Heavy Metal Pollution Assessment and Survey of Rhizosphere Bacterial Communities from Saccharum spontaneum L. in a Rehabilitated Nickel-Laterite Mine in the Philippines
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Collection
2.2. Analyses of Soil Chemical, Mineralogical, and Physicochemical Properties
2.3. Pollution Indicess to Classify the Extent of Soil Pollution
2.4. Identification of Plant Sample
2.5. DNA Preparation and Analyses
2.6. Statistical Analyses
3. Results and Discussion
3.1. Comparison of Chemical and Mineralogical Compositions of Soils from the Control and Rehabilitated Areas
3.2. Physicochemical Properties of Soils from the Control and Rehabilitated Areas
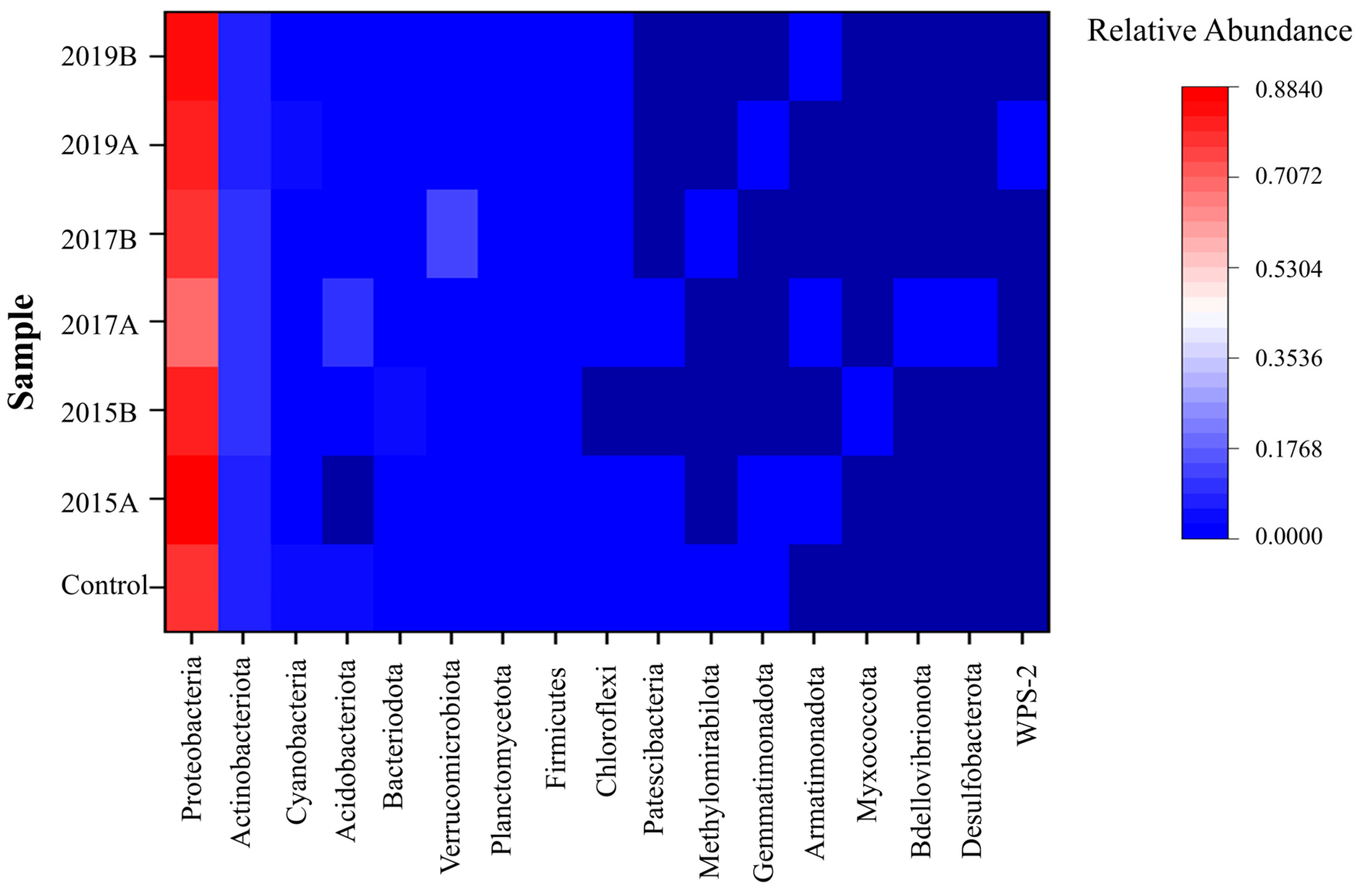
3.3. Bacterial Communities in the Rhizosphere of S. spontaneum L.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Available phosphorus |
| ASV | Amplicon sequence variants |
| CD | Contamination degree |
| CF | Contamination factor |
| EK | Exchangeable potassium |
| Igeo | Geo-accumulation index |
| MC | Moisture content |
| OC | Organic carbon |
| OM | Organic matter |
| PGPRs | Plant growth-promoting rhizobacteria |
| PLI | Pollution load index |
| TN | Total nitrogen |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence spectroscopy |
References
- U.S. Geological Survey. Mineral Commodity Summaries 2025 (ver. 1.2, March 2025); U.S. Geological Survey: Reston, VA, USA, 2025; p. 212. [CrossRef]
- Golroudbary, S.R.; Krasławski, A.; Wilson, B.P.; Lundström, M. Assessment of environmental sustainability of nickel required for mobility transition. Front. Chem. Eng. 2023, 4, 978842. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Ramos, L.M.; Gabiana, L.J.R.; Banda, M.H.T.; Delfinado, J.R.Y.; Orbecido, A.H.; Zoleta, J.B.; Park, I.; Arima, T.; et al. Development of a three-step approach to repurpose nickel-laterite mining waste into magnetite adsorbents for As (III) and As (V) removal: Synthesis, characterization, and adsorption studies. J. Environ. Chem. Eng. 2023, 11, 108992. [Google Scholar] [CrossRef]
- Calderon, A.R.M.; Alorro, R.D.; Tadesse, B.; Yoo, K.; Tabelin, C.B. Repurposing of nickeliferous pyrrhotite from mine tailings as magnetic adsorbent for the recovery of gold from chloride solution. Resour. Conserv. Recycl. 2020, 161, 104971. [Google Scholar] [CrossRef]
- Nakajima, K.; Nansai, K.; Matsubae, K.; Tomita, M.; Takayanagi, W.; Nagasaka, T. Global land-use change hidden behind nickel consumption. Sci. Total Environ. 2017, 586, 730–737. [Google Scholar] [CrossRef]
- Mudd, G.M. Global trends and environmental issues in nickel mining: Sulfides versus laterites. Ore Geol. Rev. 2010, 38, 9–26. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resources availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recy. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Carpen, H.L.; Giese, E.C. Enhancement of nickel laterite ore bioleaching by Burkholderia sp. using a factorial design. Appl. Water Sci. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Van, H.T.; Thang, P.Q.; Hoang, T.H.N.; Dao, D.C.; Nguyen, C.L.; Nguyen, L.H. Level and Potential Risk Assessment of Soil Contamination by Trace Metal from Mining Activities. Soil Sediment Contam. Int. J. 2020, 30, 92–106. [Google Scholar] [CrossRef]
- Opiso, E.M.; Aseneiro, J.P.J.; Banda, M.H.T.; Tabelin, C.B. Solid-phase partitioning of mercury in artisanal gold mine tailings from selected key areas in Mindanao, Philippines, and its implications for mercury detoxification. Waste Manag. Res. 2018, 36, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Tabelin, C.B.; Silwamba, M.; Paglinawan, F.C.; Mondejar, A.J.S.; Duc, H.G.; Resabal, V.J.; Opiso, E.M.; Igarashi, T.; Tomiyama, S.; Ito, M.; et al. Solid-phase partitioning and release-retention mechanisms of copper, lead, zinc and arsenic in soils impacted by artisanal and small-scale gold mining (ASGM) activities. Chemosphere 2020, 260, 127574. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Y.; Zhang, J.; Lu, X.; Wei, G. Naturally selected dominant weeds as heavy metal accumulators and excluders assisted by rhizosphere bacteria in a mining area. Chemosphere 2020, 243, 125365. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, P.; Korkka-Niemi, K.; Lahti, M.; Salonen, V. Groundwater and surface water contamination in the area of the Hitura nickel mine, Western Finland. Environ. Geol. 2002, 42, 313–329. [Google Scholar] [CrossRef]
- Germande, O.; Gunkel-Grillon, P.; Dominique, Y.; Feurtet-Mazel, A.; Bierque, E.; Dassié, É.P.; Daffe, G.; Pierron, F.; Baudrimont, I.; Baudrimont, M. Impact of nickel mining in New Caledonia on marbled eels Anguilla marmorata. J. Hazard. Mater. 2022, 436, 129285. [Google Scholar] [CrossRef]
- Alonzo, D.; Tabelin, C.B.; Dalona, I.M.; Abril, J.M.V.; Beltran, A.; Orbecido, A.; Villacorte-Tabelin, M.; Resabal, V.J.; Promentilla, M.A.; Suelto, M.; et al. Working with the community for the rehabilitation of legacy mines: Approaches and lessons learned from the literature. Resour. Policy 2024, 98, 105351. [Google Scholar] [CrossRef]
- Promentilla, M.A.B.; Beltran, A.B.; Orbecido, A.H.; Bernardo-Arugay, I.; Resabal, V.J.; Villacorte-Tabelin, M.; Dalona, I.M.; Opiso, E.; Alloro, R.; Alonzo, D.; et al. Systems Approach toward a Greener Eco-efficient Mineral Extraction and Sustainable Land Use Management in the Philippines. Chem. Eng. Trans. 2021, 88, 1171–1176. [Google Scholar]
- Héry, M.; Herrera, A.; Vogel, T.M.; Normand, P.; Navarro, É. Effect of carbon and nitrogen input on the bacterial community structure of Neocaledonian nickel mine spoils. FEMS Microbiol. Ecol. 2005, 51, 333–340. [Google Scholar] [CrossRef]
- Herrera, A.; Héry, M.; Stach, J.E.M.; Jaffré, T.; Normand, P.; Navarro, É. Species richness and phylogenetic diversity comparisons of soil microbial communities affected by nickel-mining and revegetation efforts in New Caledonia. Eur. J. Soil Biol. 2007, 43, 130–139. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy Metal Pollution from Gold Mines: Environmental Effects and Bacterial Strategies for Resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef]
- Collins, Y.E.; Stotzky, G. Heavy metals alter the electrokinetic properties of bacteria, yeasts, and clay minerals. Appl. Environ. Microbiol. 1992, 58, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Salgado, P.H.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef]
- Tang, L.; Gao, W.; Lu, Y.; Tabelin, C.B.; Liu, J.; Li, H.; Yang, W.; Tang, C.; Feng, X.; Jiang, J.; et al. The formation of multi-metal (loid)s contaminated groundwater at smelting site: Critical role of natural colloids. J. Hazard. Mater. 2024, 471, 134408. [Google Scholar] [CrossRef]
- Xue, S.; Ke, W.; Zeng, J.; Tabelin, C.B.; Xie, Y.; Tang, L.; Xiang, C.; Jiang, J. Pollution prediction for heavy metals in soil-groundwater systems at smelting sites. Chem. Eng. J. 2023, 473, 145499. [Google Scholar] [CrossRef]
- He, Z.; Li, S.; Wang, L.; Zhong, H. Characterization of Five Chromium-Removing Bacteria Isolated from Chromium-Contaminated Soil. Water Air Soil Pollut. 2014, 225, 1–10. [Google Scholar] [CrossRef]
- Choudhury, S.P.; Schmid, M.; Hartmann, A.; Tripathi, A.K. Diversity of 16S-rRNA and nifH genese derived from rhizosphere soil and roots of an endemic drought tolerant grass, Lasiurus sindicus. Eur. J. Soil Biol. 2009, 45, 114–122. [Google Scholar] [CrossRef]
- Oh, S.; Hassan, S.H.; Joo, J.H. Biosorption of heavy metals by lyophilized cells of Pseudomonas stutzeri. World J. Microbiol. Biotechnol. 2009, 25, 1771–1778. [Google Scholar] [CrossRef]
- Bruto, M.; Prigent-Combaret, C.; Muller, D.; Moënne-Loccoz, Y. Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci. Rep. 2014, 4, 6261. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Drogue, B.; Doré, H.; Borland, S.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Which specificity in cooperation between phytostimulating rhizobacteria and plants? Res. Microbiol. 2012, 163, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Blaha, D.; Prigent-Combaret, C.; Mirza, M.S.; Moenne-Loccoz, Y. Phylogeny of the 1-aminocyclopropane-1-carboxylic acid deaminase-encoding gene acdS in phytobeneficial and pathogenic Proteobacteria and relation with strain biogeography. FEMS Microbiol. Ecol. 2006, 56, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol. Lett. 2005, 251, 1–7. [Google Scholar] [CrossRef]
- Somers, E.; Vanderleyden, J.; Srinivasan, M. Rhizosphere bacterial signalling: A love parade beneath our feet. Crit. Rev. Microbiol. 2004, 30, 205–240. [Google Scholar] [CrossRef]
- Zamioudis, C.; Pieterse, C.M.J. Modulation of host immunity by beneficial microbes. Mol. Plant-Microbe Interact. 2012, 25, 139–150. [Google Scholar] [CrossRef]
- Couillerot, O.; Prigent-Combaret, C.; Caballero-Mellado, J.; Moënne-Loccoz, Y. Pseudomonas fluorescens and closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett. Appl. Microbiol. 2009, 48, 505–512. [Google Scholar] [CrossRef]
- Haas, D.; Defago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Saleh, S.S.; Glick, B.R. Involvement of gacS and rpoS in enhancement of the plant growth promoting capabilities of Enterobacter cloacae CAL2 and UW4. Can. J. Microbiol. 2001, 47, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Kojic, M.; Degrassi, G.; Venturi, V. Cloning and characterization of the rpoS gene from the plant growth-promoting Pseudomonas putida WCS358: RpoS is not involved in siderophore and homoserine lactone production. Biochim. Biophys. Acta 1999, 1489, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Ovadis, M.; Liu, X.; Gavriel, S.; Ismailov, Z.; Chet, I.; Chernin, L. The global regulator genes from biocontrol strain Serratia plymuthica IC1270: Cloning, sequencing, and functional studies. J. Bacteriol. 2004, 186, 4986–4993. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E. How the plant growth-promoting bacterium Azospirillum promotes plant growth—A critical assessment. Adv. Agron. 2010, 108, 77–136. [Google Scholar]
- Haas, D.; Keel, C. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 2003, 41, 117–153. [Google Scholar] [CrossRef]
- Misra, H.S.; Rajpurohit, Y.S.; Khairnar, N.P. Pyrroloquinoline-quinone and its versatile roles in biological processes. J. Biosci. 2012, 37, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Einax, J.W.; Kraft, J. Small-Scale variability of metals in soil and composite sampling. Environ. Sci. Pollut. Res. 2002, 9, 257–261. [Google Scholar] [CrossRef] [PubMed]
- GLOSOLAN 3rd meeting | Global Soil Partnership | Food and Agriculture Organization of the United Nations. 2019. Available online: https://www.fao.org/global-soil-partnership/pillars-action/5-harmonization/glosolan/presentations-glosolan-3rd-meeting/en/ (accessed on 4 April 2022).
- Wang, G.; Liu, H.-Q.; Gong, Y.; Wei, Y.; Miao, A.-J.; Yang, L.Y.; Zhong, H. Risk assessment of metals in urban soils from a typical industrial City, Suzhou, Eastern China. Int. J. Environ. Res. Public Health 2017, 14, 10–25. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. J. Geol. 1969, 2, 108–118. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control, a sedimentological approach. Water Resour. 1980, 14, 975–1001. [Google Scholar]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Fischer, M.; Renevey, N.; Thür, B.; Hoffmann, D.; Beer, M.; Hoffmann, B. Efficacy Assessment of nucleic acid decontamination reagents used in molecular diagnostic laboratories. PLoS ONE 2016, 11, e0159274. [Google Scholar] [CrossRef]
- Fan, R.; Gerson, A.R. Nickel geochemistry of a Philippine laterite examined by bulk and microprobe synchrotron analyses. Geochim. Cosmochim. Acta 2011, 75, 6400–6415. [Google Scholar] [CrossRef]
- Aquino, K.A.; Arcilla, C.A.; Schardt, C.; Tupaz, C.A.J. Mineralogical and geochemical characterization of the Sta. Cruz nickel laterite deposit, Zambales, Philippines. Minerals 2022, 12, 305. [Google Scholar] [CrossRef]
- Tupaz, C.A.J.; Watanabe, Y.; Sanematsu, K.; Echigo, T.; Arcilla, C.; Ferrer, C. Ni-co mineralization in the intex laterite deposit, Mindoro, Philippines. Minerals 2020, 10, 579. [Google Scholar] [CrossRef]
- Bowen, H. Environmental Chemistry of Elements; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Feigl, G. The impact of copper oxide nanoparticles on plant growth: A comprehensive review. J. Plant Interact. 2023, 18, 2243098. [Google Scholar] [CrossRef]
- Kazakis, N.; Kantiranis, N.; Kalaitzidou, K.; Kaprara, E.; Mitrakas, M.; Frei, R.; Vargemezis, G.; Vogiatzis, D.; Zouboulis, A.; Filippidis, A. Environmentally available hexavalent chromium in soils and sediments impacted by dispersed fly ash in Sarigkiol basin (Northern Greece). Environ. Pollut. 2018, 235, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, W.L. Zinc in soils and plant nutrition. Adv. Agron. 1972, 24, 147–186. [Google Scholar]
- Oorts, K.; Smolders, E.; Lanno, R.; Chowdhury, M.J. Bioavailability and ecotoxicity of lead in soil: Implications for setting ecological soil quality standards. Environ. Toxicol. Chem. 2021, 40, 1948–1961. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, A.; Di, R.; Lindert, S.; Heckman, J. Nickel and Soil Fertility: Review of Benefits to Environment and Food Security. Environments 2024, 11, 177. [Google Scholar] [CrossRef]
- Srivastava, P.; Bolan, N.; Casagrande, V.; Benjamin, J.; Adejumo, S.A.; Sabir, M.; Farooqi, Z.U.R. Cobalt in soils: Sources, fate, bioavailability, plant uptake, remediation, and management. In Appraisal of Metal (Loids) in the Ecosystem; Elsevier: Amsterdam, The Netherlands, 2022; pp. 81–104. [Google Scholar]
- Essandoh, P.K.; Takase, M.; Bryant, I.M. Impact of Small-Scale mining activities on physicochemical properties of soils in Dunkwa East municipality of Ghana. Sci. World J. 2021, 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Beattie, R.E.; Henke, W.; Campa, M.F.; Hazen, T.C.; McAliley, L.R.; Campbell, J.H. Variation in microbial community structure correlates with heavy-metal contamination in soils decades after mining ceased. Soil Biol. Biochem. 2018, 126, 57–63. [Google Scholar] [CrossRef]
- Maulas, K.M.; Paredes, C.S.; Tabelin, C.B.; Jose, M.A.; Opiso, E.M.; Arima, T.; Park, I.; Mufalo, W.; Ito, M.; Igarashi, T.; et al. Isolation and Characterization of Indigenous Ureolytic Bacteria from Mindanao, Philippines: Prospects for Microbially Induced Carbonate Precipitation (MICP). Minerals 2024, 14, 339. [Google Scholar] [CrossRef]
- Mondejar, A.J.S.; Paglinawan, F.; Tabelin, C.B.; Aguilos, M.; Aguilos, R.; Opiso, E.M.; Martinez, J.G.T.; Metillo, E.B.; Sumaya, N.H.N.; Villacorte-Tabelin, M. Survival, Reproduction, and Life History Traits Evaluation of Heterocephalobellus sp. and Cephalobus sp. from an Artisanal and Small-scale Gold Mine Site, Davao de Oro, Philippines as Bioindicators of Heavy Metal Contamination. Philipp. J. Sci. 2023, 152, 2213–2218. [Google Scholar]
- Xiao, E.; Ning, Z.; Xiao, T.; Sun, W.; Jiang, S. Soil bacterial community functions and distribution after mining disturbance. Soil Biol. Biochem. 2021, 157, 108232. [Google Scholar] [CrossRef]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. Elements of the Nature and Properties of Soils; Pearson Education International: Hoboken, NJ, USA, 2010. [Google Scholar]
- Marschner, H. Mechanisms of adaptation of plants to acid soils. Plant Soil 1991, 134, 1–20. [Google Scholar] [CrossRef]
- Laghlimi, M.; Baghdad, B.; Hadi, H.E.; Bouabdli, A. Phytoremediation Mechanisms of Heavy Metal Contaminated soils: A review. Open J. Ecol. 2015, 5, 375–388. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Laboratory Services Division, Bureau of Soils and Water Management. Guidelines for the Interpretation of Soil and Water Test Result. 2022. Available online: https://drive.google.com/file/d/1pX693oUSQeh8W2R2h9sw3Y1WMXJ7Q0v0/view (accessed on 10 August 2023).
- Ibrahim, U.; Kawo, A.; Yusuf, I.; Yahaya, S. Physicochemical and molecular characterization of heavy metal–tolerant bacteria isolated from soil of mining sites in Nigeria. J. Genet. Eng. Biotechnol. 2021, 19, 152. [Google Scholar] [CrossRef]
- Gerke, J. The Central Role of Soil Organic Matter in Soil Fertility and Carbon Storage. Soil Syst. 2022, 6, 33. [Google Scholar] [CrossRef]
- USDA Sustainable Agriculture Research and Extension. CH 2. What Is Organic Matter and Why Is It So Important—SARE. 2023. Available online: https://www.sare.org/publications/building-soils-for-better-crops/what-is-organic-matter-and-why-is-it-so-important/#Beneficial-Effects-of-Soil-Organisms- (accessed on 12 August 2023).
- Ngatia, L.W.; Moriasi, D.N.; Grace, J.M.; Fu, R.; Gardner, C.S.; Taylor, R.W. Land Use Change Affects Soil Organic Carbon: An Indicator of Soil Health; IntechOpen eBooks: London, UK, 2021. [Google Scholar] [CrossRef]
- Yan, X.; Yang, W.; Chen, X.; Wang, M.; Wang, W.; Ye, D.; Wu, L. Soil phosphorus pools, bioavailability and environmental risk in response to the phosphorus supply in the red soil of southern China. Int. J. Environ. Res. Public Health 2020, 17, 7384. [Google Scholar] [CrossRef]
- Arenberg, M.R.; Arai, Y. Uncertainties in soil physicochemical factors controlling phosphorus mineralization and immobilization processes. Adv. Agron. 2019, 154, 153–200. [Google Scholar] [CrossRef]
- Li, M.; Han, X.; Li, L. Total nitrogen stock in soil profile affected by land use and soil type in three counties of Mollisols. Front. Environ. Sci. 2022, 10, 945305. [Google Scholar] [CrossRef]
- Li, X.; Jin, Z.; Xiong, L.; Tong, L.; Zhu, H.; Zhang, X.; Qin, G. Effects of land reclamation on soil bacterial community and potential functions in Bauxite mining area. Int. J. Environ. Res. Public Health 2022, 19, 16921. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roychowdhury, R.; Roy, M. Phytoremediation potential of rhizobacterial isolates from Kans grass (Saccharum spontaneum) of fly ash ponds. Clean Technol. Environ. Policy 2017, 19, 1373–1385. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Ow, D.W. Promises and Prospects of Phytoremediation. Plant Physiol. 1996, 110, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Singh, S.P. A Review on phytoremediation of heavy metals and utilization of plant for metal recovery. Appl. Ecol. Environ. Res. 2005, 3, 1–18. [Google Scholar] [CrossRef]
- Bayas, Q.; Salvador, S.; Ragragio, E.; Obico, J. Taxonomic survey of nickel hyperaccumulating plants in a mining site on Luzon Island, Philippines. Philipp. J. Syst. Biol. 2018, 12, 103–108. [Google Scholar]
- Schmidt, J.E.; Kent, A.D.; Brisson, V.L.; Gaudin, A.C.M. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 2019, 7, 1–18. [Google Scholar] [CrossRef]
- Simon, E.; Guseva, K.; Darcy, S.; Alteio, L.; Pjevac, P.; Schmidt, H.; Jenab, K.; Ranits, C.; Kaiser, C. Distinct microbial communities are linked to organic matter properties in millimetre-sized soil aggregates. ISME J. 2024, 18, wrae156. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Liu, B.; Yao, J.; Ma, B.; Chen, Z.; Zhao, C.; Zhu, X.; Li, M.; Cao, Y.; Pang, W.; Li, H.; et al. Microbial community profiles in soils adjacent to mining and smelting areas: Contrasting potentially toxic metals and co-occurrence patterns. Chemosphere 2021, 282, 130992. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Weinand, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Rao, K.V.R.; Kumar, K.S.; Rao, D.B.; Rao, T.R.; Isolation and Characterization of Antagonistic Actinobacteria from Mangrove Soil. EBSCOhost. 2012. Available online: https://openurl.ebsco.com/EPDB%3Agcd%3A15%3A8055247/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A88995145&crl=c&link_origin=scholar.google.com.ph (accessed on 15 December 2024).
- Bergmann, G.T.; Bates, S.T.; Eilers, K.G.; Lauber, C.L.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol. Biochem. 2011, 43, 1450–1455. [Google Scholar] [CrossRef]
- Roesch, L.F.W.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.; Kent, A.D.; Daroub, S.H.; De Oliveira Camargo, F.A.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 1, 283–290. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, J.; Lu, H.; Yang, C.; Yang, Y.; Zhou, J.; Li, D. An integrated study to analyze soil microbial community structure and metabolic potential in two forest types. PLoS ONE 2014, 9, e93773. [Google Scholar] [CrossRef]
- Yarwood, S.A.; Wick, A.F.; Williams, M.A.; Daniels, W.L. Parent material and vegetation influence soil microbial community structure following 30-Years of rock weathering and pedogenesis. Microb. Ecol. 2014, 69, 383–394. [Google Scholar] [CrossRef]
- Ezeokoli, O.T.; Bezuidenhout, C.C.; Maboeta, M.; Khasa, D.P.; Adeleke, R. Structural and functional differentiation of bacterial communities in post-coal mining reclamation soils of South Africa: Bioindicators of soil ecosystem restoration. Sci. Rep. 2020, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, Y.; Chen, J.; Li, J.; Zhang, H. Responses of soil bacterial community structure to different artificially restored forests in open-pit coal mine dumps on the loess plateau, China. Front. Microbiol. 2023, 14, 1198313. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Marian, M.; Nishioka, T.; Koyama, H.; Suga, H.; Shimizu, M. Biocontrol potential of Ralstonia sp. TCR112 and Mitsuaria sp. TWR114 against tomato bacterial wilt. Appl. Soil Ecol. 2018, 128, 71–80. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Q.; Chen, X.; Dong, F.; Chen, H.; Liu, M.; Ali, I. Speciation Distribution of Heavy Metals in Uranium Mining Impacted Soils and Impact on Bacterial Community Revealed by High-Throughput Sequencing. Front. Microbiol. 2019, 10, 1867. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, J.; Guo, Z.; Cheng, Y.; Wu, J. Heavy metals and bacterial community determine resistance genes distribution in agricultural soils surrounding long-term mining area. Appl. Soil Ecol. 2024, 202, 105581. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Li, F.; Zhang, C.; Lyu, J. Mechanism of carbonate mineralization induced by microbes: Taking Curvibacter lanceolatus strain HJ-1 as an example. Micron 2021, 140, 102980. [Google Scholar] [CrossRef]
- Van Overbeek, L.; Duhamel, M.; Aanstoot, S.; Van Der Plas, C.L.; Nijhuis, E.; Poleij, L.; Russ, L.; Van Der Zouwen, P.; Andreo-Jimenez, B. Transmission of Escherichia coli from Manure to Root Zones of Field-Grown Lettuce and Leek Plants. Microorganisms 2021, 9, 2289. [Google Scholar] [CrossRef]
- Wen, L.; Huang, Y.; Wang, W.; Zhang, L.; Xu, J.; Li, Z.; Xu, P.; Tang, H. A novel Diaphorobacter sp. strain isolated from saponification wastewater shows highly efficient phenanthrene degradation. Environ. Res. 2022, 214, 114047. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 31906737. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Sun, M.; Ling, W.; Zhu, X. Isolation, Characterization, and Degradation Performance of the 17-Estradiol-Degrading Bacterium Novosphingobium sp. E2S. Int. J. Environ. Res. Public Health 2017, 14, 115. [Google Scholar] [CrossRef]
- Hou, H.; Wang, C.; Ding, Z.; Zhang, S.; Yang, Y.; Ma, J.; Chen, F.; Li, J. Variation in the Soil Microbial Community of Reclaimed Land over Different Reclamation Periods. Sustainability 2018, 10, 2286. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, L.; Li, Y.; Sun, Q. Shifts in Vegetation-Associated microbial community in the reclamation of coal mining subsidence land. Environ. Eng. Sci. 2020, 37, 838–848. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Kishi, L.T.; Lopes, E.M.; Omori, W.P.; De Souza, J.A.M.; Alves, L.M.C.; De Macedo Lemos, E.G. Bacterial communities in mining soils and surrounding areas under regeneration process in a former ore mine. Braz. J. Microbiol. 2018, 49, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Banning, N.C.; Gleeson, D.B.; Grigg, A.H.; Grant, C.D.; Andersen, G.L.; Brodie, E.L.; Murphy, D.V. Soil Microbial Community Successional Patterns during Forest Ecosystem Restoration. Appl. Environ. Microbiol. 2011, 77, 6158–6164. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A. Geobiotechnological Solutions to Anthropogenic Disturbances; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Lewis, D.E.; White, J.R.; Wafula, D.; Athar, R.; Dickerson, T.; Williams, H.N.; Chauhan, A. Soil functional diversity analysis of a bauxite-mined restoration chronosequence. Microb. Ecol. 2010, 59, 710–723. [Google Scholar] [CrossRef] [PubMed]
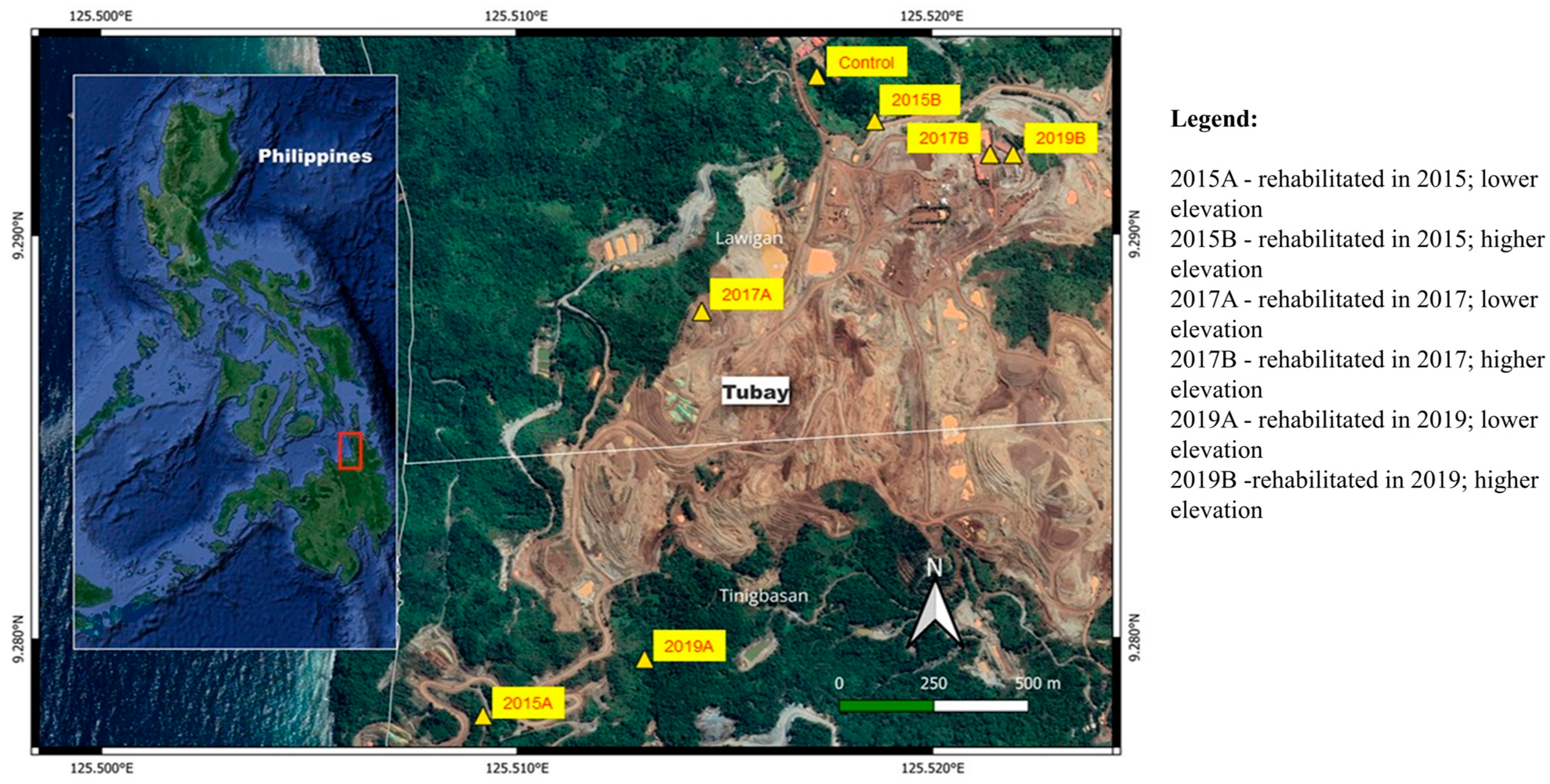



| Site | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | TiO2 | P2O5 | Ni | Cr | Co | Pb | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (wt%) | (wt%) | (wt%) | (wt%) | (wt%) | (wt%) | (wt%) | (wt%) | (wt%) | (wt%) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |
| Control | 33.2 | 3.64 | 41.7 | 0.66 | 18.0 | 0.22 | 0.07 | 0.04 | 0.83 | 0.66 | 2340 | 15.8 | 209 | 120 |
| 2015A | 63.5 | 11.1 | 1.27 | − | 21.5 | 1.20 | 0.36 | 0.11 | 0.26 | 0.32 | 924 | 13.0 | 72.3 | 80 |
| 2015B | 70.5 | 18.6 | 0.15 | 0.20 | 7.07 | 0.49 | 0.41 | 0.39 | 0.19 | 0.16 | 711 | 13.9 | 193 | 240 |
| 2017A | 6.50 | 6.48 | 79.4 | 0.86 | 2.10 | 0.11 | 0.2 | 0.07 | 1.44 | 1.16 | 3980 | 56.6 | 356 | − |
| 2017B | 10.1 | 5.64 | 74.7 | 0.99 | 4.16 | 0.10 | 0.11 | 0.05 | 1.37 | 1.00 | 3770 | 51.1 | 353 | − |
| 2019A | 13.3 | 4.59 | 71.5 | 1.15 | 4.44 | 0.36 | 0.11 | 0.12 | 1.29 | 1.25 | 3700 | − | 410 | 158 |
| 2019B | 25.5 | 4.17 | 53.5 | 0.87 | 12.03 | 0.13 | 0.08 | 0.05 | 1.54 | 0.75 | 3130 | 21.4 | 273 | 122 |
| Site | Igeo | CF | PLI | CD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | Co | Pb | Zn | Cu | Ni | Cr | Co | Pb | Zn | Cu | ||||
| 2015A | −2.27 | −1.63 | −1.93 | −0.87 | −2.12 | −1.07 | 0.31 | 0.48 | 0.39 | 0.82 | 0.35 | 0.71 | 0.48 | 3.07 | |
| 2015B | −2.73 | −2.60 | −2.31 | −0.77 | −0.70 | 0.23 | 0.23 | 0.25 | 0.30 | 0.88 | 0.92 | 1.75 | 0.54 | 4.34 | |
| 2017A | 0.20 | 0.22 | 0.18 | 1.26 | 0.30 | − * | 1.73 | 1.74 | 1.70 | 3.59 | 1.85 | − * | 1.80 | 10.6 | |
| 2017B | 0.13 | 0.00 | 0.10 | 1.11 | 0.17 | − * | 1.64 | 1.51 | 1.61 | 3.24 | 1.69 | − * | 1.67 | 9.68 | |
| 2019A | 0.04 | 0.32 | 0.07 | − * | 0.39 | −0.15 | 1.55 | 1.88 | 1.58 | − | 1.96 | 1.36 | 1.52 | 8.32 | |
| 2019B | 0.30 | −0.40 | −0.17 | −0.15 | −0.20 | −0.52 | 1.85 | 1.13 | 1.33 | 1.35 | 1.31 | 1.05 | 1.32 | 8.02 | |
| Sites | pH | Moisture Content (%) | Organic Matter (%) | Organic Carbon (%) | Available Phosphorus (ppm) | Exchangeable Potassium (ppm) | Total Nitrogen (%) |
|---|---|---|---|---|---|---|---|
| Control | 6.19 | 7.08 | 1.50 | 0.87 | 1.17 | 225 | 0.15 |
| 2015A | 6.88 | 6.10 | 0.88 | 0.51 | 5.11 | 37.5 | 0.19 |
| 2015B | 6.50 | 8.28 | 3.98 | 2.32 | 11.9 | 96 | 0.14 |
| 2017A | 6.46 | 9.86 | 2.87 | 1.67 | 2.26 | 60 | 0.23 |
| 2017B | 6.65 | 7.30 | 1.00 | 0.58 | 1.26 | 99 | 0.22 |
| 2019A | 6.73 | 6.15 | 0.25 | 0.15 | 2.12 | 78 | 0.23 |
| 2019B | 6.81 | 7.34 | 4.45 | 2.59 | 1.66 | 81 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mainit, S.W.; Tabelin, C.B.; Paglinawan, F.C.; Guihawan, J.Q.; Mondejar, A.J.S.; Resabal, V.J.T.; Madamba, M.R.S.B.; Alonzo, D.; Orbecido, A.H.; Promentilla, M.A.; et al. Heavy Metal Pollution Assessment and Survey of Rhizosphere Bacterial Communities from Saccharum spontaneum L. in a Rehabilitated Nickel-Laterite Mine in the Philippines. Minerals 2025, 15, 881. https://doi.org/10.3390/min15080881
Mainit SW, Tabelin CB, Paglinawan FC, Guihawan JQ, Mondejar AJS, Resabal VJT, Madamba MRSB, Alonzo D, Orbecido AH, Promentilla MA, et al. Heavy Metal Pollution Assessment and Survey of Rhizosphere Bacterial Communities from Saccharum spontaneum L. in a Rehabilitated Nickel-Laterite Mine in the Philippines. Minerals. 2025; 15(8):881. https://doi.org/10.3390/min15080881
Chicago/Turabian StyleMainit, Shiela W., Carlito Baltazar Tabelin, Florifern C. Paglinawan, Jaime Q. Guihawan, Alissa Jane S. Mondejar, Vannie Joy T. Resabal, Maria Reina Suzette B. Madamba, Dennis Alonzo, Aileen H. Orbecido, Michael Angelo Promentilla, and et al. 2025. "Heavy Metal Pollution Assessment and Survey of Rhizosphere Bacterial Communities from Saccharum spontaneum L. in a Rehabilitated Nickel-Laterite Mine in the Philippines" Minerals 15, no. 8: 881. https://doi.org/10.3390/min15080881
APA StyleMainit, S. W., Tabelin, C. B., Paglinawan, F. C., Guihawan, J. Q., Mondejar, A. J. S., Resabal, V. J. T., Madamba, M. R. S. B., Alonzo, D., Orbecido, A. H., Promentilla, M. A., Zoleta, J. B., Daño, D. T., Park, I., Ito, M., Arima, T., Phengsaart, T., & Villacorte-Tabelin, M. (2025). Heavy Metal Pollution Assessment and Survey of Rhizosphere Bacterial Communities from Saccharum spontaneum L. in a Rehabilitated Nickel-Laterite Mine in the Philippines. Minerals, 15(8), 881. https://doi.org/10.3390/min15080881













