Abstract
Lithium has emerged as a critical element in contemporary society. It has been classified as an indispensable feedstock in the manufacture of lithium-ion batteries for electric mobility, portable electronics, and stationary energy storage systems, which are essential for the integration of intermittent renewable energy sources. This metal also has other industrial applications and is projected to support future developments in semiconductor and aerospace technology. However, the exponential growth in global Li demand driven by energy transition and technological innovation requires a resilient and sustainable supply chain where both technological and environmental challenges should be addressed. This review discusses and analyzes some of current challenges associated with the Li supply chain given a particular emphasis on its separation methods. First, statistics of the Li market and its applications are provided, including the main sources from which to recover Li and the environmental impact associated with conventional Li extraction techniques from mineral ores and salar brines. Different separation methods (e.g., solvent extraction, adsorption, ion exchange, membrane technology) to recover Li from different sources are reviewed. Recent advances and developments in these separation strategies are described, including a brief analysis of their main limitations and capabilities. The importance and potential of recycling strategies for end-of-life batteries and industrial residues are also highlighted. A perspective on the gaps to be resolved with the aim of consolidating the Li supply chain to support the energy transition agenda is provided in this review.
1. Introduction
The global energy transition has increased the demand of raw materials and, particularly, clean energy technologies require metals such as copper, nickel and lithium (Li). Particularly, Li is the 25th most abundant metal on Earth and has important characteristics that make it a relevant feedstock for the clean energy transition [1,2]. Its concentration has been estimated at 0.005%–1.6% and its natural sources are brines of salt lakes, inorganic compounds in rocks, clays, geothermal brines and zeolites [2,3]. It is the first element from the alkali group of the periodic table with an atomic weight of 6.94 amu, and it is the lightest solid element at room temperature (0.534 g/m3), immediately after H and He [1]. Li is a soft metal due to its loosely bound valence electrons, which allow easy deformation without significant resistance. It tends to form hydroxides and exhibits chemical and physical properties that are characteristic of those of alkaline earth metals [1]. The most relevant properties of Li include its high thermal conductivity, calorific capacity, and liquid state over a wide temperature range resulting in very low density and viscosity [1,3]. Li ions are smaller than those of other alkali metal ions, generating a high charge density and reactivity especially with water, making it an excellent reducing agent [1,3]. Li ions can enhance the electrical and thermal properties of different materials, and consequently, can be applied in the energy sector [4]. Li metal exhibits the lowest electrical potential of any element (i.e., −3.04 V) [5]. It is a valuable material for the rechargeable batteries industry and is currently considered a critical element that has been called “new white gold” because of its high technological and economic relevance, but with limited availability from primary sources and critical demand for energy transition and electromobility [6].
Li can react with water, oxygen, and nitrogen contained in air under natural conditions; consequently, it does not exist in its pure state [1]. In nature, Li can be found as a mixture of stable Li7 and Li6 isotopes with abundances of 92.5 and 7.5%, respectively [7]. A variety of 124 lithium-based minerals have been identified including lithiophosphate, lithiophilite, amblygonite, lepidolite, petalite, triphylite, elbaite, and spodumene [8]. Table 1 summarizes the most common Li-containing minerals. This metal can be extracted from lepidolite and spodumene, which are the Li primary ore sources. However, it is convenient to indicate that an economical typical ore grade for Li has a concentration higher than 1% (by mass). The crustal abundance of Li is estimated to be 17–35 mg/kg [1]. It can also be found at different concentrations in other natural systems such as seawater, brine, marine sediments, and groundwater, see Figure 1. However, Li is usually diluted in nature; therefore, it must be enriched via chemical and physical routes [Balaram, Oliviera]. Various studies have indicated that economically viable Li concentrations range from <100 mg/kg in granites to 9000 mg/kg in pegmatites [9].

Table 1.
Main minerals that contain lithium.
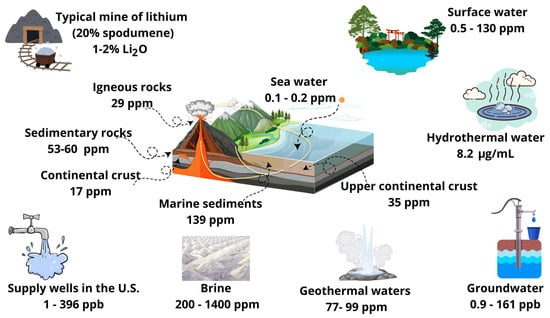
Figure 1.
Lithium concentrations reported for several geological and nature systems. This figure was prepared using data reported in [1].
Li production has increased significantly and statistics from the World Economic Forum indicate that it surpassed 100,000 tonnes for the first time in 2021, quadrupling from 2010, and its global demand is expected to reach 1.5 million tonnes of lithium carbonate equivalent by 2025 and over 3 million tonnes by 2030 [14]. Li demand is expected to increase at least 40 times by 2040 [15], and Li-ion batteries, particularly those used in electric vehicle manufacturing, will constitute over 85% of the market by 2025 [16]. Considering the panorama on current and short-term lithium needs as well as the basic requirements for its extraction from various sources, it is important to mention that Li resources worldwide are estimated to range 40–98 million tons [6,17], where approximately 70% relies on the salar deposits from South America in the so-called “Lithium Triangle” involving areas of Chile, Bolivia and Argentina [15]. It has been documented that the main global Li production accounts for mineral tailing and brine operations in China, Chile, Argentina, Brazil and Australia. Australian Li production is based on ore mining extraction, while Chilean and Argentinian production is based on brine evaporation processes. China instead has a production contribution based on the extraction of Li or mining and brines. Other countries such as Zimbabwe, United States, Portugal and Canada also contribute to Li production operations but to a minor extent [2], while the exploration and recovery of Li from brine are being developed in the United States, China and Bolivia. Other mineral-based Li projects are progressing in several countries from America (United States, Peru, Canada, Brazil), Europe (Spain, Portugal, Germany, Finland, Austria), Asia (China, Thailand, Kazakhstan, Serbia), and Africa (Zimbabwe, Ethiopia, Democratic Republic of Congo, Ghana, Nigeria, Mali, Namibia) [2].
The increases in both Li demand and price have resulted in the implementation of national strategies to address the current supply chain challenges. For instance, the U.S. Department of Energy has established an internal agenda to increase the U.S. Li reserves by funding $1.6 billion for projects aimed at supporting new commercial-scale domestic facilities for Li extraction and processing, battery recycling, manufacturing of battery components, and developing new technologies [2]. The current critical goal of technology companies in Asia, Europe, and North America is to ensure a reliable Li supply chain. Consequently, several corporations have formed strategic partnerships to consolidate and diversify Li sources to support the battery production and electromobility industries.
Given the significant growth demand for Li-ion batteries for electrical vehicles expected in the following decades, there is concern about the resilience capacity of the supply chain. There are still challenges in terms of developing new technologies to improve Li recovery and separation, minimize environmental impact, and reduce associated risks to human health and ecosystems derived from Li extraction and technological implementation, as well as other minor but relevant technical and economic issues linked to its industrial application and recycling of Li-containing products. Therefore, the objective of this review is to discuss and analyze key aspects of some of these challenges. A special emphasis is given to technologies applied for Li separation and recovery. This review provides a perspective on the gaps to be resolved with the aim of consolidating the Li supply chain to support the energy transition agenda.
2. Li Market and Its Main Applications
The inherent properties of Li and the discovery of a wide variety of technological uses have driven the exploitation of this metal since the last century [18]. The National Minerals Information Center of the U.S. government has estimated the distribution of global Li end-use market, see Figure 2 [2]. Although 80% of the current Li production is used in the battery industry, this metal has a wide spectrum of applications in the aerospace, nuclear, pharmaceutical, chemical, steel and agricultural sectors [19], see Table 2. A summary of selected Li-based compounds and their utilization is provided below.
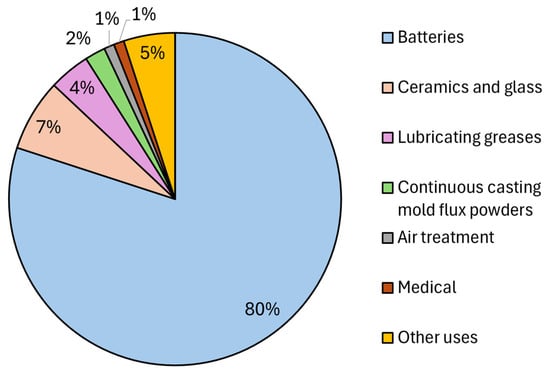
Figure 2.
Estimated distribution of lithium applications in different sectors. This figure was prepared using data reported in [2].
The consumption of Li-ion batteries has grown significantly for electric vehicles, mobile phones, tablets, computers, and as a component of power supply systems integrated with photovoltaic and wind energy to provide storage capacity [20,21]. In the Li-ion batteries, Li ion plays a key role as it moves in the organic electrolyte from cathode to anode during charging, and from the anode to cathode during discharging. The Li-ion battery consumption trend is mainly due to technological advancements and the increasing need for sustainable energy production and storage solutions [22,23]. Li-ion batteries currently represent the main source of rechargeable energy in various applications due to their high energy density (250 Wh/kg), cycling performance, and absence of memory effects [24]. Li anode battery has a theoretical capacity of 3860 mAh/g, which significantly outperforms its graphite counterpart (372 mAh/g) [24]. After 1990, Li-ion batteries have received significant attention not only because of their high ionic conductivity and energy density but also because of their high chemical stability [23]. Advanced cathode materials containing Li have also been explored as electrodes, demonstrating their potential in next-generation battery technologies [24]. It is convenient to highlight that the current technological applications have also driven the rapid deployment of Li-ion battery recycling plants. Available statistics from November 2022 indicated that more than 90 companies located in Europe, United States and Canada planned to recycle Li batteries [25]. In addition, there are initiatives of strategic cooperation between recyclers and automobile industries to supply battery materials [2].

Table 2.
Applications of several lithium compounds.
Table 2.
Applications of several lithium compounds.
| Lithium Compound | Chemical Structure | Applications | Reference |
|---|---|---|---|
| Lithium ferrite | LiFe5O8 | Electronics and medicine, the ferrofluid industry, gas sensors, contrast agents in magnetic resonance imaging, drug delivery, and rechargeable Li-ion batteries, high density data storage, catalysts, information storage systems, magnetic bulk cores, microwave absorbers, and medical diagnostics and therapy. | [26] |
| Lithium bromide | LiBr | Greases, lubricants, synthetic rubbers. | [13] |
| Lithium carbonate | Li2CO3 | Pharmaceuticals, control of thermal expansion in ceramics. | [13,27] |
| Lithium chloride | LiCl | Moisture absorber in air conditioning systems and batteries, and in the production of lightweight alloys. | [27] |
| Lithium hydroxide | LiOH | Alkaline storage batteries, manufacture of lithium soaps, and additive in industrial batteries. | [27] |
| Lithium cobaltate | LiCoO2 | Storage batteries | [24] |
| Lithium ferrophosphate | LiFePO4 | Storage batteries | [24] |
| Lithium titanate | LiTiO3 | Source of tritium in the nuclear fusion reactors. | [10] |
| Lithium zirconate | Li2ZrO3 | Source of tritium in the nuclear fusion reactors. | [10] |
| Lithium hydride | LiH | Storage of hydrogen fuel. | [28] |
Li has been widely used in the production of glass and ceramics [29,30], and lithium carbonate is the main feedstock to produce porcelain glazes. This industry was the primary Li consumer before the expansion of the battery sector [31]. Various ceramic and glass materials containing Li can be utilized in dental applications and energy storage technologies [24,32]. Li incorporation into glass ceramics enhances their mechanical properties, biocompatibility, and functionality, generating versatile materials for both restorative dentistry and battery production. For instance, glass-ceramics based on zirconia-reinforced lithium silicate and disilicate can be applied in dental restorations [32]. However, the long-term stability and potential cytotoxicity of novel Li-based materials require further investigation [33].
High-performance parts and structures can be manufactured from alloys containing Li and other metals such as manganese, copper, cadmium, and aluminum [34,35]. Particularly, aluminum-lithium (Al-Li) alloys are an interesting feedstock for the structural production of aircraft and high-speed trains because of their superior corrosion resistance, mechanical properties, and weight-reduction capabilities [34,35,36]. These alloys have also been considered essential for modern aerospace and aeronautic applications to enhance the mechanical performance and fuel efficiency of aircraft [35,37]. Despite these benefits, challenges remain particularly regarding the production cost of Al-Li alloys and their performance characterization under extreme conditions. Hydrogen storage is another potential application of Li-based compounds [28]. LiH has a promising potential for H2 adsorption because of its volumetric capacity (4.95 kg/100 L) and gravimetric density [28]. However, the drawbacks associated with high desorption temperatures (600–700 K) and material stability must be addressed to achieve large-scale industrial applications [28,38]. Other Li-based products include chemicals utilized to enhance cement properties [39], additives for the aluminum industry [37], and catalysts for polymer production [40,41].
Nuclear reactions can be carried out using high-purity Li7 and Li6 isotopes [42]. In this application, Li7 functions as a molten salt coolant for high-temperature reactors because of its heat transfer and thermodynamic properties, providing a low-shock section for neutrons [10,42]. This isotope is also a pH controller for refrigerant materials that can be applied in pressurized water nuclear reactors [10,43], while Li6 acts as a radiation shield to control nuclear reactors without emitting gamma rays [10]. Other compounds, such as Li2O, LiAlO2, LiTiO3, Li2ZrO3, and Li4SiO4, have been tested in fusion reactors [10,42,43].
Lithium hydroxide is the main Li-based compound used in catalysis, lubrication and high-voltage lithium-ion batteries [44,45]. Lithium stearate has been widely utilized as a lubricant grease thickener and high-temperature lubricant in the machinery industry [46,47]. Li-based lubricating greases are applied in automotive, railway, and aerospace sectors. Compounds containing Li can also be used in brass and autogenous welding and as additives to enhance the performance and lifetime of alkaline storage batteries [48,49].
Drying, dehumidifier and air-conditioning industries utilize concentrated brine containing lithium chloride and bromine because of its humidity absorption properties over a wide temperature range [50,51].
Li is an essential feedstock in medicine sector for producing drugs to treat bipolar disorder [52]. Lithium carbonate salt is an effective mood stabilizer, as well as an antidepressant. Sufficient scientific evidence has been published to support that Li can help regulate bone metabolism and reduce fracture risk [53], while lithium carbonate, chloride salts and their mixtures can also act as anticancer agents [54]. Li can also be utilized to manufacture protective glass shields for attenuating the gamma photons released during the radiation generated in cardiology, radiotherapy, tomography, and nuclear medical treatments [55]. For instance, thermoluminescent monitoring aids can be obtained using lithium fluoride [56] aluminum-doped lithium triborate [57], and lithium tetraborate [58].
3. Li Extraction, Environmental Impact and Toxicology
Li supply chain generates environmental pollution that may represent a threat to ecosystems and society if proper regulations and sustainable processes are not incorporated for its industrial exploitation. Therefore, it is important to discuss briefly the environmental impact generated by the main sources for Li extraction and the toxicological profile of this element.
3.1. Li Extraction and Its Environmental Impact
Li extraction is usually performed via two primary methods: ore mining from hard rocks that contributes approximately 60%, and brine (i.e., salt lake) extraction accounting for ~40% [17,59]. Hard rock mining usually ends in Li(OH), while brine extraction and processing usually end in lithium carbonate (Li2CO3).
Ore extraction involves mining hard rock sources (e.g., lepidolite, petalite, and spodumene) to concentrate Li. This recovery route is energy intensive (from 5 to 15 GJ/ton of Li2CO3) and requires both mining and processing to achieve economically viable product yields. The brine extraction is performed in saline waters with high concentrations of dissolved Li salts (e.g., lithium chloride) [60,61] and in geothermal water [62], thus avoiding ore mining. The final product is achieved by concentration via natural evaporation processes and purification process. It has been estimated that this route consumes 400–2000 L of freshwater per kg of Li2CO3 and is also energy intensive. This approach is common in arid regions (e.g., Li triangle in South America) and involves pumping brine into a series of shallow open-air evaporation and precipitation ponds to increase Li concentration over time leading to a concentrated brine [61,63]. This process begins by pumping hypersaline water into evaporation pools (through the action of sun and wind), which are characterized by being shallow and covering large surface areas, varying from several square kilometers to hectares [22,64]. The final product can contain other elements (e.g., boron, calcium, potassium, and manganese), depending on the hydrogeological source [61]. Concentrated brine is sent to a purification process involving boron solvent extraction, precipitation, ionic column exchange and carbonation. In the process, several chemicals are consumed to remove impurities. Calcium hydroxide and sodium carbonate are the most important reactants used along the process. Note that the production process requires intensive use of water, which has generated concern due to the significant hydrological alteration.
Overall, Li extraction routes have different trade-offs in terms of cost, energy consumption, environmental impact, and resource sustainability. Different studies have indicated that Li brine extraction is often more cost-effective and energy-efficient than ore mining. However, both Li extraction routes inevitably generate solid waste, pollutants and intensive water consumption that can harm ecosystems, degrade both soil and water quality, and pose risks to human health [65].
The ore extraction enables year-round Li production regardless of climate conditions, but generates tailings, dust, land disturbance and environmental impact [12,59]. It affects air quality via dust production due to rock crushing and grinding, which causes wind-based particle dispersion [66]. The leaching of mining ore, a step required for Li extraction from hard rock, can also generate soil and water pollution [67]. Also, mining activities and concentration by flotation require large amounts of water that can strain the local water resources and impact aquatic ecosystems. The management of tailings and residual materials generated during ore processing is challenging and paramount to mitigate the environmental pollution [12,62,68,69,70]. For illustration, Figure 3 shows a standard flowsheet for a Li hard-rock extraction process where the main products (including residues) and energy inputs are indicated.
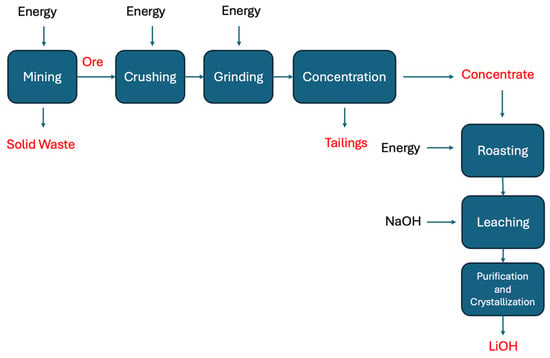
Figure 3.
Lithium extraction flowsheet process diagram of a standard hard-rock ore.
Few studies have provided quantitative data on the presence of Li in water reservoirs or soil affected by mining [71]. Li extraction from brines depends on local water resources and climate conditions, which can lead to depletion and local biodiversity disruption in arid environments, as well as affecting agriculture, soil and water availability, in addition to polluting local communities [12,15,61,72]. Yang et al. [73] analyzed the impact of Li mining from lepidolite on the pollution of the Jinjiang River in southeastern China. These authors found Li concentrations of up to 103.9 µg/L that suggested substantial Li discharge into the river via inadequately treated wastewater from the industrial zone [73].
Some studies have suggested that Li reserves in salt lakes are more abundant and can be processed with a lower environmental impact and reduced cost by 30%–50% compared to ore mining [59]. Brine extraction is often viewed as a more viable approach for consolidating Li supply [1,74]. Kelly et al. [75] reported the results of a Life Cycle Assessment (LCA) to produce battery-grade LiOH·H2O and Li2CO3 from spodumene ores in Australia and brine from the salar in Atacama, Chile. This LCA covered Li salt production and the corresponding end-products (i.e., full batteries and battery cathode materials). The calculations indicated that the freshwater consumption per kg of salt and life cycle green-house gases emissions to produce LiOH·H2O (6.9–7.3 kg CO2eq/kg) and Li2CO3 (2.7–3.1 kg CO2eq/kg) from brine-based resources were lower than those obtained for the ore-based counterpart (16–20 kg CO2eq/kg) [68]. Nikfar et al. [76] performed LCA with TRACI method to compare Li recovery from brine for producing Li2CO3 via membrane electrolysis, nanofiltration, adsorption, and solvent extraction. Calculated CO2 emissions per kg of Li2CO3 were: nanofiltration (17.7 kg CO2eq/kg) < adsorption (47.9 CO2eq/kg) < solvent extraction (52.7 kg CO2eq/kg) < membrane electrolysis (80.57 kg CO2eq/kg). These results indicate that nanofiltration is a promising alternative for mitigating the environmental impact caused by Li extraction. However, these and other environmental studies have concluded that it is paramount to develop more sustainable processes for Li recovery and processing [76]. It is convenient to note that mineral waste obtained from Li extraction activities can capture and allow the storing of CO2 from the atmosphere (via carbonation reactions related to calcium concentration), but the residues need to go to disposal alternatives that generate lower environmental impact. For instance, calcium carbonate is a solid waste resulting from the industrial procedures of Li extraction in brines and needs to be carried out to the disposal ponds.
Another important environmental challenge is the natural mobility of Li. This metal can be released into the environment via natural processes that have been identified as geogenic sources [77]. Li release from natural deposits (e.g., rocks, minerals, geothermal water, and salt flats) occurs because of the natural processes and cycles that occur on Earth [78]. One of them is weathering, in which rocks and minerals found on the Earth’s surface decompose and transform into smaller and less-structured materials over time [67]. These materials can easily be transported to new locations by natural agents (e.g., water and wind) [79]. The weathering and disintegration of rocks primarily occur due to erosion caused by the sand and gravel movement by wind or water currents [80]. Toupal et al. [67] reported high Li concentrations (>20 µg/L) in water samples near the Cínovec reservoir from Europe in comparison with the average concentration found in surface waters worldwide. They concluded that the potential cause of this result was the erosion of mica deposits due to the high Li content in the rocks and their proximity to the surface [67]. Another relevant geogenic source of Li pollution corresponds to volcanic eruptions where both volcanic rocks and ash that are dispersed can contaminate soil and water [80,81,82,83]. Note that it has been estimated that the natural concentration of Li in surface waters is <0.04 mg/L, and 0.05–1.0 mg/L in mineral water [84], depending on the climate and lithology. However, environmental Li concentrations can increase due to the exacerbated mobilization caused by anthropogenic activities that promote water system pollution, making it necessary to identify, quantify and evaluate the risks to aquatic ecosystems and public health [78,85].
Additional anthropogenic pollution sources occur at various stages of Li life cycle, including metal processing after its extraction, the manufacturing of Li-based materials, their use and final disposal [86]. The scientific community has highlighted the importance of Li pollution generated from the application, use, and disposal of Li-containing products [87]. The management and final disposal of residues containing Li are often not conducted responsibly, possibly because of inadequate (if any) regulations or a lack of awareness of proper protocols [87]. This issue is more relevant when the general population has easy access to a wide spectrum of Li-based commercial products, such as batteries, medications, and ceramics [88]. These items are commonly disposed of in regular trash, which ends up in municipal landfills causing the potential metal leaching into the soil [89,90], or they are disposed of in the drain (for example, medications) when they expire, thus generating water pollution [91,92].
3.2. Li Toxicological Profile
Li toxicology is important because its use and valuable applications generate direct and indirect environmental pollution with potential negative effects on human health and ecosystems [84,85,93]. This metal has been categorized as the fifth unregulated pollutant in 2021 by the U.S. Environmental Protection Agency [94]. The U.S. Environmental Protection Agency (EPA) has established a Health Reference Level (HRL) of 10 μg/L for Li in drinking water [95], while the World Health Organization (WHO) has not published a permissible limit for Li concentrations. However, there is a lack of information on the safety limits of Li in drinking water based on the daily human intake. A recent study used the Health Based Screening Level (HBSL) of 10 μg/L and the threshold of 60 μg/L to compare the groundwater Li concentrations from several public and domestic water supplies in the USA. This study was based on the EPA provisional reference dose of 0.002 mg/kg per day, considering that drinking water was the unique source of Li intake [82]. The authors observed that a significant proportion of public and domestic supplies exceeded these thresholds.
Recent studies have addressed the toxicological effects of short- and long-term Li exposure on different organisms [94,96]. Li meets the criteria for hormonal disrupters, affecting thyroid, hepatic, and nervous functions [27]. A meta-analysis showed that Li exerts toxicological effects on the kidneys (hyposthenuria), thyroid (hypothyroidism), and parathyroid (hyperparathyroidism) glands; however, Li-linked teratogenesis remains unclear [97]. The molecular mechanisms of toxicity in organs and glands are not completely understood yet. They seem to be related to a progressive dose-dependent disfunction associated with an alteration of the cellular redox homeostasis, DNA damage, dysregulation of cell cycles and apoptosis [98]. Further studies are necessary to elucidate the underlying mechanisms of the pathogenic effects of short- and long-term Li exposure, as well as those conditions leading to Li-toxicity predisposition [98]. Toxicokinetic experiments have demonstrated that soluble Li is instantly absorbed by the gastrointestinal tract, by the lungs via inhalation, and it can cross the placenta and reach breast milk [27]. Some studies have also suggested that toxic effects of Li consumption can occur at very close levels of therapeutic doses (1.2–2.4 mg/Kg b.w. per day) [99].
Table 3 summarizes the lethal concentration 50% (LC50) reviewed and reported for some aquatic organisms. It has been shown that Li exposure in rainbow trout (Oncorhynchus mykiss) reaches higher levels in the brain and plasma after 96 h of exposure to a nominal concentration of 1 mg/L. This result was accompanied by increased levels of arachidonic acid, altered ion composition, and dysfunction of ion transporters, lipoproteins, and prostaglandin synthesis [100]. Li-induced toxicity in Mytilus galloprovincialis after 28 days, using a concentration range of 100–750 μg/L, has been related to a redox system imbalance (oxidative stress), dysfunction of biotransforming enzymes, reduced glutathione depletion, neurotoxicity and decreased acetylcholinesterase activity [94]. It has been suggested that these toxicological effects may be potentialized by other factors. Consequently, further studies are required for determining the toxicokinetic and toxicodynamic parameters of Li exposure in other living organisms for elucidating the toxicological mechanisms of action, and to develop appropriate therapies to counteract or antagonize these effects.

Table 3.
Lethal concentration (LC50) of lithium reported for different aquatic species.
4. Methods for Li Separation
The battery industry and other relevant technological applications demand salts with more than 99% purity and residual amounts of impurities. Consequently, purification technologies are required to satisfy these industrial requirements. Figure 4 shows the different methods for Li separation from various sources such as brine, wastewater, ore, waste and residual products. Li purification can be performed using several technologies including chemical precipitation [106,107], ion exchange [108], adsorption [109], membrane technology [110], and solvent extraction [111].
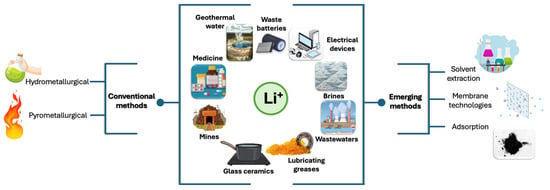
Figure 4.
Conventional and novel emerging methods to separate lithium.
Chemical precipitation is widely used to separate Li from the fluids generated during mining activities, waste recycling processes, and wastewater treatment [112,113]. In this process, a specific agent is added to the fluid containing ions other than Li to generate an insoluble compound [114]. This separation technique is characterized by its efficacy, minimal requirement for expensive or sophisticated equipment, and low energy consumption [115]. However, the pretreatment of Li sources can be required in some cases before applying this method [116]. This pretreatment may include other processes (e.g., leaching or evaporation) to ensure that the metal is dissolved and/or concentrated in the solution, allowing precipitation to occur [117,118]. Leaching is applied when Li is present in solid matrices such as batteries or ores [119]. Chemical precipitation can be alkaline or acidic, depending on the reagent used, and it can also be performed with the assistance of bacterial or fungal cultures [120,121].
Membrane technology has been established as an effective and versatile separation method for the selective recovery of ions in various industrial and environmental scenarios [122]. Membranes act as barriers that allow for the controlled transport of certain ions and restrict the passage of other ions, facilitating their separation [123]. This approach is useful for desalination, wastewater treatment, mining, and recovery of valuable metals [122]. Membrane-based processes have been suggested as sustainable and competitive alternatives to conventional methods, such as chemical precipitation or solvent extraction, because of their high efficacy and adaptability to different operating conditions [124,125,126]. They have been diversified into a wide range of configurations designed to efficiently separate and recover specific compounds [127,128]. The most prominent operations include osmosis (reverse or direct), nanofiltration, dialysis (diffusion or cross-ion), electrodialysis, ion-imprinted membrane, supported liquid membrane, and membrane distillation [129,130]. The main advantage of membranes is their high selectivity, which allows separation or blockage of the passage of a specific ion [131]. This is particularly useful for solutions that contain several ions with similar chemical characteristics. For example, Li+ and Mg2+ are difficult to separate efficiently using conventional methods such as solvent extraction [132] and, consequently, membrane-based processes can be an effective alternative.
Adsorption is a separation method to recover various components present in fluids [121]. It is useful for Li extraction industry because of its selectivity, efficiency, and moderate-to-low environmental impact compared to other purification methods, making it a cost-effective alternative [76,133,134,135]. This technique allows the exploitation of sources with low Li concentrations, thereby expanding its feasibility for diverse applications [121]. Adsorption relies on the use of organic and inorganic materials (usually porous) that can selectively retain Li ions from fluids (e.g., wastewater or brine) [136]. After the saturation of the adsorbent surface with the target metal, desorption can be performed to regenerate the material and recuperate the adsorbate [137]. Adsorbents for Li separation include Li-ion sieves [138,139], polymers [140], metal–organic frameworks (MOFs) [141], functionalized nanotubes [142], porous carbon-based materials [143], composites [144], and ion-exchange resins [145,146].
Solvent extraction can be of importance for recovering Li from various sources, including brines, clay minerals, and even spent Li-ion batteries. Solvent extraction separates Li from fluids using a combination of solvents [132]. This technique is based on the selective separation of Li ions from the aqueous phase (brine or leachates) with an organic phase, usually composed of an extractant and a diluent. The organic phase selectively separates Li from the rest of the ions in the brine, particularly Mg and Ca. After mixing, the organic phase settles over the brine from where it is separated from this matrix mechanically. The loaded Li in the organic phase requires at least one stripping or discharging step where Li ion exchanged for protons provided by an acid, usually HCl giving the chlorine nature of the brine. In some systems, an additional solvent is added that serves as a co-extractant [147]. The extractant aids in the formation of complexes that are insoluble in the aqueous phase but highly soluble in the organic phase [148]. The co-extracting agent increases the extraction efficacy by collaborating with the extracted complex compound due to its solvation with it via the extracted metal, while the diluent stabilizes the organic phase and improves mass transfer [149,150]. The main advantages of this separation route are its productivity, easily scalable and reduced energy consumption [151].
Electrochemical methods are emerging alternatives for Li separation that are characterized by high efficiency, low energy consumption, and selectivity in extracting Li from various sources including brines, spent Li-ion batteries, and wastewater [152]. These methods rely on the principles of electrolysis, where electric currents drive chemical reactions to separate Li ions from other substances. Electrochemical processes (e.g., aqueous electrolysis, electrochemical ion exchange, electrochemical ion pumping or capacitive deionization) can achieve the effective separation of Li from other ions, even in complex mixtures [130,152,153]. It is important to note that in some cases, electrochemical technologies are used in conjunction with other methods described here to optimize the recovery process, which can further improve the overall separation efficacy and economic benefits [153]. In this review, only the electrodialysis in membranes method will be addressed. The discussion of other electrochemical methods is out of the scope of this manuscript but interested readers are referred to other reviews covering this topic [152,153].
The selection of the appropriate separation method depends on several factors, such as economic metrics, environmental impact, and recovery efficacy. However, the concentration of Li in the raw source is fundamental to determine the most suitable separation technology [154]. For instance, chemical precipitation is only viable for Li concentrations above 1300 mg Li/L [154]. Novel organic solvents offer advantages for Li extraction from dilute sources, such as sea bittern (e.g., ≤10 mg/L), particularly for selective separation [154]. Other methods, such as evaporation or conventional solvent extraction, exhibit low separation efficacy with seawater-based sources (0.17–2 mg/L), whereas electrochemical, membrane, and adsorption methods effectively overcome the challenges associated with low Li concentrations and enable processing Li-containing materials in a wide range of concentrations [143,155]. A combination of technologies (e.g., electrosorption, electrodialysis, reaction-coupled separation) can address the challenges associated with a wide range of initial Li concentrations depending on the raw material conditions [156]. For example, hybrid reverse osmosis combined with electrodialysis has been studied for Li recovery from industrial wastewater containing 7800 mg/L of LiCl [122], while electrodialysis achieved a recovery of 85% from initial concentration of 500 mg/L of LiCl solution [157].
An overview of recent advances and developments for chemical precipitation, adsorption, membranes and solvent extraction technologies is provided in the following subsections.
4.1. Chemical Precipitation
Chemical precipitation offers several operational advantages, including simplicity, scalability, and relatively low processing costs. However, the process effectiveness depends on various factors such as pH, temperature, concentration of competing ions, and the nature of the precipitating agent used. Several studies have focused on optimizing precipitation conditions to improve Li separation efficiency. A selection of key manuscripts that explore different aspects of chemical precipitation techniques for Li recovery are reviewed in this section highlighting the advancements, challenges, and practical applications of this approach.
Table 4 provides a survey of studies on Li recovery using chemical precipitation. Biswal et al. [158] compared acid leaching using inorganic acids (i.e., nitric and sulfuric acids) with bioleaching based on fungi Aspergillus niger MM1 and SG1 to treat a complex mixture of dust extracted from spent Li-based batteries. They determined that Li concentration obtained with the fungus (22.7–24.2 mg/L) was even higher than that obtained with nitric acid (20.7 mg/L), which resulted in greater availability of Li for precipitation with Na2CO3, ultimately recovering Li up to 73.6% in the carbonate form. Shi et al. [159] also recovered Li from spent battery powder using Na2CO3, which was obtained from nickel-manganese-cobalt (NMC) lithium-ion batteries. The metal was leached using hydrogen peroxide and sulfuric acid, and Li was precipitated at 95 °C for 1.5 h, recovering up to 99.85%. CO2 is another precipitating agent that can be used to obtain lithium carbonate. Velázquez et al. [160] and Tawonezvi et al. [161] used this gas to precipitate Li from the leachate of spent battery powder and successfully recovered 80.4 and 91% Li, respectively. Note that this metal can also be precipitated using Na3PO4 or H3PO4. Mahandra and Ghahreman [119] reported the leaching of Li from spent battery powder with H2O2 and acetic acid solution achieving a high Li selectivity of 94.1% with only 6% of impurity elements (i.e., Al, Fe); then, Li recovery with Na3PO4 in a double precipitation process, first at 60 °C and then at 90 °C, reached 99.5% as Li2CO3, in a process with reduced acid consumption. Chen et al. [162] applied H3PO4 to precipitate lithium phosphate from a leachate of battery powder. The separation process reported in this study involved a single precipitation step at 25 °C, achieving a metal recovery of up to 92.7%.

Table 4.
Recent studies on lithium recovery by chemical precipitation.
The temperature is an operating parameter that significantly influences Li separation via chemical precipitation, as higher temperatures result in higher recovery yields. Han et al. [167] demonstrated that the separation yield using CO2 ranged from 41.6 to 45.5% at 50 °C, which significantly outperformed the 22.6–25.6% yield obtained at 25 °C. Battaglia et al. [170] also observed the temperature effect by comparing Li recovery yields via the carbonate form from a dilute LiCl solution at 50 and 80 °C. They determined that the separation yield was 55% at 50 °C, which increased to 77% at 80 °C. The increase in temperature enhances the solubility of compounds, facilitating their interaction, and thus favoring their precipitation [168]. However, it should be noted that this effect also depends on other important factors such as stirring speed, precipitating agent and its concentration [167,168]. The temperature effect is particularly noticeable for certain chemicals, such as sodium carbonate, which require relatively high temperatures because of their low solubility to achieve competitive results, in contrast to CO2, which can be used effectively at low temperatures [171].
The agitation speed also plays an important role in Li separation performance, particularly affecting the size of precipitated particles. As the agitation speed increased, the particle size tended to decrease, likely due to more frequent particle collisions. Gu et al. [173] studied this effect on Li precipitation using sodium carbonate and leachate from spent batteries. The results indicated that increasing the agitation speed from 200 to 800 rpm reduced the particle size from 169 to 116 μm. pH also significantly affects Li precipitation. If the pH is not optimal, Li solubility in the solution may be too high, preventing the formation of the desired precipitate or favoring the formation of unwanted compounds from the precipitating agents. Carbonic acid can be formed under acidic conditions or phosphate compounds may undergo hydrolysis [177].
The composition of Li source is fundamental to achieving a successful separation. Salt lake brine, seawater or Li-batteries are mixed systems that commonly contain a variety of coexisting ions like Na, K, Ca, Mg, Co, Ni, and Mn that affect the separation process [178,179]. Li (radius ≈ 0.076 nm) has similar ionic properties to Mg (radius ≈ 0.072 nm) and, since their ionic size is nearly identical [180], their separation is difficult. The presence of co-ions can significantly affect the solubility and precipitation kinetics of Li salts and can lead to the formation of unwanted by-products or precipitates, thereby reducing the purity and yield of Li products [179,181]. Gu et al. [173] observed that Ni and Co ions were incorporated into the crystal lattice of Li2CO3, affecting the growth and morphology of lithium carbonate. Ca ions can precipitate as calcium carbonate, which competes with Li for precipitation and decreases the efficiency of Li recovery [182]. Magnesium ions may cause the formation of magnesium hydroxide, which can co-precipitate with Li compounds, complicating the separation process [182]. In terms of the application of strategies to control the presence of these competing ions is important to improve the process performance, Quintero et al. [182] removed Ca impurities from industrial Li-rich brines using oxalic acid as precipitating agent, reducing Ca from 300 to 30 mg/L. A high purity Li2CO3 with controlled concentrations of Mg (i.e., 0.05% as Mg(OH)2) was obtained via a co-precipitation step of Mg salts (initial 1.14%) using NaOH.
The complexity of impurity management and the requirement for cost-effective reagents implies additional challenges to improve the separation efficiency and Li quality [175,181]. To overcome these drawbacks, advanced chemical precipitation methods have been proposed [175]. Chang et al. [175] obtained high-purity salts (i.e., Li2CO3, Co2O3, Ni(OH)2, and MnO2) successfully recovered from Li-ion batterie cathodes, achieving purities > 99% (by mass) for Co and Li salts where the impurities such as Al, Cu, and Fe were effectively removed. The process consisted of the leaching of material using sulfuric acid and hydrogen peroxide. The precipitation step was conducted using reagents such as NaOH, Na2CO3, KMnO4, and NaClO.
The particle size of the powder samples plays a crucial role in the precipitation process. The diverse types of Li-ores and spent Li-ion batteries with complex compositions generate additional challenges for effective leaching and separation [183]. Smaller particles have a larger surface area, which enhances the rate of precipitation by increasing the interaction between the solute and the precipitating agent [176]. Excessively fine particles can also cause agglomeration, leading to poor separation and filtration issues, which reduce the overall process efficiency [184]. It has been shown that controlling the particle size distribution within an optimal range can improve the kinetics of Li precipitation and facilitate easier handling and separation [176]. This stage is paramount since Li precursors for battery manufacturing (mostly as Li2CO3 salt) must achieve stringent quality conditions, explicitly, a purity ≥ 99.5% and a particle size distribution defined by d10 ≥ 1 μm, 3 μm ≤ d50 ≤ 8 μm, and 9 μm ≤ d90 ≤ 15 μm [185].
Intensified chemical precipitation processes have been tested for Li separation [166]. Ultrasound has also been explored to optimize the reagent and improve the precipitation efficiency. The application of ultrasound during precipitation step is also a promising approach to reduce particle size and achieve the desired distribution [185]. Biswal et al. [158] analyzed the impact of ultrasonic waves and proved an increase in Li recovery yield, even with low initial metal concentrations, while also enabling the production of high-purity compounds in a shorter time (35 min). This technique eliminates the need for prior pre-concentration steps such as evaporation before precipitation. They recovered approximately 45% of Li in the carbonate form with an initial concentration of 5 g/L using traditional agitation, whereas the recovery rate increased to over 60% by applying ultrasonic-based precipitation [158].
Chemical precipitation has also been applied to recover Li from other sources, such as brine and wastewater. Zhao et al. [166] and Alsabbagh et al. [168] employed wastewater and Dead Sea brine for Li recovery, respectively, using sodium phosphate. Zhao et al. [166] implemented a preliminary step to remove other metals via precipitation, ultimately achieving 84.26% Li recovery at 60 °C, while Alsabbagh et al. [168] recovered Li with >40% yield at 40 °C. Wang et al. [164] utilized brine from Taijinaier Salt Lake in China for Li recovery using MgAlCO3-layered double hydroxide materials to obtain lithium carbonate, achieving 91.94% yield where a prior step was incorporated to remove impurity ions. Several studies on Li separation via chemical precipitation have focused on spent Li-ion batteries because they represent one of the primary Li sources that can be commercially implemented on an industrial scale [186].
Although well-established chemical precipitation processes for obtaining Li compounds from minerals are used at the industrial level, further studies are required to optimize their operating conditions, improve Li recovery efficiencies, reduce costs, and develop more environmentally friendly separation routes [118]. Some drawbacks of current technologies can be illustrated using examples of the production of lithium phosphate from lepidolite [169] and lithium hydroxide from bauxite clay [171]. Li extraction from lepidolite requires energy-intensive processing because it involves concentration followed by roasting at temperatures of up to 1000 °C, and afterwards leaching with acids that have poor selectivity, making it difficult to purify the resulting liquors. An alternative approach is alkaline leaching with NaOH; however, the recovery efficiency remains low due to the high sodium content, which interferes with carbonate precipitation. Mulwanda et al. [169] opted for NaOH leaching, testing various concentrations, agitation speeds, and temperatures to obtain a reduced aluminum solution, which allowed them to precipitate Li in the form of phosphate. This phosphate can then be further treated to produce lithium ferrophosphate or lithium carbonate. Guan et al. [171] proposed NaOH to address the challenges caused by the high aluminum content in ores and to achieve competitive Li extraction. After precipitation, calcination was performed, followed by leaching, to selectively isolate the target metal.
4.2. Membrane-Based Technologies
A variety of membrane technologies have been used to separate Li, which can be classified in pressure-driven membrane process, electrically driven membrane process, and thermally driven membrane process [129].
Pressure-driven membrane technology used in Li recovery is categorized into three types based on membrane pore size and transmembrane pressure difference: ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO) [187]. UF membranes, with pore sizes between 1 nm and 0.05 μm, can separate macromolecules and colloids with a molecular mass higher than 500 kD [188]. NF membranes have characteristics that lie between those of UF and RO membranes, typically possessing pore sizes around 1 nm [187,188].
Reverse osmosis is a highly effective technique for increasing the salt concentration of a solution because membranes allow water to pass through, while retaining salts with high efficacy [187,189]. However, this requires external pressure for operation, being energy intensive and prone to fouling, which can reduce separation efficacy, and increase operational costs and maintenance demands. Direct osmosis is an alternative to reverse osmosis for treating effluents with complex compositions such as geothermal brine [124]. This membrane-based process does not rely on external pressure; instead, it uses an extraction solution to generate the necessary osmotic gradient that drives water flow through the semipermeable membrane [190]. Nanofiltration operates based on both Donnan exclusion and steric hindrance, being capable of rejecting multivalent ions but permitting monovalent ions to pass [187,191]. Dialysis is a separation method that does not rely on an osmotic pressure gradient but on a concentration gradient [192]. Ceramic membranes can be used in this process because they offer high selectivity; however, their use requires precautions because of their fragility. Polymeric membranes are more flexible and resistant but offer lower selectivity.
Electrically powered membrane processes use electricity as the main driving force and rely on ion-exchange membranes that are integrated into a polymer matrix with a fixed charge. These membranes are highly selective, allowing only ions with an opposite charge to pass through, while preventing ions with the same charge from penetrating [188]. Ion-exchange membranes are widely applied in operations such as electrodeionization, diffusion dialysis, membrane electrolysis, and electrodialysis [188].
Temperature-driven membrane technologies separate substances based on temperature differences, causing substances to diffuse or migrate via the membrane at different rates [188]. Examples of these technologies include membrane crystallization, membrane distillation, and systems that combine membranes with multi-effect distillation. These processes are also effective for recovering Li [188].
A hybrid technology that combines solvent extraction and membranes is the supported liquid membrane (SLM) that uses a membrane that absorbs an organic phase to separate two aqueous phases, transferring a substance from the feed phase to the stripping phase [191]. For hydrophilic metal ion separation, the organic phase contains an extracting molecule that selectively binds to the target metal, forming lipophilic metal–organic complexes. SLM is an attractive alternative for Li separation due to its low energy consumption, reduced solvent use, high selectivity, and integrated extraction and stripping in one stage [191].
An ion-imprinted membrane (IIM) can be applied in Li separation. It is typically created by grafting an ion-imprinted polymer (IIP) on the membrane surface that reacts with template molecules (e.g., Li), then the template is removed, creating binding sites with molecular recognition properties. IIPs exhibit high affinity for the template molecules. The use of powdery Li ion-sieves in column operations leads to significant pressure drops and adsorbent loss, limiting their industrial application. Li ion-sieve membranes have been developed to combine the benefits of ion-sieves (high surface area and selectivity) with those of membranes (immobilized adsorbents and low energy consumption) with synergistic benefits [187,191]. The hybrid membrane distillation crystallization (MDC) has been used to recover Li from high-concentration brines. It offers faster crystallization rates and better-controlled nucleation kinetics. In MDC, water vapor passes through a hydrophobic membrane under a thermal gradient and condenses on the permeate side, allowing Li to be concentrated and recovered in the crystallizer [191]. Electrodialysis (ED) is a widely used electro-membrane separation process where cation and anion exchange membranes are arranged alternately and an electrical field drives cations and anions towards their respective electrodes [191]. To address the separation of ions with same charge (e.g., Li and Mg), selective ED with monovalent ion-exchange membranes have been developed, enabling the separation of monovalent ions from divalent ones.
Table 5 shows recent studies on Li separation using membrane-based processes. He et al. [193] achieved a remarkable selectivity coefficient of 430 when recovering Li from a solution containing MgCl2 via the application of (PPS/PAH)2.5—poly(styrene sulfonate) and—poly(allylamine hydrochloride) membranes, which were positively charged to reject Mg2+ ions in a nanofiltration process via the Donnan exclusion mechanism. Mg2+ and Li+ ions have similar hydrated radii and can pass through membranes, making size-based separation difficult. These authors showed that the efficacy of tested separation process depends on factors such as brine concentration and pH. It was found that a high total brine concentration and low pH of 2.7 favored Li separation. He et al. [194] used nanofiltration via a membrane with a PES substrate and an intermediate layer formed by NH2—multi-walled carbon nanotubes modified with polydopamine to separate Li from the leachate of spent Li ternary batteries, which contained high concentrations of Ni2+, Co2+, and Mn2+. This process achieved Li recovery of 79.2% due to an improvement in the Donnan effect provided by the advanced membrane structure [195].

Table 5.
Recent studies on lithium recovery by membrane technologies.
The integration of different membrane-based processes is an effective approach to optimize Li recovery. Pramanik et al. [123] combined nanofiltration with membrane distillation, taking advantage of the fact that the latter method increases the salt concentration to saturate the solution using sustainable energy. These authors evaluated two filtration membranes (NF90 and NF270) to recover Li from a simulated brine containing Li and Mg. They observed that Li rejection was higher in the presence of Mg, reaching rejection values of 77 and 56% with NF90 and NF270 membranes, respectively, after 24 h at pH 5. Li-enriched solution was concentrated to 80% after distillation using a hydrophobic polytetrafluoroethylene membrane. The objective of this two-step process was to maximize Mg removal (with nanofiltration) because its presence can form deposits on the distillation membrane, which decreases its performance and accelerates membrane deterioration during prolonged use in brines. The authors recognized that these membranes still present limitations, particularly steric hindrance and Donnan exclusion. They emphasized the need to optimize these aspects to significantly increase the Li recovery efficiency, promoting a simpler process that facilitates the separation of these valuable metals. Qiu et al. [122] used three different membrane-based processes to concentrate Li from industrial wastewater. They used cation exchange resins (CH-93) to separate Ca2+ and Mg2+, so that they would not interfere with subsequent methods. Once these cations were removed, reverse osmosis was applied to obtain a preconcentrated solution containing Li, which was then used in electrodialysis (with CIMS/ACS/CMTE membranes) to further enrich the solution with LiCl. They obtained 28.5 g/L of Li with reverse osmosis alone, and up to 87.09 g/L after completing the electrodialysis process [122].
Regarding reverse and direct osmosis, previous studies have demonstrated the performance of direct osmosis to separate Li [194,198]. Pham et al. [200] used NaCl and MgCl2 solutions and a simulated brine containing Mg2+, K+, B3+, and Na+ to assess Li recovery. The authors designed the direct osmosis systems to concentrate Li from the simulated brine and LiCl solution where membranes composed of thin films and cellulose triacetate were applied for this purpose. The results showed that when using MgCl2 as extraction solution and LiCl as feed solution, Li concentration increased up to five times when using the thin film composite membrane since this salt presents a higher osmotic pressure. Li concentration was four times higher for the same membrane when simulated brine was used for both the feed and extraction solutions. Note that MgCl2 solution does not generate a significant reverse solute, whereas NaCl can be easily separated and reused, which contributes to reducing the process costs. Mustika et al. [202] applied direct osmosis to concentrate Li from a synthetic geothermal brine composed of various salts (NaCl, KCl, MgCl2, CaCl2, LiCl) using an asymmetric cellulose triacetate membrane and NaCl extraction solution at different concentrations to evaluate its impact on process performance. The results showed that the highest NaCl concentration (i.e., 5 M) significantly increased the water flux, reaching to 38.16 L/m2·h, due to the increased ionic activity of NaCl solution. This concentration, combined with a flow rate of 4 L/h at 42 °C, generated Li rejection of ~80%.
Ounissi et al. [199] combined the advantages of using different types of membrane in dialysis to develop a flexible system with high Li selectivity. The membrane was produced using Li ionic conductive glass-ceramics and a matrix composed of PECH-DABCO+PES-NH2. This membrane was evaluated using diffusion dialysis (DD) and cross-ion dialysis (CID). DD is the simplest process because it is based on the passage or retention of ions according to their size, resulting in a slow process; but, it is more efficient than the conventional evaporation used to treat brines. Li concentrated up to 22.1% from a lithium chloride solution after 27 days using DD. In contrast, Li enrichment reached 33% in 24 h using CID with HCl as the receiving solution. Acidic media favor ionic transfer by improving ion mobility and maximizing diffusion.
These studies indicated that the use of membrane-based processes for Li recovery and enrichment is an effective approach. Some improvements are still required, especially in membrane fouling, material degradation, cleaning and maintenance of membranes after prolonged periods of use, as this implies an increase in operating costs in addition to the generation of solid waste, affecting the sustainability of this separation process [205]. This is because Li-sources are corrosive containing complex chemicals and may contain high metal concentrations, which complicate membrane treatment [187,188]. Metal ions can deposit on membrane surfaces or react chemically, causing damage or reduced material performance. Corrosive environments accelerate membrane degradation, and the presence of organic solvents and chemicals leads to contamination [188]. High temperatures and osmotic pressures also threaten membrane integrity. To address these challenges, corrosion-resistant materials like ceramic, specialized synthetic polymer membranes, and membrane surface modifications are crucial for maintaining membrane performance and extending service life [187,188].
4.3. Adsorption
Adsorption is an effective method to separate Li due to its efficiency and low energy consumption where a variety of adsorbents such as activated carbon, zeolites, metal–organic frameworks (MOFs), or ion-exchange resins, have been tested. Adsorption offers several advantages, including the ability to recover Li from low-concentration sources, ease of implementation, and the potential for regeneration of the adsorbent, making it a sustainable option for large-scale applications. The performance of adsorption is influenced by factors such as adsorbent properties, ionic strength, temperature, and the presence of competing ions. A selection of studies that focus on the development and optimization of adsorption-based methods for Li recovery is reported in Table 6.
Ion sieves represent one of the best options from an environmental perspective for Li recovery, as they do not require large amounts of water, have low energy consumption, and are regenerable, leading to a reduction in solid waste generation. The most studied adsorbents are Li sieves based on titanium and manganese oxides, because of their high adsorption capacities and reduced toxicity [206]. These materials allow effective Li recovery from aqueous solutions with high purity and efficacy without requiring additional purification steps [138]. Wang et al. [207] synthesized manganese-based sieves to adsorb Li+ ions from leachates of spent battery powders, achieving an adsorption capacity of 31.62 mg/g with Li separation efficiency of 99.9%, even in the presence of other ions such as Co2+ and Ni2+. Wang et al. [139] recovered 36.34 mg/g of Li using titanium-based sieves from a brine containing Mg2+, Ca2+, K+, and Na+. This study proved that pH is a critical factor in this separation process. Li adsorption using this material was higher at pH 8.8 due to the reduction in surface charge, thereby improving the electrostatic interactions between the cations and the adsorbent surface. The authors emphasized the importance of maintaining pH < 8.8 to prevent the formation of magnesium precipitates. The adsorption properties of Li sieves can be improved via surface doping. Molybdenum-doped titanium sieves have an adsorption capacity of 78 mg/g [206]. It was concluded that molybdenum can improve the dispersion of active sites for Li adsorption. One of the main drawbacks of manganese-based sieves for industrial scale applications is pollution caused by metal leaching. Metallic cation doping is also an alternative method for improving the structural stability of materials and reducing Mn dissolution. Gao et al. [208] developed a manganese oxide sieve doped with iron that also exhibited magnetic properties. This material was tested to separate Li and achieved an adsorption capacity of 34.8 mg/g. It retained 70% of its original adsorption capacity with a Mn loss of only 0.51% after five adsorption cycles, whereas the undoped sieve exhibited a significantly higher Mn loss of 2.48% [208].

Table 6.
Recent studies on lithium recovery using adsorbents and ion exchange membranes.
Table 6.
Recent studies on lithium recovery using adsorbents and ion exchange membranes.
| Lithium Recovery Source | Lithium Source Pretreatment | Adsorbent | Adsorbent Preparation Conditions | Adsorption Conditions | Adsorption Capacity, mg/g | Reference |
|---|---|---|---|---|---|---|
| Spent Li-ion battery powders | Leaching by NH3·H2O–NH4HCO3 solutions with H2O2 | Manganese type lithium ion-sieve powder | --- | Initial concentration of Li+ 2.5 g/L, 2 g/L of Ni2+ and Co2+, 150 g/L of NH4+ at 30 °C for 6 h | 31.62 | [207] |
| Enriched salt lake brine from Qaidam basin in China | --- | Titanium type lithium ion sieve (HxTiO3) powder | 0.5 g of H2TiO3 and 7.5 g of tetrabutylamine, 2.5 mol/L of LiOH at 170 °C for 24 h. Pickled with 0.5 M HCl at 30 °C for 24 h | 0.1 g of HxTiO3, 20 mL of brine, at 25 °C for 24 h, initial pH 8.8 | 36.34 | [139] |
| Concentrated brine from the Qaidam area of China | Adjust to pH 8.8 with Ca(OH)2. Precipitate removal | Iron-doped lithium ion sieve powder | Precursor: LiOH, TiO2/Fe2O3 molar ratio of 0.15 at 600 °C for 3 h. 2 g of precursor, 0.5 M HCl at 25 °C for 24 h | 0.1 g of iron-doped lithium ion sieves, brine (1.6 g/L) at 25 °C for 55 h | 34.8 | [209] |
| Lithium solution | --- | Lithium ion imprinted polymers (LIP) Non-lithium ion imprinted polymers (NLIPs) | 1 dose of liquor: 0.3 g of DB14C4, 0.0689 g of LiNO3 and 0.17 mL of α-MAA in 60 mL of methanol and DMF at 25 °C for 30 min. 1 dose of liquor and 0.5 g of MWCNTs (for 5 min), 3.96 g of EGDMA and 125 mg of AIBN | 15 mg of LIP, 300 mg/L of Li+, pH 6, 25 °C for 2 | 9.45 0.52 | [210] |
| Synthetic brine (LiCl and NaCl) | --- | Commercial ion-exchange resins Lewatit TP 207 TP 208 K2629 | Matrix of crosslinked polyestirene TP 207 and TP 208 are macroporous cation exchange weakly acidic resins. K2629 is microporous, strongly acidic resin | 50 mL of synthetic brine (100 mg/L), 25 °C, 20 min | 4.30 4.34 3.24 | [211] |
| LiOH solution (1.8 g/L) | --- | Molybdenum-titanium oxide lithium ion sieve (Mo/Ti-LIS) powder | TiO2, LiOH∙H2O, MoO2 at 600 °C for 3 h. Pickled with 0.5 M HCl at 25 °C for 24 h | 0.1 g of Mo/Ti-LIS, 100 rpm, 25 °C, 50 h | 78 | [206] |
| LiOH solution | --- | Fe3O4-doped magnetic lithium ion-sieve powder | Mixture: 0.1 g of magnetic Fe3O4 nanospheres, 3.9 g of MnCl2 in 50 mL of water, 30 min of ultrasonication. Mixture containing 2.5 g of LiOH and 2.5 mL of H2O2 in 50 mL of water under stirring for 2 h. Final mixture at 170 °C for 10 h | 0.4 g of adsorbent in 500 mL of LiOH (50 mg/L), pH 10.1, 24 h | 29.33 | [212] |
| Lithium solution | --- | Pristine titanate nanotubes (pTNTs) Urea-Titanate nanotubes (UTNTs) Melamine-Titanate nanotubes (MTNTs) Polyacrylonitrile-Titanate nanotubes (PANTNTs) | pTNT: 2 g of anatase titanium (IV) oxide powder, 60 mL of 10 M NaOH at 130 °C for 26 h. UTNTs, MTNTs and PANTNTs: 1 g of urea, melamine or polyacrylonitrile in 20 mL of ethanol at 40 °C for 20 min and then 1 g of pTNTs at 80 °C for 2 h | 0.15 g of powder adsorbent, 250 mL of Li solution (30 mg/L) at 25 °C, pH 8 | 34.4 35.31 40.26 31.81 | [142] |
| Lithium solution | --- | Magnetic carbon-based lithium ion-imprinted material (Li+-IIP-Fe3O4@C) | Magnetic carbon nanospheres salinization by γ-methacryloxypropyltrimethoxysilane and functionalized with methacrylic acid. Grafting with 2-hydroxymethyl-12-crown-4 and LiClO4 | 5 mg of adsorbent, 10 mL of lithium solution (300 mg/L), 25 °C | 22.94 | [140] |
| Lithium solution | --- | Fe-doped manganese oxide lithium ion-sieves (LiMn2-xFexO4) | Calcination of LiOH, MnO2 and Fe2O3 at 450 °C for 6 h. Calcined obtained with 1 M HCl for 6 h | 0.150 L of LiCl (200 mg/L), 0.15 g of adsorbent, 2 h, pH 12 | 34.8 | [208] |
| West Taijinar salt lake brine | Adjust to pH 8.8 with NH3 1 M | Shaped titanium-based lithium ion sieve (HTO-P) | Titanium-based lithium ion sieve powder was pressed into a sheet and crushed (0.64 mm) | 0.15 g of HTO-P, 30 mL of brine, pH 8.8, 30 °C, 24 h | 19.22 | [213] |
| Seawater reverse osmosis brine (43 mg-Li/L, 17 mg-Sr/L) | --- | Li-ion-imprinted polymer (IIP) | Solution 1: 20 mL of acetonitrile, 74.5 mg of dicyclohexane-18-crown-6, 21.3 mg of LiCl at 25 °C and sonicated. 0.8 g of EGDMA (Crosslinker), 0.13 g of tertbutyl acrylate (monomer), 0.3 g of potassium persulphate with solution 1 at 25 °C | 10 mg of IIP, 30 mL of seawater brine, 45 °C, pH 10, 24 h | 300 | [214] |
| LiOH solution | --- | Porous Li1.33Mn1.67O4 (H-LMO) disc | LMO: Li2CO3/MnCO3 molar ratio of 1.33/1.67, 500 °C, 4 h. LMO disc: Mixture of LMO and 25% (by mass) petroleum pitch at 150 °C for 30 min. Pressing with a disc-shaped mold (20 mm) at 150 °C for 30 min. Extraction of Li+ with 0.3 M HCl for 6 days | 1.5 g of adsorbent, 0.5 L of Li+ solution (200 mg/L), 72 h at 25 °C. | 24 | [215] |
| LiCl solution | --- | Ionic Liquid-Cellulose nanocrystals-Date pits (IL-CNC@DP) | IL-CNC: C7H8INO and DMSO at 65 °C; cellulose nanocrystals at 65 ° for 1 h. IL-CNC@DP: IL-CNC and 5 g of date pit | 0.05 g of adsorbent, 50 mL of LiCl (100 mg/L) 25 °C, pH 6 | 105 | [144] |
| LiOH solution | --- | Ti-LIS Ti-LIS-Zr E-12/Ti-LIS-Zr | For lithium ion sieve (LIS) pellets: Li2CO3, TiO2 and Zr(NO3)4 at 650 °C for 4 h. 0.25 M HCl for 24 h | 0.5 g of adsorbent, 50 mL of LiOH (1.8 g/L), 24 h | 56.3 93.2 47.5 | [216] |
| LiNO3 solution | --- | MIL-100(Fe) MOF (Metal–organic framework) | Iron powder, H3BTC, HF, HNO3 in 10 mL of water at 150 °C for 12 h in teflon-lined autoclave | 20 mg of adsorbent, 2 of mL LiNO3 (12 g/L), 15 °C, 8 h | 83.9 | [141] |
| Lithium solution Qarhan salt lake brine | --- | Hydrogen manganese oxide-sepiolite (HMO-SEP) | MO-SEP: MnSO4·H2O and Na2S2O8 by hydrothermal method LMO-SEP: 2.0462 g of MO-SEP and 25 mL of LiNO3 (0.5 M) for 1 h HMO-SEP: LMO-SEP and 0.2 M HCl for 48 h | 25 °C, 8 h, 300 mg, 2.5 m/L of Li, pH 12 50 mg of adsorbent, 20 mL of brine, pH 12, 25 °C, 24 h | 52.41 47.25 | [217] |
| LiCl solution | --- | Commercial Dowex G26 resin as powder | --- | 1.5 L of Li, 1 g of adsorbent, 0.16 mL/min, 3 h, pH 9 | 12 | [145] |
| Lithium solution | --- | Tungsten-doped Ti-based Li-ion sieve (HTO-W) | LiOH·H2O, TiO2 and WO3 in 20 mL water at 160 °C for 24 h. Calcination at 600 °C for 2 h. 0.2 M HCl at 30 °C for 24 h | LiCl solution (425 mg/L), 30 °C, 3 h | 43.01 | [218] |
| Li2CO3 solution | Simulated lithium solution (2.5 g/L): Li2CO3 and H2SO4 | Porous carbon beads-Fe3O4 Porous carbon beads-FeCl3 | Beads: Coal tar pitch and THF/DMF in isopropyl alcohol. Beads carbonization at 800 °C, 2 h. Beads activation at 850 °C, 2 h. Spherical droplets with coal tar pitch in isopropyl alcohol and water. Magnetization impregnated with FeCl3 | 30 mg of adsorbent, 10 mL of lithium solution, 5 h | 73.8 79.8 | [143] |
| Simulated seawater | --- | HTO/MXene@PVC hybrid film | Precursor: MXene, Li2TiO3, LIS, 13 mL of NMP sonication for 15 min. HTO/MXene@PVC precursor: precursor and 3 g of PVC at 80 °C. HTO/MXene@PVC LIS: 0.1 M HCl for 24 h | 200 mg/L of Li+, for 24 h at 30 °C, pH 9 | 25.4 | [219] |
| Synthetic Li-ion battery waste leachate (2.45 g/L) | --- | Commercial chelating resin with aminomethylphosphonic acid functional group Lewatit® MDS TP 260 | --- | Simulated moving bed with 6 jacketed glass columns arranged in 1-1-1-3, 15 mm diameter, switch time 25 min, 1.64 L/h | 100% | [146] |
Nomenclature—AIBN: 2,20-azobisisobutyronitrile; H3BTC: Benzene-1,3,5-tricarboxylic acid; DB14C4: Dibenzo14-crown-4; DMF: N,N-dimethyl formamide; DMSO: Dimethyl sulphoxide; EGDMA: Ethyleneglycol dimethacrylate; HTO: Titanium-based LIS; LIS: Lithium-ion sieve; LMO-SEP: Lithium manganese oxide-Sepiolite; α-MAA: α-Methacrylic; MO-SEP: Manganese oxide-Sepiolite; MWCNTs: multi-wall carbon nanotubes; PVC: Poly(vinyl chloride); THF: Tetrahydrofuran.
Adsorbents based on ion-imprinted polymers have gained popularity as promising materials with specific binding sites for ion separation. Huang and Wang [210] achieved an adsorption capacity of 1363 μmol/g with imprinted polymers, compared to 75 μmol/g obtained with non-imprinted polymers. This result demonstrated a significant improvement in Li adsorption due to the introduction of DB14C4, which favored the formation of unique coordination cavities between the chelating agent and Li ions. Alshuiael and Al-Ghouti [214] also synthesized ion-imprinted polymers to adsorb Li from reverse osmosis seawater brine, recovering up to 2500 µg of Li per gram of adsorbent. A high adsorption capacity was achieved because of the increase in temperature at pH 10, which significantly influenced the adsorption rate. The increase in pH promotes the ionization of the carboxyl groups of the polymers, enhancing their interactions with the target ions. However, this operating parameter also reduced the solution viscosity, which in turn improved the diffusion of adsorbate ions [214].
MOF-based adsorbents are promising for the separation of various ions because of their excellent adsorption capacities. Huangfu et al. [141] used MIL-100(Fe) to separate Li from LiNO3 solution obtaining an adsorption capacity of 83.9 mg/g in 2 h, and the adsorbent structure was stable over four adsorption cycles. Kamran and Park [142] evaluated titanium nanotubes (TNTs) to separate Li. These nanotubes are considered exceptional porous materials because of their tubular structure that is stabilized by hydrothermal treatment and can be chemically modified. The authors functionalized TNTs with urea, melamine, and polyacrylonitrile. The batch studies were performed at room temperature, basic conditions using Li concentration of 30 mg/L with both functionalized and unfunctionalized TNTs to separate Li in the absence of other ions. They determined that the melamine-functionalized adsorbent exhibited the highest Li adsorption capacity (i.e., 40 mg/g) in comparison to the non-functionalized adsorbent (31 mg/g). For recovery of the adsorbed Li ions, 0.3 M HCl was evaluated as eluting agent, achieving 3 cycles with the same initial adsorption capacity [142]. Various porous carbon-based materials have been applied for Li recovery. Liu et al. [143] reported Li adsorption from solutions with low pH using magnetized porous carbon beads. This study showed that the mesopore volume of the tested adsorbent favored Li separation, with an adsorption capacity of 80 mg/g, which prevailed after five adsorption cycles [143]. Other Li adsorbents include biomass waste and composites. Wahib et al. [144] studied date pits impregnated with nanocrystals of cellulose and an ionic liquid (IL-CNC@DP) to separate Li achieving 90% efficiency with an adsorption capacity of 99 mg/g at pH 6. Chen et al. [219] reported an adsorbent based on a titanium hybrid membrane incorporating MXene, which is a 2D advanced material known for its excellent properties in terms of surface area, conductivity, and strength. The adsorption capacity of this novel material was lower than 26 mg/g, but it exhibited excellent reusability, maintaining over 90% of its adsorption properties after several adsorption–desorption cycles [219].
Ion-exchange resins are versatile materials for the selective recovery of valuable metals in various industrial contexts, see Table 6. Arroyo et al. [211] Cunha et al. [145] and Wesselborg et al. [147] used different resins to demonstrate their effectiveness for separating Li from various sources. The results indicated that resin TP207 is an interesting alternative for processing brines [211]. Dowex G26 resin was effective to handle leachates from lepidolite and spodumene ores achieving a maximum loading capacity of 1.8 meq of Li/g and a recovery of 71%, with an elution of up to 82% [145]. Wesselborg et al. [146] analyzed a continuous resin-based process for Li separation from battery cathode leachates and achieved high purity in the refined product. This method outperformed the solvent extraction and precipitation methods.
Recent advances in material design and process optimization have enabled the handling of competing ions during Li separation [117,220]. As stated, the importance of adsorption, compared to other Li separation techniques, relies on its versatility and flexibility [221]. This allows the use of a wide range of adsorbents to separate Li from fluids, involving physical interactions, electrostatic forces, ion exchange, and chemisorption [190,216,222,223,224]. Different studies have documented that physisorption is relevant for Li separation from sources such as brine or polluted water [225]. Shoghi et al. [226] used H4Ti5O12 spinel nanotubes for Li recovery and determined that the adsorption mechanism followed physisorption where Dubinin-Radushkevitch isotherm fitted experimental data. This result was confirmed by the calculation of the adsorption energy, which was ~5 kJ/mol indicating the presence of van der Waals forces for the interaction between Li ions and the adsorbent surface [226]. Liu et al. [143] showed that Li adsorption process from a brine using magnesium oxide-based adsorbent implied physisorption. These authors proved that adsorbent regeneration can be performed using an HCl solution [143]. Note that the materials functionalized with active groups can form specific chemical bonds with Li ions even in systems with high concentrations of other competitive ions [223]. Marthi et al. [227] analyzed Li adsorption mechanism using H2TiO3 and the results indicated the relevant role of electrostatic forces in the separation process, while Zhou et al. [216] studied sieves doped with Zr and epoxy granular compounds, demonstrating that Li adsorption process involved predominantly chemisorption. Another study reported that Li adsorption on Mn- and Al-based composites could be governed by a combination of chemisorption and physisorption [228].
Aluminum-based materials can be used as ion sieves for Li extraction because their laminar structure allows them to selectively exchange ions even in the presence of other cations such as Mg, Ca, or Na. For example, the recovery of lithium from brines can be performed using lithium-aluminum double hydroxide (LADH-Cl) with high magnesium chloride content. The results demonstrated that the lithium adsorption efficiency depends mainly on the crystalline defects of LADH-Cl matrix [229].
Overall, understanding the Li adsorption mechanisms helps to identify the potential routes for adsorbent regeneration [221]. Adsorbent regeneration enables the recovery of adsorbed Li and the material recycling in multiple adsorption cycles, which helps to reduce operational costs by avoiding the need for new adsorbents and minimizing the environmental impact related to the waste management of spent adsorbents [230].
Regarding environmental impact, adsorption is among the technologies with the lowest-to-moderate effects [76,134,135]. One of the key advantages of adsorption is its reduced chemical usage. Adsorption typically operates under milder conditions (e.g., ambient temperature and pressure) resulting in lower energy consumption compared to the energy-intensive processes of hydrometallurgy, electrochemical, and solvent extraction [76,134,135]. Adsorption processes also generate less waste. The use of eco-friendly adsorbents, such as activated carbon prepared from biowastes, enhances the sustainability of separation process.
4.4. Solvent Extraction
Li recovery by solvent extraction depends on several factors such as ratio of aqueous to organic phases, extraction time, temperature and pH [231]. The concentration and type of extracting agent are relevant parameters for Li separation [232]. The solvents used in this process are diverse and include organic compounds such as di-(2-ethylhexyl) phosphoric acid (D2EHPA), Cyanex 923 [147], and tributylphosphate (TBP) [233], deep eutectic solvents (DES) [234,235], and ionic liquids (ILs) [235]. Organic solvents are widely used due to their availability and well-understood properties [236]. Organophosphorus acids are also an alternative because they can be used to remove alkaline earth and/or transition metal impurities. Lithium can be recovered via precipitation using Na2CO3 from solutions containing Li+, Na+, and K+, among other cations. Other more chemicals for Li separation include the use of complexes such as FeCl3-TBP or ortho-substituted phenols. It is convenient to indicate that the high volatility of traditional solvents can generate health risks and environmental concerns during Li separation [132]. These risks can be mitigated using appropriate methodologies and equipment such as sedimentation basins, traps, safety tanks, condensation systems, activated carbon filters, and encapsulation [237].
A summary of the different Li separation studies using solvent extraction is presented in Table 7. Harvianto et al. [238] successfully extracted up to 99% of Li from a solution using thenoyltrifluoroacetone (TTA) and trioctylphosphine oxide (TOPO) with kerosene. Jang et al. [239] and Lee and Chung [240] also used organic solvents (D2EHPA and TBP) in kerosene to perform Li extraction from shale gas wastewater and simulated geothermal fluid, respectively.

Table 7.
Recent studies on lithium recovery by solvent extraction.
The solutions containing only Li are useful for preliminary tests to assess the extraction efficacy of single or multiple solvents [238]. The industrial applications involve complex solutions containing various dissolved salts, which increases the difficulty of performing selective extraction of a specific ion [109,132]. For example, the shale gas wastewater contains Li and other cations such as Ca, Mg, Sr, and Ba, which can be effectively removed by D2EHPA due to their high affinity for divalent cations. Jang et al. [239] used this solvent in the first stage to remove these cations, which interfered with Li extraction process. In the second stage, the same solvent was utilized because of its high affinity for Li [239]. This second stage involved combining TBP as a synergistic additive to facilitate the formation of Li-D2EHPA complex. The positive effect of TBP on Li extraction occurs when it is present in an appropriate amount. Note that an excess of TBP (>0.3 M) can lead to undesired solvent interactions, reducing extraction efficacy by limiting the ability of D2EHPA to interact with Li ions. Consequently, Li separation can be very low under these conditions. Lee and Chung [240] achieved an extraction efficacy of up to 87.7% using 1.5 M D2EHPA and 0.3 M TBP to treat a synthesized Soultz-sous-Forêts geothermal fluid. The other ions (e.g., Mg, Ca, Sr, Ba) that can interfere with this extraction process were removed by chemical precipitation using Na2CO3 and Ca(OH)2 without generating Li losses in the first stage.
DES (Deep Eutectic Solvents) are created by combining compounds that act as hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD) [252]. They are considered to be more environmentally friendly because of their low toxicity, biodegradability, reduced volatility, and low cost [253]. Li separation can be performed effectively using DES. Xue et al. [250] synthesized DES for Li separation by combining menthol (HBA) with three carboxylic acids (HBD) (i.e., decanoic, octanoic, and lauric acids). They determined that Li extraction efficacy was directly related to the alkyl chain length of the carboxylic acids, as the viscosity supported stronger hydrogen bonding and the efficiency of the equipment used during the extraction process, since there are not economically viable high-efficiency extractors on the market. Consequently, a mixture of lauric acid and menthol (in 1/2 molar ratio) exhibited the highest extraction efficiency, reaching 80.69% at 25 °C under stirring 660 rpm for 30 min at pH 12. The results indicated that pH was a critical parameter in solvent-based Li extraction. Li extraction efficacy was low at acidic pH, while Li separation also reduced at pH > 12 due to emulsification.
Li extraction efficacy using solvents, such as ILs, is also influenced by the carbon chain size [248]. This trend can be illustrated using the results reported for Li separation from a simulated brine using ILs with different chain lengths: [Omim]3PW12O40, [Hmim]3PW12O40, and ([Bmim]3PW12O40. These ILs were used in combination with DMP (diluent) and TBP (extractant). [Bmim]3PW12O40 showed a higher extraction efficacy (99.23%). Note that, unlike DES, a longer carbon chain length reduces the transfer of Li ions from the aqueous phase to the organic phase because of the increase in viscosity, as this extraction mechanism is based on ion exchange. Shi et al. [132] used [C4mim][NTf2] and TBP for Li separation from brine. The authors concluded that IL showed selectivity for Li ions, which is particularly useful because brine contains magnesium that often complicates Li separation.
Li separation based on solvent extraction is simple compared to other methods, as it typically involves short operating times (only a few minutes) at low temperature (20–30 °C), being a cost-effective option [248,254]. These advantages have been demonstrated in previous studies. Raiguel et al. [249] and Ni et al. [111] reported Li separation > 90% using Mextral 54-100/Cyanex 923 on Shellsol D70 and HBTA/TOPO, respectively, at 25 and 20 °C with 5 min of operation. The solution used in the separation process could be regenerated after Li recovery. This recovery process typically involves the addition of compounds such as HCl to facilitate the exchange of metal ions by H+ [242]. The regeneration is then achieved by removing excess H+ using NaCl and NaOH. Xiang et al. [241] proved this concept via the reuse of the organic phase composed of methyl isobutyl ketone (MIBK) and TBP/FeCl3 for 14 cycles, maintaining separation efficiency > 98%. Although its advantages, solvent extraction still rises concerns regarding the selectivity under complex ion solutions, the solvent degradation, and the organic contamination of solutions that could end up into natural reservoirs, for instance, reinjection of the solution to the same salt lake from where the brine is extracted.
5. Lithium Recycling from Spent Products and Waste Streams
Although exploring and implementing effective processes to recover Li from its natural sources is crucial, it is also important to research and develop technologies to obtain it from industrial waste and discarded Li-containing commercial products [255]. The objective of this section is to provide a brief discussion to highlight the importance of recycling and waste valorization for lithium recovery.
Waste management and recycling of Li-containing materials will allow optimal use of this critical material, not only in the face of concerns about the potential depletion of reserves but also by contributing to its pollution control, promoting a more sustainable Li valorization in the context of circular economy [190,256]. Studies dedicated to estimating the trends of long-term Li production and consumption concluded that Li reserves could be depleted between 23 and 471 years where the supply of this resource could be maintained for up to 100 years to meet the expected electromobility demand [257,258]. These studies have also highlighted the uncertainty in Li availability, emphasizing the importance of controlling the dispersive use of this mineral to protect natural resources and promote its reuse via circular economy.
The use of industrial waste and residual streams for Li recovery is a strategic alternative to address the growing demand for batteries and electronic devices [144]. Different industrial waste and residual streams contain Li in quantities that can be attractive for commercial implementation, and proper disposal and recycling can contribute to mitigating the environmental impact associated with Li supply chain [259]. One prominent source of Li recovery is wastewater generated during the manufacturing of Li-ion batteries. This wastewater can contain other compounds such as N-methyl-2-pyrrolidone and various suspended particles [260]. Li separation from this wastewater may require advanced processes such as membrane-based and electrochemical technologies [122,144]. Li can also be found in other residual streams, such as those generated by mining activities [261], albeit in smaller quantities. Li concentrations of 500 mg/L have been observed in water from the Smackover formation in the U.S. [254], and 0.896 mg/L in a reservoir located in the southeastern Arabian Peninsula [262]. The water produced from oil and gas operations is also a promising source to recover Li [134]. Some studies have indicated that pretreatment operations are crucial to processing this wastewater from an economic standpoint [179]. However, information regarding its industrial extraction is scarce due to commercial competition, suggesting that the technology required is still underdeveloped. The thermal brines utilized in geothermal power plants also contain Li that can be potentially recovered [263]. An example is the plant located in the Salton Sea that recovers ~15,000 tons of Li salt annually [264]. Li can be separated from wastewater generated by chemical industries that use brine for potassium or magnesium extraction [265], as well as from the polymer and advanced plastics industries that utilize Li-based compounds to modify thermal properties of some commercial products [266]. The integration of these residual streams into the Li supply chain not only enables the recovery of this strategic feedstock, but also fosters a more sustainable production model in which industrial waste and potential contaminants can be converted into valuable resources, strengthening the transition towards a greener and more resource-efficient economy [267,268,269].
It is important to recall that the largest amount of recoverable Li from waste products comes from spent batteries, which contain compounds such as lithium-iron phosphate and lithium-cobalt oxide [162,175,207]. Their processing via hydrometallurgical methods using acid leaching and selective precipitation [270], or pyrometallurgical methods applying high temperatures to separate metals, allows for the recovery of not only Li but also other important secondary elements (e.g., manganese, nickel, cobalt) that are essential for other processes [271]. Discarded products containing glass and ceramics that correspond to materials widely used in the domestic, electronics, and construction industries are also promising Li sources [240]. These products are commonly enriched with lithium oxide to enhance the thermal and mechanical properties. They can contain 3%–6% of lithium oxide exceeding the composition found in batteries that contain 2%–3% of various lithium compounds [272,273]. These residues can be processed via a separation sequence that integrates crushing, mechanical separation, and chemical leaching [219]. This recycling route appears less complex than that used for battery recycling. It is convenient to indicate that some glass-ceramic products may require pre-leaching treatment at high temperature, which can reach up to 1100 °C, making their recycling more challenging [272].
Discarded electronic devices (e.g., cameras, laptops, and mobile phones) that have reached the end of their life cycle or are defective can be used for Li recovery. These products are composed of internal batteries and structural components that contain Li. This metal can be recovered via product disassembly, followed by separation processes such as ion exchange and electrochemical extraction [274,275]. This separation is challenging because of the complexity of the device composition and diversity of the materials they contain [276]. The lithium soap used in the lubricant industry can be recovered, minimizing the environmental risks associated with the improper disposal of used lubricants, which are often discarded in landfills [277,278]. Li recovery from discarded and expired drugs is an emerging area in waste management. Previous studies have indicated that Li recovery from expired lithium carbonate medication can achieve > 85% [279].
Circular economy-based initiatives for Li recycling can help to maximize the use of Li resources, minimize waste generation and control environmental pollution, closing the life cycle of its products [71]. The development of innovative technologies in this field is essential to consolidate a sustainable industrial model that meets the growing global Li demand and reduces pressure on traditional Li sources, thereby promoting a balance between economic development and environmental conservation [73]. It is also important to promote public policies for circular economy-based Li valorization [280].
6. Conclusions
Lithium has become a cornerstone of modern technology due to its unique electrochemical properties making it indispensable in rechargeable batteries and power devices ranging from smartphones to electric vehicles, in addition to other industrial applications including ceramics, glass, medicine, lubricants, among others. Specifically, Li-ion batteries have revolutionized energy storage for electric vehicles and portable electronics, and they are anticipated to play a primary role in grid-scale energy storage, enabling the widespread integration of renewable energy sources. Future research may unlock additional Li applications in high-tech sectors, including semiconductors and aerospace engineering. The global energy transition to renewable systems will exponentially increase Li demand; consequently, its supply chain should be consolidated. However, the rapid Li valorization and exploitation have created both sustainability and technological challenges that need to be resolved.
Different studies have indicated that conventional Li extraction processes from ores and brines are resource-intensive causing water depletion, ecosystem disruption, and greenhouse gas emissions. Adsorption, solvent extraction, ion exchange and membrane-based processes can be effective for Li separation, but they still have technical and economic drawbacks that must be addressed. Li selectivity in separation processes (e.g., Mg presence in brines or systems with a high content of Na+, Ca+, Mg+, K+,) is a relevant challenge that should be handled in current industrial applications. The sustainable Li production requires efficient and clean separation and purification processes to meet a growing global demand without negatively impacting the environment. Bio-based and hybrid techniques that combine multiple processes for improved separation performance are alternatives for solving the current gaps. The integration of artificial intelligence and machine learning can also accelerate the discovery and optimization of novel materials (e.g., adsorbents) and processes for Li recovery. An emphasis should be given to developing novel low-cost materials used in separation methods with better properties and chemical stability to operate under acidic and high salt conditions. It is convenient to indicate that further studies should focus on a reliable comparison of available Li separation technologies. This analysis requires a detailed life cycle assessment to valorize and compare different metrics (e.g., eco-indicators) with the aim of establishing the advantages and limitations of each alternative. This evaluation should also be based on a proper set of cases, with characteristics representative of different operating scenarios, to obtain reliable results.
Effective recycling strategies can also contribute to satisfying Li demand and mitigating the environmental impact caused by the exploitation of traditional sources. The valorization of industrial waste and residues via innovative recovery methods should be further investigated and play an active role in the new electromobility ecosystem. Some alternatives for Li recycling may present technical barriers, particularly from low-concentration sources, thus requiring research to enhance their efficacy and cost-effectiveness. The high initial costs of advanced recovery technologies can hinder their widespread adoption. Scaling these processes and achieving economic parity with respect to the conventional extraction methods remains a challenge. Li recovery from spent batteries is a promising supply source of this critical metal that can help reduce dependence on primary mining and salar activities. The diversification of Li sources via waste management can enhance the resilience of its global supply chain, reducing vulnerabilities associated with geopolitical tensions and market fluctuations. It is also necessary to develop and apply new sustainable and effective technologies for Li pollution remediation and to recover other pollutants associated with the supply chain.
A multidisciplinary approach combining technological innovation, robust policy frameworks, and international collaboration is essential to ensure that Li can support a cleaner and greener future while minimizing its carbon footprint. By adopting sustainable practices and advancing research, society can fully harness the transformative potential of this relevant metal, while safeguarding the environment for future generations.
Author Contributions
Conceptualization and methodology, M.d.R.M.-V.; validation, A.B.-P.; investigation, B.P.E.-V., M.d.R.M.-V., H.A.G.-P. and H.E.R.-Á.; writing—original draft preparation, B.P.E.-V., H.E.R.-Á., A.R.V.-L., A.I.M.-T., M.L.-M. and N.S.; writing—review and editing, A.B.-P.; supervision, M.d.R.M.-V. and A.B.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript has been funded by the European Union via the EULAC Energytran project. EULAC ENERGYTRAN - EULAC FOR ENERGY TRANSITION: RESEARCH INFRASTRUCTURES COOPERATION FOR ENERGY TRANSITION BETWEEN EUROPE AND LATINAMERICAN AND THE CARIBBEAN COUNTRIES. (Funding number: 1011311725). The views and opinions expressed are solely those of the authors and do not necessarily reflect those of the European Union. Neither the European Union nor the funding authority can be held responsible for them.
Acknowledgments
Authors acknowledge the funding provided by the Horizon Europe programme of European Commission.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balaram, V.; Santosh, M.; Satyanarayanan, M.; Srinivas, N.; Gupta, H. Lithium: A review of applications, occurrence, exploration, extraction, recycling, analysis, and environmental impact. Geosci. Front. 2024, 15, 101868. [Google Scholar] [CrossRef]
- United States Geological Survey 2025; Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2025; 212p. [CrossRef]
- RSC (Royal Society of Chemistry). Available online: https://www.rsc.org/periodic-table/element/3/lithium (accessed on 10 June 2025).
- da Silva, L.; Wu, J.; Cadena, E.; Groombridge, A.S.; Dewulf, J. Towards environmentally sustainable battery anode materials: Life cycle assessment of mixed niobium oxide (XNOTM) and lithium-titanium-oxide (LTO). Sustain. Mater. Technol. 2023, 37, ie00654. [Google Scholar]
- Bi, Z.; Guo, X. Solidification for solid-state lithium batteries with high energy density and long cycle life. Energy Mater. 2022, 2, 200011. [Google Scholar] [CrossRef]
- Gavrilescu, M. Microbial recovery of critical metals from secondary sources. Bioresour. Technol. 2022, 344, 126208. [Google Scholar] [CrossRef]
- Tomascak, P. Developments in the understanding and application of lithium isotopes in the Earth and planetary sciences. Rev. Mineral. Geochem. 2004, 55, 153–195. [Google Scholar] [CrossRef]
- Mihalasky, M.; Briggs, D.; Baker, M.; Jaskula, B.; Cheriyan, K.; Deloach-Overton, S. Lithium Occurrences and Processing Facilities of Argentina, and Salars of the Lithium Triangle, Central South America. U.S. Geological Survey Data Release, 2020. Available online: https://www.usgs.gov/data/lithium-occurrences-and-processing-facilities-argentina-and-salars-lithium-triangle-central?utm_source=chatgpt.com (accessed on 25 July 2025).
- Hudson-Edwards, K. Geochemistry and mineralogy of wastes from lithium-bearing granite-pegmatite mining: Resource potential and environmental risks. Front. Geochem. 2024, 2, 1378996. [Google Scholar] [CrossRef]
- Oliviera, G.A.; Bustillos, J.O.; Ferreira, J.; Bergamaschi, V.; Moraes, R.; Gimenez, M.; Miyamoto, F.; Seneda, J. Applications of lithium in nuclear energy. In Proceedings of the International Nuclear Atlantic Conference (INAC 2017), Belo Horizonte, Brazil, 22–27 October 2017. [Google Scholar]
- Aylmore, M.G.; Merigot, K.; Quadir, Z.; Rickard, W.; Evans, N.; McDonald, B.; Catovic, E.; Spitalny, P. Applications of advanced analytical and mass spectroscopy techniques to the characterization of micaceous lithium-bearing ores. Miner. Eng. 2018, 116, 182–195. [Google Scholar] [CrossRef]
- Krishnan, R.; Gopan, G. A comprehensive review of lithium extraction: From historical perspectives to emerging technologies, storage, and environmental considerations. Clean. Eng. Technol. 2024, 20, 100749. [Google Scholar] [CrossRef]
- Kesler, S.; Gruber, P.W.; Medina, P.; Keoleian, G.; Everson, M.; Wallington, T. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- WEF (World Economic Forum) 2023. Available online: https://www.weforum.org/stories/2023/01/chart-countries-produce-lithium-world/ (accessed on 27 July 2025).
- Halkes, R.T.; Hughes, A.; Wall, F.; Petavratzi, E.; Pell, R.; Lindsay, J.J. Life cycle assessment and water use impacts of lithium production from salar deposits: Challenges and opportunities. Resour. Conserv. Recycl. 2024, 207, 107554. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2025; IEA: Paris, France, 2025; Available online: https://www.iea.org/reports/global-ev-outlook-2025 (accessed on 25 July 2025).
- Dugamin, E.J.; Richard, A.; Cathelineau, M.; Boiron, M.C.; Despinois, F.; Brisset, A. Groundwater in sedimentary basins as potential lithium resource: A global prospective study. Sci. Rep. Nat. 2021, 11, 21091. [Google Scholar] [CrossRef]
- Miatto, A.; Reck, B.K.; West, J.; Graedel, T.E. The rise and fall of American lithium. Resour. Conserv. Recycl. 2020, 162, 105034. [Google Scholar] [CrossRef]
- He, M.; Luo, C.; Yang, H.; Kong, F.; Li, Y.; Deng, L.; Zhang, X.; Yang, K. Sources and a proposal for comprehensive exploitation of lithium brine deposits in the Qaidam Basin on the northern Tibetan Plateau, China: Evidence from Li isotopes. Ore Geol. Rev. 2020, 117, 103277. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Lin, H.; Jin, Y.; Tao, M.; Zhou, Y.; Shan, P.; Zhao, D.; Yang, Y. Magnetic resonance imaging techniques for lithium-ion batteries: Principles and applications. Magn. Reson. Lett. 2024, 4, 200113. [Google Scholar] [CrossRef]
- Bustos-Gallardo, B.; Bridge, G.; Prieto, M. Harvesting Lithium: Water, brine and the industrial dynamics of production in the Salar de Atacama. Geoforum 2021, 119, 177–189. [Google Scholar] [CrossRef]
- Das, A.; Sahu, S.; Mohapatra, M.; Verma, S.; Bhattacharyya, A.J.; Basu, S. Lithium-ion conductive glass-ceramic electrolytes enable safe and practical Li batteries. Mater. Today Energy 2022, 29, 101118. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, S.; Zhang, X.; Guo, S.; Li, P.; Yan, S. Glass and glass ceramic electrodes and solid electrolyte materials for lithium ion batteries: A review. J. Non-Cryst. Solids 2023, 619, 122581. [Google Scholar] [CrossRef]
- Zagorodny, J.P. Gestión Integral de Las Baterías Fuera de Uso de Vehículos Eléctricos en el Marco de Una Estrategia de Economía Circular; Serie Medio Ambiente y Desarrollo, N° 173 (LC/TS.2023/36); Comisión Económica para América Latina y el Caribe (CEPAL): Santiago, Chile, 2023. [Google Scholar]
- Askarzadeh, N.; Shokrollhi, H. A review on synthesis, characterization and properties of lithium ferrites. Results Chem. 2024, 10, 101679. [Google Scholar] [CrossRef]
- Chevalier, N.; Guillou, P.; Viguié, C.; Fini, J.B.; Sachs, L.M.; Michel-Caillet, C.; Mhaouty-Kodja, S. Lithium and endocrine disruption: A concern for human health? Environ. Int. 2024, 190, 108861. [Google Scholar] [CrossRef]
- Arharbi, A.; Ez-Zahraouy, H. Fluorine, nitrogen doping, and triaxial strain effects on the structural stability and hydrogen storage properties of lithium hydride LiH: DFT investigation. Int. J. Hydrogen Energy 2024, 65, 262–270. [Google Scholar] [CrossRef]
- Biswas, P.; Suresh, M.B.; Jana, D.C.; Saha, B.P.; Johnson, R. Processing of lithium aluminium silicate glass-ceramics and investigations of fracture behaviour and its correlation with the microstructural features. Ceram. Int. 2024, 50, 4708–4714. [Google Scholar] [CrossRef]
- Lohbauer, U.; Niero, D.C.; Lubauer, J.; Abdelmaseh, S.; Cicconi, M.R.; Hurle, K.; de Ligny, D.; Goetz-Neunhoeffer, F. Glass science behind lithium silicate glass-ceramics. Dent. Mater. 2024, 40, 842–857. [Google Scholar] [CrossRef]
- Sánchez-López, M.D. Geopolitics of the Li-ion battery value chain and the Lithium Triangle in South America. Lat. Am. Policy 2023, 14, 22–45. [Google Scholar] [CrossRef]
- Leelaponglit, S.; Angkananuwat, C.; Krajangta, N.; Paaopanchon, C.; Ackapolpanich, T.; Champakerdsap, C.; Klaisiri, A. Comparison of mechanical properties between zirconia-reinforced lithium silicate glass-ceramic and lithium disilicate glass-ceramic: A literature review. Oral Sci. Rep. 2024, 45, 13–21. [Google Scholar] [CrossRef]
- Gomes, G.H.; Oliveira, G.; Rodas, A.; Barbosa, M.T.; Costa, F.; Rodrigues, M.F.; Santos, C.; Barboza, J. In vitro biodegradation, blood and cytocompatibility studies of a bioactive lithium silicate glass-ceramic. Mater. Chem. Phys. 2024, 314, 128828. [Google Scholar] [CrossRef]
- Lee, I.; Kim, H. A study on high speed machining distortion characteristics of aluminum lithium alloys wing rib. J. Korean Soc. Manuf. Process. Eng. 2014, 13, 111–118. [Google Scholar]
- Kablov, E.N.; Antipov, V.V.; Oglodkova, J.S.; Oglodkov, M.S. Development and application prospects of aluminum-lithium alloys in aircraft and space technology. Metallurgies 2021, 65, 72–81. [Google Scholar] [CrossRef]
- Abdalwahid, K.; Enass, R.; Ali, L. Influence of alloying element on corrosion behavior of (Al-Li) alloys used in aerospace industries. J. Eng. Appl. Sci. 2019, 14, 9950–9954. [Google Scholar] [CrossRef][Green Version]
- Wanhill, R.J.H. Aerospace applications of aluminum–lithium alloys. In Aluminum-Lithium Alloys; Elsevier: Amsterdam, The Netherlands, 2014; pp. 503–535. [Google Scholar]
- Muduli, R.C.; Kale, P. Silicon nanostructures for solid-state hydrogen storage: A review. Int. J. Hydrogen Energy 2023, 48, 1401–1439. [Google Scholar] [CrossRef]
- Tan, H.; Li, M.; He, X.; Su, Y.; Yang, J.; Zhao, H. Effect of wet grinded lithium slag on compressive strength and hydration of sulphoaluminate cement system. Constr. Build. Mater. 2021, 267, 120465. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Anothumakkool, B.; Kurungot, S.; Winter, M.; Nair, J.R. In situ polymerization process: An essential design tool for lithium polymer batteries. Energy Environ. Sci. 2021, 14, 2708–2788. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, B.; Jing, M.; Shen, X.; Wang, L.; Xu, H.; Yan, X.; He, X. In situ catalytic polymerization of a highly homogeneous PDOL composite electrolyte for long-cycle high-voltage solid-state lithium batteries. Adv. Energy Mater. 2022, 12, 2201762. [Google Scholar] [CrossRef]
- Pei, H.; Yan, F.; Liu, H.; He, B.; Li, J. The selective complexation of crown ethers for lithium isotope separation: A critical review. Sep. Purif. Technol. 2024, 341, 126857. [Google Scholar] [CrossRef]
- Badea, S.L.; Niculescu, V.C.; Iordache, A.M. New trends in separation techniques of lithium isotopes: A review of chemical separation methods. Mater. 2023, 16, 3817. [Google Scholar] [CrossRef]
- Guo, X.; Wang, H. Preparation Method and Application of Lithium Hydroxy Fatty Acid. Patent CN110205179B, 17 August 2021. [Google Scholar]
- Xue, M.; Yan, D.; Xu, X.; Zhu, Z.; Wu, Z.; Sheng, Q. Applications of Lithium Anilide in Catalyzing Hydroboration Reaction of Imine and Borane. Patent WO/2020/073178, 16 April 2020. [Google Scholar]
- Lin, K.; Zhao, Z.; Li, Y.; Zeng, Z.; Wei, X.; Fan, X.; Zhu, M. Well-dispersed graphene enhanced lithium complex grease toward high-efficient lubrication. Chin. J. Mech. Eng. 2023, 36, 133. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Yang, T.; Su, H.; Wang, X.; Zhang, S.; Lou, W. Rheological behaviors and tribological properties of nano-silica grease: A study compared with lithium grease and polyurea grease. Tribol. Int. 2023, 186, 108657. [Google Scholar] [CrossRef]
- Sahul, M.; Sahul, M.; Harsani, M.; Domankova, M. On the microstructure and mechanical properties of AW2099 aluminium lithium alloy joints produced with electron beam welding. Mater. Lett. 2020, 276, 128276. [Google Scholar] [CrossRef]
- Han, C.; Jiang, P.; Geng, S.; Mi, G.; Wang, C.; Li, Y. Nucleation mechanisms of equiaxed grains in the fusion zone of aluminum-lithium alloys by laser welding. J. Mater. Res. Technol. 2021, 14, 2219–2232. [Google Scholar] [CrossRef]
- Bai, H.; Wee, M.G.V.; Chinnappan, A.; Li, J.; Shang, R.; Ramakrishna, S. Effect of polyvinylpyrrolidone and lithium chloride composite desiccant-coated heat exchangers on dehumidification studies. Appl. Therm. Eng. 2024, 248, 123318. [Google Scholar] [CrossRef]
- Yang, C.H.; Chen, Y.S.; Lin, S.T.; Chen, J.L.; Hung, C.S.; Chen, H.P.; Hu, C.C.; Cheng, C.C.; Chen, H.; Li, M.H. Air/water vapor control with lithium chloride/polyvinyl alcohol/sulfone composite films. Surf. Coat. Technol. 2024, 482, 130739. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Pressman, P.; Hayes, A.W.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Lithium and hormesis: Enhancement of adaptive responses and biological performance via hormetic mechanisms. J. Trace Elem. Med. Biol. 2023, 78, 127156. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.; Leung, M.; Lau, W.; Chan, E.; Hayes, J.; Osborn, D.; Cheung, C.; Wong, I.; Man, K. Lithium and the risk of fractures in patients with bipolar disorder: A population-based cohort study. Psychiatry Res. 2024, 339, 116075. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Vázquez, E.; Quintas-Granados, L.; Cortés, H.; González-Del Carmen, M.; Leyva-Gómez, G.; Rodríguez-Morales, M.; Bustamante-Montes, L.; Silva-Adaya, D.; Pérez-Pasencia, C.; Jacobo-Herrera, N.; et al. Lithium: A promising anticancer agent. Life 2023, 13, 537. [Google Scholar] [CrossRef]
- Almatari, M.; Agar, O.; Altunsoy, E.E.; Kilicoglu, O.; Sayyed, M.I.; Tekin, H.O. Photon and neutron shielding characteristics of samarium doped lead alumino borate glasses containing barium, lithium and zinc oxides determined at medical diagnostic energies. Results Phys. 2019, 12, 2123–2128. [Google Scholar] [CrossRef]
- Hoey, O.; Moudud, H.; Struelens, L.; Schoonjans, W.; Dabin, J.; Castillo, D.; Block, S.; Vanhavere, F. Evaluation of different types of lithium fluoride thermoluminescent detectors for ring dosimetry in nuclear medicine. Radiat. Meas. 2022, 159, 106866. [Google Scholar] [CrossRef]
- Saray, A.A.; Kaviani, P.; Shahbazi-Gahrouei, D. Dosimetric characteristics of lithium triborate (LiB3O5) nanophosphor for medical applications. Radiat. Meas. 2021, 140, 106502. [Google Scholar] [CrossRef]
- Khalilzadeh, N.; Saion, E.B.; Mirabolghasemi, H.; Soltani, N.; Shaari, A.; Hashim, M.; Ali, N.; Dehzangi, A. Formation and characterization of ultrafine nanophosphors of lithium tetraborate (Li2B4O7) for personnel and medical dosimetry. J. Mater. Res. Technol. 2016, 5, 206–212. [Google Scholar] [CrossRef][Green Version]
- Ren, P.; Yin, Z.; Wang, G.; Zhao, H.; Ji, P. The sustainable supply of lithium resources from the Qinghai-Tibet plateau salt group: The selection of extraction methods and the assessment of adsorbent application prospects. Desalination 2024, 583, 117659. [Google Scholar] [CrossRef]
- Mas-Fons, A.; Horta Arduin, R.; Loubet, P.; Pereira, T.; Parvez, A.M.; Sonnemann, G. Carbon and water footprint of battery-grade lithium from brine and spodumene: A simulation-based LCA. J. Clean. Prod. 2024, 452, 142108. [Google Scholar] [CrossRef]
- Rentier, E.S.; Hoorn, C.; Seijmonsbergen, A.C. Lithium brine mining affects geodiversity and sustainable development goals. Renew. Sustain. Energy Rev. 2024, 202, 114642. [Google Scholar] [CrossRef]
- Kölbel, L.; Kölbel, T.; Herrmann, L.; Kaymakci, E.; Ghergut, I.; Poirel, A.; Schneider, J. Lithium extraction from geothermal brines in the Upper Rhine Graben: A case study of potential and current state of the art. Hydrometallurgy 2023, 221, 106131. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.H.; Moon, S.J.; Jung, J.T.; Wang, H.H.; Ali, A.; Quist-Jensen, C.A.; Macedonio, F.; Drioli, E.; Lee, Y.M. Lithium recovery from artificial brine using energy-efficient membrane distillation and nanofiltration. J. Membr. Sci. 2020, 598, 117683. [Google Scholar] [CrossRef]
- Xu, S.; Song, J.; Bi, Q.; Chen, Q.; Zhang, W.M.; Qian, Z.; Zhang, L.; Xu, S.; Tang, N.; He, T. Extraction of lithium from Chinese salt-lake brines by membranes: Design and practice. J. Membr. Sci. 2021, 635, 119441. [Google Scholar] [CrossRef]
- Ambrose, H.; Kendall, A. Understanding the future of lithium: Part 2, temporally and spatially resolved life-cycle assessment modeling. J. Ind. Ecol. 2019, 224, 90–100. [Google Scholar] [CrossRef]
- Ibarra-Gutiérrez, S.; Bouchard, J.; Laflamme, M.; Fytas, K. Perspectives of lithium mining in Quebec, potential and advantages of integration into a local battery production chain for electric vehicles. Mater. Proc. 2021, 5, 33. [Google Scholar]
- Toupal, J.; Vann, D.R.; Zhu, C.; Gieré, R. Geochemistry of surface waters around four hard-rock lithium deposits in Central Europe. J. Geochem. Explor. 2022, 234, 106937. [Google Scholar] [CrossRef]
- Bazamad, M.; Tangestani, M.H.; Asadi, S.; Staubwasser, M. Investigating the geochemical behavior and exploration potential of lithium in brines; a case study of Bam salt plug, Zagros Zone, southern Iran. Sci. Rep. 2023, 13, 21567. [Google Scholar] [CrossRef]
- Necke, T.; Stein, J.; Kleebe, H.; Balke-Grünewald, B. Lithium extraction and zeolite synthesis via mechanochemical treatment of the silicate minerals lepidolite, spodumene, and petalite. Minerals 2023, 13, 1030. [Google Scholar] [CrossRef]
- Yang, X.; Wen, H.; Lin, Y.; Zhang, H.; Liu, Y.; Fu, J.; Liu, Q.; Jiang, G. Emerging research needs for characterizing the risks of global lithium pollution under carbon neutrality strategies. Environ. Sci. Technol. 2023, 57, 5103–5106. [Google Scholar] [CrossRef]
- Liu, W.; Agusdinata, F.B.; Myint, S.W. Spatiotemporal patterns of lithium mining and environmental degradation in the Atacama Salt Flat, Chile. Int. J. Appl. Earth Observ. Geoinf. 2019, 80, 145–156. [Google Scholar] [CrossRef]
- Mousavinezhad, S.; Nili, S.; Fahimi, A.; Vahidi, E. Environmental impact assessment of direct lithium extraction from brine resources: Global warming potential, land use, water consumption, and charting sustainable scenarios. Resour. Conserv. Recycl. 2024, 205, 107583. [Google Scholar] [CrossRef]
- Yang, X.; Wen, H.; Liu, Y.; Huang, Y.; Zhang, Q.; Wang, W.; Zhang, H.; Fu, J.; Li, G.; Liu, Q.; et al. Lithium pollution and its associated health risks in the largest lithium extraction industrial area in China. Environ. Sci. Technol. 2024, 58, 11637–11648. [Google Scholar] [CrossRef]
- Ma, G.; Jiang, J.; Wei, Y.; Cai, A.; Wang, L.; Zhou, H. Lithium extraction from salt lake via rocking-chair flow electrode capacitive deionization with monovalent selective membrane. Desalination 2025, 600, 118516. [Google Scholar] [CrossRef]
- Kelly, J.; Wang, M.; Dai, Q.; Winjobi, O. Energy, greenhouse gas, and water life cycle analysis of lithium carbonate and lithium hydroxide monohydrate from brine and ore resources and their use in lithium ion battery cathodes and lithium ion batteries. Resour. Conserv. Recycl. 2021, 174, 105762. [Google Scholar] [CrossRef]
- Nikfar, S.; Fahimi, A.; Vahidi, E. Unlocking sustainable lithium: A comparative life cycle assessment of innovative extraction methods for brine. Resour. Conserv. Recycl. 2025, 212, 107977. [Google Scholar] [CrossRef]
- Blumenstiel, D.; McDonald, M.; Arriaza, B.; Amarasiriwardena, D. Exposure to geogenic lithium in ancient Andeans: Unraveling lithium in mummy hair using LA-ICP-MS. J. Archaeol. Sci. 2020, 113, 105062. [Google Scholar] [CrossRef]
- Adeel, M.; Zain, M.; Shakoor, N.; Ahmad, M.A.; Azeem, I.; Aziz, M.A.; Tulcan, R.X.S.; Rathore, A.; Tahir, M.; Horton, R.; et al. Global navigation of Lithium in water bodies and emerging human health crisis. npj Clean Water 2023, 6, 33. [Google Scholar] [CrossRef]
- Hewitt, A.E.; Balks, M.R.; Lowe, D.J. Soils in the Ross Sea Region of Antarctica. In The Soils of Aotearoa New Zealand; World Soils Book Series; Springer: Cham, Switzerland, 2021; pp. 267–287. [Google Scholar]
- Coffey, D.M.; Munk, L.A.; Ibarra, D.E.; Butler, K.L.; Boutt, D.F.; Jenckes, J. Lithium storage and release from lacustrine sediments: Implications for lithium enrichment and sustainability in continental brines. Geochem. Geophys. Geosyst. 2021, 22, e2021GC009916. [Google Scholar] [CrossRef]
- Figueroa, L.; Barton, S.; Schull, W.; Razmilic, B.; Zumaeta, O.; Young, A.; Kamiya, Y.; Hoskins, J.; Ilgren, E. Environmental lithium exposure in the North of Chile—I. Natural water sources. Biol. Trace Elem. Res. 2012, 149, 280–290. [Google Scholar] [CrossRef]
- Sarchi, C.; Lucassen, F.; Meixner, A.; Caffe, P.J.; Becchio, R.; Kasemann, S.A. Lithium enrichment in the Salar de Diablillos, Argentina, and the influence of Cenozoic volcanism in a basin dominated by Paleozoic basement. Miner. Deposita 2023, 58, 1351–1370. [Google Scholar] [CrossRef]
- Alam, M.A.; Muñoz, A. A critical evaluation of the role of a geothermal system in lithium enrichment of brines in the salt flats: A case study from Laguna Verde in the Atacama Region of Chile. Geothermics 2024, 119, 102970. [Google Scholar] [CrossRef]
- Shakoor, N.; Adeel, M.; Ahmad, M.A.; Zain, M.; Waheed, U.; Javaid, R.A.; Haider, F.U.; Azeem, I.; Zhou, P.; Li, Y.; et al. Reimagining safe lithium applications in the living environment and its impacts on human, animal, and plant system. Environ. Sci. Ecotechnol. 2023, 15, 100252. [Google Scholar] [CrossRef] [PubMed]
- Aral, A.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment—A literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef]
- Millot, R.; Négrel, P. Lithium isotopes in the Loire River Basin (France): Hydrogeochemical characterizations at two complementary scales. Appl. Geochem. 2021, 125, 104831. [Google Scholar] [CrossRef]
- King, S.; Boxall, N.J. Lithium battery recycling in Australia: Defining the status and identifying opportunities for the development of a new industry. J. Clean. Prod. 2019, 215, 1279–1287. [Google Scholar] [CrossRef]
- Robinson, B.H.; Yalamanchali, R.; Reiser, R.; Dickinson, N.M. Lithium as an emerging environmental contaminant: Mobility in the soil-plant system. Chemosphere 2018, 197, 1–6. [Google Scholar] [CrossRef]
- Ishchenko, V. Heavy metals in municipal waste: The content and leaching ability by waste fraction. J. Environ. Sci. Health Part A 2019, 54, 1448–1456. [Google Scholar] [CrossRef]
- Kilgo, M.K.; Anctil, A.; Kennedy, M.S.; Powell, B.A. Metal leaching from Lithium-ion and Nickel-metal hydride batteries and photovoltaic modules in simulated landfill leachates and municipal solid waste materials. Chem. Eng. J. 2022, 431, 133825. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Proctor, K.; Jagadeesan, K.; Watkins, S.; Standerwick, R.; Barden, R.; Barnett, J. Diagnosing down-the-drain disposal of unused pharmaceuticals at a River Catchment level: Unrecognized sources of environmental contamination that require nontechnological solutions. Contam. Aquat. Terr. Environ. 2021, 5, 11657–11666. [Google Scholar] [CrossRef]
- Boafo, J.; Obodai, J.; Stemn, E.; Nkrumah, P.N. The race for critical minerals in Africa: A blessing or another resource curse? Resour. Policy 2024, 93, 105046. [Google Scholar] [CrossRef]
- Viana, T.; Ferreira, N.; Henriques, B.; Leite, C.; De Marchi, L.; Amaral, J.; Freitas, R.; Pereira, E. How safe are the new green energy resources for marine wildlife? The case of lithium. Environ. Pollut. 2020, 267, 115458. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.P.; Yadav, V.; Rajput, A.; Gupta, H.; Saravaia, H.; Kulshrestha, V. Sulfonated poly (ether ether ketone) composite cation exchange membrane for selective recovery of lithium by electrodialysis. Desalination 2020, 496, 114755. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Drinking Water Contaminant Candidate List 5-Final; Environmental Protection Agency: Washington, DC, USA, 2022.
- Bradley, D.; Stillings, L.L.; Jaskula, B.W.; Munk, L.; McCauley, A.D. Lithium; Report 1802K; Professional Paper; USGS Publications Warehouse: Reston, VA, USA, 2017; p. 34.
- McKnight, R.F.; Adida, M.; Budge, K.; Stockton, S.; Goodwin, G.M.; Geddes, J.R. Lithium toxicity profile: A systematic review and meta-analysis. Lancet 2012, 379, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sands, M.; Lin, M.; Guelfo, J.; Irudayaraj, J. In Vitro Toxicity of Lithium Bis(Trifluoromethanesulfonyl)Imide (LiTFSI) on Human Renal and Hepatoma Cells. Toxicol. Rep. 2024, 12, 280–288. [Google Scholar] [CrossRef]
- Cipriani, A.; Hawton, K.; Stockton, S.; Geddes, J.R. Lithium in the prevention of suicide in mood disorders: Updated systematic review and meta-analysis. BMJ Br. Med. J. 2013, 346, f3646. [Google Scholar] [CrossRef]
- Tkatcheva, V.; Poirier, D.; Chong-Kit, R.; Furdui, V.L.; Burr, C.; Leger, R.; Parmar, J.; Switzer, T.; Maedler, S.; Reiner, E.J.; et al. Lithium an emerging contaminant: Bioavailability, effects on protein expression, and homeostasis disruption in short-term exposure of rainbow trout. Aquat. Toxicol. 2015, 161, 85–93. [Google Scholar] [CrossRef]
- Dwyer, F.J.; Burch, S.A.; Ingersoll, C.G.; Hunn, J.B. Toxicity of trace element and salinity mixtures to striped bass (Morone saxatilis) and Daphnia magna. Environ. Toxicol. Chem. 1992, 11, 513–520. [Google Scholar] [CrossRef]
- Hamilton, S.J. Hazard Assessment of Inorganics to Three Endangered Fish in the Green River, Utah. Ecotoxicol. Environ. Saf. 1995, 30, 134–142. [Google Scholar] [CrossRef]
- Kim, D.; Choi, J.H.; Hong, Y.P.; Ryoo, K.S. Use of loess as adsorbent for recovery of Li⁺ from seawater. Bull. Korean Chem. Soc. 2017, 38, 5–11. [Google Scholar] [CrossRef]
- Fraga, N.; Benito, D.; Briaudeau, T.; Izagirre, U.; Ruiz, P. Toxicopathic effects of lithium in mussels. Chemosphere 2022, 307, 136022. [Google Scholar] [CrossRef]
- Peltzer, P.M.; Cuzziol Boccioni, A.P.; Attademo, A.M.; Simoniello, M.F.; Lener, G.; Lajmanovich, R.C. Ecotoxicological characterization of lithium as a “timebomb” in aquatic systems: Tadpoles of the South American toad Rhinella arenarum (Hensel, 1867) as model organisms. Toxics 2024, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Le, Q. Synthesis and performance of cerium oxide as anode materials for lithium ion batteries by a chemical precipitation method. J. Alloys Compd. 2016, 669, 142973. [Google Scholar] [CrossRef]
- Wu, L.; Hu, J.; Chen, S.; Yang, X.; Liu, L.; Foord, J.S.; Pobedinskas, P.; Haenen, K.; Hou, H.; Yang, J. Lithium nitrate mediated dynamic formation of solid electrolyte interphase revealed by in situ Fourier transform infrared spectroscopy. Electrochim. Acta 2023, 466, 142973. [Google Scholar] [CrossRef]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of iron, aluminium, manganese and copper from leach solutions of lithium-ion battery waste using ion exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Chen, J.; Lin, S.; Yu, J. High-selective cyclic adsorption and magnetic recovery performance of magnetic lithium-aluminum layered double hydroxides (MLDHs) in extracting Li+ from ultrahigh Mg/Li ratio brines. Sep. Purif. Technol. 2021, 255, 117710. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Seriche, G.; González, C.; Piaggio, G.; Barros, L.; Gallardo, F.; Díaz-Quezada, S.; Zamora, D.; Barraza, B.; Ruby-Figueroa, R.; et al. Membrane distillation-crystallization applied to a multi-ion hypersaline lithium brine for water recovery and crystallization of potassium and magnesium salts. Desalination 2024, 586, 117895. [Google Scholar] [CrossRef]
- Ni, C.; Liu, C.; Han, Z.; Wang, J.; Liang, Y.; Zhong, H.; He, Z. Sustainable and efficient recovery of lithium from rubidium raffinate via solvent extraction. J. Environ. Chem. Eng. 2024, 12, 113374. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Z.; Xu, W.; Xiong, J.; He, L. A closed-loop process for selective lithium recovery from brines via electrochemical and precipitation. Desalination 2021, 519, 115302. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Meng, Q.; Dong, P.; Ning, P.; Li, Q. Recovery of valuable metals from mixed spent lithium-ion batteries by multi-step directional precipitation. RSC Adv. 2021, 11, 268–277. [Google Scholar] [CrossRef]
- Shin, J.; Jeong, J.M.; Lee, J.B.; Cho, H.J.; Kim, Y.H.; Ryu, T. Preparation of lithium carbonate from waste lithium solution through precipitation and wet conversion methods. Hydrometallurgy 2022, 210, 105863. [Google Scholar] [CrossRef]
- Xiao, C.; Zeng, L. Thermodynamic study on recovery of lithium using phosphate precipitation method. Hydrometallurgy 2018, 178, 283–286. [Google Scholar] [CrossRef]
- Huang, Y.; Han, G.; Liu, J.; Chai, W.; Wang, W.; Yang, S.; Su, S. A stepwise recovery of metals from hybrid cathodes of spent Li-ion batteries with leaching-flotation-precipitation process. J. Power Sour. 2016, 325, 555–564. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Zhang, Y.; Zhang, Y.; Zheng, S. Lithium adsorption from brine by iron-doped titanium lithium ion sieves. Particuology 2018, 41, 40–47. [Google Scholar] [CrossRef]
- Lv, Y.; Xing, P.; Ma, B.; Liu, Y.; Wang, C.; Zhang, W.; Chen, Y. Efficient extraction of lithium and rubidium from polylithionite via alkaline leaching combined with solvent extraction and precipitation. ACS Sustain. Chem. Eng. 2020, 8, 14462–14470. [Google Scholar] [CrossRef]
- Mahandra, H.; Ghahreman, A. A sustainable process for selective recovery of lithium as lithium phosphate from spent LiFePO4 batteries. Resour. Conserv. Recycl. 2021, 175, 105883. [Google Scholar] [CrossRef]
- Manikandan, S.; Inbakandan, D.; Nachiyar, C.V.; Namasivayam, S.K.R. Towards sustainable metal recovery from e-waste: A mini review. Sustain. Chem. Environ. 2023, 2, 100001. [Google Scholar] [CrossRef]
- Nikkhah, H.; Ipekci, D.; Xiang, W.; Stoll, Z.; Xu, P.; Li, B.; McCutcheon, J.R.; Beykal, B. Challenges and opportunities of recovering lithium from seawater, produced water, geothermal brines, and salt lakes using conventional and emerging technologies. Chem. Eng. J. 2024, 498, 155349. [Google Scholar] [CrossRef]
- Qiu, Y.; Ruan, H.; Tang, C.; Yao, L.; Shen, J.; Scotto, A. Study on recovering high-concentration lithium salt from lithium-containing wastewater using a hybrid reverse osmosis (RO)–electrodialysis (ED) process. ACS Sustain. Chem. Eng. 2019, 7, 13481–13490. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Asif, M.B.; Kentish, S.; Nghiem, L.D.; Hai, F.I. Lithium enrichment from a simulated salt lake brine using an integrated nanofiltration-membrane distillation process. J. Environ. Chem. Eng. 2019, 7, 103395. [Google Scholar] [CrossRef]
- Mahto, A.; Aruchamy, K.; Meena, R.; Kamali, M.; Kotrappanavar, N.S.; Aminabhavi, T.M. Forward osmosis for industrial effluents treatment—Sustainability considerations. Sep. Purif. Technol. 2021, 254, 117568. [Google Scholar] [CrossRef]
- Martins, G.C.; Choo, Y.; Park, M.J.; Shon, H.K.; Naidu, G. Rare earth europium recovery using selective metal-organic framework incorporated mixed-matrix membrane. Chemosphere 2024, 364, 143272. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kim, S.H.; Yoo, C.H.; Lee, H.J.; Nguyen, B.T.D.; Lee, G.G.; Kim, J.F.; Lee, J.S. Valorization of battery manufacturing wastewater: Recovery of high-value metal ions through reaction-enhanced membrane cascade. Chem. Eng. J. 2024, 493, 152247. [Google Scholar] [CrossRef]
- Cao, D.Q.; Jin, Y.; Liu, H.; Lei, S.C.; Song, Y.X.; Han, J.L.; Hao, X.D.; Ma, M.G.; Zhang, Z.; Wu, R. Concentration properties of biopolymers via dead-end forward osmosis. Int. J. Biol. Macromol. 2024, 270, 132338. [Google Scholar] [CrossRef] [PubMed]
- Humudat, Y.R.; Abdul-Majeed, M.A.; Khraibet, A.C. Membrane filtration enhanced by magnetic field for reducing endotoxin from dialysis water. Desalination Water Treat. 2024, 318, 100315. [Google Scholar] [CrossRef]
- Han, B.; Chevrier, S.M.; Yan, Q.; Gabriel, J.C.P. Tailorable metal–organic framework based thin film nanocomposite membrane for lithium recovery from wasted batteries. Sep. Purif. Technol. 2024, 334, 125943. [Google Scholar] [CrossRef]
- Ma, W.; Han, G.; Li, J.; An, X.; Gao, F.; Du, X.; Li, J.; Liu, Z.; Guan, G.; Hao, X. Hierarchical electroactive ion permselective membrane with electrochemical switched ion pump effect for continuous lithium-ion recovery. J. Membr. Sci. 2024, 700, 122719. [Google Scholar] [CrossRef]
- Alkenani, A.; Saleh, T.A. Synthesis of amine-modified graphene integrated membrane as protocols for simultaneous rejection of hydrocarbons pollutants, metal ions, and salts from water. J. Mol. Liq. 2022, 367, 120291. [Google Scholar] [CrossRef]
- Shi, C.; Jing, Y.; Jia, Y. Solvent extraction of lithium ions by tri-n-butyl phosphate using a room temperature ionic liquid. J. Mol. Liq. 2016, 215, 640–646. [Google Scholar] [CrossRef]
- Luo, X.; Guo, B.; Luo, J.; Deng, F.; Zhang, S.; Luo, S.; Crittenden, J. Recovery of lithium from wastewater using development of Li ion-imprinted polymers. ACS Sustain. Chem. Eng. 2015, 3, 460–467. [Google Scholar] [CrossRef]
- Almousa, M.; Lim, Y.; AlMubaidin, M.; Alshami, A.; Al-Tayyem, B.; Tomomewo, O.; Khalifa, H. Comparative feasibility of lithium extraction technologies in U.S. oilfields. Desalination Water Treat. 2025, 322, 101128. [Google Scholar] [CrossRef]
- Hosseini, N.; Saadmohammadi, A.; Kianoush, P. Innovative approaches to lithium extraction in Iran: Assessing resource potential and sustainable practices for a competitive future. Results Eng. 2025, 27, 105745. [Google Scholar] [CrossRef]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-Din, W.; Hameed, M.A.; Abrar, M.M.; Maitlo, A.A.; Noreen, S.; et al. Current and emerging adsorbent technologies for wastewater treatment: Trends, limitations, and environmental implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Santos, D.H.S.; Santos, J.P.T.S.; Duarte, J.L.S.; Oliveira, L.M.T.M.; Tonholo, J.; Meili, L.; Zanta, C.L.P.S. Regeneration of activated carbon adsorbent by anodic and cathodic electrochemical process. Process. Saf. Environ. Prot. 2022, 159, 1150–1163. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Wan, P.; Gasem, K.; Wang, K.; He, T.; Adidharma, H.; Fan, M. Extraction of lithium with functionalized lithium ion-sieves. Prog. Mater. Sci. 2016, 84, 276–313. [Google Scholar] [CrossRef]
- Wang, S.; Li, P.; Zhang, X.; Zheng, S.; Zhang, Y. Selective adsorption of lithium from high Mg-containing brines using HxTiO3 ion sieve. Hydrometallurgy 2017, 174, 21–28. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, E.H.; Yan, G.; Yang, Y.Z.; Liu, W.F.; Liu, X.G. A lithium ion-imprinted adsorbent using magnetic carbon nanospheres as a support for the selective recovery of lithium ions. New Carbon Mater. 2020, 35, 696–706. [Google Scholar] [CrossRef]
- Huangfu, C.; Yu, S.; Tong, B.; Yang, A.; Lyu, J.; Guo, X. Efficient lithium extraction from aqueous solutions by MIL-100(Fe): A study on adsorption kinetics, thermodynamics and mechanism. Sep. Purif. Technol. 2023, 322, 124365. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Functionalized titanate nanotubes for efficient lithium adsorption and recovery from aqueous media. J. Solid State Chem. 2020, 283, 121157. [Google Scholar] [CrossRef]
- Liu, J.; An, X.; Huang, L.; Zhou, H.; Liang, D.; Xie, Q.; Fan, M. In-situ magnetization of porous carbon beads for lithium-ion adsorption from strongly acidic solution. J. Clean. Prod. 2024, 435, 140625. [Google Scholar] [CrossRef]
- Wahib, S.A.; Da’na, D.A.; Zaouri, N.; Hijji, Y.M.; Al-Ghouti, M.A. Adsorption and recovery of lithium ions from groundwater using date pits impregnated with cellulose nanocrystals and ionic liquid. J. Hazard. Mater. 2022, 421, 126657. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Monteiro, J.; Futuro, A.; Fiúza, A. Lithium sorption by ion exchange. Miner. Eng. 2024, 214, 108786. [Google Scholar] [CrossRef]
- Wesselborg, T.; Asumalahti, S.; Virolainen, S.; Sainio, T. Continuous multicolumn ion exchange process for spent lithium-ion battery leachate: Recovery and purification of a Li+Ni+Co mixture. Sep. Purif. Technol. 2025, 353, 128351. [Google Scholar] [CrossRef]
- Yang, S.; Liu, G.; Wang, J.; Cui, L.; Chen, Y. Recovery of lithium from alkaline brine by solvent extraction with functionalized ionic liquid. Fluid Phase Equilibria 2019, 493, 129–136. [Google Scholar] [CrossRef]
- Neves, H.P.; Ferreira, G.M.D.; de Lemos, L.R.; Rodrigues, G.D.; Leã, V.A.; Mageste, A.B. Liquid-liquid extraction of rare earth elements using systems that are more environmentally friendly: Advances, challenges and perspectives. Sep. Purif. Technol. 2022, 282, 120064. [Google Scholar] [CrossRef]
- Riaño, S.; Foltova, S.S.; Binnemans, K. Separation of neodymium and dysprosium by solvent extraction using ionic liquids combined with neutral extractants: Batch and mixer-settler experiments. RSC Adv. 2019, 10, 307–316. [Google Scholar] [CrossRef]
- Waengwan, P.; Eksangsri, T. Recovery of lithium from simulated secondary resources (LiCO3) through solvent extraction. Sustainability 2020, 12, 7179. [Google Scholar] [CrossRef]
- Niu, Z.; Xu, T.; Zhang, L.; Ji, L.; Li, L. Mechanism and process study of lithium extraction by 2-ethylhexyl salicylate extraction system. J. Clean. Prod. 2024, 446, 141351. [Google Scholar] [CrossRef]
- Yuan, H.; Li, M.; Cui, L.; Wang, L.; Chen, F. Electrochemical extraction technologies of lithium: Development and challenges. Desalination 2025, 598, 118419. [Google Scholar] [CrossRef]
- Qu, B.; Liang, J.; Huo, D.; Li, H. Application of electrochemical methods in the recycling of spent lithium-ion batteries. J. Energy Storage 2025, 127, 117180. [Google Scholar] [CrossRef]
- Choudhary, N.; Rajpurohit, D.; Saha, A.; Yadav, S.; Tothadi, S.; Ganguly, B.; Paital, A. Lithium sequestration from dilute solutions and sea bittern inspired by the self-assembled complexation. Chem. Eng. J. 2023, 470, 144408. [Google Scholar] [CrossRef]
- Yu, H.; Wang, C.; Phuntsho, S.; He, T.; Naidu, G.; Han, D. Highly selective lithium recovery from seawater desalination brine using Li2TiO3 membrane-coated capacitive deionization. Water Res. 2025, 285, 124113. [Google Scholar] [CrossRef]
- Alera, A.; Benitez, J.P.; Fernandez, R.; Pascual, C.; Policarpio, F.; Lopez, E. Recent advances in lithium extraction. Eng. Proc. 2024, 67, 52. [Google Scholar]
- Hwang, C.W.; Jeong, M.H.; Kim, Y.J.; Son, W.K.; Kang, K.S.; Lee, C.S.; Hwang, T.S. Process design for lithium recovery using bipolar membrane electrodialysis system. Sep. Purif. Technol. 2016, 166, 34–40. [Google Scholar] [CrossRef]
- Biswal, B.K.; Jadhav, U.U.; Madhaiyan, M.; Ji, L.; Yang, E.H.; Cao, B. Biological leaching and chemical precipitation methods for recovery of Co and Li from spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 9, 12343–12352. [Google Scholar] [CrossRef]
- Shi, P.; Yang, S.; Wu, G.; Chen, H.; Chang, D.; Jie, Y.; Fang, G.; Mo, C.; Chen, Y. Efficient separation and recovering of lithium and manganese from spent lithium-ion batteries powder leaching solution. Sep. Purif. Technol. 2023, 309, 123063. [Google Scholar] [CrossRef]
- Velázquez, L.E.R.; Palos, L.; Mostefa, M.L.P.; Muhr, H. Recovery of lithium from Li-ion battery leachate by gas-liquid precipitation. J. Cryst. Growth 2024, 631, 127625. [Google Scholar] [CrossRef]
- Tawonezvi, T.; Zide, D.; Nomnqa, M.; Madondo, M.; Petrik, L.; Bladergroen, B.J. Recovery of NixMnyCoz(OH)2 and Li2CO3 from spent Li-ion cathode leachates using non-Na precipitant-based chemical precipitation for sustainable recycling. Chem. Eng. J. Adv. 2024, 17, 100582. [Google Scholar] [CrossRef]
- Chen, X.; Fan, B.; Zhou, T.; Kong, J. An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries. J. Clean. Prod. 2016, 112, 3562–3570. [Google Scholar] [CrossRef]
- Guo, X.; Cao, X.; Huang, G.; Tian, Q.; Sun, H. Recovery of lithium from the effluent obtained in the process of spent lithium-ion batteries recycling. J. Environ. Manag. 2017, 198, 84–89. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Y.; Du, B.; Zhao, Y.; Wang, M. Recovery of both magnesium and lithium from high Mg/Li ratio brines using a novel process. Hydrometallurgy 2018, 175, 102–108. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Cao, H.; Zheng, X.; Gerven, T.V.; Hu, Y.; Sun, Z. Lithium carbonate recovery from lithium-containing solution by ultrasound assisted precipitation. Ultrason. Sonochem. 2019, 52, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; He, M.; Cao, H.; Zheng, X.; Gao, W.; Sun, Y.; Zhao, H.; Liu, D.; Zhang, Y.; Sun, Z. Investigation of solution chemistry to enable efficient lithium recovery from low-concentration lithium-containing wastewater. Front. Chem. Sci. Eng. 2020, 14, 639–650. [Google Scholar] [CrossRef]
- Han, B.; Haq, R.A.U.; Louhi-Kultanen, M. Lithium carbonate precipitation by homogeneous and heterogeneous reactive crystallization. Hydrometallurgy 2020, 195, 105386. [Google Scholar] [CrossRef]
- Alsabbagh, A.; Aljarrah, S.; Almahasneh, M. Lithium enrichment optimization from Dead Sea end brine by chemical precipitation technique. Miner. Eng. 2021, 170, 107038. [Google Scholar] [CrossRef]
- Mulwanda, J.; Senanayake, G.; Oskierski, H.; Altarawneh, M.; Dlugogorski, B.Z. Leaching of lepidolite and recovery of lithium hydroxide from purified alkaline pressure leach liquor by phosphate precipitation and lime addition. Hydrometallurgy 2021, 201, 105538. [Google Scholar] [CrossRef]
- Battaglia, G.; Berkemeyer, L.; Cipollina, A.; Cortina, J.L.; de Labastida, M.F.; López-Rodríguez, J.; Winter, D. Recovery of lithium carbonate from dilute Li-rich brine via homogenous and heterogeneous Precipitation. Ind. Eng. Chem. Res. 2022, 61, 13589–13602. [Google Scholar] [CrossRef]
- Guan, J.; Zhou, Z.; Li, N.; Liu, Z.; Wen, H.; Li, J.; Hu, L.; Liu, L.; Luo, C. Extracting lithium from the H2SO4 leaching solution of bauxitic claystone via co-precipitation methods without addition of Al source. Chem. Eng. J. Adv. 2022, 9, 100223. [Google Scholar] [CrossRef]
- Pavón, S.; Kah, M.; Hippman, S.; Bertau, M. Lithium recovery from production waste by thermal pre-treatment. Sustain. Chem. Pharm. 2022, 28, 100725. [Google Scholar] [CrossRef]
- Gu, K.; Feng, W.; Wei, H.; Dang, L. The factors influencing lithium carbonate crystallization in spent lithium-ion battery leachate. Processes 2024, 12, 753. [Google Scholar] [CrossRef]
- Lawagon, C.P.; Nisola, G.M.; Cuevas, R.A.I.; Kim, H.; Lee, S.P.; Chung, W.J. Li1−xNi0.33Co1/3Mn1/3O2/Ag for electrochemical lithium recovery from brine. Chem. Eng. J. 2018, 348, 1000–1011. [Google Scholar] [CrossRef]
- Chang, H.; Lin, J.; Cheng, T.; Lai, C. Advanced absolute chemical precipitation for high-purity metal recovery in all-types of lithium-ion battery recycling. Sep. Purif. Technol. 2025, 361, 131454. [Google Scholar] [CrossRef]
- Shokri, Z.; Mousavi, S.; Omidkhah, M. Advancing sustainable lithium recovery from spent laptop lithium-ion battery cathodes through high pulp density bioleaching followed by Li2CO3 precipitation: Process intensification and environmental insights. J. Environ. Chem. Eng. 2025, 13, 117014. [Google Scholar] [CrossRef]
- Lei, F.; Xu, X.; Wang, Q.; Xie, H.; Gan, W. Selective and efficient recovery of lithium from mother liquor from lithium carbonate precipitation process. JOM 2024, 76, 539–5407. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, Q.; Zheng, F.; Lu, J. Enhancement in CO2 adsorption by zeolite synthesized from co-combustion ash of coal and rice husk modified with lithium ion. J. Energy Inst. 2023, 110, 101348. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, B.; Zeng, B.; Qiu, S.; Zhong, X.; Wang, R. Recovery of transition metals (Ni, Co, and Mn) and Li from the sulfate leach solutions of spent ternary lithium-ion batteries by stepwise solvent extraction and precipitation. Hydrometallurgy 2025, 236, 106519. [Google Scholar] [CrossRef]
- Wang, K.; Arcisauskaite, V.; Jiao, J.; Zhang, J.; Zhang, T.; Zhou, Z.-N. Structural prediction, analysis and decomposition mechanism of solid M(NH2BH3)n (M = Mg, Ca and Al). RSC Adv. 2014, 28, 14624–14632. [Google Scholar] [CrossRef]
- Xiao, W.; Peng, H.; Wang, H.; Bian, Z. Impact of interfering ions on λ-MnO2 for lithium recovery from brine. Sep. Purif. Technol. 2025, 365, 132657. [Google Scholar] [CrossRef]
- Quintero, C.; Dahlkamp, J.; Fierro, F.; Thennis, T.; Zhang, Y.; Videla, A.; Rojas, R. Development of a co-precipitation process for the preparation of magnesium hydroxide containing lithium carbonate from Li-enriched brines. Hydrometallurgy 2020, 198, 105515. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, R.; Huang, Y.; Wang, X.; Chen, L.; Sun, X.; Wang, M.; Zhang, Y.; Wu, K. High efficiency leaching of black powder from spent lithium-ion battery by ternary deep eutectic solvent and recovery of metals by precipitation and electrodeposition. Sep. Purif. Technol. 2025, 364, 132438. [Google Scholar] [CrossRef]
- Wang, W.Y.; Yen, C.H.; Lin, J.L.; Xu, R.B. Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J. Mater. Cycles Waste Manag. 2019, 21, 300–307. [Google Scholar] [CrossRef]
- Aprilianto, D.; Perdana, I.; Aluicius, I.; Adi-Kusumo, F.; Rochmadi; Petrus, H.T. Controlled particle size distribution in ultrasound-assisted lithium carbonate precipitation. ACS Omega 2025, 10, 32226–32245. [Google Scholar] [CrossRef]
- Mrozik, W.; Rajaeifar, M.A.; Heidrich, O.; Christensen, P. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- Sahu, L.; Yadav, D.; Ingole, P. Recent developments and innovations in thin-film nanocomposite nanofiltration: The next generation selective membrane for heavy metal ion removal from water. Chem. Eng. J. 2025, 513, 162579. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, F.; Jia, H.; Chen, Z.; Ni, B. Applications of membrane technology in the resource recovery of power lithium-ion battery precursor wastewater: A review. Environ. Res. 2025, 275, 121372. [Google Scholar] [CrossRef] [PubMed]
- Tawalbeh, M.; Qalyoubi, L.; Al-Othman, A.; Qasim, M.; Shirazi, M. Insights on the development of enhanced antifouling reverse osmosis membranes: Industrial applications and challenges. Desalination 2023, 553, 116460. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Zou, P.; Ye, R.; Wang, L. Synthesis and application of anthraquinone quaternary phosphonium salt electroplating copper leveler based on expanded electrostatic adsorption area strategy and physicochemical salt bridge mechanism. Appl. Suf. Sci. 2024, 664, 160259. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.; Li, J. Membrane-based technologies for lithium recovery from water lithium resources: A review. J. Membr. Sci. 2019, 291, 117317. [Google Scholar] [CrossRef]
- Sonoc, A.C.; Jeswiet, J.; Murayama, N.; Shibata, J. A study of the application of Donnan dialysis to the recycling of lithium ion batteries. Hydrometallurgy 2018, 175, 133–143. [Google Scholar] [CrossRef]
- He, R.; Xu, S.; Wang, R.; Bai, B.; Lin, S.; He, T. Polyelectrolyte-based nanofiltration membranes with exceptional performance in Mg2+/Li+ separation in a wide range of solution conditions. J. Membr. Sci. 2022, 663, 121027. [Google Scholar] [CrossRef]
- Bunani, S.; Arda, M.; Kabay, N.; Yoshizuka, K.; Nishihama, S. Effect of process conditions on recovery of lithium and boron from water using bipolar membrane electrodialysis (BMED). Desalination 2017, 416, 10–15. [Google Scholar] [CrossRef]
- He, S.; Zuo, Q.; Shi, H.; Geng, Z.; Liu, C.; Ding, L.; Li, X.; Shao, P.; Yang, L.; Luo, X. Direct recovery of high-purity lithium via nanofiltration membranes from leaching solution of spent lithium batteries. Resour. Conserv. Recycl. 2024, 210, 107846. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Chen, Q.B.; Yuan, J.S.; Liu, J.; Zhao, Y.Y.; Feng, W.X. Preliminary study on recovering lithium from high Mg2+/Li+ ratio brines by electrodialysis. Sep. Purif. Technol. 2017, 172, 168–177. [Google Scholar] [CrossRef]
- Nie, X.Y.; Sun, S.Y.; Sun, Z.; Song, X.; Yu, J.G. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 2017, 403, 128–135. [Google Scholar] [CrossRef]
- Ípekçi, D.; Altiok, E.; Bunani, S.; Yoshizuka, K.; Nishihama, S.; Arda, M.; Kabay, N. Effect of acid-base solutions used in acid-base compartments for simultaneous recovery of lithium and boron from aqueous solution using bipolar membrane electrodialysis (BMED). Desalination 2018, 448, 69–75. [Google Scholar] [CrossRef]
- Ounissi, T.; Dammak, L.; Fauvarque, J.F.; Hmida, E.S.B.H. Ecofriendly lithium-sodium separation by diffusion processes using lithium composite membrane. Sep. Purif. Technol. 2021, 275, 119134. [Google Scholar] [CrossRef]
- Pham, M.T.; Nishihama, S.; Yoshizuka, K. Concentration of lithium by forward osmosis. Hydrometallurgy 2020, 197, 105485. [Google Scholar] [CrossRef]
- Jarma, Y.; Cermikli, E.; Ipekci, D.; Altiok, E.; Kabay, N. Comparison of two electrodialysis stacks having different ion exchange and bipolar membranes for simultaneous separation of boron and lithium from aqueous solution. Desalination 2021, 500, 114850. [Google Scholar] [CrossRef]
- Mustika, P.C.B.W.; Astuti, W.; Sumardi, S.; Petrus, H.T.B.M. Separation characteristic and selectivity of lithium from geothermal brine using forward osmosis. J. Sustain. Metall. 2022, 8, 1769–1784. [Google Scholar] [CrossRef]
- Jiang, Z.; Kong, W.; Zhao, F.; Han, Q.; Liu, Y.; Wang, S.; Wang, H. Li1.5Al0.5Ge1.5(PO4)3 membrane electrodialysis for lithium enrichment. J. Membr. Sci. 2023, 670, 121353. [Google Scholar] [CrossRef]
- Jeon, S.; Min, T.; Kim, C.; Lim, S.; Yoon, H. Chemical free pH control for efficient lithium recovery via redox-couple mediated bipolar membrane electrodialysis. Desalination 2025, 614, 119145. [Google Scholar] [CrossRef]
- Ma, Q.; Mu, J.; Lv, X.; Meng, J.; Cui, H.; Qiu, Y.; Ruan, H.; Shen, J. Sustainable recovery of ionic resources from resin regeneration wastewater: Long-term evaluation, membrane fouling analysis, and cleaning. ACS EST Water 2022, 3, 1855–1864. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Zhang, Y.; Zhang, Y.; Qiao, S.; Zheng, S. High adsorption performance of the Mo-doped titanium oxide sieve for lithium ions. Hydrometallurgy 2019, 187, 30–37. [Google Scholar] [CrossRef]
- Wang, H.; Huang, K.; Zhang, Y.; Chen, X.; Jin, W.; Zheng, S.; Zhang, Y.; Li, P. Recovery of lithium, nickel, and cobalt from spent lithium-ion battery powders by selective ammonia leaching and an adsorption separation system. ACS Sustain. Chem. Eng. 2017, 5, 11489–11495. [Google Scholar] [CrossRef]
- Gao, J.M.; Du, Z.; Zhao, Q.; Guo, Y.; Cheng, F. Enhanced Li+ adsorption by magnetically recyclable iron-doped lithium manganese oxide ion-sieve: Synthesis, characterization, adsorption kinetics and isotherm. J. Mater. Res. Technol. 2021, 13, 228–240. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, S.; Wang, Z.; Cui, W.; Zhang, H.; Yang, L.; Zhang, Y.; Li, P. Superior lithium adsorption and required magnetic separation behavior of iron-doped lithium ion-sieves. Chem. Eng. J. 2018, 332, 160–168. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, R. An efficient lithium ion imprinted adsorbent using multi-wall carbon nanotubes as support to recover lithium from water. J. Clean. Prod. 2018, 205, 201–209. [Google Scholar] [CrossRef]
- Arroyo, F.; Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H. Lithium recovery from desalination brines using specific ion-exchange resins. Desalination 2019, 468, 114073. [Google Scholar] [CrossRef]
- Xue, F.; Wang, B.; Chen, M.; Yi, C.; Ju, S.; Xing, W. Fe3O4-doped lithium ion-sieves for lithium adsorption and magnetic separation. Sep. Purif. Technol. 2019, 228, 115750. [Google Scholar] [CrossRef]
- Zhu, X.; Yue, H.; Sun, W.; Zhang, L.; Cui, Q.; Wang, H. Study on adsorption extraction process of lithium ion from West Taijinar brine by shaped titanium-based lithium ion sieves. Sep. Purif. Technol. 2021, 274, 119099. [Google Scholar] [CrossRef]
- Alshuiael, A.M.; Al-Ghouti, M.A. Development of a novel tailored ion-imprinted polymer for recovery of lithium and strontium from reverse osmosis concentrated brine. Sep. Purif. Technol. 2022, 295, 121320. [Google Scholar] [CrossRef]
- Ryu, J.C.; Shin, J.; Lim, C.; Kim, K.H.; Ryu, T.; Lee, Y.S. Lithium ion adsorption characteristics of porous Li1.33Mn1.67O4 adsorbent prepared using petroleum-based pitch as a binder. Hydrometallurgy 2022, 209, 105837. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, X.; Yan, X.; Chen, Y.; Lang, W. Zr-doped titanium lithium ion sieve and EP-granulated composite: Superb adsorption and recycling performance. Particuology 2022, 69, 100–110. [Google Scholar] [CrossRef]
- Liu, L.; Kuang, Q.; Xu, S.; Pan, W.; Liu, Y.; Zhou, J.; Tang, A.; Xue, J. Enhanced lithium-ion adsorption by recyclable lithium manganese oxide-sepiolite composite microsphere from aqueous media: Fabrication, structure, and adsorption characteristics. J. Molec. Liq. 2023, 380, 121780. [Google Scholar] [CrossRef]
- Jin, Z.H.; Ma, T.T.; Liu, Y.Y.; Jia, Z.Q.; Tan, H.W.; Peng, W.J. Preparation of tungsten-doped Ti-based lithium ion sieves with excellent adsorption performance by hydrothermal method. J. Alloys Compd. 2024, 1005, 176058. [Google Scholar] [CrossRef]
- Chen, Z.; Du, J.; Shi, J. Mxene-based lithium-ion sieve polymer membrane for sustainable lithium adsorption. Sep. Purif. Technol. 2025, 354, 129316. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep. Purif. Technol. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Adrah, K.; Dawood, S.; Rathnayake, H. Mechanistic understanding of sieving lithium ions using a biobased sorbent technology for sustainable lithium reclamation and cleansing brines. ACS Omega 2024, 9, 21917–21929. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Lei, X.; Lu, C.; Liu, J.; Zhang, G.; Zhang, B.; Zhang, Q. A magnetic ion exchange resin with high efficiency of removing Cr (VI). Colloids Surf. A 2020, 604, 125279. [Google Scholar] [CrossRef]
- Atif, M.; Haider, H.Z.; Bongiovanni, R.; Fayyaz, M.; Razzaq, T.; Gul, S. Physisorption and chemisorption trends in surface modification of carbon black. Surf. Interfaces 2022, 31, 102080. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Zhan, L.; Wu, D.; Zhang, S.; Pang, R.; Xie, B. Removal of emerging contaminants (bisphenol A and antibiotics) from kitchen wastewater by alkali-modified biochar. Sci. Total Environ. 2022, 805, 150158. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qi, Y.; Yang, L.; Wu, L.; Li, P.; Gao, F.; Qi, X.; Zhang, Z. Adsorptive removal of imidacloprid by potassium hydroxide activated magnetic sugarcane bagasse biochar: Adsorption efficiency, mechanism and regeneration. J. Clean. Prod. 2021, 292, 126005. [Google Scholar] [CrossRef]
- Shoghi, A.; Ghasemi, S.; Askari, M.; Khosravi, A.; Hasan-Zadeh, A.; Almolhoda, A. Spinel H4Ti5O12 nanotubes for Li recovery from aqueous solutions: Thermodynamics and kinetics study. J. Environ. Chem. Eng. 2021, 9, 104679. [Google Scholar] [CrossRef]
- Marthi, R.; Asgar, H.; Gadikota, G.; Smith, Y. On the structure and lithium adsorption mechanism of layered H2TiO3. Energy Environ. Catal. Appl. 2021, 13, 8361–8369. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Chen, G.; Li, H.; Duan, Y.; Sun, Y.; Tiraferri, A.; Liu, B. Efficient recovery of lithium from spent lithium-ion battery raffinate by Mn and Al-based adsorbents: Pretreatment, adsorption mechanism, and performance comparison. Sep. Purif. Technol. 2025, 354, 128652. [Google Scholar] [CrossRef]
- Kotsupalo, N.P.; Ryabtsev, A.D.; Poroshina, I.A.; Kurakov, A.A.; Mamylova, E.V.; Menzheres, L.T.; Korchagin, A. Effect of structure on the sorption properties of chlorine-containing form of double aluminum lithium hydroxide. Inorg. Synth. Ind. Inorg. Chem. 2013, 86, 482–487. [Google Scholar] [CrossRef]
- Baskar, A.; Bolan, N.; Hoang, S.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Zante, G.; Braun, A.; Masmoudi, A.; Barrillon, R.; Trébouet, D.; Boltoeva, M. Solvent extraction fractionation of manganese, cobalt, nickel and lithium using ionic liquids and deep eutectic solvents. Miner. Eng. 2020, 156, 106512. [Google Scholar] [CrossRef]
- Ivanović, M.; Razboršek, M.I.; Kolar, M. Innovative extraction techniques using deep eutectic solvents and analytical methods for the isolation and characterization of natural bioactive compounds from plant material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef]
- Wesselborg, T.; Virolainen, S.; Sainio, T. Recovery of lithium from leach solutions of battery waste using direct solvent extraction with TBP and FeCl3. Hydrometallurgy 2021, 202, 105593. [Google Scholar] [CrossRef]
- Hanada, T.; Goto, M. Synergistic deep eutectic solvents for lithium extraction. ACS Sustain. Chem. Eng. 2021, 9, 2152–2160. [Google Scholar] [CrossRef]
- Yu, L.Y.; Wu, K.J.; He, C.H. Tailoring hydrophobic deep eutectic solvent for selective lithium recovery from dilute aqueous solutions. Sep. Purif. Technol. 2022, 281, 119928. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Best Management Practices (BMPs) for Soils Treatment Technologies: Suggested Operational Guidelines to Prevent Cross-Media Transfer of Contaminants; EPA-530-R-97-007; U.S. Environmental Protection Agency: Washington, DC, USA, 1997.
- Harvianto, G.R.; Kim, S.H.; Ju, C.S. Solvent extraction and stripping of lithium ion from aqueous solution and its application to seawater. Rare Met. 2016, 35, 948–953. [Google Scholar] [CrossRef]
- Jang, E.; Jang, Y.; Chung, E. Lithium recovery from shale gas produced water using solvent extraction. Appl. Geochem. 2017, 78, 343–350. [Google Scholar] [CrossRef]
- Lee, J.; Chung, E. Lithium recovery from a simulated geothermal fluid by a combined selective precipitation and solvent extraction method. Geothermics 2022, 102, 102388. [Google Scholar] [CrossRef]
- Xiang, W.; Liang, S.; Zhou, Z.; Qin, W.; Fei, W. Lithium recovery from salt lake brine by counter-current extraction using tributyl phosphate/FeCl3 in methyl isobutyl ketone. Hydrometallurgy 2017, 171, 27–32. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Shi, D.; Peng, X.; Song, F.; Nie, F.; Han, W. Recovery of lithium from alkaline brine by solvent extraction with β-diketone. Hydrometallurgy 2018, 175, 35–42. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Duan, M.; Huang, X. Liquid-liquid extraction of lithium from aqueous solution using novel ionic liquid extractants via COSMO-RS and experiments. Fluid Ph. Equilib 2018, 459, 129–137. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Fan, J.; Liu, X.; Hu, Y.; Hu, Y.; Zhou, Z.; Ren, Z. Recovery of lithium ions from salt lake brine with a high magnesium/lithium ratio using heteropolyacid ionic liquid. ACS Sustain. Chem. Eng. 2019, 7, 3062–3072. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, D.; Li, L.; Peng, X.; Song, F.; Rui, H. Solvent extraction of lithium from ammoniacal solution using thenoyltrifluoroacetone and neutral ligands. J. Mol. Liq. 2019, 274, 746–751. [Google Scholar] [CrossRef]
- Zhang, L.; Lijuan, L.; Rui, H.; Shi, D.; Peng, X.; Ji, L.; Song, X. Lithium recovery from effluent of spent lithium battery recycling process using solvent extraction. J. Hazard. Mater. 2020, 398, 122840. [Google Scholar] [CrossRef]
- Punt, T.; Akdogan, G.; Bradshaw, S.; van Wyk, P. Development of a novel solvent extraction process using citric acid for lithium-ion battery recycling. Miner. Eng. 2021, 173, 107204. [Google Scholar] [CrossRef]
- Han, Z.; Wu, S.; Wu, X.; Guan, W.; Cao, Z.; Li, Q.; Wang, M.; Zhang, G. Recycling of lithium and fluoride from LiF wastewater from LiF synthesis industry by solvent extraction. J. Environ. Chem. Eng. 2023, 11, 110557. [Google Scholar] [CrossRef]
- Raiguel, S.; Nguyen, V.T.; Rodrigues, I.R.; Deferm, C.; Riaño, S.; Binnemans, K. Recovery of lithium from simulated nanofiltration-treated seawater desalination brine using solvent extraction and selective precipitation. Solvent Extr. Ion. Exch. 2023, 41, 425–448. [Google Scholar] [CrossRef]
- Xue, K.; Fan, D.; Wang, X.; Dong, Z.; Zhu, Z.; Chi, P.; Meng, F.; Wang, Y.; Qi, J. Lithium extraction from aqueous medium using hydrophobic deep eutectic solvents. J. Environ. Chem. Eng. 2023, 11, 110490. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, C.; Xiao, Y.; Gong, W.; Wang, Y.; Zhang, H.; Ku, P.; Nie, X. Water acidification aggravates lithium-induced toxicity represented by energy supply, oxidative stress, and cell fate in Daphnia magna neonates. Sci. Total Environ. 2024, 955, 177143. [Google Scholar] [CrossRef]
- Stephens, N.M.; Smith, E.A. Structure of deep eutectic solvents (DESs): What we know, what we want to know, and why we need to know it. Langmuir 2022, 38, 14017–14024. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadehi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Kumar, A.; Fukuda, H.; Hatton, T.A.; Lienhard, J.H. Lithium recovery from oil and gas produced water: A need for a growing energy industry. ACS Energy Lett. 2019, 4, 1471–1474. [Google Scholar] [CrossRef]
- Pham, T.K.; Shin, J.H.; Karima, N.C.; Jun, Y.S.; Jeong, S.K.; Cho, N.; Lee, Y.W.; Cho, Y.; Lim, S.N.; Ahn, W. Application of recycled Si from industrial waste towards Si/rGO composite material for long lifetime lithium-ion battery. J. Power Sources 2021, 506, 230244. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, J.I.; Zhou, X.Y.; Guo, R.; Yu, Y.F.; Hu, Z.; Zhao, H.Y.; Yang, S.H.; Wu, Y.W. Evaluation of optimal waste lithium-ion battery recycling technology driven by multiple factors. J. Energy Storage 2024, 86, 111229. [Google Scholar] [CrossRef]
- Sanginesi, F.; Millacci, G.; Giaccherini, A.; Buccianti, A.; Fusi, L.; Di Benedetto, F.; Pardi, L. Long term lithium availability and electric mobility: What can we learn from resource assessment? J. Geochem. Explor. 2023, 249, 107212. [Google Scholar] [CrossRef]
- Fleming, M.; Kannan, S.G.; Eggert, R. Long-run availability of mineral resources: The dynamic case of lithium. Resour. Policy 2024, 97, 105226. [Google Scholar] [CrossRef]
- Tripathy, A.; Bhuyan, A.; Padhy, R.K.; Mangla, S.K.; Roopak, R. Drivers of lithium-ion batteries recycling industry toward circular economy in industry 4.0. Comput. Ind. Eng. 2023, 179, 109157. [Google Scholar] [CrossRef]
- Ni, H.; Arslan, M.; Liang, Z.; Wang, C.; Luo, Z.; Qian, J.; Wu, Z.; El-Din, M.G. Mixotrophic denitrification processes in basalt fiber bio-carriers drive effective treatment of low carbon/nitrogen lithium slurry wastewater. J. Hazard. Mater. 2022, 364, 128036. [Google Scholar] [CrossRef]
- Le, W.; Zhao, W.; Zhu, Y.; Wei, Z.; Liu, Z.; Liu, D.; Jiao, Q. Stable aluminum-lithium alloy fuels for solid propellants by facile surface modifying. Chem. Eng. J. 2024, 497, 154451. [Google Scholar] [CrossRef]
- Schaller, J.; Headley, T.; Prigent, S.; Breuer, R. Potential mining of lithium, beryllium and strontium from oilfield wastewater after enrichment in constructed wetlands and ponds. Sci. Total Environ. 2014, 493, 910–913. [Google Scholar] [CrossRef]
- Weinand, J.M.; Vandenberg, G.; Risch, S.; Behrens, J.; Pflugradt, N.; Linßen, J.; Stolten, D. Low-carbon lithium extraction makes deep geothermal plants cost-competitive in future energy systems. Adv. Appl. Energy 2023, 11, 100148. [Google Scholar] [CrossRef]
- Toprak, S.; Öncel, C.; Yılmaz, S.; Baba, A.; Koç, G.A.; Demir, M.M. Lithium extraction from geothermal brine using γ-MnO2 A case study for Tuzla geothermal power plant. Heliyon 2024, 10, e39656. [Google Scholar] [CrossRef]
- Kumari, A.; Choubey, P.K.; Gupta, R.; Jha, M.K. Recovery of lithium (Li) salts from industrial effluent of recycling plant. In Rare Metal Technology; Springer International Publishing: Cham, Switzerland, 2021; pp. 91–100. [Google Scholar]
- Qiu, B.; Liu, M.; Qu, X.; Zhou, F.; Xie, H.; Wang, D.; Lee, L.S.; Yin, H. Waste plastics upcycled for high-efficiency H2O2 production and lithium recovery via Ni-Co/carbon nanotubes composites. Nat. Commun. 2024, 15, 6473. [Google Scholar] [CrossRef]
- Khan, M.T.; Shah, I.A.; Ihsanullah, I.; Naushad, M.; Ali, S.; Shah, S.H.A.; Mohammad, A.W. Hospital wastewater as a source of environmental contamination: An overview of management practices, environmental risks, and treatment processes. J. Water Process Eng. 2021, 41, 101990. [Google Scholar] [CrossRef]
- Shi, J.; Huang, W.; Han, J.; Xu, C. Pollution control of wastewater from the coal chemical industry in China: Environmental management policy and technical standards. Renew. Sustain. Energy Rev. 2021, 143, 110883. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.; Niu, Y.; Xu, D.; Wang, J.; Han, J.; Wang, H. Removal of microplastics and attached heavy metals from secondary effluent of wastewater treatment plant using interpenetrating bipolar plate electrocoagulation. Sep. Purif. Technol. 2022, 290, 120905. [Google Scholar] [CrossRef]
- Lindsey, B.; Belitz, K.; Cravotta, C.; Toccalino, P.; Dubrovsky, N. Lithium in groundwater used for drinking-water supply in the United States. Sci. Total Environ. 2021, 767, 144691. [Google Scholar] [CrossRef]
- Rajaeifar, M.A.; Raugei, M.; Steubing, B.; Hartwell, A.; Anderson, P.A.; Heidrich, O. Life cycle assessment of lithium-ion battery recycling using pyrometallurgical technologies. J. Ind. Ecol. 2021, 25, 1560–1571. [Google Scholar] [CrossRef]
- Kim, Y.; Han, Y.; Kim, S.; Jeon, H.S. Green extraction of lithium from waste lithium aluminosilicate glass-ceramics using a water leaching process. Process Saf. Environ. Prot. 2021, 148, 765–774. [Google Scholar] [CrossRef]
- Tao, X.; Li, B.; Zhang, H.; Peng, A.; Wang, J.; Zheng, Y.; Yang, L.; Luo, X.; Luo, S.; Shao, P. High-efficiency, environment-friendly extraction of lithium from waste LAS glass-ceramics by roasting with KOH at low temperature. Resour. Conserv. Recycl. 2024, 209, 107775. [Google Scholar] [CrossRef]
- Jang, Y.; Hou, C.H.; Park, S.; Kwon, K.; Chung, E. Direct electrochemical lithium recovery from acidic lithium-ion battery leachate using intercalation electrodes. Resour. Conserv. Recycl. 2021, 175, 105837. [Google Scholar] [CrossRef]
- Morita, Y.; Saito, Y.; Yoshioka, T.; Shiratori, T. Estimation of recoverable resources used in lithium-ion batteries from portable electronic devices in Japan. Resour. Conserv. Recycl. 2021, 175, 105884. [Google Scholar] [CrossRef]
- Gu, F.; Guo, J.; Yao, X.; Summers, P.A.; Widijatmoko, S.D.; Hall, P. An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China. J. Clean. Prod. 2017, 161, 765–780. [Google Scholar] [CrossRef]
- Madanhire, I.; Mbohwa, C. Lubricating Grease Handling and Waste Management. In Mitigating Environmental Impact of Petroleum Lubricants; Springer: Cham, Switzerland, 2016; pp. 189–206. [Google Scholar]
- Lu, B.; Liu, J.; Yang, J. Substance flow analysis of lithium for sustainable management in mainland China: 2007–2014. Resour. Conserv. Recycl. 2017, 119, 109–116. [Google Scholar] [CrossRef]
- Hou, H.; Li, D.; Liu, X.; Yao, Y.; Dai, Z.; Yu, C. Recovery of expired lithium carbonate tablets for LiFePO4/C cathode. Waste Biomass Valorization 2019, 11, 3097–3105. [Google Scholar] [CrossRef]
- Dunn, J.; Kendall, A.; Slattery, M. Electric vehicle lithium-ion battery recycled content standards for the US—Targets, costs, and environmental impacts. Resour. Conserv. Recycl. 2022, 185, 106488. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).