to be 11.2 m2/g [32] and 8.5 m2/g, respectively. This decrease suggests that surfactant adsorption leads to micropore blockage, reducing the available surface area for nitrogen adsorption. Aroke and Hamidu [43] reported a similar trend in their study on the textural properties of kaolinite modified with HDTMA in amounts corresponding to 100% and 200% of the kaolin CEC (9.5 meq/100 g). The surface area significantly decreased from 11.9754 m2/g (kaolinite) to 3.0132 m2/g (100%) and 3.8225 m2/g (200%) after modification. This decline in surface area further confirms that organic surfactant adsorption blocks pores and restricts access to the inner surface for nitrogen molecules. Overall, their results confirm the successful modification of kaolin with the highest HDTMA loading, where surfactant ions predominantly reside on the external clay surface, contributing to the observed decrease in specific surface area.
3.1.3. Thermal Analysis
Thermal analysis was used to study the thermal properties of the starting material (kaolin) and organokaolinites. The differential thermal analysis (DTA) and thermogravimetric (TG) analysis curves of kaolin, HKR-50 and HKR-90 are presented in Figure 4. The mass loss for starting kaolin and organokaolinites in different temperature regions is presented in Table 1.
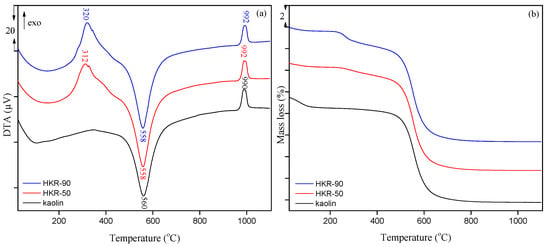
Figure 4.
Thermal analysis of kaolin and organokaolinites: (a) DTA and (b) TG curves.

Table 1.
Mass loss (%) for starting kaolin, HKR-50 and HKR-90 in different temperature regions.
In the first temperature region (25–180 °C), mass loss is due to dehydration and should decrease with increasing hydrophobicity of the mineral. This tendency was achieved for both organokaolinites, where the mass loss was 0.4% for HKR-50 and HKR-90 in comparison with starting kaolin (1.0%). The second temperature region (180–450 °C), is related to the oxidation of the organic phase (HDTMA) on the kaolinite surface, so the mass loss increased with increasing amounts of surfactant in organokaolinites (from 0.5% for kaolin to 1.1% for HKR-50 and 1.8% for HKR-90). The dehydroxylation of kaolinite was characteristic of the third temperature region (450–800 °C). In this region, the structural OH groups of kaolinite are destroyed and deposited as water from the mineral, forming amorphous metakaolin (Al2O3 × 2SiO2 × 2H2O → Al2O3 × 2SiO2 + 2H2O) [24,44]. It was also noticed that an insignificant increase in the mass loss with the presence of surfactant in organokaolinites (8.4% for HKR-50 and HKR-90 compared to 8.2% for kaolin), which may have occurred due to the oxidation of the remaining firmly bound HDTMA to the kaolinite surface. Amorphous metakaolin recrystallises to stable crystal structures above 800 °C and is not accompanied by a change in mass loss [44,45].
The DTA curve of kaolin contains three peaks. One endothermic broad peak of low intensity in the temperature region from 25 to 180 °C originates from dehydration. Then, a much sharper and more intense endothermic peak at 560 °C is related to the dehydroxylation whereby metakaolin was formed. The last peak in the DTA curve of kaolin is a sharp exothermic peak at 990 °C corresponding to metakaolin’s recrystallization [44]. All of the mentioned peaks are also present in the DTA curves of HKR-50 and HKR-90, except in the temperature range from 180 to 450 °C, where an exothermic peak at 312 °C for HKR-50 and 320 °C for HKR-90 was observed due to the oxidation of surfactant on the surface of kaolinite. With the increase in the amount of HDTMA in organokaolinite, a shift towards higher temperatures was observed, indicating that more energy is required for the oxidation of the organic phase in HKR-90 due to a higher amount of HDTMA on the kaolinite surface compared with HKR-50. The presence and shift of the aforementioned peaks towards higher temperatures, as well as the increase in mass loss in the temperature range from 180 to 450 °C, confirmed the presence of HDTMA in both organokaolinites.
3.1.4. FTIR Analysis
DRIFT spectroscopy offers a few advantages over other infrared techniques including simple sample preparation, greater numbers of substantial bands, and the ability to detect both major and minor components from the same spectra [46]. The DRIFT spectra of the starting kaolin and organokaolinites are given in Figure 5. The DRIFT technique has led to a better understanding of the outer layers and properties of materials [47,48]. Compared with the starting kaolin, the infrared spectra of HKR-50 and HKR-90 have all the characteristic peaks of the untreated kaolinite without any changes in their positions. The characteristic bands for hydroxyl stretching vibrations and adsorbed water stretching vibrations were located between 4000 and 3000 cm−1 [49]. Specifically, the stretching vibrations of the outer and inner hydroxyl groups of kaolinite were found between 3700 and 3600 cm−1 [25,49,50,51]. Absorption bands at 3690, 3669, 3652, and 3620 cm−1 are characteristic of kaolinite, and the presence of all four bands suggests good crystallinity [49,50,51]. The band at 3620 cm−1 corresponds to the stretching vibration of the inner hydroxyl group, while the remaining three correspond to the stretching vibrations of the outer hydroxyl groups [50]. The OH vibration band at 1615 cm−1 was present in all spectra of kaolin and organokaolinites and was associated with physically adsorbed water [22]. Characteristic vibrations bands of kaolinite Si-O (1114, 1027, 1004, 463, 426 cm−1), Al-OH (940, 913 and 690 cm−1) and Si-O-Al (793, 754 and 530 cm−1) appeared in the DRIFT spectrum of raw kaolin and in spectra of organokaolinites with the same intensity of bands [22,32,51]. The presence of additional absorption bands typical for HDTMA in the DRIFT spectra of both organokaolinites provided substantial proof of the successful modification of kaolinite with HDTMA ions. Bands between 3000 and 2800 cm−1 were considered the signature of -CH2 asymmetric and symmetric stretching vibrations of the alkyl chain, and the band at 1468 cm−1 is characteristic of the bending vibration of the CH2 group [32,52,53]. The position of stretching vibration bands is usually used for a better understanding of how surfactants were arranged on the surface of a mineral [54]. Namely, Kung and Hayes [55] noticed that at a lower surface coverage, adsorbed HDTMA molecules appeared more like monomers, with absorption bands at 2860 and 2930 cm−1. At a higher surface coverage, surfactant molecules had a micelle surface configuration, causing a shift of the vibration bands to lower frequencies (2853 and 2923 cm−1). Also, if the initial concentration of surfactant is below the CMC value, monomers exist in solution, so surfactant ions usually form monolayer at the mineral surface. When the initial surfactant concentration exceeds the CMC, as in this study, micelles exist in solution, so surfactant molecules will associate to form aggregates (e.g., admicelles) or a bilayer at the mineral surface [36,56]. In the DRIFT spectra of the organokaolinites (Figure 5), asymmetric and symmetric stretching bands of CH2 originating from HDTMA were clearly visible, and their intensities increased with the amount of surfactant. The organokaolinite with a lower HDTMA content (HKR-50) showed stretching bands at 2925 and 2854 cm−1. In contrast, the absorption bands of HKR-90 were shifted further to lower values, 2923 (asymmetric) and 2852 cm−1 (symmetric stretching), similar to those reported for micelle configurations [51]. The band positions for HKR-90 corresponded to the arrangement of the surfactant on the kaolinite surface as micelles, while the position of absorption bands for HKR-50 laid between the values reported by Kung and Hayes [55] for monomer and micelle configurations, which may suggest a local bilayer arrangement of the surfactant on the kaolinite surface.
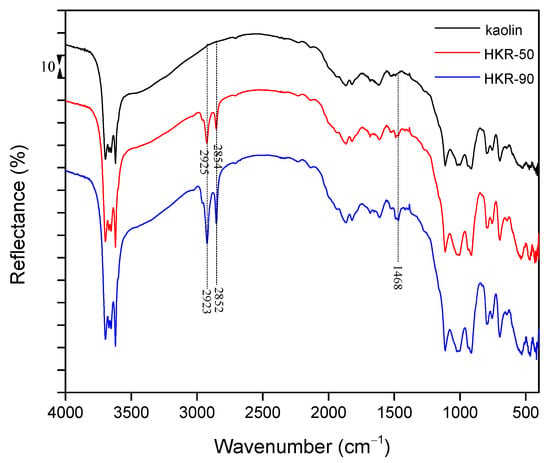
Figure 5.
DRIFT spectra of kaolin and organokaolinites.
Also, the DRIFT technique has already been used for quantifying the hydrophilicity degree of various porous, silica-based materials, such as mesoporous silicas, clays with silica pillars, and porous silica xerogels [47,57]. In this research, the DRIFT technique was used to determine the influence of HDTMA ions on the hydrophilicity, i.e., hydrophobicity, of the modified kaolinite compared with the starting material.
Kubelka–Munk conversion was applied to all recorded DRIFT spectra. Also, the normalization of the spectra was performed using the most intense band at 920 cm−1 to facilitate a better comparison of the bands of interest between samples (as illustrated by the black lines in Figure 6). All bands, except for the hydroxyl stretching and adsorbed water vibration bands, were included in the calculation of hydrophilicity. These bands are presented in Figure 6 and denoted with numbers from 1 to 12. The degree of hydrophilicity (%A) of kaolin and organokaolinites was determined using Equation (2):
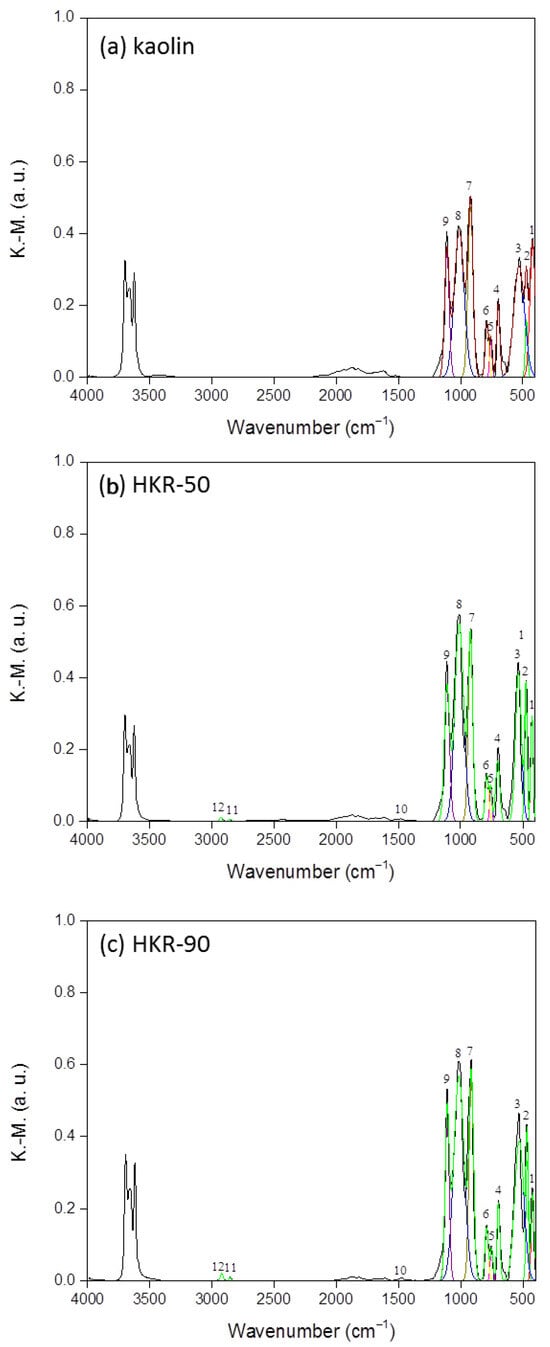
Figure 6.
K.-M. DRIFT spectra of (a) kaolin, (b) HKR-50 and (c) HKR-90. 1:Si-O; 2: Si-O; 3: Si-O-Al; 4: Al-OH; 5: Si-O-Al; 6: Si-O-Al; 7: Al-O; 8: Si-O; 9: Si-O; 10: C-H; 11: -CH2; 12: -CH2.
This was calculated as the ratio of the sum of the areas of the bands (A) corresponding to hydrophilic groups to the total areas of both hydrophilic and hydrophobic groups present in the samples. Higher values of the degree of hydrophilicity correspond to a less hydrophobic material and vice versa [47,58]. To calculate the areas of the bands which were used in Equation (2), a Gaussian function was utilized for the deconvolution and fitting of the normalised DRIFT spectra, as presented in Figure 6 by colored lines in all three spectra (the relative areas of each group and the absorption maxima (ṽ) are not presented in this research). The degree of hydrophilicity (%A) for the kaolin was 79.30%, while for HKR-50 and HKR-90, it was 78.42% and 73.93%, respectively. These results showed that hydrophobicity increased with increasing amounts of the surfactant on the kaolinite surface.
3.1.5. SEM Analysis
As a visual confirmation of the presence of the surfactant on the kaolinite surface after modification, SEM images of the initial kaolin and HKR-90 were recorded and are presented in Figure 7.
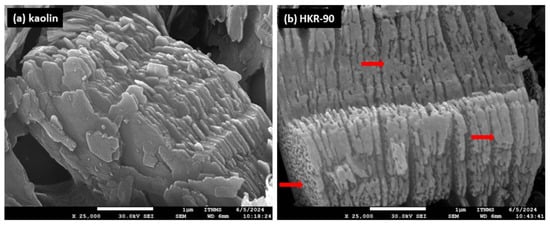
Figure 7.
SEM images: (a) kaolin and (b) HKR-90 with 25,000 times magnification of all samples and a scale bar of 1 μm.
The initial material kaolin (a) consisted of a large amount of scaly kaolinites of different sizes and a smaller amount of layered mica and quartz with characteristic regular shapes, confirming the results of the XRPD analysis regarding the presence of these minerals. Also, for organokaolinite, all the previously mentioned minerals were observed. Still, changes were observed on the surface of the kaolinite in the form of group-distributed surfactants, uneven deposits that were formed based on the amount of surfactant used to modify the mineral. It can be observed that for HKR-50 (Figure S1), part of the kaolinite surface was not covered with HDTMA ions while a significant portion of the surface was modified, which corresponded to the amount of surfactant used in the preparation of this material. In the case of HKR-90, a large part of the kaolinite surface was covered with HDTMA ions (red arrow in Figure 7b), reflecting the highest amount of surfactant used in its synthesis. The results obtained in this way were another confirmation of the successful modification of the kaolinite surface. To provide a more comprehensive explanation of the results, an additional EDS analysis was conducted. Several points were selected during the experiment to identify all present minerals. The primary focus was on identifying kaolinite and examining changes in the elemental composition of kaolin before and after modification.
Selected sites of kaolinite in kaolin and HKR-90 are presented in Figure 8. As can be seen, in kaolinite (Figure 8, Table (a)), the element Si had slightly higher values compared with the element Al, and such a trend existed in HKR-90 (Figure 8, Table (b)). The elements potassium and iron appeared in traces. In organokaolinite, bromine occurred in a small percentage, probably because it remained “stuck” among the surfactants bound to the surface of kaolinite as micelle. SEM and EDS analyses confirmed that no difference in morphology was observed between kaolinite and organokaolinite, which confirmed that the modification occurred only on the surface of the clay mineral and that the HDTMA did not change the structure of layered kaolinite.
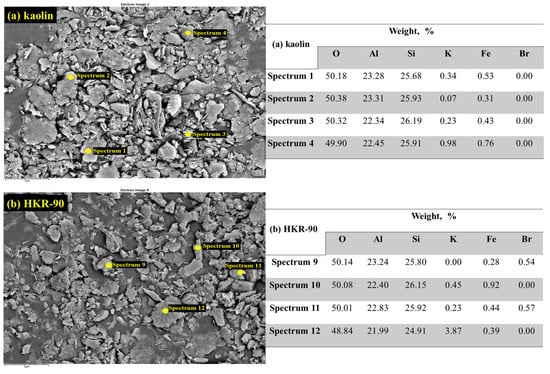
Figure 8.
EDS of kaolin (a) and organokaolinites and HKR-90 (b).
3.1.6. The Point of Zero Charge
The pH is an important factor affecting different forms of ionizable organic compounds, such as OCHRA and ZEN, in solution. Also, the ionization of chemically active sites on an adsorbent surface can be different depending on the pH of a solution. In this study, pH-metric measurements were used to investigate how the surface charge of the adsorbent changed at different pH values. This was done by determining the point of zero charge (pHpzc). The pHpzc is defined as the pH at which the net surface charge of the adsorbent is zero, meaning that the number of positively and negatively charged sites is balanced [59]. The surface charge is positive at pH < pHpzc and negative at pH > pHpzc [60]. The kaolinite, as a 1:1 layer aluminosilicate, contained an octahedral sheet whose surface properties were pH-dependent due to the protonation and deprotonation of hydroxyl groups on broken edges and the sheet surface. In contrast, the tetrahedral sheet possessed a permanent negative charge, independent of pH [22,61]. When pH > pHpzc, the deprotonation of hydroxyl groups occurred (Al-OH + OH− →Al-O− and Si-OH + OH− → SiO−), while at pH < pHpzc, protonation occurred (Al-OH + H+→ Al-OH2+), leading to a more positively charged kaolin surface [22,61,62]. The results of the pHpzc determination for the kaolin and two organokaolinites as a function of the concentration of electrolytes are presented in Figure 9a–c.
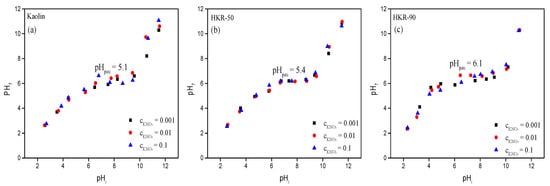
Figure 9.
pHf versus pHi at different background electrolyte (KNO3) concentrations.
The pHf vs. pHi curves of kaolin, HKR-50 and HKR-90 exhibited similar shapes across all adsorbents. The plateaus of each curve represent the pHpzc values of the kaolin and organokaolinites, which differed between materials and also varied in length. Within the plateau range (for kaolin and HKR-50 from 5.5 to 9.5 and for HKR-90 from 4.0 to 10.0), all three materials exhibited amphoteric properties and acted as buffers, meaning that the addition of OH− or H+ ions did not significantly change the equilibrium pH, which remained equal to pHpzc. This indicated that the surface of the materials (kaolin, HKR-50, and HKR-90) was positively charged at pH values below the plateau region, uncharged within the plateau range, and negatively charged at pH values above the plateau region. The obtained pHpzc value of 5.1 for the raw kaolin was similar to values reported by Schroth and Sposito [63] for two kaolinites (pHpzc = 5.0 for KGa1 and pHpzc = 5.4 for KGa2). Similarly as in this study, Mudzielwana et al. [64] reported an increase in pHpzc from 6.5 (unmodified kaolin) to 7.5 (kaolin modified with HDTMA), attributing this shift to the adsorption of HDTMA ions, which masked negative surface sites and enhanced the overall positive charge. A comparison of the pHpzc values of kaolin and organokaolinites indicated that the presence of HDTMA on the kaolinite surface increased the pHpzc of kaolin. This suggested that the organokaolinites had different surface functional groups with varying acidic and basic characteristics compared with the natural kaolin. Furthermore, the extended plateau range observed in HKR-90 implies that the surface remained uncharged over a broader pH range, corresponding to an enhanced buffering capacity. Finally, the adsorption of OCHRA and ZEN by the organokaolinites was studied at pH 3 and 7 (see below), and the pHpzc results indicate that at pH 3, the surfaces of the adsorbents had a higher number of positively charged active sites, while at pH 7, an approximately equal number of positively and negatively charged active sites was present. The pHpzc data confirm that surface charge varied significantly with pH and between samples, which is a key parameter influencing future adsorption behavior.
3.1.7. The Zeta Potential
To have a better understanding of the interparticle forces operating in suspensions of clay particles, the zeta potential of kaolin, HKR-50 and HKR-90 in distilled water and buffer solutions (pH 3 and 7) was determined, and the results are presented in Figure 10. The zeta potential properties of charged particles in colloidal systems may help in understanding how mycotoxin adsorption occurs on sample surfaces. The adsorbed surfactant—HDTMA—created a rich organic layer on the previously negative kaolin surface, changing the surface charge from more negative to less negative or even positive values with increasing HDTMA concentration. The zeta potential of the kaolin in distilled water (pH ~5.7) showed a negative value of −27.6 mV [32]. The presence of HDTMA ions on the clay surface in an amount equal to 50% of the CEC value (HKR-50) led to a slightly less negative zeta potential of −23.0 mV, probably due to the formation of a local bilayer, leaving part of the kaolinite surface uncovered with surfactant, which only resulted in a small change in the zeta potential. A positive zeta potential of +9.96 mV was observed for HKR-90, where the surfactant molecules were arranged in a micellar structure considerably covering the external kaolinite surface. In the phosphate buffer, the increase in pH from 3 to 7 caused a shift of the zeta potential of kaolin to more negative values (from -19.6 mV at pH 3 to −23.2 mV at pH 7), which is characteristic for the mineral kaolinite and is strongly dependent on pH-dependent charges [65]. The positive charges (Al-OH2+) formed by the protonation of the edge Al-OH sites on the kaolinite surface caused a shift of the negative zeta potential toward less negative values with the decrease in the pH. Opposite to this, the increase in the pH caused the adsorption of -OH groups at the edge sites on the surface of the kaolinite, which became more negative alongside the zeta potential. Compared with the zeta potential of the kaolin in the buffer solutions (pH 3 and 7), its modification with two amounts of HDTMA resulted in the zeta potential becoming less negative or even positive at both pH values. For HKR-50, the zeta potential changed from −5.6 mV at pH 3 to −13.7 mV at pH 7, while for HKR-90, these changes were from +2.1 mV at pH 3 to −9.2 mV at pH 7. The changes in the zeta potential provide more evidence of surfactant adsorption, which decreased the number of pH-dependent charges on the organokaolinites’ surfaces.
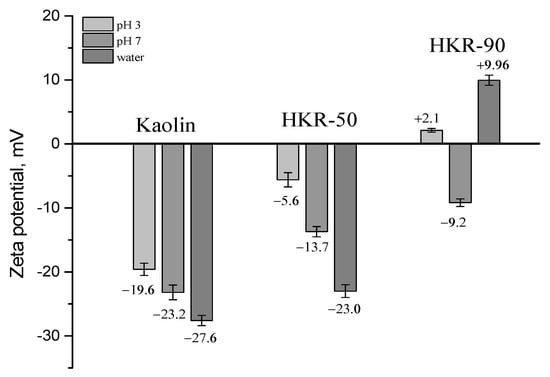
Figure 10.
Zeta potential of kaolin and organokaolinite in water and at pH 3 and 7 [32].
3.2. Adsorption of Mycotoxins
The adsorption of OCHRA and ZEN was carried out under conditions that resemble the gastrointestinal tract of animals, the stomach (pH 3) and intestines (pH 7). Preliminary results showed that less than 10% of OCHRA and ZEN were adsorbed onto the unmodified kaolin at pH 3 and pH 7 [35]. To improve the adsorption efficiency of kaolin for mycotoxins, the kaolin was modified with a new surfactant, HDTMA, aiming to obtain a safer material with enhanced properties. Characterization results revealed that the modification increased hydrophobicity and shifted the zeta potential to more positive values while preserving the intact layered structure of the kaolinite, as only the surface was modified with HDTMA. OCHRA is a hydrophobic molecule with carboxylic (pKa1 = 3.5) and phenolic (pKa2 = 7.0) functional groups. At pH 3, OCHRA predominantly exists in its neutral form, while at pH 7, it is fully in the anionic form. Similarly, ZEN is a hydrophobic molecule containing two phenolic hydroxyl groups, with a pKa1 value of 7.6. As a result, ZEN exists primarily in the neutral form at pH 3, and at pH 7, it remains mostly neutral, with approximately 80% in the neutral form and 20% in the anionic form [32]. The adsorption of OCHRA and ZEN versus different concentrations (0.3–5.0 g/L) of HKR-50 and HKR-90 in suspension at a constant initial mycotoxins concentration (2.0 mg/L) was followed in a buffer solution at pH 3 and 7, and the results are presented in Figure 11.
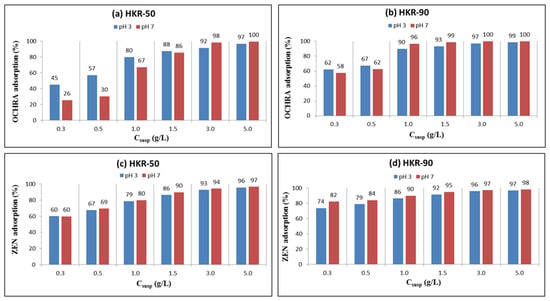
Figure 11.
Adsorption of OCHRA (a,b) and ZEN (c,d) by organokaolinites at two pH values.
The results showed that for HKR-50 and HKR-90, the presence of HDTMA ions on the kaolinite surface significantly enhanced the adsorption of OCHRA and ZEN compared with the unmodified material. At both pH values, the adsorption of mycotoxins generally increased with increasing concentration of each adsorbent in suspension. For HKR-50, at the lowest amount of adsorbent (0.3 g/L), the adsorption of OCHRA was much higher at pH 3 (45%) compared with pH 7 (26%). As the concentration of HKR-50 in the suspension increased, the difference in OCHRA adsorption at pH 3 and 7 became less pronounced. For HKR-90, differences in OCHRA adsorption at the two pH values were minimal. For example, at the lowest amount of adsorbent in suspension (0.3 g/L), the adsorption of OCHRA was 62% at pH 3 and 58% at pH 7. On the other hand, the adsorption of ZEN was practically independent of pH values, but adsorption was noticed to increase with increasing amounts of HDTMA on the kaolinite surface. At the lowest amount of both adsorbents (0.3 g/L), the adsorption of ZEN was 60% for HKR-50 at pH 3 and 7, while for HKR-90, its adsorption was 74% at pH 3 and 82% at pH 7. Based on these results, HDTMA ions create active sites on the surface of kaolinite for the adsorption of these mycotoxins, so with an increase in the amount of surfactant, the number of sites increases and thus the adsorption of OCHRA and ZEN increases.
OCHRA and ZEN adsorption isotherms for HKR-50 and HKR-90 at two pHs are presented in Figure 12 and Figure 13. Typically, isotherms are presented by plotting the concentration of the mycotoxin remaining in solution after equilibrium versus the amount of mycotoxins adsorbed per unit of weight of the adsorbent. The adsorption of both mycotoxins followed nonlinear adsorption isotherms at pH 3 and 7, and Langmuir, Freundlich and Sips models of isotherms were used to fit the OCHRA and ZEN experimental data.
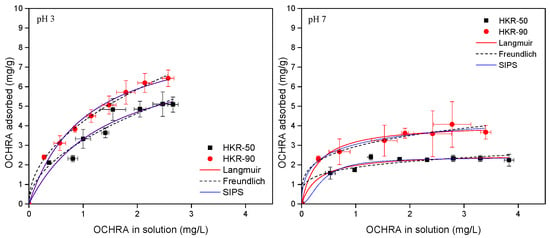
Figure 12.
OCHRA adsorption by HKR-50 and HKR-90 at pH 3 and pH 7.
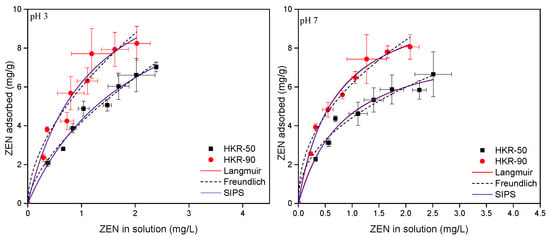
Figure 13.
ZEN adsorption by HKR-50 and HKR-90 at pH 3 and pH 7.
Langmuir’s model assumes a monolayer coverage of the adsorbate on a homogeneous adsorbent surface and that equilibrium is established when there is no additional adsorption. The mathematical expression describing this type of isotherm is shown in Equation (3):
where (mg/g) is the amount of mycotoxins adsorbed by organokaolinites, (mg/L) is the equilibrium concentration of mycotoxins in solution, KL (L/mg) is a Langmuir constant, and Qm (mg/g) is the maximum adsorption capacity.
The Freundlich type of isotherm is an empirical type of dependence that describes adsorption by heterogeneous adsorbents. The mathematical expression Equation (4) describes this type of isotherm [16,32]:
where KF () is the Freundlich isotherm constant, 1/n is the Freundlich exponent, and other parameters have been defined as in Equation (3).
The Sips isotherm is a three-parameter model that combines the Langmuir and Freundlich models and that approaches the Freundlich model at low concentrations. In contrast, at higher concentrations, this isotherm predicts monolayer adsorption characteristic of the Langmuir model [66,67]. The Sips isotherm can be expressed as Equation (5) [68,69]:
where (mg/g) is the maximum amount of mycotoxin adsorbed per unit mass of adsorbent, (L/mg) is the Sips constant, and parameter s characterises the heterogeneity of the system, with values ranging from 0 to 1 [70,71]. When s equals 1, the Sips equation reduces to the Langmuir equation, indicating a homogeneous adsorption process. Conversely, when s is greater than 1, it suggests cooperative adsorption [67,71].
The parameters for the Langmuir, Freundlich and Sips isotherms for OCHRA and ZEN are presented in Table 2 and Table 3. High values of the correlation coefficient of the plots (r2) indicate that all three models well-described the adsorption of OCHRA and ZEN by the organokaolinites. Furthermore, based on the lowest values of the sum of squares of errors (SSE), the Langmuir model exhibited the best agreement with the experimental data for the adsorption of both ZEN and OCHRA onto HKR-50 and HKR-90 at pH 3 and 7 (Table 2 and Table 3). The values of parameter n from the Freundlich isotherm for OCHRA and ZEN at pH 3 and 7 were greater than 1, indicating a favourable adsorption process. Nonlinear isotherms were obtained for the adsorption of OCHRA and ZEN by both organokaolinites at pHs that indicated a complex adsorption mechanism. At pH 3, the surface of organokaolinites features several active sites: hydrophobic sites formed by electrostatic interactions between kaolinite and the surfactant, positively charged sites from the polar regions of the surfactant (bilayer or micelles), negatively charged basal oxygens, and protonated hydroxyl groups (Al-OH2+) located on the surface and edges of the kaolinite’s octahedral sheet. The Langmuir isotherms showed adsorption capacities for OCHRA of 8.1 mg/g and 8.9 mg/g for HKR-50 and HKR-90 at pH 3, respectively. Slightly higher maximum adsorbed amounts were observed for ZEN 13.2 mg/g and 13.4 mg/g for HKR-50 and HKR-90 under the same conditions. The similar OCHRA and ZEN capacities obtained for both organokaolinites at pH 3 indicated that even in HKR-50, there were enough active sites available for the adsorption of both mycotoxins. Also, the Sips adsorption isotherms at pH 3 for both mycotoxins converged to Langmuir isotherms, and the parameter s was approximately equal to 1, assuming that the adsorption was homogeneous. Thus, it was assumed that despite the presence of different active sites on the surface of the organokaolinites at pH 3, hydrophobic interactions were dominant, leading to a more uniform OCHRA and ZEN adsorption pattern and approaching homogeneity on a heterogeneous surface. At pH 7, the surface of the organokaolinites contained distinct active sites: hydrophobic sites derived from the surfactant’s long chain, positively charged sites from the polar regions of the surfactant (bilayer or micelles), and negatively charged basal oxygens along with deprotonated hydroxyl groups (Al-O−) on the surface and edges of the kaolinite’s octahedral sheet. On the other hand, at pH 7, OCHRA was completely in anionic form while ZEN was mainly in neutral (80%) and partially in anionic form, meaning that different forms of mycotoxins in solution lead to different adsorption behaviors at pH 7. From the Langmuir model, the maximum adsorption capacities for OCHRA at pH 7 were much lower than at pH 3 (2.6 mg/g and 4.1 mg/g for HKR-50 and HKR-90, respectively). This behavior may be explained by the fact that in addition to the hydrophobic interactions and electrostatic interactions between the polar “heads” of the surfactant and negatively charged OCHRA, there were also repulsive interactions between the surface of the kaolinite, which remained uncovered with HDTMA, and the negatively charged OCHRA, which resulted in a lower maximum adsorption capacity at pH 7. A slightly higher OCHRA adsorption was observed at pH 7 for HKR-90, and this was due to the higher surfactant amount. The Sips parameter s was lower than 1 for HKR-50 (0.817) and HKR-90 (0.512), confirming heterogenous adsorption. For ZEN, from the Langmuir isotherms, the following maximum adsorption capacities were obtained: 8.5 mg/g for HKR-50 and 10.8 mg/g for HKR-90 at pH 7. Since ZEN is predominantly a neutral molecule, hydrophobic interactions played a major role in its adsorption. However, there was also an attractive interaction between the positively charged “heads” of HDTMA and the partially negatively charged ZEN. At the same time, the repulsive interactions between the uncovered negatively charged kaolinite and anionic form of ZEN were less pronounced, causing only a slight decrease in adsorption capacities at pH 7 compared to pH 3. At pH 7, as for OCHRA, ZEN adsorption was slightly higher with HKR-90 than with HKR-50, resulting from the higher HDTMA amount used in kaolinite modification. The Sips parameter s for HKR-50 was 1.050 and that for HKR-90 was 0.9432, which were different from 1, indicating that the ZEN adsorption was more heterogeneous than at pH 3. Generally, for the adsorption of ZEN (pH 3 and 7) and OCHRA (pH 3) onto HKR-50 and HKR-90, the experimental equilibrium adsorption capacities were lower than the theoretical values predicted by the Langmuir model (Table 2 and Table 3) since under the applied experimental conditions, the plateaus were not reached. For the adsorption of OCHRA by both organokaolinites, at pH 7, the adsorption plateau was reached, so the experimental adsorption capacities were similar to the theoretical values predicted by the Langmuir model (Table 2).

Table 2.
Calculated parameters of isotherm models for OCHRA adsorption.

Table 3.
Calculated parameters of isotherm models for ZEN adsorption.
The present adsorption results were compared with results from our previous study Spasojević et al. [32], in which OCHRA and ZEN adsorption was followed onto kaolin modified with ODMBA in amounts of 50% and 90% of the CEC of kaolin (OKR-50 and OKR-90). For the OKRs, nonlinear adsorption isotherms suggested a complex adsorption mechanism involving hydrophobic interactions and electrostatic interactions between the positively charged “heads” of ODMBA (bilayer or micelle) and the anionic forms of OCHRA and ZEN. From the Langmuir model, much higher OCHRA adsorption capacities were reported at pH 3 (26.7 mg/g for OKR-50 and 39.2 mg/g for OKR-90) than for the HKR-50 and HKR-90 samples. At pH 7, these values were similar to those for HKR-50 and HKR-90 (4.0 mg/g for OKR-50 and 6.9 mg/g for OKR-90). For ZEN adsorption by OKR-50 and OKR-90 at pH 3 and 7, the Langmuir model adsorption capacities were similar at pH 3 to those for HKR-50 and HKR-90 (12.0 mg/g for OKR-50 and 13.5 mg/g for OKR-90) while at pH 7, these values were higher and increased to 15.3 mg/g for OKR-50 and 14.4 mg/g for OKR-90. Although both ODMBA and HDTMA are long-chain organic cations, the observed differences in OCHRA and ZEN adsorption by organokaolinite HKRs and OKRs could be attributed to the structural variations between these two surfactants. Namely, in the study of Li et al. [27], the effects of different organic cations on ZEN adsorption were investigated by comparing unmodified montmorillonite with montmorillonite modified with different surfactants, containing different alkyl chain lengths, with or without a benzyl group, and containing one or two long alkyl chains. Their findings showed that the presence of a benzyl group or more than one alkyl chain in a surfactant molecule significantly increased ZEN adsorption. Thus, it was expected that since ODMBA contained a benzyl functional group and a longer alkyl chain (by two carbon atoms) compared with HDTMA, the adsorption of both mycotoxins would be higher by OKRs. The presence of the benzyl group in ODMBA allowed for additional π–π interactions, especially with OCHRA, which has two aromatic rings while ZEN has only one. Additionally, the longer alkyl chain in ODMBA further enhanced the hydrophobic interactions. Together, these effects significantly strengthened the overall hydrophobic interactions at pH 3, leading to a higher adsorption affinity for OCHRA. At pH 7, as OCHRA predominantly exists in its anionic form, in addition to hydrophobic and electrostatic interactions, electrostatic repulsion played a significant role, causing a decrease in OCHRA adsorption capacity. In contrast, for ZEN adsorption at pH 7, the hydrophobic and electrostatic attractive interactions outweighed repulsive forces, resulting in slightly higher adsorption capacities than at pH 3. These findings indicated that the adsorption of OCHRA was dependent on the type of surfactant used for the modification of the kaolinite surface, as well as on the form of the molecule in solution. On the other hand, although it was expected, ZEN adsorption was less dependent on the type of surfactant and its form in solution.
In general, the adsorption behavior of the organokaolinites toward OCHRA and ZEN confirmed that HDTMA ions are directly involved in mycotoxin adsorption and that the adsorption mechanism is very complex. A comprehensive understanding of kaolinite’s mineralogical characteristics, its modification with surfactants, the properties of organokaolinites, and the physicochemical characteristics of mycotoxins was essential for identifying the key factors influencing the adsorption of OCHRA and ZEN.
3.3. Acute Oral Toxicity Study in Rats
Although the HDTMA-modified kaolinite showed a lower adsorption capacity for both mycotoxins, probably due to the absence of an aromatic functional group, an acute oral toxicity study supported its safety as a feed additive. After 24 h, the LD50 value of HKR-90 was 2400 mg/kg in male and female rats, respectively. Also, HKR-90 showed a 11.43% lower acute toxicity in comparison with unmodified kaolin (2100 mg/kg) (Table 4).

Table 4.
Effects of kaolin and HKR-90 on 24 h of survival in male and female rats.
In all treated animals, during the 7 days of observation, no significant changes in the general health could be seen. All rats were in good shape, and their hair, skin, visible mucosa and muscle tonicity were without any changes. Their movements and coordination were preserved. While LD50 data for ODMBA-modified kaolin are not included in this study, the structural features of the surfactant, particularly the presence of a benzyl group, suggest potentially increased toxicity compared with HDTMA. Therefore, despite the superior adsorption performance of the OKR series, the HKR series appears to be a safer candidate for potential use as a feed additive, balancing adsorption efficiency with its toxicological properties.
4. Conclusions
Organokaolinites were obtained by modifying raw kaolin with two levels of HDTMA (50% and 90% of kaolin CEC). The obtained materials (HKR-50 and HKR-90) were used for the in vitro adsorption of OCHRA and ZEN. The characterization of the organokaolinites revealed that HDTMA ions were predominantly located on the surface of the kaolinite, enhancing the hydrophobicity of materials with no alteration to its interlayer structure. The results showed that at lower amounts of surfactant (sample HKR-50), a local bilayer arrangement was present on the organokaolinite surface, while at higher amounts of HDTMA (sample HKR-90), micelle configurations existed at the mineral surface. Also, it was confirmed that the modification of kaolin with HDTMA ions led to a transition of the surface from hydrophilic to hydrophobic, forming a better adsorbent for mycotoxins. The adsorption of OCHRA and ZEN increased with increasing amounts of HDTMA on the kaolinite’s surface, showing that HDTMA ions were the active sites relevant for mycotoxin adsorption. The adsorption capacities of OCHRA and ZEN were similar for HKR-50 and HKR-90 at pH 3, with a higher adsorption observed for ZEN; at pH 7, the adsorption of OCHRA and ZEN was lower than at pH 3, especially for OCHRA, but slightly increased with increasing of the amount of HDTMA at the kaolinite surface. Nonlinear isotherm models fitted the data well for both mycotoxins at pH 3 and 7, indicating a complex adsorption mechanism that includes hydrophobic interactions between the hydrophobic parts of both mycotoxins and the hydrophobic organokaolinite surface, interactions between the positively charged “head” of HDTMA and anionic forms of OCHRA and ZEN. At the same time, repulsive interactions between the uncovered negatively charged kaolinite surface and the anionic form of OCHRA and ZEN may result in a decrease in adsorption capacities at pH 7, with this being more pronounced for OCHRA. A safety assessment of the raw kaolin and selected organokaolinite (HKR-90) was performed, and toxicological tests confirmed the safety of both materials, as no adverse effects were observed in rats. The obtained results highlight the potential of synthesized organokaolinites as efficient mycotoxin adsorbents for application in animal feed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min15070731/s1, Figure S1: SEM image of HKR-50 with 25,000 times magnification and scale bar 1 μm.
Author Contributions
Conceptualization, A.D.; methodology M.O. (Milica Ožegović), M.M. and D.S.; formal analysis, M.O. (Milica Ožegović), M.M. and D.S.; investigation, M.O. (Milica Ožegović), V.J., G.E.R. and M.O. (Milena Obradović); resources, M.M., D.S., G.E.R., V.J. and I.S.I.; writing—original draft preparation, M.O. (Milica Ožegović); writing—review and editing, A.D., G.E.R., V.J., L.I. and I.S.I.; visualization, M.O. (Milica Ožegović); supervision, A.D. and L.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (contract numbers: 451-03-136/2025-03/200023, 451-03-137/2025-03/200146 and 451-03-137/2025-03/200116). This study was partially supported by the Medical Faculty of the Military Medical Academy, University of Defence in Belgrade, Serbia (MFVMA01/23–25).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guerre, P. Worldwide Mycotoxins Exposure in Pig and Poultry Feed Formulations. Toxins 2016, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Animal Nutrition in a 360-Degree View and a Framework for Future R&D Work: Towards Sustainable Livestock Production. Anim. Prod. Sci. 2016, 56, 1561–1568. [Google Scholar] [CrossRef]
- Elliott, C.T.; Connolly, L.; Kolawole, O. Potential Adverse Effects on Animal Health and Performance Caused by the Addition of Mineral Adsorbents to Feeds to Reduce Mycotoxin Exposure. Mycotoxin Res. 2020, 36, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Colović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Ðuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef]
- Sanchis, V.; Magan, N. Environmental Conditions Affecting Mycotoxins. In Mycotoxins in Food Detection and Control; Woodhead Publishing: Cambridge, UK, 2004; pp. 174–189. [Google Scholar]
- Neme, K.; Mohammed, A. Mycotoxin Occurrence in Grains and the Role of Postharvest Management as a Mitigation Strategies. A Review. Food Control. 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. A Review of the Mycotoxin Adsorbing Agents, with an Emphasis on Their Multi-Binding Capacity, for Animal Feed Decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Influence of Temperature and Water Activity on Deleterious Fungi and Mycotoxin Production during Grain Storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Havenaar, R.; Visconti, A. Assessment of the Multi-Mycotoxin-Binding Efficacy of a Carbon/Aluminosilicate-Based Product in an in Vitro Gastrointestinal Model. J. Agric. Food Chem. 2007, 55, 4810–4819. [Google Scholar] [CrossRef]
- Srivastava, P.; Singh, B.; Angove, M. Competitive Adsorption Behavior of Heavy Metals on Kaolinite. J. Colloid Interface Sci. 2005, 290, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, M.C. Theoretical Study of Heavy Metal Cd, Cu, Hg, and Ni(II) Adsorption on the Kaolinite(0 0 1) Surface. Appl. Surf. Sci. 2014, 317, 718–723. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, P.; Liu, D.; Annabi-Bergaya, F.; Zhou, J.; Chen, F.; Liu, Z. Effects of Microstructure of Clay Minerals, Montmorillonite, Kaolinite and Halloysite, on Their Benzene Adsorption Behaviors. Appl. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Liu, D.; Ning, Y.; Yang, S.; Yang, Z. Adsorption of Benzene Vapor on Natural Silicate Clay Minerals under Different Moisture Contents and Binary Mineral Mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124072. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Alene, A.N. A Comparative Study of Acidic, Basic, and Reactive Dyes Adsorption from Aqueous Solution onto Kaolin Adsorbent: Effect of Operating Parameters, Isotherms, Kinetics, and Thermodynamics. Emerg. Contam. 2022, 8, 59–74. [Google Scholar] [CrossRef]
- Shaban, M.; Sayed, M.I.; Shahien, M.G.; Abukhadra, M.R.; Ahmed, Z.M. Adsorption Behavior of Inorganic- and Organic-Modified Kaolinite for Congo Red Dye from Water, Kinetic Modeling, and Equilibrium Studies. J. Sol-Gel Sci. Technol. 2018, 87, 427–441. [Google Scholar] [CrossRef]
- Obradović, M.; Daković, A.; Smiljanić, D.; Ožegović, M.; Marković, M.; Rottinghaus, G.E.; Krstić, J. Ibuprofen and Diclofenac Sodium Adsorption onto Functionalized Minerals: Equilibrium, Kinetic and Thermodynamic Studies. Microporous Mesoporous Mater. 2022, 335, 111795. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Li, J.; Du, H.; Li, Z. Efficient with Low-Cost Removal and Adsorption Mechanisms of Norfloxacin, Ciprofloxacin and Ofloxacin on Modified Thermal Kaolin: Experimental and Theoretical Studies. J. Hazard. Mater. 2022, 430, 128500. [Google Scholar] [CrossRef]
- Hounfodji, J.W.; Kanhounnon, W.G.; Kpotin, G.; Atohoun, G.S.; Lainé, J.; Foucaud, Y.; Badawi, M. Molecular Insights on the Adsorption of Some Pharmaceutical Residues from Wastewater on Kaolinite Surfaces. Chem. Eng. J. 2021, 407, 127176. [Google Scholar] [CrossRef]
- Kang, F.; Ge, Y.; Hu, X.; Goikavi, C.; Waigi, M.G.; Gao, Y.; Ling, W. Understanding the Sorption Mechanisms of Aflatoxin B1 to Kaolinite, Illite, and Smectite Clays via a Comparative Computational Study. J. Hazard. Mater. 2016, 320, 80–87. [Google Scholar] [CrossRef]
- Ogbebor, O.J.; Okieimen, F.E.; Ogbeifun, D.E.; Okwu, U.N. Organo-Modifikovani Kaolin Kao Punilac Prirodnog Kaučuka. Chem. Ind. Chem. Eng. Q. 2015, 21, 477–484. [Google Scholar] [CrossRef]
- Mi, F.; He, Z.; Fang, B.; Ning, F.; Jiang, G. Molecular Insights into the Effects of Surface Property and Pore Size of Non-Swelling Clay on Methane Hydrate Formation. Fuel 2022, 311, 122607. [Google Scholar] [CrossRef]
- Hu, P.; Yang, H. Insight into the Physicochemical Aspects of Kaolins with Different Morphologies. Appl. Clay Sci. 2013, 74, 58–65. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Chen, Z.; Megharaj, M.; Naidu, R. Kaolinite-Supported Nanoscale Zero-Valent Iron for Removal of Pb2+ from Aqueous Solution: Reactivity, Characterization and Mechanism. Water Res. 2011, 45, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Moya, J.S.; Cabal, B.; Lopez-Esteban, S.; Bartolomé, J.F.; Sanz, J. Significance of the Formation of Pentahedral Aluminum in the Reactivity of Calcined Kaolin/Metakaolin and Its Applications. Ceram. Int. 2024, 50, 1329–1340. [Google Scholar] [CrossRef]
- Bougeard, D.; Smirnov, K.S.; Geidel, E. Vibrational Spectra and Structure of Kaolinite: A Computer Simulation Study. J. Phys. Chem. B 2000, 104, 9210–9217. [Google Scholar] [CrossRef]
- Turer, D. Effect of Heavy Metal and Alkali Contamination on the Swelling Properties of Kaolinite. Environ. Geol. 2007, 52, 421–425. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, L.; Zhou, Y.; Wang, T.; Zhang, Y. Preparation and Characterization of Montmorillonite Intercalation Compounds with Quaternary Ammonium Surfactant: Adsorption Effect of Zearalenone. J. Nanomater. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Bish, D.L. Rietveld Refinement of the Kaolinite Structure at 1.5 K. Clays Clay Miner. 1993, 41, 738–744. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Zenasni, M.A.; Meroufel, B.; Merlin, A.; George, B. Adsorption of Congo Red from Aqueous Solution Using CTAB-Kaolin from Bechar Algeria. J. Surf. Eng. Mater. Adv. Technol. 2014, 04, 332–341. [Google Scholar] [CrossRef]
- Sharafee Shamsudin, M.; Taufik Mohd Din, A.; Sellaoui, L.; Badawi, M.; Bonilla-Petriciolet, A.; Ismail, S. Characterization, Evaluation, and Mechanism Analysis of the Functionalization of Kaolin with a Surfactant for the Removal of Diclofenac from Aqueous Solution. Chem. Eng. J. 2023, 465, 142833. [Google Scholar] [CrossRef]
- Spasojević, M.; Daković, A.; Rottinghaus, G.E.; Obradović, M.; Krajišnik, D.; Marković, M.; Krstić, J. Influence of Surface Coverage of Kaolin with Surfactant Ions on Adsorption of Ochratoxin A and Zearalenone. Appl. Clay Sci. 2021, 205, 106040. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Pillai, S.D.; Guan, H.; Bowman, R.; Couroux, E.; Hielscher, F.; Totten, J.; Espinosa, I.Y.; Kretzschmar, T. Surfactant-Modified Zeolite Can Protect Drinking Water Wells from Viruses and Bacteria. Eos 2002, 83, 193–201. [Google Scholar] [CrossRef]
- Rakić, V.; Rajić, N.; Daković, A.; Auroux, A. The Adsorption of Salicylic Acid, Acetylsalicylic Acid and Atenolol from Aqueous Solutions onto Natural Zeolites and Clays: Clinoptilolite, Bentonite and Kaolin. Microporous Mesoporous Mater. 2013, 166, 185–194. [Google Scholar] [CrossRef]
- Spasojević, M.P.; Daković, A.; Rottinghaus, G.E.; Radosavljević-Mihajlović, A.S.; Marković, M.A.; Krajišnik, D.R. Zearalenone and Ochratoxin A: Adsorption by Kaolin Modified with Surfactant. Metall. Mater. Eng. 2019, 25, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.J.; Hunter, D.B.; Bowman, R.S. Fourier Transform Raman Spectroscopy of Sorbed HDTMA and the Mechanism of Chromate Sorption to Surfactant-Modified Clinoptilolite. Environ. Sci. Technol. 1998, 32, 1948–1955. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: New York, NY, USA, 1999; ISBN 0125989202. [Google Scholar]
- Obradović, M.; Daković, A.; Smiljanić, D.; Marković, M.; Ožegović, M.; Krstić, J.; Vuković, N.; Milojević-Rakić, M. Bentonite Modified with Surfactants—Efficient Adsorbents for the Removal of Non-Steroidal Anti-Inflammatory Drugs. Processes 2024, 12, 96. [Google Scholar] [CrossRef]
- Jaćević, V.; Dumanović, J.; Lazarević, M.; Nepovimova, E.; Resanović, R.; Milovanović, Z.; Wu, Q.; Kuča, K. Antidotal Potency of the Novel, Structurally Different Adsorbents in Rats Acutely Intoxicated with the T-2 Toxin. Toxins 2020, 12, 643. [Google Scholar] [CrossRef]
- Jacevic, V.; Kuca, K.; Milovanovic, Z.; Bocarov-Stancic, A.; Rancic, I.; Bokonjic, D.; Dragojevic-Simic, V.; Segrt, Z. Gastroprotective Effects of Amifostine in Rats Treated by T-2 Toxin. Toxin Rev. 2018, 37, 123–127. [Google Scholar] [CrossRef]
- Litchfield, J.T.; Wilcoxon, F. A Simplified Method of Evaluating Dose-Effect Experiments. J. Pharmacol. Exp. Ther. 1948, 96, 99–113. [Google Scholar] [CrossRef]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Preparation, Characterization of Surfactants Modified Clay Minerals and Nitrate Adsorption. Appl. Clay Sci. 2010, 48, 92–96. [Google Scholar] [CrossRef]
- Aroke, U.O.; Hamidu, L.A.J. Instrumental Characterization of Unmodified and HDTMA-Br Modified Kaolinite Clay: SEM-EDX, Quantachrome and TGA-DTA. Path Sci. 2020, 6, 2001–2009. [Google Scholar] [CrossRef]
- Mitrović, A.A.; Komljenović, M.M.; Ilić, B.R. Research of Possibilities for Use Domestic Kaolin Clays for Production of Metakaolin. Hem. Ind. 2009, 63, 107–113. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S. Reexamining Calcination of Kaolinite for the Synthesis of Metakaolin Geopolymers—Roles of Dehydroxylation and Recrystallization. J. Non-Cryst. Solids 2017, 460, 74–80. [Google Scholar] [CrossRef]
- Parker, R.W.; Frost, R.L. The Application of Drift Spectroscopy to the Multicomponent Analysis of Organic Chemicals Adsorbed on Montmorillonite. Clays Clay Miner. 1996, 44, 32–40. [Google Scholar] [CrossRef]
- Marković, M.; Daković, A.; Rottinghaus, G.E.; Petković, A.; Kragović, M.; Krajišnik, D.; Milić, J. Ochratoxin A and Zearalenone Adsorption by the Natural Zeolite Treated with Benzalkonium Chloride. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 7–17. [Google Scholar] [CrossRef]
- Diehl, D.; Ellerbrock, R.H.; Schaumann, G.E. Influence of Drying Conditions on Wettability and DRIFT Spectroscopic C-H Band of Soil Samples. Eur. J. Soil Sci. 2009, 60, 557–566. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Zhou, A.; Nnachi, E.N.; Huo, S.; Zhang, Q. The Kaolinite Crystallinity and Influence Factors of Coal-measure Kaolinite Rock from Datong Coalfield, China. Minerals 2022, 12, 54. [Google Scholar] [CrossRef]
- Saikia, B.J.; Parthasarathy, G. Fourier Transform Infrared Spectroscopic Characterization of Kaolinite from Assam and Meghalaya, Northeastern India. J. Mod. Phys. 2010, 1, 206–210. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Irassar, E.F.; Scian, A.N. Thermal Treatment of Kaolin: Effect on the Pozzolanic Activity. Procedia Mater. Sci. 2012, 1, 343–350. [Google Scholar] [CrossRef]
- Wang, G.; Xi, Y.; Lian, C.; Sun, Z.; Zheng, S. Simultaneous Detoxification of Polar Aflatoxin B1 and Weak Polar Zearalenone from Simulated Gastrointestinal Tract by Zwitterionic Montmorillonites. J. Hazard. Mater. 2019, 364, 227–237. [Google Scholar] [CrossRef]
- Smiljanić, D.; Daković, A.; Obradović, M.; Ožegović, M.; Marković, M.; Rottinghaus, G.E.; de Gennaro, B. Influence of the Type and the Amount of Surfactant in Phillipsite on Adsorption of Diclofenac Sodium. Catalysts 2023, 13, 71. [Google Scholar] [CrossRef]
- Li, Z.; Gallus, L. Surface Configuration of Sorbed Hexadecyltrimethylammonium on Kaolinite as Indicated by Surfactant and Counterion Sorption, Cation Desorption, and FTIR. Colloids Surf. A Physicochem. Eng. Asp. 2005, 264, 61–67. [Google Scholar] [CrossRef]
- Kung, K.H.S.; Hayes, K.F. Fourier Transform Infrared Spectroscopic Study of the Adsorption of Cetyltrimethylammonium Bromide and Cetylpyridinium Chloride on Silica. Langmuir 1993, 9, 263–267. [Google Scholar] [CrossRef]
- Krajišnik, D.; Daković, A.; Milojević, M.; Malenović, A.; Kragović, M.; Bogdanović, D.B.; Dondur, V.; Milić, J. Properties of Diclofenac Sodium Sorption onto Natural Zeolite Modified with Cetylpyridinium Chloride. Colloids Surf. B Biointerfaces 2011, 83, 165–172. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. Chemical Tailoring of Porous Silica Xerogels: Local Structure by Vibrational Spectroscopy. Chem.-A Eur. J. 2004, 10, 392–398. [Google Scholar] [CrossRef]
- Pires, J.; Pinto, M.; Estella, J.; Echeverría, J.C. Characterization of the Hydrophobicity of Mesoporous Silicas and Clays with Silica Pillars by Water Adsorption and DRIFT. J. Colloid Interface Sci. 2008, 317, 206–213. [Google Scholar] [CrossRef]
- Smiljanić, D.; Daković, A.; Obradović, M.; Ožegović, M.; Izzo, F.; Germinario, C.; de Gennaro, B. Application of Surfactant Modified Natural Zeolites for the Removal of Salicylic Acid—A Contaminant of Emerging Concern. Materials 2021, 14, 7728. [Google Scholar] [CrossRef]
- Daković, A.; Kragović, M.; Rottinghaus, G.E.; Sekulić, Ž.; Milićević, S.; Milonjić, S.K.; Zarić, S. Influence of Natural Zeolitic Tuff and Organozeolites Surface Charge on Sorption of Ionizable Fumonisin B1. Colloids Surf. B Biointerfaces 2010, 76, 272–278. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Q.; Kou, J.; Sun, C.; Li, H. Aggregation Mechanism of Colloidal Kaolinite in Aqueous Solutions with Electrolyte and Surfactants. PLoS ONE 2020, 15, e0238350. [Google Scholar] [CrossRef]
- Tombácz, E.; Szekeres, M. Surface Charge Heterogeneity of Kaolinite in Aqueous Suspension in Comparison with Montmorillonite. Appl. Clay Sci. 2006, 34, 105–124. [Google Scholar] [CrossRef]
- Schroth, B.; Sposito, G. Surface Charge Properties of Kaolinite. Clays Clay Miner. 1997, 45, 85–91. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.W.; Ndungu, P. Performance Evaluation of Surfactant Modified Kaolin Clay in As(III) and As(V) Adsorption from Groundwater: Adsorption Kinetics, Isotherms and Thermodynamics. Heliyon 2019, 5, e02756. [Google Scholar] [CrossRef]
- Au, P.I.; Leong, Y.K. Rheological and Zeta Potential Behaviour of Kaolin and Bentonite Composite Slurries. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 530–541. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Smiljanić, D.; de Gennaro, B.; Izzo, F.; Langella, A.; Daković, A.; Germinario, C.; Rottinghaus, G.E.; Spasojević, M.; Mercurio, M. Removal of Emerging Contaminants from Water by Zeolite-Rich Composites: A First Approach Aiming at Diclofenac and Ketoprofen. Microporous Mesoporous Mater. 2020, 298, 110057. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and Multilayer Adsorption Isotherm Models for Sorption from Aqueous Media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Ignjatovic, L.; Tasic, A.; Sredovic-Ignjatovic, I.; Nastasovic, A. Investigation of Phenol Adsorption on Macroporous Polymeric Adsorbents. Zast. Mater. 2015, 56, 199–205. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Dhedan, S.K. Equilibrium Isotherms and Kinetics Modeling of Methylene Blue Adsorption on Agricultural Wastes-Based Activated Carbons. Fluid Phase Equilib. 2012, 317, 9–14. [Google Scholar] [CrossRef]
- Keren, Y.; Borisover, M.; Bukhanovsky, N. Sorption Interactions of Organic Compounds with Soils Affected by Agricultural Olive Mill Wastewater. Chemosphere 2015, 138, 462–468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).