Abstract
Phosphorite, or phosphate rock, has garnered increasing attention in recent years as a promising unconventional resource for rare earth elements (REEs). This paper presents a processing scheme aimed at recovering both REEs and phosphate values from amine flotation tailings generated during phosphate beneficiation in Florida. In these tailings, REEs are primarily present as monazite and xenotime, often associated with heavy minerals. The proposed flowsheet includes gravity separation to pre-concentrate REE- and phosphate-bearing minerals, followed by flotation to further upgrade both REEs and phosphate, and finally sulfuric acid leaching to extract REEs and phosphate from the flotation concentrate. Gravity separation using a shaking table increased the total REE content from approximately 202 ppm to 657 ppm, with a concentrate yield of 12.51%, REE recovery of around 41%, and P2O5 recovery of 33%. Fatty acid flotation of the shaking table concentrate produced a final concentrate containing 1106 ppm REEs and 14.90% P2O5, with recoveries of approximately 86% for REEs and 90% for P2O5. Subsequent pyrolysis with concentrated sulfuric acid followed by water leaching achieved recoveries of about 85% for REEs and 93% for P2O5. While the process demonstrated effective concentration and leaching of REE minerals and apatite, the major challenge to further improving separation and extraction efficiency lies in the fine-grained nature of the valuable minerals and their interlocking with gangue minerals.
1. Introduction
Phosphorite, or phosphate rock, has been used as a feedstock for phosphate fertilizer production for more than 150 years. In modern manufacturing, phosphate rock is produced through beneficiation of phosphate ore, which involves removing large volumes of waste materials, including phosphatic clay and flotation tailings. The resulting phosphate concentrate then undergoes a “wet-process,” in which it is digested by mineral acids (typically sulfuric acid) to produce phosphoric acid. This process generates two major by-products: phosphogypsum and phosphoric acid sludge. Finally, phosphoric acid is converted into various phosphate fertilizers [1].
In recent years, phosphorite has attracted renewed interest for its potential as an unconventional resource for rare earth elements (REEs) [2,3,4,5,6,7,8,9,10,11,12]. Kanazawa [13] estimated that global phosphate resources contain approximately 50 million tons of REEs. Other studies [14,15] have shown that phosphate rock typically contains an average of 0.046 wt% REEs, and with around 250 million tons of phosphate rock mined globally each year, more than 110,000 tons of REEs are introduced into the phosphate industry annually—most of which are lost during processing. In Florida alone, estimated annual REE losses include approximately 5800 tons in phosphatic clay, 3400 tons in phosphogypsum, 1100 tons in phosphoric acid sludge, 780 tons in flotation tails, and 620 tons in phosphate fertilizer. If these REEs could be efficiently recovered, the total amount could meet the entire U.S. demand—and yttrium (Y) recovery alone could potentially satisfy global demand.
Central Florida hosts large sedimentary phosphate rock deposits that have been mined and processed for over a century. In current beneficiation operations, phosphate ore is first washed and classified to remove micro-fine clay (−150 mesh), producing three size fractions: pebble (+16 mesh), coarse flotation feed (−16 + 35 mesh), and fine flotation feed (−35 + 150 mesh). The coarse and fine feeds undergo the “Crago” double flotation process—direct flotation using fatty acid as a collector to concentrate phosphate minerals, followed by reverse flotation using amine to remove residual silica [16]. The resulting phosphate concentrate is then sent to the acid plant, where it is digested by sulfuric acid using the dihydrate wet-process to produce phosphoric acid. This entire process generates substantial waste streams, including phosphatic clay, fatty acid and amine flotation tails, and phosphogypsum.
Numerous studies have confirmed the presence of REEs in Florida phosphate resources [17,18,19,20]. As early as 1989, Kremer and Chokshi [21] of Mobile Research & Development Corporation conducted a comprehensive investigation, finding that phosphate ore (matrix) contained approximately 282 ppm total REEs. Their study revealed that, after beneficiation and chemical processing, REEs are distributed across various waste streams: 40% in phosphatic clay, 37.5% in phosphogypsum, 12.5% in phosphoric acid, and 10% in flotation tails. More recent work by Zhang [4] and Poul et al. [22] estimated that the total annual REE content in Florida phosphate ore exceeds 30,000 tons—significantly surpassing current U.S. demand. Notably, compared to traditional REE ores such as bastnäsite, Florida phosphate resources contain higher proportions of heavy REEs, which are both rarer and more critical for high-tech applications and clean energy technologies. Therefore, recovering REEs from Florida phosphates could play a key role in establishing a secure and resilient domestic REE supply chain.
As a founding member of the U.S. Department of Energy’s Critical Materials Innovation (CMI) Hub, the FIPR Institute has been actively conducting research on REE recovery from Florida phosphate resources. This paper presents a processing scheme aimed at recovering both REEs and phosphate values from amine flotation tailings. The proposed approach includes gravity separation to pre-concentrate REE-bearing minerals, flotation of the gravity concentrate to further upgrade REEs and phosphate, and concentrated sulfuric acid pyrolysis followed by water leaching to extract REEs and phosphate from the final concentrate.
2. Materials and Methods
2.1. Mineral Beneficiation
Investigations revealed that REEs in the flotation tailings exist primarily as monazite and xenotime, often associated with heavy minerals. Most of the phosphorus is hosted in francolite, which is typically associated with coarse gangue minerals [23,24]. Given the low concentrations of REEs and P2O5 (commonly used to represent phosphorus content in minerals) in the tailings, it is essential to employ a cost-effective and efficient beneficiation method to eliminate as much gangue material as possible.
Gravity separation using a shaking table was selected for pre-concentrating REE-bearing minerals and phosphate, based on the following considerations:
- The specific gravities of monazite (4.6–5.7), xenotime (4.4–5.1), and phosphate rock (3.16–3.22) are significantly higher than that of quartz (~2.65), which is the predominant gangue mineral in the tailings.
- The particle size distribution of the tailings is well-suited for gravity separation.
- Shaking tables offer a low-cost and simple method for gravity-based separation.
Accordingly, a shaking table was used to remove a large portion of gangue minerals from the tailings. The shaking table separation was conducted at a feed rate of 50 kg/h. The resulting concentrate was then ground and further upgraded via flotation. Flotation was conducted using a Denver mechanical flotation cell with a volume of one liter and feed charge of 500 g at 1200 rpm. Slurry density in the flotation cell was 30%.
2.2. Leaching of REEs and Phosphorus
To recover REEs and phosphorus from the final concentrate, a process involving concentrated sulfuric acid pyrolysis followed by water leaching (CSAP–WL) was applied. In this method, the concentrate was first mixed with concentrated sulfuric acid, then roasted at 350 °C for 4 h. After cooling, water was added and the mixture was stirred for 2 h to leach REEs and phosphorus.
During roasting, the REE minerals and phosphate minerals (primarily fluorapatite) react with sulfuric acid according to the following reactions:
where RE denotes a rare earth element.
2REPO4 + 3H2SO4 → RE2(SO4)3 + 2H3PO4
Ca5(PO4)3F + 5H2SO4 → 5CaSO4 + 3H3PO4 + HF↑
3. Results and Discussion
3.1. Pre-Concentration with Shaking Table
A representative sample of amine flotation tails was collected from beneficiation plant at the Four Corners Mine, one of the phosphate mines in central Florida. The sample was assayed for main components and REE content, Table 1.

Table 1.
Main chemical components in amine tails sample.
Quantitative mineral analysis was conducted using a Mineral Liberation Analyzer (MLA). Two rare earth minerals were identified by MLA, they are monazite and xenotime, both at very low concentrations. Major non rare earth minerals include quartz, fluorapatite, feldspar, zircon, and rutile, Table 2.

Table 2.
Quantitative mineral analysis of amine tails using MLA [15].
The size range of the mineral particles was also analyzed using MLA, Table 3.

Table 3.
Size distribution analysis for the major valuable minerals in amine tails [15].
A shaking table was used to concentrate the REE-bearing minerals and apatite from the amine flotation tailings. The operating conditions of the shaking table were optimized first and determined as follows:
- Transverse angle: 5°
- Stroke length: 7 mm
- Stroke frequency: 310 revolutions per minute
- Wash water: 3.5 gallons per minute
- Tailings feed: 583 g per minute
Then, 500 kg of the sample was used in shaking table concentration testing, and the separation results are presented in Table 4.

Table 4.
Results of shaking table separation.
The pre-concentration step removed up to 87.49 wt% of the solids from the feed, yielding a concentrate with significantly higher REE and P2O5 contents compared to the original tailings. However, the recoveries of REEs and P2O5 were relatively low—40.73% and 33.12%, respectively. These results suggest that a substantial portion of the valuable minerals in the amine flotation tailings exist as fine particles interlocked with gangue minerals. Without sufficient grinding to liberate these minerals, effective separation cannot be achieved.
3.2. Flotation
The shaking table concentrate was first ground in a ball mill to improve the liberation of REE-bearing minerals and apatite, followed by flotation for further beneficiation. Sodium carbonate was used to adjust the pulp pH, sodium silicate served as a gangue depressant, and sodium oleate was employed as the collector for both REE and phosphate minerals. The flotation pulp concentration was maintained at 30 wt%.
Operating conditions were optimized through a series of flotation tests, with the results presented in Figure 1, Figure 2, Figure 3 and Figure 4. Based on these tests, the optimal conditions were determined as follows: grinding fineness of 52.70 wt% passing 200 mesh (0.074 mm), pulp pH of 9.5, depressant dosage of 0.55 kg/t (based on tailings feed), and collector dosage of 1.2 kg/t (also based on tailings feed).
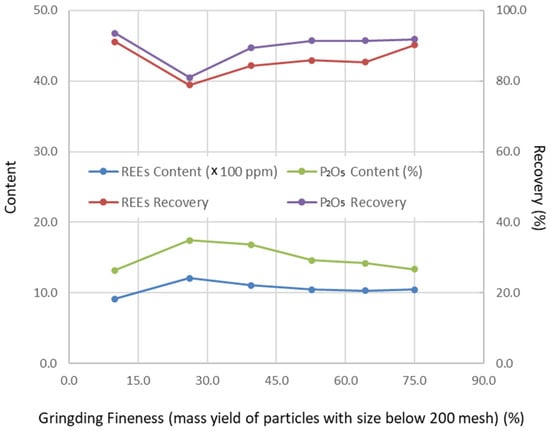
Figure 1.
Change in floatation results with grinding fineness (pulp pH 9.3, depressor addition 0.5 kg/t tails feed, collector addition 1.2 kg/t tails feed).
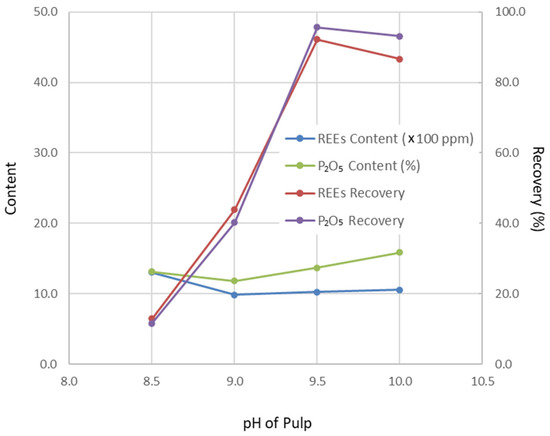
Figure 2.
Change in flotation results with pH of pulp (grinding fineness 52.70 wt% of −200 mesh particles, depressor addition 0.5 kg/t tails feed, collector addition 1.2 kg/t tails feed).
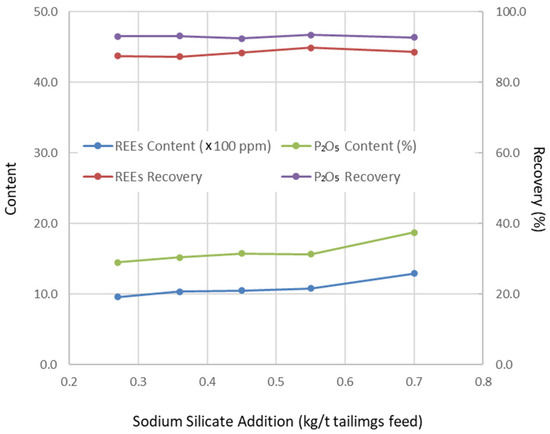
Figure 3.
Change in flotation results with depressor addition (grinding fineness 52.70 wt% of −200 mesh particles, pulp pH 9.5, collector addition 1.2 kg/t tails feed).
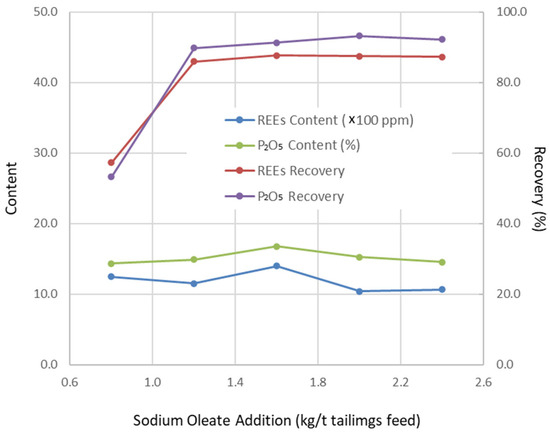
Figure 4.
Change in flotation results with collector addition (grinding fineness 52.70 wt% of −200 mesh particles, pulp pH 9.5, depressor addition 0.55 kg/t tails feed).
A rougher flotation test was then conducted under these optimized conditions, and the results are summarized in Table 5.

Table 5.
Results of flotation.
The results presented in Table 5 indicate that the shaking table concentrate can be further upgraded through flotation after grinding. However, the P2O5 content in the resulting flotation concentrate was only 14.90%, significantly lower than the typical 28% P2O5 found in the standard “Crago” flotation concentrate used for phosphoric acid production. Test observations revealed that the P2O5 grade of the flotation concentrate could not be substantially improved, even when grinding fineness was increased beyond 52.70 wt% passing 200 mesh (0.074 mm). This limitation is likely due to the presence of valuable minerals as micro-fine particles, tightly interlocked with gangue in the amine flotation tailings. The chemical composition and REE content of the flotation concentrate are provided in Table 6.

Table 6.
Main chemical components and rare earths in flotation concentrate.
3.3. Leaching
The CSAP–WL leaching method described above is a widely used process for decomposing REE minerals, including monazite. To evaluate its effectiveness, the impact of sulfuric acid dosage on the leaching efficiencies of REEs and phosphorus was investigated. The results are presented in Figure 5.

Figure 5.
Change in leaching efficiency with sulfuric acid addition.
As shown in Figure 5, the leaching efficiency of REEs initially increased with the rising stoichiometric ratio of H2SO4 to CaO, reaching a maximum of 85.54% at a ratio of 3.61, after which it plateaued. In contrast, the leaching efficiency of P2O5 exhibited a different trend: it remained relatively stable, ranging from 88.5% to 90.5% as the H2SO4/CaO ratio increased from 1.85 to 3.26. Beyond this point, P2O5 recovery increased steadily, reaching 95.33% at the highest tested ratio of 4.60.
The sulfuric acid consumption during leaching was significantly higher than the theoretical requirement for phosphate decomposition, primarily due to the high impurity content in the flotation concentrate.
It is important to note that the downstream processing of REEs from the leachate, including extraction and production of mixed rare earth oxides, will be detailed in separate papers to be published in the near future.
4. Conclusions
The research results presented in this paper demonstrate that REEs and phosphates in amine flotation tails can be effectively concentrated and leached using a combined separation and leaching process. Gravity separation using a shaking table increased the REE and P2O5 contents from approximately 202 ppm and 3% in the tails to 657 ppm and 8%, respectively, with a concentrate yield of 12.51%, achieving recoveries of about 41% for REEs and 33% for P2O5. After grinding, the shaking table concentrate was further upgraded by flotation, producing a concentrate containing 1151 ppm REEs and 14.90% P2O5, with recoveries of 85.91% for REEs and 89.81% for P2O5. Leaching tests showed that REEs and phosphorus could be recovered from the flotation concentrate via concentrated sulfuric acid pyrolysis followed by water leaching, resulting in approximately 85% recovery of REEs and 93% recovery of P2O5.
However, several challenges remain with the current processing approach. First, the REE minerals and apatite are finely disseminated within the amine flotation tails, with many particles interlocked with gangue minerals. This led to relatively low recoveries in the shaking table separation—40.73% for REEs and 33.15% for P2O5—and the limited upgrading of grades in the flotation concentrate. The final flotation concentrate, with 1150.82 ppm REEs and 14.90% P2O5, does not yet meet industry standards. Given that the amine flotation tails contain over 90 wt% gangue minerals (based on a P2O5 content of 3.02%), it is not economically viable to grind the entire tailings before removing the majority of gangue due to the high energy requirements.
The second issue is the high sulfuric acid consumption required in the roasting–leaching step. The acid additions tested across a stoichiometric ratio range of 1.85 to 4.60 (H2SO4 to CaO) were all substantially higher than the theoretical amount needed to decompose phosphates. The efficient leaching of REEs and P2O5 was only achieved at ratios above 3.26. This increased acid demand is primarily due to impurities such as calcite, dolomite, and Fe-bearing minerals consuming acid during roasting. Additionally, REE minerals require a high concentration of sulfuric acid for effective decomposition.
Author Contributions
Conceptualization, P.Z. and H.L.; methodology, H.L. and P.Z.; validation, H.L. and A.M.; formal analysis, A.M.; investigation, H.L. and Z.J.; resources, D.D. and P.Z.; data curation, A.M.; writing—original draft preparation, H.L. and P.Z.; writing—review and editing, P.Z.; visualization, H.L.; supervision, P.Z.; project administration, P.Z. and A.M.; funding acquisition, D.D. and P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the US Department of Energy through CMI Hub, grant number SC-14-392.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research is part of a major project conducted under the Critical Materials Innovation (CMI) Hub (formerly the Critical Materials Institute), funded by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Materials & Manufacturing Technologies Office. We gratefully acknowledge the guidance and leadership of Bruce Moyer, CMI focus area lead, and David DePaoli, CMI project lead. Significant matching funds were provided by the Florida Industrial and Phosphate Research Institute, Florida Polytechnic University. Special thanks are extended to The Mosaic Company for their invaluable technical support, substantial in-kind contributions, and assistance with sample collection. We sincerely appreciate the help of Mosaic employees and former employees Nicole Christiansen, Paul Kucera, Marcos Ortiz, Chris Dennis, Glen Oswald, Chaucer Hwang, Cameron Weed, and Gary Whitt.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Becker, P. Phosphates and Phosphoric Acid: Raw Materials, Technology, and Economics of the Wet Process; Marcel Dekker: New York, NY, USA, 1983. [Google Scholar]
- Pan, Y.; Fleet, M.E.; Macrae, N.D. Oriented monazite inclusions in apatite porphyroblasts from the Hemlo gold deposit, Ontario, Canada. Miner. Mag. 1993, 57, 697–708. [Google Scholar] [CrossRef]
- Li, S.; Malik, M.; Azimi, G. Extraction of Rare Earth Elements from Phosphogypsum Using Mineral Acids: Process Development and Mechanistic Investigation. Ind. Eng. Chem. Res. 2022, 61, 102–114. [Google Scholar] [CrossRef]
- Zhang, P. Comprehensive recovery and sustainable development of phosphate resources. Procedia Eng. 2014, 83, 37–51. [Google Scholar] [CrossRef]
- Christmann, P. A forward look into rare earth supply and demand: A role for sedimentary phosphate deposits? Procedia Eng. 2014, 83, 19–26. [Google Scholar] [CrossRef]
- Zielinski, S.; Szczepanik, A.; Buca, M.; Kunecki, M. Recovery of lanthanides from kola apatite in phosphoric acid manufacture. J. Chem. Technol. Biotechnol. 1993, 56, 355–360. [Google Scholar] [CrossRef]
- Preston, J. The recovery of rare earth oxides from a phosphoric acid byproduct. Part 4. The preparation of magnet-grade neodymium oxide from the light rare earth fraction. Hydrometallurgy 1996, 42, 151–167. [Google Scholar] [CrossRef]
- Preston, J.; Cole, P.; Craig, W.; Feather, A. The recovery of rare earth oxides from a phosphoric acid by-product. Part 1: Leaching of rare earth values and recovery of a mixed rare earth oxide by solvent extraction. Hydrometallurgy 1996, 41, 1–19. [Google Scholar] [CrossRef]
- Preston, J.; Cole, P.; du Preez, A.; Fox, M.; Fleming, A. The recovery of rare earth oxides from a phosphoric acid by-product. Part 2: The preparation of high-purity cerium dioxide and recovery of a heavy rare earth oxide concentrate. Hydrometallurgy 1996, 41, 21–44. [Google Scholar] [CrossRef]
- Preston, J.; du Preez, A.; Cole, P.; Fox, M. The recovery of rare earth oxides from a phosphoric acid by-product. Part 3. The separation of the middle and light rare earth fractions and the preparation of pure europium oxide. Hydrometallurgy 1996, 42, 131–149. [Google Scholar] [CrossRef]
- Filippov, L.O.; Dehaine, Q.; Filippova, I.V. Rare earths (La, Ce, Nd) and rare metals (Sn, Nb, W) as by-products of kaolin production–Part 3: Processing of fines using gravity and flotation. Miner. Eng. 2016, 95, 96–106. [Google Scholar] [CrossRef]
- Laurino, J.P.; Mustacato, J.; Huba, Z.J. Rare Earth Element Recovery from Acidic Extracts of Florida Phosphate Mining Materials Using Chelating Polymer 1-Octadecene, Polymer with 2,5-Furandione, Sodium Salt. Minerals 2019, 9, 477. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloys Compd. 2006, 408, 1339–1343. [Google Scholar] [CrossRef]
- Grosz, A.E.; Meier, A.L.; Clardy, B.F.; Howard, J.M. Rare Earth Elements in the Cason Shale of Northern Arkansas: A Geochemical Reconnaissance; Arkansas Geological Commission: North Little Rock, AR, USA, 1995. [Google Scholar]
- Jasinski, S.M. Mineral Commodity Summaries Phosphate Rock; US Geological Survey, Department of the Interior: Washington, DC, USA, 2017; pp. 124–125.
- Zhang, P.; Yu, Y.; Bogan, M. Challenging the “Crago” double float process ii. Amine-type-fatty acid flotation of silicious phosphates. Miner. Eng. 1997, 10, 983–994. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare earths recovery and gypsum upgrade from Florida phosphogypsum. Miner. Met. Process. 2017, 34, 201–206. [Google Scholar] [CrossRef]
- Claude, L.W.; Henry, M.J.R. Method for determination of small amounts of rare earths and thorium in phosphate rocks. Anal. Chem. 1953, 25, 432–435. [Google Scholar] [CrossRef]
- Giesekke, E.W. Florida phosphate rock. In SME Mineral Processing Handbook; Section 21; Weiss, N.L., Ed.; SME: Englewood, CO, USA, 1985; pp. 1–18. [Google Scholar]
- Zhang, P. Recovery of critical elements from Florida phosphate: Phase 1. Characterization of rare earths. In Proceedings of the ECI International Conference: Rare earth Minerals Metals—Sustainable Technologies for the Future, San Diego, CA, USA, 12–17 August 2012. [Google Scholar]
- Kremer, R.A.; Chokshi, J.C. Fate of Rare Earth Elements in Mining/Beneficiation of Florida Phosphate Rock and Conversion to DAP Fertilizer; Research Report; Mobil Mining and Minerals Company: Nichols, FL, USA, 1989. [Google Scholar]
- Poul, E.; Mclauglin, I.P.; Breit, N.G.; du Bray, A.E.; Alan, E.K. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef]
- Zhang, P. Rare Earths in Phosphate: Characterization & Extraction. In Rare Earth Elements: Sustainable Recovery, Processing, and Purification; Haramalidis, A., Eggert, R., Eds.; American Geophysical Union (AGU) and John Wiley and Sons: Hoboken, NJ, USA, 2025; pp. 99–165. [Google Scholar]
- Hassan, E.; Bogan, M. Characterization of Future Florida Phosphate Resource; FIPR Publication: Bartow, FL, USA, 1994. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).