Biomineralization Mediated by Iron-Oxidizing Microorganisms: Implication for the Immobilization and Transformation of Heavy Metals in AMD
Abstract
1. Introduction
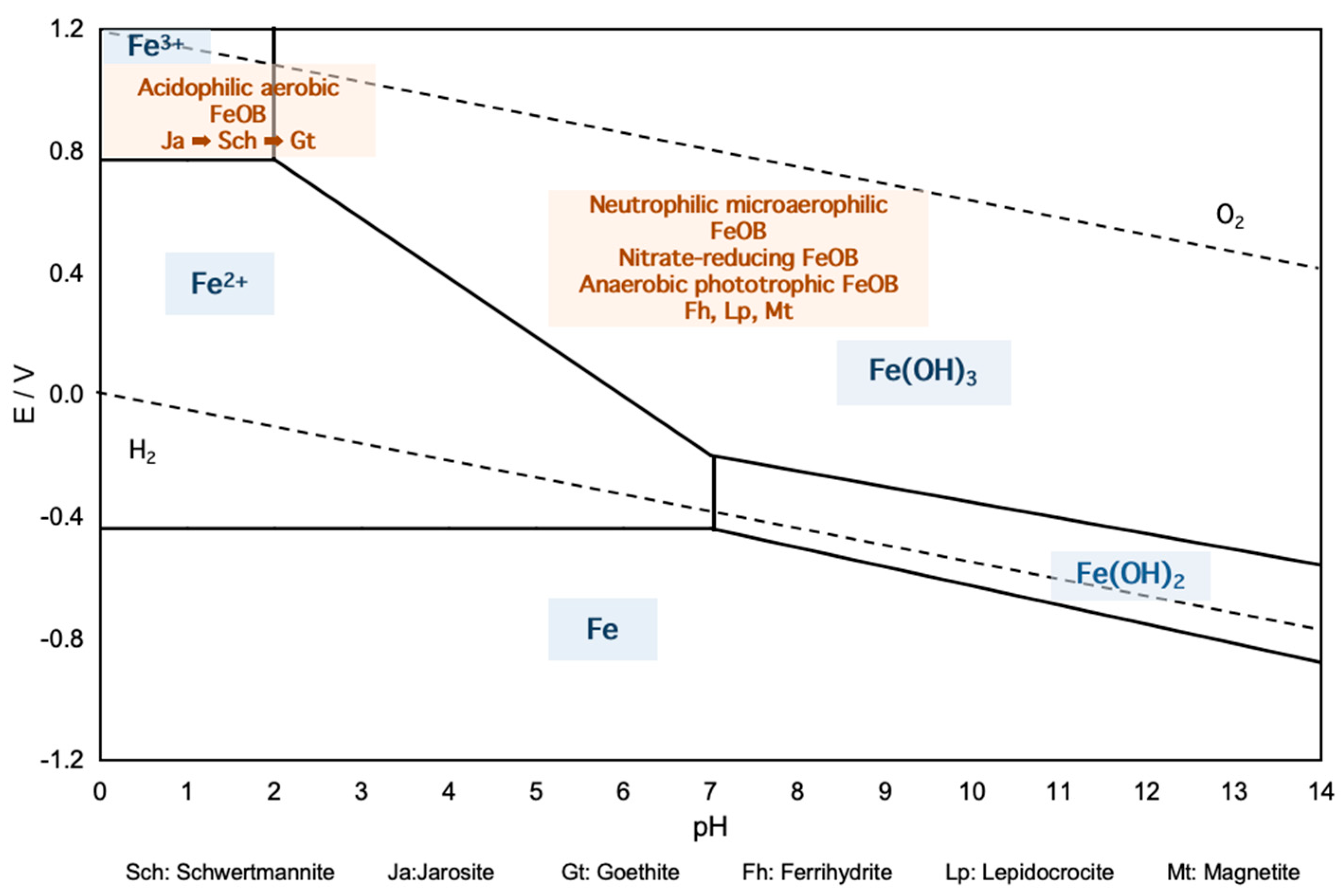
2. Microbially Mediated Fe(II) Oxidation and Mineralization
2.1. Mechanisms of Microbial Fe(II) Oxidation
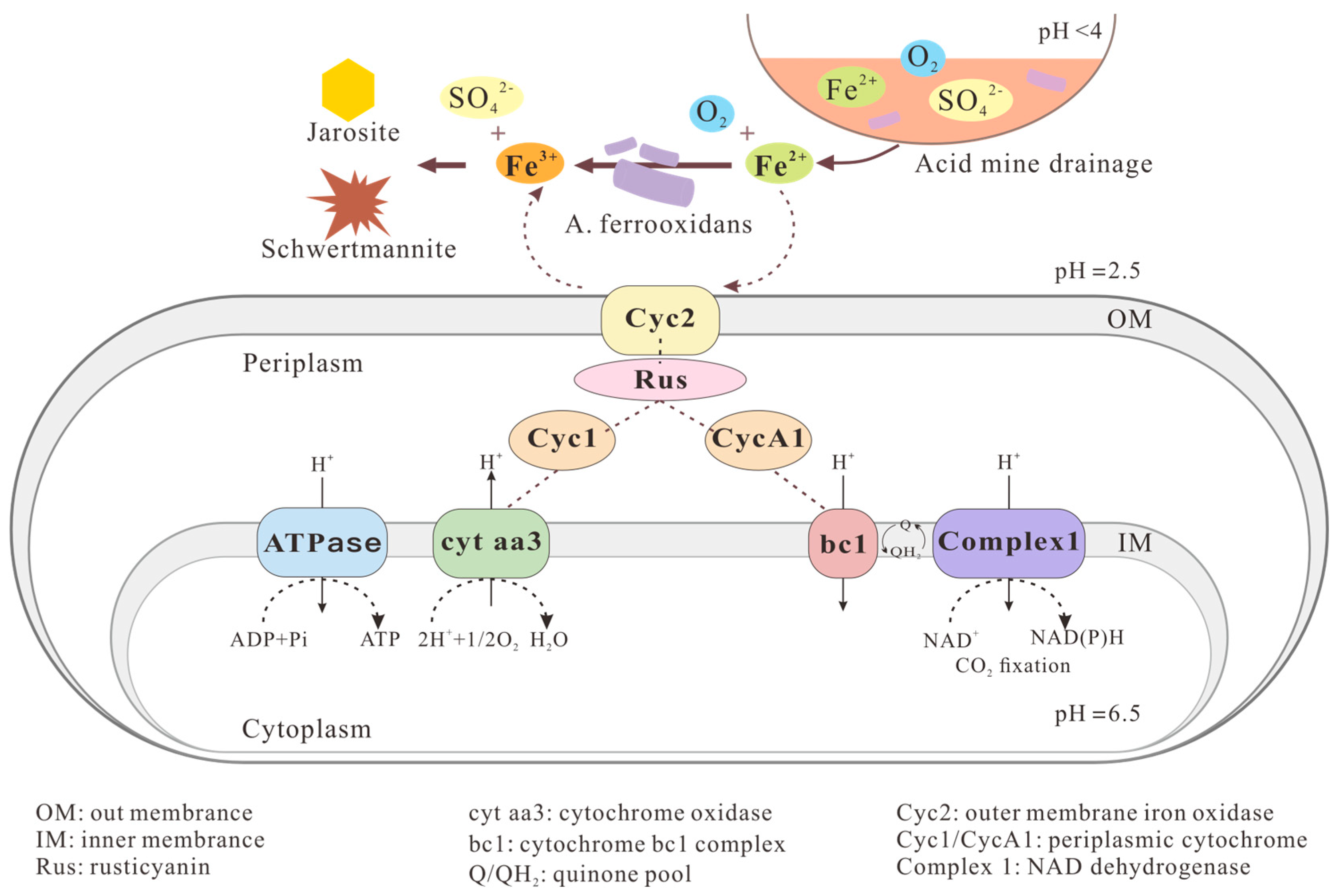
2.1.1. Acidophilic Aerobic Fe(II)-Oxidizers
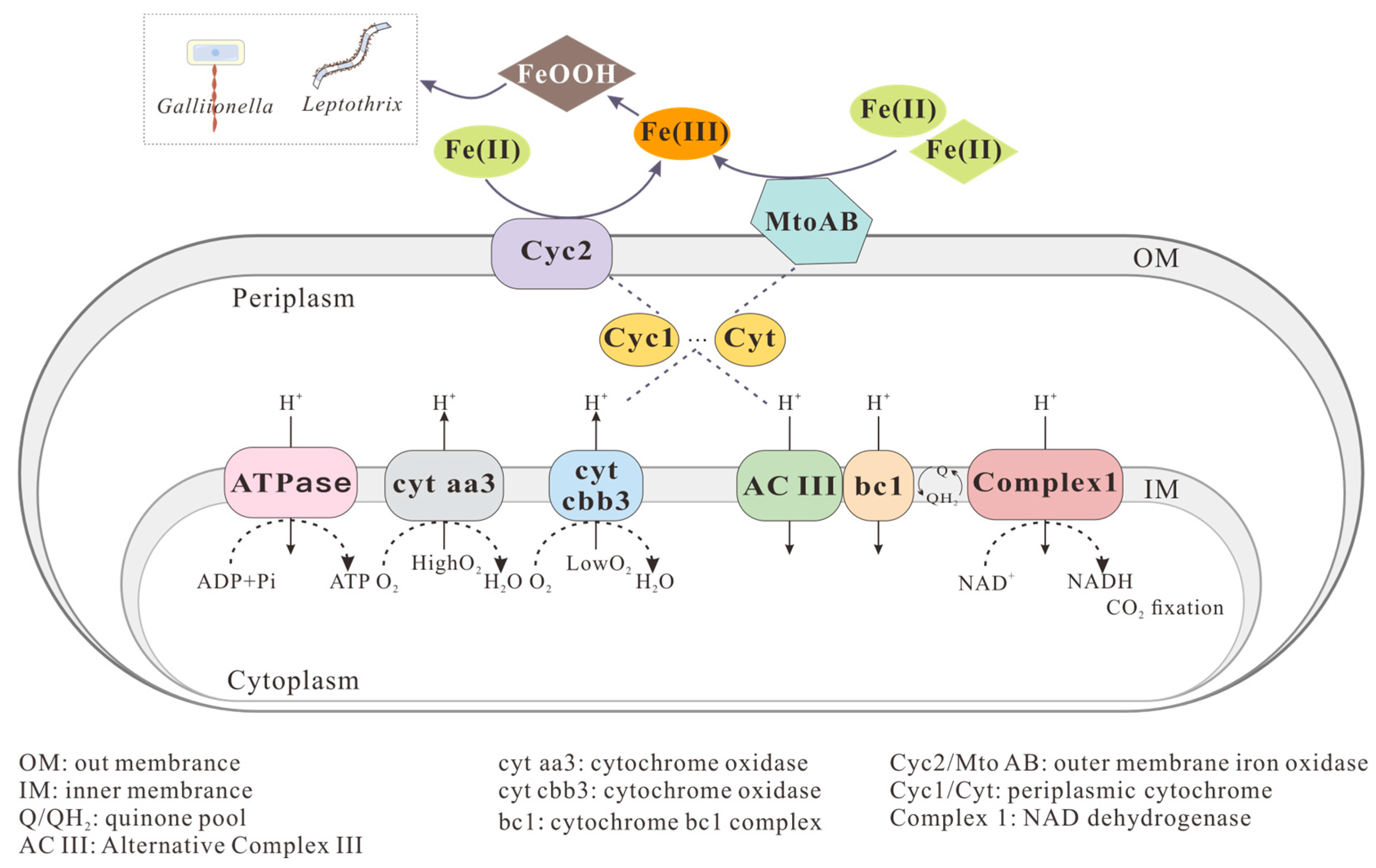
2.1.2. Neutrophilic Microaerophilic Fe(II)-Oxidizers
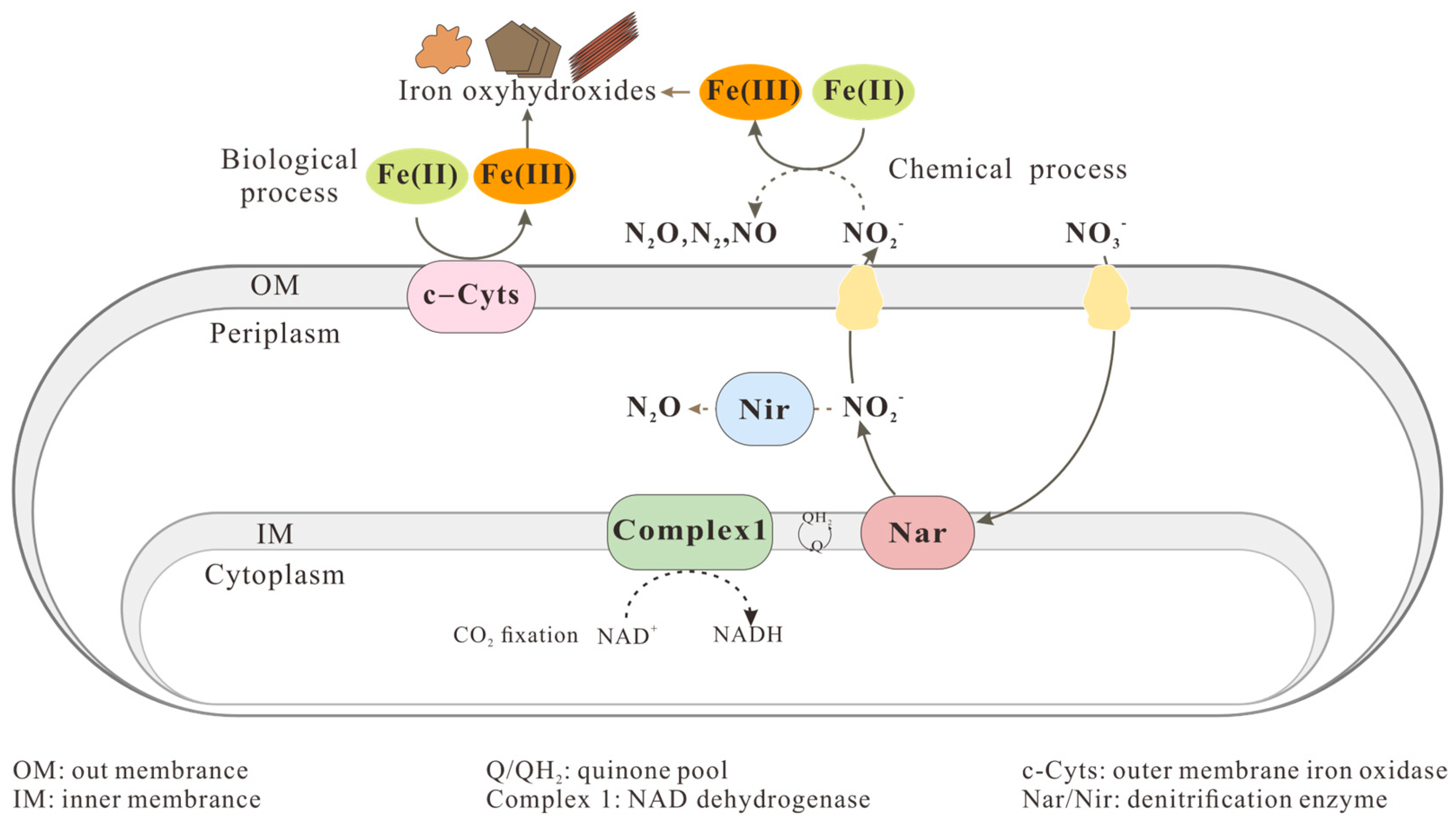
2.1.3. Nitrate-Reducing Fe(II)-Oxidizers
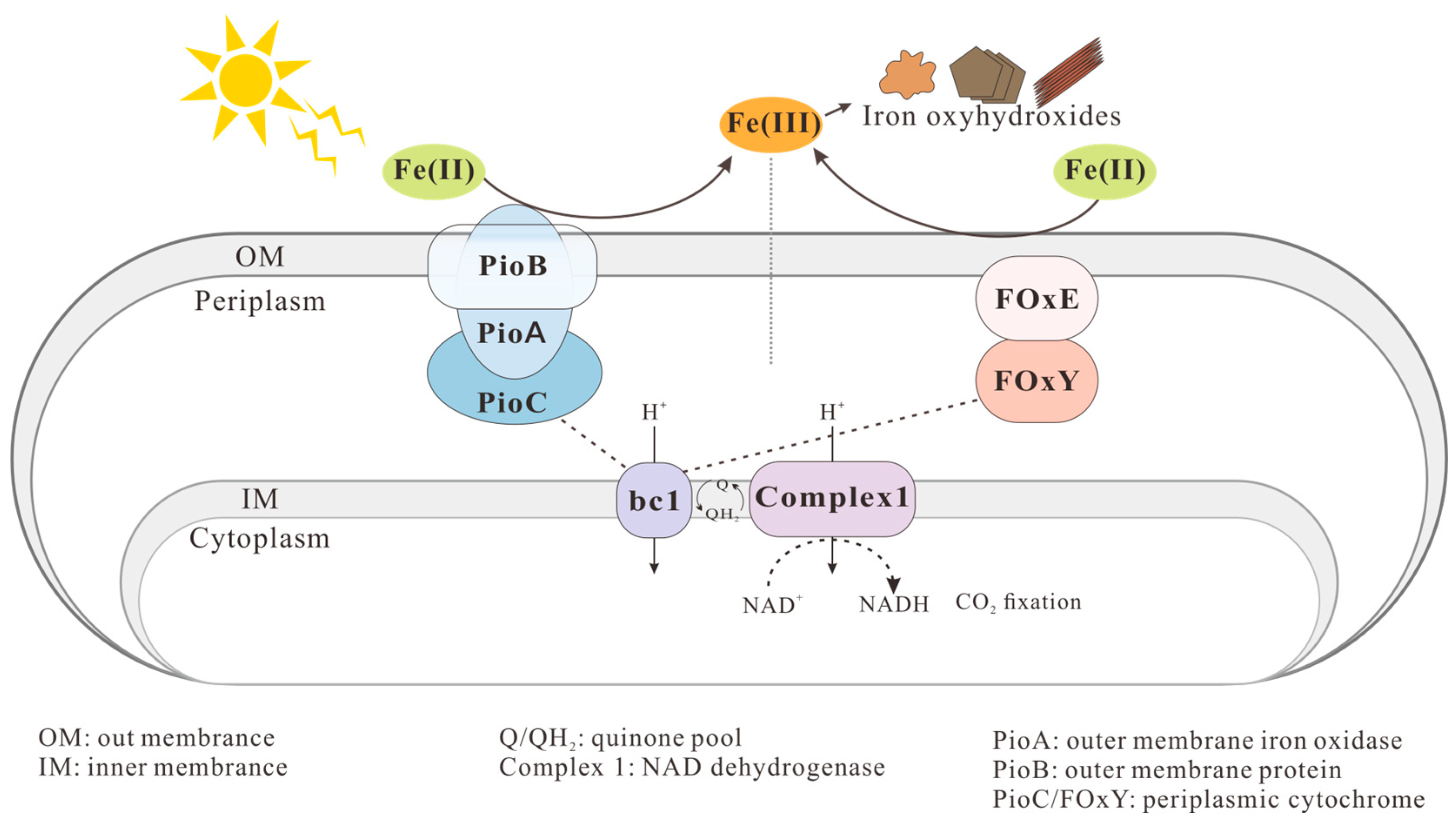
2.1.4. Anaerobic Phototrophic Fe(II)-Oxidizers
2.2. Iron Mineral Formation and Characteristics
2.2.1. Formation of Fe(III) Minerals by Acidophilic Aerobic Fe(II)-Oxidizers
2.2.2. Formation of Fe(III) Minerals by Neutrophilic Microaerophilic Fe(II)-Oxidizers
2.2.3. Formation of Fe(III) Minerals by Nitrate-Reducing Fe(II)-Oxidizers
2.2.4. Formation of Fe(III) Minerals by Phototrophic Fe(II)-Oxidizers
3. Influencing Factors of Fe(II) Bio-Oxidation and Mineralization
3.1. Impact of pH and Temperature
3.2. Effects of Organic Matter
3.3. Characteristics of Coexisting Ions
4. Heavy Metal Immobilization by Biogenic Iron Minerals
4.1. Adsorption and Coprecipitation of Heavy Metals
4.2. Formation of Mixed Fe(III)-As Minerals
5. Knowledge Gaps and Future Recommendations
- (1)
- Current research has confirmed that anaerobic nitrate-reducing Fe(II)-oxidizing bacteria mediate the NRFO process through both biological and chemical mechanisms. However, the specific pathways and relative contributions of these biological/chemical mechanisms need to be further elucidated. Additionally, these microorganisms can alleviate crust formation by secreting EPSs, but whether this phenomenon is widespread and whether other anti-crusting mechanisms exist still require further investigation. Previous studies on anaerobic phototrophic Fe(II)-oxidizing microbes have primarily focused on strains TIE-1 and SW2, demonstrating their reliance on the Pio and Fox operons, respectively. Nevertheless, the electron transfer pathways in these microorganisms remain unclear. Future research should employ genomic, transcriptomic, and metabolomic approaches to explore Fe(II)-oxidation-related functional genes and proteins, thereby refining the Fe(II) oxidation pathways and electron transfer mechanisms.
- (2)
- Current research on microbially mediated Fe2+ oxidation and mineralization primarily focuses on heavy metal immobilization, particularly arsenic species [As(III)/As(V)], while largely neglecting its implications for natural water quality and soil evolution. Therefore, research on the biomineralization processes mediated by FeOB at the field scale are necessary.
- (3)
- In natural environments, FeOB typically coexist with heterotrophic bacteria and sulfur-cycling microorganisms. Moreover, different types of FeOB with varying metabolic requirements may co-occur in certain habitats. Current research has primarily focused on laboratory experiments using single-strain cultures, leaving the interspecies interactions within these microbial communities poorly understood. Future studies should investigate the electron transfer mechanisms and elemental cycling processes among mixed microbial consortia.
- (4)
- As mentioned above, adsorption is an effective method for heavy metal removal, and thus the selection of appropriate adsorbents is crucial. Nanomaterials are widely used in heavy metal adsorption due to their stable mechanical structure, high adsorption capacity, and cost-effectiveness [179]. However, traditional methods for synthesizing nanomaterials may release volatile compounds, leading to secondary pollution. Therefore, biogenic minerals offer an environmentally friendly alternative for nanomaterial synthesis. Further optimization of biogenic minerals is still needed. Under the premise of not harming bacterial activity, mild, efficient, and sustainable modification strategies should be adopted to regulate mineral morphology, structure, and surface properties, thereby enhancing the adsorption capacity and stability of biogenic minerals.
6. Implications
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIM | Biologically induced mineralization |
| FeOB | Iron-oxidizing bacteria |
| AMD | Acid mine drainage |
| A. ferrooxidans | Acidithiobacillus ferrooxidans |
| NRFO | Nitrate reduction coupled with Fe(II) oxidation |
| EPSs | Extracellular polymeric substances |
| DOM | Dissolved organic matter |
References
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms Pumping Iron: Anaerobic Microbial Iron Oxidation and Reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.; Fleming, E.J.; McBeth, J.M. Iron-Oxidizing Bacteria: An Environmental and Genomic Perspective. Annu. Rev. Microbiol. 2010, 64, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Melton, E.D.; Swanner, E.D.; Behrens, S.; Schmidt, C.; Kappler, A. The Interplay of Microbially Mediated and Abiotic Reactions in the Biogeochemical Fe Cycle. Nat. Rev. Microbiol. 2014, 12, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Posth, N.R.; Canfield, D.E.; Kappler, A. Biogenic Fe(III) Minerals: From Formation to Diagenesis and Preservation in the Rock Record. Earth-Sci. Rev. 2014, 135, 103–121. [Google Scholar] [CrossRef]
- Tagliabue, A.; Bowie, A.R.; Boyd, P.W.; Buck, K.N.; Johnson, K.S.; Saito, M.A. The Integral Role of Iron in Ocean Biogeochemistry. Nature 2017, 543, 51–59. [Google Scholar] [CrossRef]
- Ehrlich, H. Geomicrobiology: Its Significance for Geology. Earth-Sci. Rev. 1998, 45, 45–60. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Microbes and Metals. Appl. Microbiol. Biotechnol. 1997, 48, 687–692. [Google Scholar] [CrossRef]
- Ehrlich, H.L. How Microbes Influence Mineral Growth and Dissolution. Chem. Geol. 1996, 132, 5–9. [Google Scholar] [CrossRef]
- Ehrlich, H.L. How Microbes Mobilize Metals in Ores: A Review of Current Understandings and Proposals for Further Research. Min. Metall. Explor. 2002, 19, 220–224. [Google Scholar] [CrossRef]
- Li, H.; Ji, H.; Cui, X.; Che, X.; Zhang, Q.; Zhong, J.; Jin, R.; Wang, L.; Luo, Y. Kinetics, Thermodynamics, and Equilibrium of As(III), Cd(II), Cu(II) and Pb(II) Adsorption Using Porous Chitosan Bead-Supported MnFe2O4 Nanoparticles. Int. J. Min. Sci. Technol. 2021, 31, 1107–1115. [Google Scholar] [CrossRef]
- Wang, C.-C.; Zhang, Q.-C.; Yan, C.-A.; Tang, G.-Y.; Zhang, M.-Y.; Ma, L.Q.; Gu, R.-H.; Xiang, P. Heavy Metal(Loid)s in Agriculture Soils, Rice, and Wheat across China: Status Assessment and Spatiotemporal Analysis. Sci. Total Environ. 2023, 882, 163361. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent Advances in Soil Remediation Technology for Heavy Metal Contaminated Sites: A Critical Review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef] [PubMed]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery From Various Environments-A Review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar] [CrossRef]
- Shi, J.; Wu, X.; Zhao, X.; Zhou, J.; Liu, S.; Li, B.; Zhang, J.; Li, W.; Zeng, X.; Wang, X.; et al. Remediation of Heavy Metal-Contaminated Estuarine Sediments by Strengthening Microbial in-Situ Mineralization. Appl. Geochem. 2024, 169, 106051. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A Critical Review on Various Remediation Approaches for Heavy Metal Contaminants Removal from Contaminated Soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef]
- Mann, S. Mineralization in Biological Systems. In Inorganic Elements in Biochemistry; Connett, P.H., Folłmann, H., Lammers, M., Mann, S., Odom, J.D., Wetterhahn, K.E., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 125–174. [Google Scholar]
- Bazylinski, D.; Frankel, R. Biologically Controlled Mineralization in Prokaryotes. In Biomineralization; Dove, P., DeYoreo, J., Weiner, S., Eds.; Shorte Course on Biomineralization: Napa Valley, CA, USA, 2003; Volume 54, pp. 217–247. ISBN 1529-6466. [Google Scholar]
- Lowenstam, H.A. Minerals Formed by Organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef]
- Weiner, S.; Dove, P. An Overview of Biomineralization Processes and the Problem of the Vital Effect. In Biomineralization; Dove, P., DeYoreo, J., Weiner, S., Eds.; Shorte Course on Biomineralization: Napa Valley, CA, USA, 2003; Volume 54, pp. 1–29. ISBN 1529-6466. [Google Scholar]
- Ehrlich, H.; Bailey, E.; Wysokowski, M.; Jesionowski, T. Forced Biomineralization: A Review. Biomimetics 2021, 6, 46. [Google Scholar] [CrossRef]
- Bian, Z.; Dong, W.; Li, X.; Song, Y.; Huang, H.; Hong, K.; Hu, K. Enrichment of Terbium(III) under Synergistic Effect of Biosorption and Biomineralization by Bacillus Sp. DW015 Sporosarcina pasteurii. Microbiol. Spectr. 2024, 12, e00760-24. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Su, J.; Li, X.; Wen, G.; Li, X. Simultaneous Removal of Calcium, Phosphorus, and Bisphenol A from Industrial Wastewater by Stutzerimonas Sp. ZW5 via Microbially Induced Calcium Precipitation (MICP): Kinetics, Mechanism, and Stress Response. J. Hazard. Mater. 2024, 473, 134700. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Zhou, R.; Si, Y. β-Tricalcium Phosphate Enhanced Biomineralization of Cd2+ and Pb2+ by Sporosarcina ureilytica HJ1 and Sporosarcina pasteurii HJ2. J. Hazard. Mater. 2024, 474, 134624. [Google Scholar] [CrossRef]
- Zhao, X.; Do, H.; Zhou, Y.; Li, Z.; Zhang, X.; Zhao, S.; Li, M.; Wu, D. Rahnella Sp. LRP3 Induces Phosphate Precipitation of Cu (II) and Its Role in Copper-Contaminated Soil Remediation. J. Hazard. Mater. 2019, 368, 133–140. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, J. Phosphate Microbial Mineralization Consolidation of Waste Incineration Fly Ash and Removal of Lead Ions. Ecotoxicol. Environ. Saf. 2020, 191, 110224. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Zhang, Z. Bacillus megaterium Y5 Fixed Mechanism on Lead. J. Jilin Agric. Univ. 2024, 1–9. [Google Scholar] [CrossRef]
- Yang, F.; Zhai, W.; Li, Z.; Huang, Y.; Manzoor, M.; Yang, B.; Hou, Y.; Lei, L.; Tang, X. Immobilization of Lead and Cadmium in Agricultural Soil by Bioelectrochemical Reduction of Sulfate in Underground Water. Chem. Eng. J. 2021, 422, 130010. [Google Scholar] [CrossRef]
- Wang, Y.; Tsang, Y.F.; Wang, H.; Sun, Y.; Song, Y.; Pan, X.; Luo, S. Effective Stabilization of Arsenic in Contaminated Soils with Biogenic Manganese Oxide (BMO) Materials. Environ. Pollut. 2020, 258, 113481. [Google Scholar] [CrossRef] [PubMed]
- Nelson, Y.; Lion, L.; Shuler, M.; Ghiorse, W. Effect of Oxide Formation Mechanisms on Lead Adsorption by Biogenic Manganese (Hydr)Oxides, Iron (Hydr)Oxides, and Their Mixtures. Environ. Sci. Technol. 2002, 36, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Ahoranta, S.H.; Kokko, M.E.; Papirio, S.; Özkaya, B.; Puhakka, J.A. Arsenic Removal from Acidic Solutions with Biogenic Ferric Precipitates. J. Hazard. Mater. 2016, 306, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Sand, W.; Gehrke, T. Extracellular Polymeric Substances Mediate Bioleaching/Biocorrosion via Interfacial Processes Involving Iron(III) Ions and Acidophilic Bacteria. Res. Microbiol. 2006, 157, 49–56. [Google Scholar] [CrossRef]
- Vera, M.A.; Rohwerder, T.; Bellenberg, S.; Sand, W.; Denis, Y.; Bonnefoy, V. Characterization of Biofilm Formation by the Bioleaching Acidophilic Bacterium Acidithiobacillus ferrooxidans by a Microarray Transcriptome Analysis. Adv. Mater. Res. 2009, 71–73, 175–178. [Google Scholar] [CrossRef]
- Rodríguez-Galán, M.; Baena-Moreno, F.; Vázquez, S.; Arroyo-Torralvo, F.; Vilches, L.; Zhang, Z. Remediation of Acid Mine Drainage. Environ. Chem. Lett. 2019, 17, 1529–1538. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Resource Utilization of Acid Mine Drainage (AMD): A Review. Water 2022, 14, 2385. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. The Microbiology of Acidic Mine Waters. Res. Microbiol. 2003, 154, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B. Importance of Microbial Ecology in the Development of New Mineral Technologies. Hydrometallurgy 2001, 59, 147–157. [Google Scholar] [CrossRef]
- Johnson, D.B. Biodiversity and Ecology of Acidophilic Microorganisms. FEMS Microbiol. Ecol. 1998, 27, 307–317. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Kusano, T. Molecular Genetics of Thiobacillus ferrooxidans. Microbiol. Rev. 1994, 58, 39–55. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Li, Y.; Zhang, Y.; Wang, X. Progress in Structure Regulation and Environmental Applications of Biologically Synthesized Schwertmannite. Mod. Chem. Ind. 2025, 45, 19–23. [Google Scholar] [CrossRef]
- Bonnefoy, V.; Holmes, D.S. Genomic Insights into Microbial Iron Oxidation and Iron Uptake Strategies in Extremely Acidic Environments. Environ. Microbiol. 2012, 14, 1597–1611. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, M.; Zhang, S.; Zhao, D.; Duan, J.; Wang, W.; Yan, L. Iron and Sulfur Oxidation Pathways of Acidithiobacillus ferrooxidans. World J. Microbiol. Biotechnol. 2019, 35, 60. [Google Scholar] [CrossRef]
- Wang, R. Basic Study on the Electron Transport Chain of Ferrous Pxidation in Acidithiobacillus ferrooxidans. Master’s Thesis, Shandong University, Jinan, China, 2022. [Google Scholar]
- Zhang, C.; Zhang, S.; Liu, T.; Zhao, Z.; Yan, L. Condition Optimization and Identification of Secondary Minerals Produced by Leptospirillum Ferrodiazotrophum. Microbiol. China 2024, 51, 3954–3969. [Google Scholar] [CrossRef]
- Tyson, G.W.; Lo, I.; Baker, B.J.; Allen, E.E.; Hugenholtz, P.; Banfield, J.F. Genome-Directed Isolation of the Key Nitrogen Fixer Leptospirillum ferrodiazotrophum Sp. Nov. from an Acidophilic Microbial Community. Appl. Environ. Microbiol. 2005, 71, 6319–6324. [Google Scholar] [CrossRef]
- Goltsman, D.S.A.; Denef, V.J.; Singer, S.W.; VerBerkmoes, N.C.; Lefsrud, M.; Mueller, R.S.; Dick, G.J.; Sun, C.L.; Wheeler, K.E.; Zemla, A.; et al. Community Genomic and Proteomic Analyses of Chemoautotrophic Iron-Oxidizing “Leptospirillum Rubarum” (Group II) and “Leptospirillum Ferrodiazotrophum” (Group III) Bacteria in Acid Mine Drainage Biofilms. Appl. Environ. Microbiol. 2009, 75, 4599–4615. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Liu, Y.; Yang, B.; Wu, B.; Li, H.; Liu, H.; Yu, S.; Liu, S.; Liao, R. Research Progress and Application in Biomineralization of Acidophilic Iron-Oxidizing Bacteria. J. Biol. 2024, 41, 69–76. [Google Scholar]
- Sand, W.; Rohde, K.; Sobotke, B.; Zenneck, C. Evaluation of Leptospirillum ferrooxidans for Leaching. Appl. Environ. Microbiol. 1992, 58, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, J.; Yan, W. Bioming Microbes—Recent Research Progress of Bacteria Belonging to the Genus Leptospirillum. Microbiol. China 2006, 02, 151–155. [Google Scholar] [CrossRef]
- Quatrini, R.; Appia-Ayme, C.; Denis, Y.; Jedlicki, E.; Holmes, D.S.; Bonnefoy, V. Extending the Models for Iron and Sulfur Oxidation in the Extreme Acidophile Acidithiobacillus ferrooxidans. BMC Genom. 2009, 10, 394. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and Its Potential Application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef]
- Li, X.; Mou, S.; Chen, Y.; Liu, T.; Dong, J.; Li, F. Microaerobic Fe(II) Oxidation Coupled to Carbon Assimilation Processes Driven by Microbes from Paddy Soil. Sci. China Earth Sci. 2019, 49, 1948–1959. [Google Scholar] [CrossRef]
- Maisch, M.; Lueder, U.; Laufer, K.; Scholze, C.; Kappler, A.; Schmidt, C. Contribution of Microaerophilic Iron(II)-Oxidizers to Iron(III) Mineral Formation. Environ. Sci. Technol. 2019, 53, 8197–8204. [Google Scholar] [CrossRef]
- Emerson, D.; Moyer, C. Isolation and Characterization of Novel Iron-Oxidizing Bacteria That Grow at Circumneutral pH. Appl. Environ. Microbiol. 1997, 63, 4784–4792. [Google Scholar] [CrossRef]
- Hedrich, S.; Schlömann, M.; Johnson, D.B. The Iron-Oxidizing Proteobacteria. Microbiology 2011, 157, 1551–1564. [Google Scholar] [CrossRef]
- Emerson, D. Microbial Oxidation of Fe(II) and Mn(II) at Circumneutral pH. In Environmental Microbe-Metal Interactions; Wiley: Hoboken, NJ, USA, 2000; pp. 31–52. ISBN 978-1-68367-249-4. [Google Scholar]
- Zhou, N.; Keffer, J.L.; Polson, S.W.; Chan, C.S. Unraveling Fe(II)-Oxidizing Mechanisms in a Facultative Fe(II) Oxidizer, Sideroxydans lithotrophicus Strain ES-1, via Culturing, Transcriptomics, and Reverse Transcription-Quantitative PCR. Appl. Environ. Microbiol. 2022, 88, e0159521. [Google Scholar] [CrossRef]
- Li, J.; Tong, H.; Chen, M.; Liu, C.; Jiang, Q.; Yi, X. Formation of Fe(III) Minerals by Microaerophilic Fe(II)-Oxidizing Bacteria and Its Effect on Immobilization of Heavy Metals: A Review. Ecol. Environ. Sci. 2024, 33, 310–320. [Google Scholar] [CrossRef]
- He, S.; Barco, R.A.; Emerson, D.; Roden, E.E. Comparative Genomic Analysis of Neutrophilic Iron(II) Oxidizer Genomes for Candidate Genes in Extracellular Electron Transfer. Front. Microbiol. 2017, 8, 1584. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Belchik, S.M.; Edwards, M.J.; Liu, C.; Kennedy, D.W.; Merkley, E.D.; Lipton, M.S.; Butt, J.N.; Richardson, D.J.; et al. Identification and Characterization of MtoA: A Decaheme c-Type Cytochrome of the Neutrophilic Fe(II)-Oxidizing Bacterium Sideroxydans lithotrophicus ES-1. Front. Microbiol. 2012, 3, 37. [Google Scholar] [CrossRef]
- Zhou, N.; Kupper, R.J.; Catalano, J.G.; Thompson, A.; Chan, C.S. Biological Oxidation of Fe(II)-Bearing Smectite by Microaerophilic Iron Oxidizer Sideroxydans lithotrophicus Using Dual Mto and Cyc2 Iron Oxidation Pathways. Environ. Sci. Technol. 2022, 56, 17443–17453. [Google Scholar] [CrossRef]
- McAllister, S.M.; Polson, S.W.; Butterfield, D.A.; Glazer, B.T.; Sylvan, J.B.; Chan, C.S. Validating the Cyc2 Neutrophilic Iron Oxidation Pathway Using Meta-Omics of Zetaproteobacteria Iron Mats at Marine Hydrothermal Vents. mSystems 2020, 5, e00553-19. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Liu, T.; Li, F.; Sun, W.; Young, L.Y.; Huang, W. Metagenomic Analysis of Fe(II)-Oxidizing Bacteria for Fe(III) Mineral Formation and Carbon Assimilation under Microoxic Conditions in Paddy Soil. Sci. Total Environ. 2022, 851, 158068. [Google Scholar] [CrossRef]
- Lin, C.; Gong, J. Recent Progress in Research on Neutrophilic, Microaerophilic Iron(II)-Oxidizing Bacteria. Acta Ecol. Sin. 2012, 32, 5889–5899. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, K.; Liu, T.; Chen, G.; Kappler, A.; Li, X.; Zeng, R.J.; Yang, Y.; Yue, F.; Hu, S.; et al. Novel Insight into Microbially Mediated Nitrate-Reducing Fe(II) Oxidation by Acidovorax Sp. Strain BoFeN1 Using Dual N–O Isotope Fractionation. Environ. Sci. Technol. 2023, 57, 12546–12555. [Google Scholar] [CrossRef]
- Smith, R.L.; Kent, D.B.; Repert, D.A.; Böhlke, J.K. Anoxic Nitrate Reduction Coupled with Iron Oxidation and Attenuation of Dissolved Arsenic and Phosphate in a Sand and Gravel Aquifer. Geochim. Cosmochim. Acta 2017, 196, 102–120. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, H.; Kukkadapu, R.; Agrawal, A.; Liu, D.; Zhang, J.; Edelmann, R.E. Biological Oxidation of Fe(II) in Reduced Nontronite Coupled with Nitrate Reduction by Pseudogulbenkiania Sp. Strain 2002. Geochim. Cosmochim. Acta 2013, 119, 231–247. [Google Scholar] [CrossRef]
- Straub, K.L.; Benz, M.; Schink, B.; Widdel, F. Anaerobic, Nitrate-Dependent Microbial Oxidation of Ferrous Iron. Appl. Environ. Microbiol. 1996, 62, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, T.; Li, X.; Li, F.; Luo, X.; Wu, Y.; Wang, Y. Biological and Chemical Processes of Microbially Mediated Nitrate-Reducing Fe(II) Oxidation by Pseudogulbenkiania Sp Strain 2002. Chem. Geol. 2018, 476, 59–69. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, K.; Chen, D.; Wang, Y.; Yin, Y.; Li, F. Formation of Fe (III) -Minerals by Microbially Mediated Coupling of Nitrate Reduction and Fe (II) Oxidation: A Review. Ecol. Environ. Sci. 2019, 28, 620–628. [Google Scholar] [CrossRef]
- Peng, R.; Shen, J.; Huang, Y.; Liu, H. Isolation, Characterization and Function of a Nitrate-Dependent Iron-Oxidizing Bacterium Aquabacterium olei HJ-4. Saf. Environ. Eng. 2022, 29, 204–211. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; Kleerebezem, R.; de Vet, W.W.J.M.; van Loosdrecht, M.C.M. Reduced Iron Induced Nitric Oxide and Nitrous Oxide Emission. Water Res. 2011, 45, 5945–5952. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, C.-W.; ThomasArrigo, L.K.; Zhao, S.-C.; Kretzschmar, R.; Zhao, F.-J. Nitrite Accumulation Is Required for Microbial Anaerobic Iron Oxidation, but Not for Arsenite Oxidation, in Two Heterotrophic Denitrifiers. Environ. Sci. Technol. 2020, 54, 4036–4045. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Xu, Y.; Zhou, W.; Huang, K.; Tang, Z.; Zhao, F.-J. Nitrate Stimulates Anaerobic Microbial Arsenite Oxidation in Paddy Soils. Environ. Sci. Technol. 2017, 51, 4377–4386. [Google Scholar] [CrossRef]
- Feng, Q.; Li, C.; Hu, N.; Li, S.; He, Y.; Li, Y.; Guo, T.; Hu, W.; Dong, Y. Research Progress in the Microbially Catalyzed Reactions Associated with Coupled Nitrogen and Iron Transformation in Lake Ecosystems. Saf. Environ. Eng. 2024, 31, 271–281. [Google Scholar] [CrossRef]
- Widdel, F.; Schnell, S.; Heising, S.; Ehrenreich, A.; Assmus, B.; Schink, B. Ferrous Iron Oxidation by Anoxygenic Phototrophic Bacteria. Nature 1993, 362, 834–836. [Google Scholar] [CrossRef]
- Huang, L.; Li, L.; Jiang, H. Formation and Iron Oxidation Mechanisms of BIFs:Research Progress Review and Outlook. Earth Sci. Front. 2023, 30, 333–346. [Google Scholar] [CrossRef]
- Straub, K.; Rainey, F.; Widdel, F. Rhodovulum iodosum Sp. Nov, and Rhodovulum robiginosum Sp. Nov., Two New Marine Phototrophic Ferrous-Iron-Oxidizing Purple Bacteria. Int. J. Syst. Bacteriol. 1999, 49, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Swanner, E.D.; Wu, W.; Schoenberg, R.; Byrne, J.; Michel, F.M.; Pan, Y.; Kappler, A. Fractionation of Fe Isotopes during Fe(II) Oxidation by a Marine Photoferrotroph Is Controlled by the Formation of Organic Fe-Complexes and Colloidal Fe Fractions. Geochim. Cosmochim. Acta 2015, 165, 44–61. [Google Scholar] [CrossRef]
- Melton, E.D.; Schmidt, C.; Kappler, A. Microbial Iron(II) Oxidation in Littoral Freshwater Lake Sediment: The Potential for Competition between Phototrophic vs. Nitrate-Reducing Iron(II)-Oxidizers. Front. Microbio. 2012, 3, 197. [Google Scholar] [CrossRef]
- Crowe, S.A.; Jones, C.; Katsev, S.; Magen, C.; O’Neill, A.H.; Sturm, A.; Canfield, D.E.; Haffner, G.D.; Mucci, A.; Sundby, B.; et al. Photoferrotrophs Thrive in an Archean Ocean Analogue. Proc. Natl. Acad. Sci. USA 2008, 105, 15938–15943. [Google Scholar] [CrossRef]
- Heising, S.; Richter, L.; Ludwig, W.; Schink, B. Chlorobium ferrooxidans Sp. Nov., a Phototrophic Green Sulfur Bacterium That Oxidizes Ferrous Iron in Coculture with a “Geospirillum” Sp. Strain. Arch. Microbiol. 1999, 172, 116–124. [Google Scholar] [CrossRef]
- Croal, L.R.; Johnson, C.M.; Beard, B.L.; Newman, D.K. Iron Isotope Fractionation by Fe(II)-Oxidizing Photoautotrophic Bacteria. Geochim. Cosmochim. Acta 2004, 68, 1227–1242. [Google Scholar] [CrossRef]
- Jiao, Y.; Kappler, A.; Croal, L.R.; Newman, D.K. Isolation and Characterization of a Genetically Tractable Photoautotrophic Fe(II)-Oxidizing Bacterium, Rhodopseudomonas palustris Strain TIE-1. Appl. Environ. Microbiol. 2005, 71, 4487–4496. [Google Scholar] [CrossRef]
- Xiong, J. Photosynthesis: What Color Was Its Origin? Genome Biol. 2007, 7, 245. [Google Scholar] [CrossRef]
- Bryce, C.; Franz-Wachtel, M.; Nalpas, N.C.; Miot, J.; Benzerara, K.; Byrne, J.M.; Kleindienst, S.; Macek, B.; Kappler, A. Proteome Response of a Metabolically Flexible Anoxygenic Phototroph to Fe(II) Oxidation. Appl. Environ. Microbiol. 2018, 84, e01166-18. [Google Scholar] [CrossRef]
- Kappler, A.; Pasquero, C.; Konhauser, K.O.; Newman, D.K. Deposition of Banded Iron Formations by Anoxygenic Phototrophic Fe(II)-Oxidizing Bacteria. Geology 2005, 33, 865. [Google Scholar] [CrossRef]
- Posth, N.R.; Konhauser, K.O.; Kappler, A. Microbiological Processes in Banded Iron Formation Deposition. Sedimentology 2013, 60, 1733–1754. [Google Scholar] [CrossRef]
- Melton, E.D.; Schmidt, C.; Behrens, S.; Schink, B.; Kappler, A. Metabolic Flexibility and Substrate Preference by the Fe(II)-Oxidizing Purple Non-Sulphur Bacterium Rhodopseudomonas Palustris Strain TIE-1. Geomicrobiol. J. 2014, 31, 835–843. [Google Scholar] [CrossRef]
- Laufer, K.; Niemeyer, A.; Nikeleit, V.; Halama, M.; Byrne, J.M.; Kappler, A. Physiological Characterization of a Halotolerant Anoxygenic Phototrophic Fe(II)-Oxidizing Green-Sulfur Bacterium Isolated from a Marine Sediment. FEMS Microbiol. Ecol. 2017, 93, fix054. [Google Scholar] [CrossRef]
- Bose, A.; Gardel, E.J.; Vidoudez, C.; Parra, E.A.; Girguis, P.R. Electron Uptake by Iron-Oxidizing Phototrophic Bacteria. Nat. Commun. 2014, 5, 3391. [Google Scholar] [CrossRef]
- Jiao, Y.; Newman, D.K. The Pio Operon Is Essential for Phototrophic Fe(II) Oxidation in Rhodopseudomonas palustris TIE-1. J Bacteriol. 2007, 189, 1765–1773. [Google Scholar] [CrossRef]
- Bird, L.J.; Bonnefoy, V.; Newman, D.K. Bioenergetic Challenges of Microbial Iron Metabolisms. Trends Microbiol. 2011, 19, 330–340. [Google Scholar] [CrossRef]
- Saraiva, I.H.; Newman, D.K.; Louro, R.O. Functional Characterization of the FoxE Iron Oxidoreductase from the Photoferrotroph Rhodobacter ferrooxidans SW2. J. Biol. Chem. 2012, 287, 25541–25548. [Google Scholar] [CrossRef]
- Bryce, C.; Blackwell, N.; Schmidt, C.; Otte, J.; Huang, Y.; Kleindienst, S.; Tomaszewski, E.; Schad, M.; Warter, V.; Peng, C.; et al. Microbial Anaerobic Fe(II) Oxidation–Ecology, Mechanisms and Environmental Implications. Environ. Microbiol. 2018, 20, 3462–3483. [Google Scholar] [CrossRef]
- Croal, L.R.; Jiao, Y.; Newman, D.K. The Fox Operon from Rhodobacter Strain SW2 Promotes Phototrophic Fe(II) Oxidation in Rhodobacter capsulatus SB1003. J. Bacteriol. 2007, 189, 1774–1782. [Google Scholar] [CrossRef]
- Gupta, D.; Guzman, M.S.; Rengasamy, K.; Stoica, A.; Singh, R.; Ranaivoarisoa, T.O.; Davenport, E.J.; Bai, W.; McGinley, B.; Meacham, J.M.; et al. Photoferrotrophy and Phototrophic Extracellular Electron Uptake Is Common in the Marine Anoxygenic Phototroph Rhodovulum sulfidophilum. ISME J. 2021, 15, 3384–3398. [Google Scholar] [CrossRef]
- Laufer, K.; Nordhoff, M.; Røy, H.; Schmidt, C.; Behrens, S.; Jørgensen, B.B.; Kappler, A. Coexistence of Microaerophilic, Nitrate-Reducing, and Phototrophic Fe(II) Oxidizers and Fe(III) Reducers in Coastal Marine Sediment. Appl. Environ. Microbiol. 2016, 82, 1433–1447. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Li, M.-M.; Zeng, J.; Liu, X.-X.; Zhu, J.-Y.; Hu, Y.-H.; Qiu, G.-Z. Acidithiobacillus ferrooxidans Enhanced Heavy Metals Immobilization Efficiency in Acidic Aqueous System through Bio-Mediated Coprecipitation. Trans. Nonferrous Met. Soc. China 2017, 27, 1156–1164. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, L. Thermodynamics and Kinetics of Adsorption of Arsenite in Acid Mine Drainage by Biogenic Secondary Iron Minerals. Acta Pedol. Sin. 2012, 49, 481–490. [Google Scholar]
- Zhong, P.; Wu, Z.; Ji, C.; Xiao, Q. Research Progress on Source Control Technologies of Biological Mineralization for Acid Mine Drainage. Hydrometall. China 2022, 41, 289–294. [Google Scholar] [CrossRef]
- Bigham, J.M.; Schwertmann, U.; Traina, S.J.; Winland, R.L.; Wolf, M. Schwertmannite and the Chemical Modeling of Iron in Acid Sulfate Waters. Geochim. Cosmochim. Acta 1996, 60, 2111–2121. [Google Scholar] [CrossRef]
- Hedrich, S.; Johnson, D.B. A Modular Continuous Flow Reactor System for the Selective Bio-Oxidation of Iron and Precipitation of Schwertmannite from Mine-Impacted Waters. Bioresour. Technol. 2012, 106, 44–49. [Google Scholar] [CrossRef]
- Regenspurg, S.; Brand, A.; Peiffer, S. Formation and Stability of Schwertmannite in Acidic Mining Lakes. Geochim. Cosmochim. Acta 2004, 68, 1185–1197. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Gan, M. Controllable Biosynthesis of Nanoscale Schwertmannite and the Application in Heavy Metal Effective Removal. Appl. Surf. Sci. 2020, 529, 147012. [Google Scholar] [CrossRef]
- Feng, K.; Wang, X.; Zhou, B.; Xu, M.; Liang, J.; Zhou, L. Hydroxyl, Fe2+, and Acidithiobacillus ferrooxidans Jointly Determined the Crystal Growth and Morphology of Schwertmannite in a Sulfate-Rich Acidic Environment. ACS Omega 2021, 6, 3194–3201. [Google Scholar] [CrossRef]
- Feng, K.; Wang, X.; Ding, B.; Xu, M.; Liang, J.; Zhou, L. Acidithiobacillus ferrooxidans Mediates Morphology Evolution of Schwertmannite in the Presence of Fe2+. Chem. Geol. 2022, 598, 120828. [Google Scholar] [CrossRef]
- Song, Y.; Yang, L.; Wang, H.; Sun, X.; Bai, S.; Wang, N.; Liang, J.; Zhou, L. The Coupling Reaction of Fe2+ Bio-Oxidation and Resulting Fe3+ Hydrolysis Drastically Improve the Formation of Iron Hydroxysulfate Minerals in AMD. Environ. Technol. 2021, 42, 2325–2334. [Google Scholar] [CrossRef]
- Sun, M.; Su, L.; Li, J. Diversity, Biomineralization, and Metabolic Characteristics of Neutrophilic Microaerophilic Iron-Oxidizing Bacteria Inseafloor Hydrothermal Environment. Acta Microbiol. Sin. 2022, 62, 2119–2135. [Google Scholar] [CrossRef]
- Chan, C.S.; Emerson, D.; Iii, G.W.L. The Role of Microaerophilic Fe-oxidizing Micro-organisms in Producing Banded Iron Formations. Geobiology 2016, 14, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, F.; Li, X. Diversity and Biomineralization of Microaerophilic Iron-Oxidizing Bacteria in Paddy Soil. Ecol. Environ. Sci. 2016, 25, 547–554. [Google Scholar] [CrossRef]
- McAllister, S.M.; Moore, R.M.; Gartman, A.; Luther, G.W.; Emerson, D.; Chan, C.S. The Fe(II)-Oxidizing Zetaproteobacteria: Historical, Ecological and Genomic Perspectives. FEMS Microbiol. Ecol. 2019, 95, fiz015. [Google Scholar] [CrossRef]
- Chan, C.S.; Fakra, S.C.; Emerson, D.; Fleming, E.J.; Edwards, K.J. Lithotrophic Iron-Oxidizing Bacteria Produce Organic Stalks to Control Mineral Growth: Implications for Biosignature Formation. ISME J. 2011, 5, 717–727. [Google Scholar] [CrossRef]
- Tong, H.; Liu, C.; Hao, L.; Swanner, E.D.; Chen, M.; Li, F.; Xia, Y.; Liu, Y.; Liu, Y. Biological Fe(II) and As(III) Oxidation Immobilizes Arsenic in Micro-Oxic Environments. Geochim. Cosmochim. Acta 2019, 265, 96–108. [Google Scholar] [CrossRef]
- Xiu, W.; Guo, H.; Shen, J.; Liu, S.; Ding, S.; Hou, W.; Ma, J.; Dong, H. Stimulation of Fe(II) Oxidation, Biogenic Lepidocrocite Formation, and Arsenic Immobilization by Pseudogulbenkiania Sp. Strain 2002. Environ. Sci. Technol. 2016, 50, 6449–6458. [Google Scholar] [CrossRef]
- Pantke, C.; Obst, M.; Benzerara, K.; Morin, G.; Ona-Nguema, G.; Dippon, U.; Kappler, A. Green Rust Formation during Fe(II) Oxidation by the Nitrate-Reducing Acidovorax Sp. Strain BoFeN1. Environ. Sci. Technol. 2012, 46, 1439–1446. [Google Scholar] [CrossRef]
- Sun, J.; Chillrud, S.N.; Mailloux, B.J.; Stute, M.; Singh, R.; Dong, H.; Lepre, C.J.; Bostick, B.C. Enhanced and Stabilized Arsenic Retention in Microcosms through the Microbial Oxidation of Ferrous Iron by Nitrate. Chemosphere 2016, 144, 1106–1115. [Google Scholar] [CrossRef]
- Kiskira, K.; Papirio, S.; Mascolo, M.C.; Fourdrin, C.; Pechaud, Y.; Van Hullebusch, E.D.; Esposito, G. Mineral Characterization of the Biogenic Fe(III)(Hydr)Oxides Produced during Fe(II)-Driven Denitrification with Cu, Ni and Zn. Sci. Total Environ. 2019, 687, 401–412. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-H.; Shin, T.J.; Hur, H.-G.; Kim, M.G. Adsorption and Incorporation of Arsenic to Biogenic Lepidocrocite Formed in the Presence of Ferrous Iron during Denitrification by Paracoccus denitrificans. Environ. Sci. Technol. 2018, 52, 9983–9991. [Google Scholar] [CrossRef]
- Miot, J.; Benzerara, K.; Morin, G.; Kappler, A.; Bernard, S.; Obst, M.; Férard, C.; Skouri-Panet, F.; Guigner, J.-M.; Posth, N.; et al. Iron Biomineralization by Anaerobic Neutrophilic Iron-Oxidizing Bacteria. Geochim. Cosmochim. Acta 2009, 73, 696–711. [Google Scholar] [CrossRef]
- Klueglein, N.; Zeitvogel, F.; Stierhof, Y.-D.; Floetenmeyer, M.; Konhauser, K.O.; Kappler, A.; Obst, M. Potential Role of Nitrite for Abiotic Fe(II) Oxidation and Cell Encrustation during Nitrate Reduction by Denitrifying Bacteria. Appl. Environ. Microbiol. 2014, 80, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; He, Y.; Li, J.; Kappler, A.; Pan, Y. Iron Isotope Fractionation in Anoxygenic Phototrophic Fe(II) Oxidation by Rhodobacter ferrooxidans SW2. Geochim. Cosmochim. Acta 2022, 332, 355–368. [Google Scholar] [CrossRef]
- Wu, W.; Swanner, E.D.; Hao, L.; Zeitvogel, F.; Obst, M.; Pan, Y.; Kappler, A. Characterization of the Physiology and Cell–Mineral Interactions of the Marine Anoxygenic Phototrophic Fe(II) Oxidizer Rhodovulum iodosum–Implications for Precambrian Fe(II) Oxidation. FEMS Microbiol. Ecol. 2014, 88, 503–515. [Google Scholar] [CrossRef]
- Swanner, E.D.; Bayer, T.; Wu, W.; Hao, L.; Obst, M.; Sundman, A.; Byrne, J.M.; Michel, F.M.; Kleinhanns, I.C.; Kappler, A.; et al. Iron Isotope Fractionation during Fe(II) Oxidation Mediated by the Oxygen-Producing Marine Cyanobacterium Synechococcus PCC 7002. Environ. Sci. Technol. 2017, 51, 4897–4906. [Google Scholar] [CrossRef]
- Gauger, T.; Byrne, J.M.; Konhauser, K.O.; Obst, M.; Crowe, S.; Kappler, A. Influence of Organics and Silica on Fe(II) Oxidation Rates and Cell–Mineral Aggregate Formation by the Green-Sulfur Fe(II)-Oxidizing Bacterium Chlorobium ferrooxidans KoFox–Implications for Fe(II) Oxidation in Ancient Oceans. Earth Planet. Sci. Lett. 2016, 443, 81–89. [Google Scholar] [CrossRef]
- Hegler, F.; Schmidt, C.; Schwarz, H.; Kappler, A. Does a Low-pH Microenvironment around Phototrophic FeII Oxidizing Bacteria Prevent Cell Encrustation by FeIII Minerals? FEMS Microbiol. Ecol. 2010, 74, 592–600. [Google Scholar] [CrossRef]
- Miot, J.; Benzerara, K.; Obst, M.; Kappler, A.; Hegler, F.; Schädler, S.; Bouchez, C.; Guyot, F.; Morin, G. Extracellular Iron Biomineralization by Photoautotrophic Iron-Oxidizing Bacteria. Appl. Environ. Microbiol. 2009, 75, 5586–5591. [Google Scholar] [CrossRef] [PubMed]
- Schädler, S.; Burkhardt, C.; Hegler, F.; Straub, K.L.; Miot, J.; Benzerara, K.; Kappler, A. Formation of Cell-Iron-Mineral Aggregates by Phototrophic and Nitrate-Reducing Anaerobic Fe(II)-Oxidizing Bacteria. Geomicrobiol. J. 2009, 26, 93–103. [Google Scholar] [CrossRef]
- Gauger, T.; Konhauser, K.; Kappler, A. Protection of Phototrophic Iron(II)-Oxidizing Bacteria from UV Irradiation by Biogenic Iron(III) Minerals: Implications for Early Archean Banded Iron Formation. Geology 2015, 43, 1067–1070. [Google Scholar] [CrossRef]
- Jin, D.; Wang, X.; Liu, L.; Liang, J.; Zhou, L. A Novel Approach for Treating Acid Mine Drainage through Forming Schwertmannite Driven by a Mixed Culture of Acidiphilium multivorum and Acidithiobacillus ferrooxidans Prior to Lime Neutralization. J. Hazard. Mater. 2020, 400, 123108. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, W.; Nie, Z.; Liu, H.; Liu, Y.; Wang, C.; Xia, J.; Shu, W. Fe/S Oxidation-Coupled Arsenic Speciation Transformation Mediated by AMD Enrichment Culture under Different pH Conditions. J. Environ. Sci. 2024, 137, 681–700. [Google Scholar] [CrossRef]
- Wang, H.; Bigham, J.M.; Tuovinen, O.H. Formation of Schwertmannite and Its Transformation to Jarosite in the Presence of Acidophilic Iron-Oxidizing Microorganisms. Mater. Sci. Eng. C 2006, 26, 588–592. [Google Scholar] [CrossRef]
- Kappler, A.; Newman, D.K. Formation of Fe(III)-Minerals by Fe(II)-Oxidizing Photoautotrophic Bacteria. Geochim. Cosmochim. Acta 2004, 68, 1217–1226. [Google Scholar] [CrossRef]
- Chaudhuri Swades, K.; Lack Joseph, G.; Coates John, D. Biogenic Magnetite Formation through Anaerobic Biooxidation of Fe(II). Appl. Environ. Microbiol. 2001, 67, 2844–2848. [Google Scholar] [CrossRef]
- Nurmi, P.; Özkaya, B.; Sasaki, K.; Kaksonen, A.H.; Riekkola-Vanhanen, M.; Tuovinen, O.H.; Puhakka, J.A. Biooxidation and Precipitation for Iron and Sulfate Removal from Heap Bioleaching Effluent Streams. Hydrometallurgy 2010, 101, 7–14. [Google Scholar] [CrossRef]
- Burgos, W.D.; Borch, T.; Troyer, L.D.; Luan, F.; Larson, L.N.; Brown, J.F.; Lambson, J.; Shimizu, M. Schwertmannite and Fe Oxides Formed by Biological Low-pH Fe(II) Oxidation versus Abiotic Neutralization: Impact on Trace Metal Sequestration. Geochim. Cosmochim. Acta 2012, 76, 29–44. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, R.; Wang, N.; Wu, P.; Zhang, Y.; An, L.; Zhang, Y. Effects of Initial pH and Carbonate Rock Dosage on Bio-Oxidation and Secondary Iron Mineral Synthesis. Toxics 2023, 11, 224. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, B.; Huo, M.; Cui, C.; Zhou, L. Effect of Temperature on Activity of Acidithiobacillus ferrooxidan and Formation of Biogenic Secondary Iron Minerals. Environ. Sci. 2013, 34, 3264–3271. [Google Scholar] [CrossRef]
- Dopffel, N.; Jamieson, J.; Bryce, C.; Joshi, P.; Mansor, M.; Siade, A.; Prommer, H.; Kappler, A. Temperature Dependence of Nitrate-Reducing Fe(II) Oxidation by Acidovorax Strain BoFeN1–Evaluating the Role of Enzymatic vs. Abiotic Fe(II) Oxidation by Nitrite. FEMS Microbiol. Ecol. 2022, 97, fiab155. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Geng, K.; Wang, C.; Wu, X.; Wei, C. Impact of Fulvic Acid and Acidithiobacillus ferrooxidan Inoculum Amount on the Formation of Secondary Iron Minerals. Int. J. Environ. Res. Public Health 2023, 20, 4736. [Google Scholar] [CrossRef]
- Bao, Y.; Lai, J.; Wang, Y.; Fang, Z.; Su, Y.; Alessi, D.S.; Bolan, N.S.; Wu, X.; Zhang, Y.; Jiang, X.; et al. Effect of Fulvic Acid Co-Precipitation on Biosynthesis of Fe(III) Hydroxysulfate and Its Adsorption of Lead. Environ. Pollut. 2022, 295, 118669. [Google Scholar] [CrossRef]
- Huang, H.; Ji, Y.; Wang, C.; Geng, K.; Wu, X.; Wei, C. Effect of Rotation Speed and Fulvic Acid Concentration on Biogenic Secondary High-Iron Mineral Synthesis. Water 2024, 16, 2092. [Google Scholar] [CrossRef]
- Peng, C.; Bryce, C.; Sundman, A.; Borch, T.; Kappler, A. Organic Matter Complexation Promotes Fe(II) Oxidation by the Photoautotrophic Fe(II)-Oxidizer Rhodopseudomonas palustris TIE-1. ACS Earth Space Chem. 2019, 3, 531–536. [Google Scholar] [CrossRef]
- Zhou, N.; Luther, G.W.; Chan, C.S. Ligand Effects on Biotic and Abiotic Fe(II) Oxidation by the Microaerophile Sideroxydans lithotrophicus. Environ. Sci. Technol. 2021, 55, 9362–9371. [Google Scholar] [CrossRef]
- Peng, C.; Sundman, A.; Bryce, C.; Catrouillet, C.; Borch, T.; Kappler, A. Oxidation of Fe(II)−Organic Matter Complexes in the Presence of the Mixotrophic Nitrate-Reducing Fe(II)-Oxidizing Bacterium Acidovorax Sp. BoFeN1. Environ. Sci. Technol. 2018, 52, 5753–5763. [Google Scholar] [CrossRef]
- Li, W.; Feng, Q.; Li, Z.; Jin, T.; Zhang, Y.; Southam, G. Inhibition of Iron Oxidation in Acidithiobacillus ferrooxidans by Low-Molecular-Weight Organic Acids: Evaluation of Performance and Elucidation of Mechanisms. Sci. Total Environ. 2024, 927, 171919. [Google Scholar] [CrossRef]
- Hädrich, A.; Taillefert, M.; Akob, D.M.; Cooper, R.E.; Litzba, U.; Wagner, F.E.; Nietzsche, S.; Ciobota, V.; Rösch, P.; Popp, J.; et al. Microbial Fe(II) oxidation by Sideroxydans lithotrophicus ES-1 in the presence of Schlöppnerbrunnen fen-derived humic acids. FEMS Microbiol. Ecol. 2019, 95, fiz034. [Google Scholar] [CrossRef]
- Liu, F.; Gao, S.; Wang, M.; Bu, Y.; Cui, C.; Zhou, L. Effect of Magnesium Ions on the Formation of Secondary Iron Minerals Facilitated by Acidithiobacillus ferrooxidans. China Environ. Sci. 2014, 34, 713–719. [Google Scholar]
- Liu, F.; Gao, S.; Cui, C.; Liang, J.; Zhou, L. Effect of Calcium Ions on Secondary Iron Minerals Formation in Sulfate-Rich Acidic Environment. China Environ. Sci. 2015, 35, 1142–1148. [Google Scholar]
- Song, Y.; Wang, H.; Liang, J.; Zhou, L.; Cao, Y.; Zhou, J. Factors Affecting the Formation of Secondary Iron Minerals Mediated by Acidithiobacillus ferrooxidans. Acta Sci. Circumstantiae 2018, 38, 1024–1030. [Google Scholar] [CrossRef]
- Song, Y.; Chen, T.; Wang, H.; Yang, J.; Cao, Y.; Zhou, L. Effect of Anions on the Oxidation Activity of Acidithiobacillus ferrooxidans and the Formation of Secondary Iron Minerals. China Environ. Sci. 2018, 38, 574–580. [Google Scholar] [CrossRef]
- Xiong, H.; Liao, Y.; Zhou, L. Influence of Chloride and Sulfate on Formation of Akaganéite and Schwertmannite through Ferrous Biooxidation by Acidithiobacillus ferrooxidans Cells. Environ. Sci. Technol. 2008, 42, 8681–8686. [Google Scholar] [CrossRef] [PubMed]
- Muehe, E.M.; Scheer, L.; Daus, B.; Kappler, A. Fate of Arsenic during Microbial Reduction of Biogenic versus Abiogenic As–Fe(III)–Mineral Coprecipitates. Environ. Sci. Technol. 2013, 47, 8297–8307. [Google Scholar] [CrossRef] [PubMed]
- Maillot, F.; Morin, G.; Juillot, F.; Bruneel, O.; Casiot, C.; Ona-Nguema, G.; Wang, Y.; Lebrun, S.; Aubry, E.; Vlaic, G.; et al. Structure and Reactivity of As(III)- and As(V)-Rich Schwertmannites and Amorphous Ferric Arsenate Sulfate from the Carnoulès Acid Mine Drainage, France: Comparison with Biotic and Abiotic Model Compounds and Implications for As Remediation. Geochim. Cosmochim. Acta 2013, 104, 310–329. [Google Scholar] [CrossRef]
- Liao, Y.; Liang, J.; Zhou, L. Adsorptive Removal of As(III) by Biogenic Schwertmannite from Simulated As-Contaminated Groundwater. Chemosphere 2011, 83, 295–301. [Google Scholar] [CrossRef]
- Jiang, F.; Xue, C.; Zeng, L.; Zheng, Y.; Wang, Y.; Jin, X.; Yi, X.; Dang, Z. Effects of Fe(II) Bio-Oxidation Rate and Alkali Control on Schwertmannite Microstructure and Adsorption of Oxyanions: Characteristics, Performance and Mechanism. Sci. Total Environ. 2024, 930, 172844. [Google Scholar] [CrossRef]
- Jia, Y.; Demopoulos, G.P. Adsorption of Arsenate onto Ferrihydrite from Aqueous Solution: Influence of Media (Sulfate vs. Nitrate), Added Gypsum, and pH Alteration. Environ. Sci. Technol. 2005, 39, 9523–9527. [Google Scholar] [CrossRef]
- Carlson, L.; Bigham, J.M.; Schwertmann, U.; Kyek, A.; Wagner, F. Scavenging of As from Acid Mine Drainage by Schwertmannite and Ferrihydrite: A Comparison with Synthetic Analogues. Environ. Sci. Technol. 2002, 36, 1712–1719. [Google Scholar] [CrossRef]
- Park, J.H.; Han, Y.-S.; Ahn, J.S. Comparison of Arsenic Co-Precipitation and Adsorption by Iron Minerals and the Mechanism of Arsenic Natural Attenuation in a Mine Stream. Water Res. 2016, 106, 295–303. [Google Scholar] [CrossRef]
- Burton, E.D.; Johnston, S.G.; Watling, K.; Bush, R.T.; Keene, A.F.; Sullivan, L.A. Arsenic Effects and Behavior in Association with the Fe(II)-Catalyzed Transformation of Schwertmannite. Environ. Sci. Technol. 2010, 44, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.C.; Davis, J.A.; Waychunas, G.A. Surface Chemistry of Ferrihydrite: Part 2. Kinetics of Arsenate Adsorption and Coprecipitation. Geochim. Cosmochim. Acta 1993, 57, 2271–2282. [Google Scholar] [CrossRef]
- Xiu, W.; Guo, H.; Liu, Q.; Liu, Z.; Zou, Y.; Zhang, B. Arsenic Removal and Transformation by Pseudomonas Sp. Strain GE-1-Induced Ferrihydrite: Co-Precipitation Versus Adsorption. Water Air Soil Pollut. 2015, 226, 167. [Google Scholar] [CrossRef]
- Li, B.; Pan, X.; Zhang, D.; Lee, D.-J.; Al-Misned, F.A.; Mortuza, M.G. Anaerobic Nitrate Reduction with Oxidation of Fe(II) by Citrobacter freundii Strain PXL1–a Potential Candidate for Simultaneous Removal of As and Nitrate from Groundwater. Ecol. Eng. 2015, 77, 196–201. [Google Scholar] [CrossRef]
- Hohmann, C.; Winkler, E.; Morin, G.; Kappler, A. Anaerobic Fe(II)-Oxidizing Bacteria Show As Resistance and Immobilize As during Fe(III) Mineral Precipitation. Environ. Sci. Technol. 2010, 44, 94–101. [Google Scholar] [CrossRef]
- Omoregie, E.O.; Couture, R.-M.; Van Cappellen, P.; Corkhill, C.L.; Charnock, J.M.; Polya, D.A.; Vaughan, D.; Vanbroekhoven, K.; Lloyd, J.R. Arsenic Bioremediation by Biogenic Iron Oxides and Sulfides. Appl. Environ. Microbiol. 2013, 79, 4325–4335. [Google Scholar] [CrossRef]
- Luo, X.; Jiang, X.; Xue, S.; Tang, X.; Zhou, C.; Wu, C.; Qian, Z.; Wu, K. Arsenic Biomineralization by Iron Oxidizing Strain (Ochrobactrum Sp.) Isolated from a Paddy Soil in Hunan, China. Land Degrad. Dev. 2021, 32, 2082–2093. [Google Scholar] [CrossRef]
- Hohmann, C.; Morin, G.; Ona-Nguema, G.; Guigner, J.-M.; Brown, G.E.; Kappler, A. Molecular-Level Modes of As Binding to Fe(III) (Oxyhydr)Oxides Precipitated by the Anaerobic Nitrate-Reducing Fe(II)-Oxidizing Acidovorax Sp. Strain BoFeN1. Geochim. Cosmochim. Acta 2011, 75, 4699–4712. [Google Scholar] [CrossRef]
- Luo, X.; Wu, C.; Lin, Y.; Li, W.; Deng, M.; Tan, J.; Xue, S. Soil Heavy Metal Pollution from Pb/Zn Smelting Regions in China and the Remediation Potential of Biomineralization. J. Environ. Sci. 2023, 125, 662–677. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, M.; Tong, H.; Meng, F.; Lv, Y.; Liu, C. Effect of Zn on Fe(II) Oxidation and Nitrate Reduction by a Denitrifying Bacterium, Pseudomonas stutzeri LS-2. Acta Microbiol. Sin. 2021, 61, 1463–1473. [Google Scholar] [CrossRef]
- Okibe, N.; Koga, M.; Morishita, S.; Tanaka, M.; Heguri, S.; Asano, S.; Sasaki, K.; Hirajima, T. Microbial Formation of Crystalline Scorodite for Treatment of As(III)-Bearing Copper Refinery Process Solution Using Acidianus Brierleyi. Hydrometallurgy 2014, 143, 34–41. [Google Scholar] [CrossRef]
- Vega-Hernandez, S.; Weijma, J.; Buisman, C.J.N. Immobilization of Arsenic as Scorodite by a Thermoacidophilic Mixed Culture via As(III)-Catalyzed Oxidation with Activated Carbon. J. Hazard. Mater. 2019, 368, 221–227. [Google Scholar] [CrossRef]
- Gonzalez-Contreras, P.; Weijma, J.; Weijden, R.V.D.; Buisman, C.J.N. Biogenic Scorodite Crystallization by Acidianus sulfidivorans for Arsenic Removal. Environ. Sci. Technol. 2010, 44, 675–680. [Google Scholar] [CrossRef]
- Okibe, N.; Koga, M.; Sasaki, K.; Hirajima, T.; Heguri, S.; Asano, S. Simultaneous Oxidation and Immobilization of Arsenite from Refinery Waste Water by Thermoacidophilic Iron-Oxidizing Archaeon, Acidianus brierleyi. Miner. Eng. 2013, 48, 126–134. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Z.; Wang, J.; Chen, M. Thiosulfate Driving Bio-Reduction Mechanisms of Scorodite in Groundwater Environment. Chemosphere 2023, 311, 136956. [Google Scholar] [CrossRef]
- Drahota, P.; Filippi, M. Secondary Arsenic Minerals in the Environment: A Review. Environ. Int. 2009, 35, 1243–1255. [Google Scholar] [CrossRef]
- Tanaka, M.; Sasaki, K.; Okibe, N. Behavior of Sulfate Ions during Biogenic Scorodite Crystallization from Dilute As(III)-Bearing Acidic Waters. Hydrometallurgy 2018, 180, 144–152. [Google Scholar] [CrossRef]
- Paktunc, D.; Bruggeman, K. Solubility of Nanocrystalline Scorodite and Amorphous Ferric Arsenate: Implications for Stabilization of Arsenic in Mine Wastes. Appl. Geochem. 2010, 25, 674–683. [Google Scholar] [CrossRef]
- Vega-Hernandez, S.; Weijma, J.; Buisman, C.J.N. Particle Size Control of Biogenic Scorodite during the GAC-Catalysed As(III) Oxidation for Efficient Arsenic Removal in Acid Wastewaters. Water Resour. Ind. 2020, 23, 100128. [Google Scholar] [CrossRef]
- Tanaka, M.; Okibe, N. Factors to Enable Crystallization of Environmentally Stable Bioscorodite from Dilute As(III)-Contaminated Waters. Minerals 2018, 8, 23. [Google Scholar] [CrossRef]
- Zeng, G.; Feng, S.; Jia, M.; Yan, C.; Liu, H.; Xu, H. Performance Enhancement of Biogenic Hydroxyapatite for Cd Adsorption: Role of Inorganic Mineral and Extracellular Polymeric Substances. Environ. Res. 2025, 282, 122043. [Google Scholar] [CrossRef] [PubMed]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid Mine Drainage Formation and Arsenic Mobility under Strongly Acidic Conditions: Importance of Soluble Phases, Iron Oxyhydroxides/Oxides and Nature of Oxidation Layer on Pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef]
- Tomiyama, S.; Igarashi, T.; Tabelin, C.B.; Tangviroon, P.; Ii, H. Acid Mine Drainage Sources and Hydrogeochemistry at the Yatani Mine, Yamagata, Japan: A Geochemical and Isotopic Study. J. Contam. Hydrol. 2019, 225, 103502. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, Treatment and Case Studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Méndez-García, C.; Peláez, A.; Mesa, V.; Sánchez, J.; Golyshina, O.; Ferrer, M. Microbial Diversity and Metabolic Networks in Acid Mine Drainage Habitats. Front. Microbiol. 2015, 6, 475. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, X.; Hou, B.; Zhang, S.; Zhang, J.; Li, C.; Wang, W. Occurrence and Environmental Impact of Coal Mine Goaf Water in Karst Areas in China. J. Clean. Prod. 2020, 275, 123813. [Google Scholar] [CrossRef]
| Typical Minerals | Formula | References |
|---|---|---|
| Schwertmannite | Fe8O8(OH)8−2x(SO4)x, 1 ≤ x ≤ 1.75 | [101,102,129] |
| Jarosite | MFe3(SO4)2(OH)6, M = K+, Na+, NH4+, H3O+ | [130,131] |
| Ferrihydrite | Simplified:Fe(OH)3 | [113,132] |
| Lepidocrocite | γ-FeOOH | [118,132] |
| Goethite | α-FeOOH | [105,106,122] |
| Magnetite | Fe3O4 | [116,117,133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, C.; Gao, X.; Zhu, M.; Li, H.; Wang, X. Biomineralization Mediated by Iron-Oxidizing Microorganisms: Implication for the Immobilization and Transformation of Heavy Metals in AMD. Minerals 2025, 15, 868. https://doi.org/10.3390/min15080868
Li S, Li C, Gao X, Zhu M, Li H, Wang X. Biomineralization Mediated by Iron-Oxidizing Microorganisms: Implication for the Immobilization and Transformation of Heavy Metals in AMD. Minerals. 2025; 15(8):868. https://doi.org/10.3390/min15080868
Chicago/Turabian StyleLi, Siyu, Chengcheng Li, Xubo Gao, Mengyun Zhu, Huihui Li, and Xue Wang. 2025. "Biomineralization Mediated by Iron-Oxidizing Microorganisms: Implication for the Immobilization and Transformation of Heavy Metals in AMD" Minerals 15, no. 8: 868. https://doi.org/10.3390/min15080868
APA StyleLi, S., Li, C., Gao, X., Zhu, M., Li, H., & Wang, X. (2025). Biomineralization Mediated by Iron-Oxidizing Microorganisms: Implication for the Immobilization and Transformation of Heavy Metals in AMD. Minerals, 15(8), 868. https://doi.org/10.3390/min15080868