The Petrology of Tuffisite in a Trachytic Diatreme from the Kızılcaören Alkaline Silicate–Carbonatite Complex, NW Anatolia
Abstract
1. Introduction
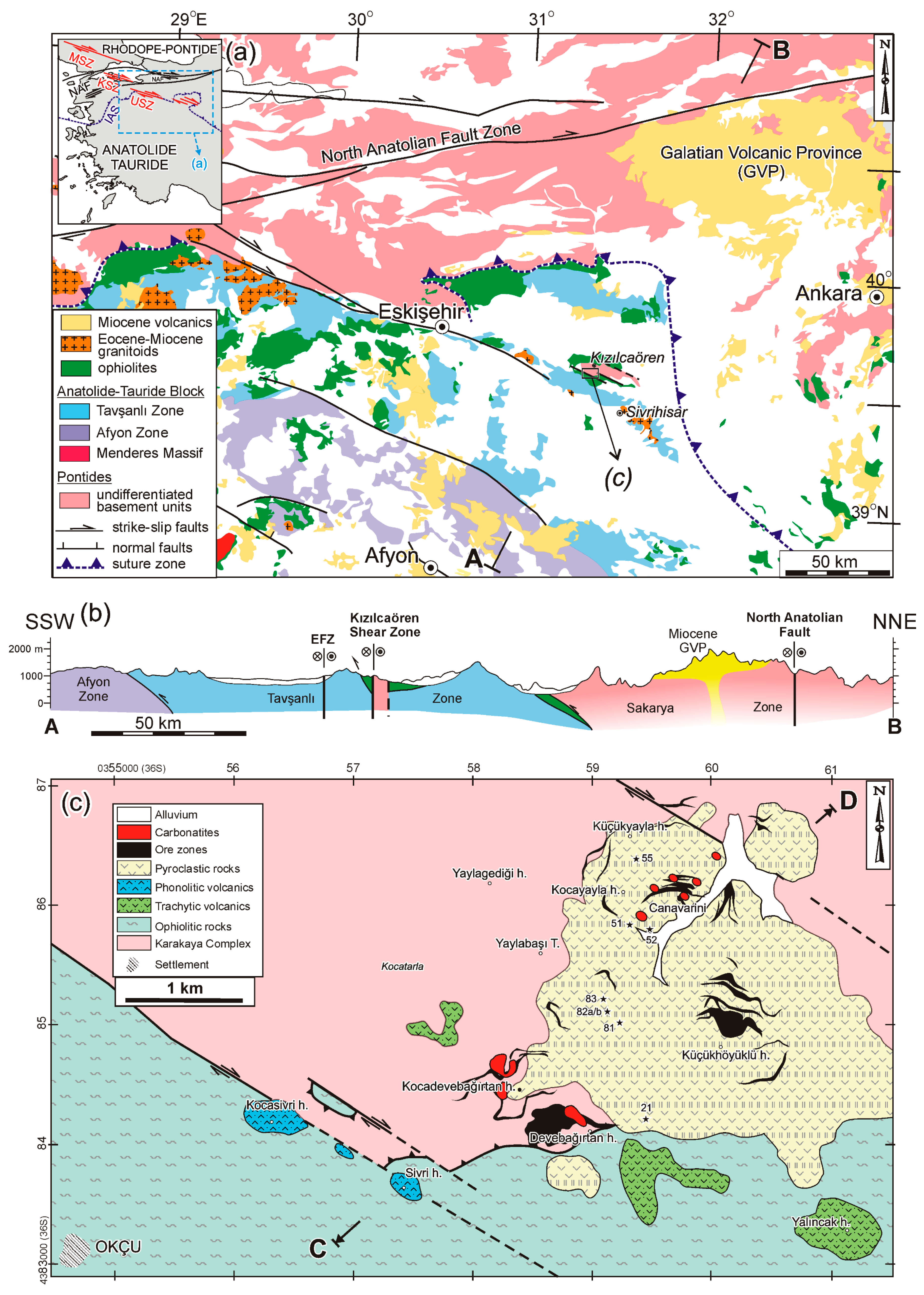
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Field Relations with Strike-Slip Faulting
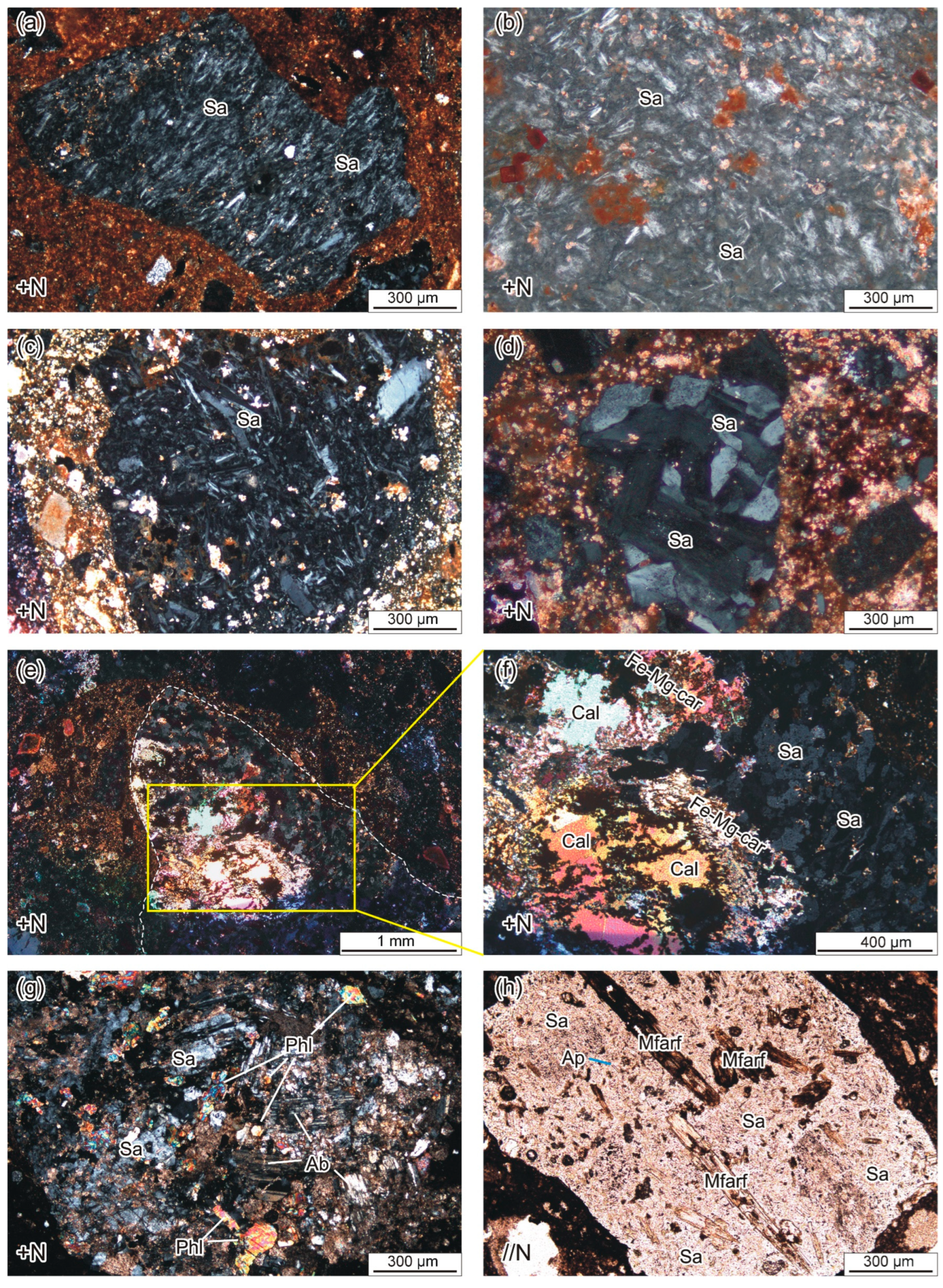
4.2. Petrography
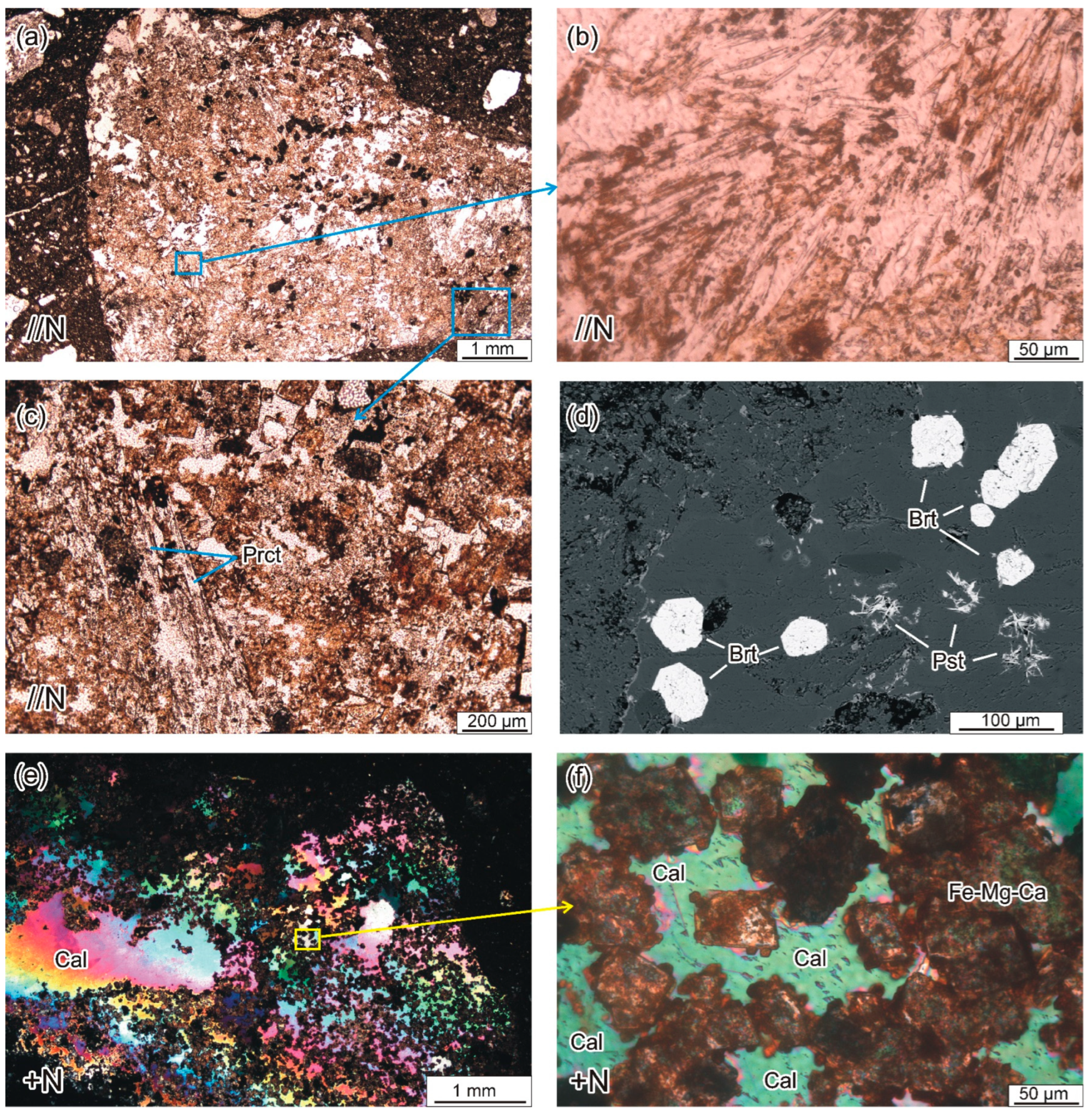
4.2.1. Cognate Inclusions
4.2.2. Juvenile Components
4.3. Mineral Chemistry
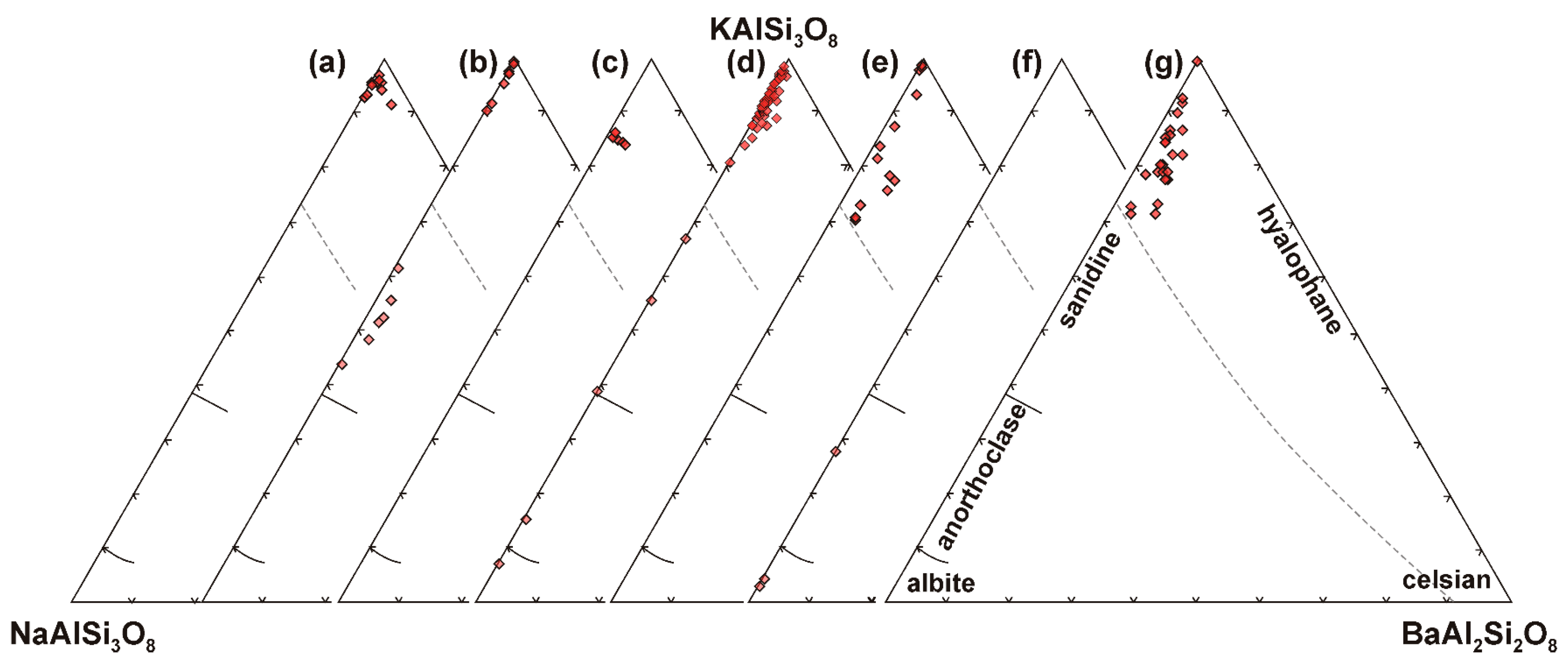
4.3.1. Feldspar
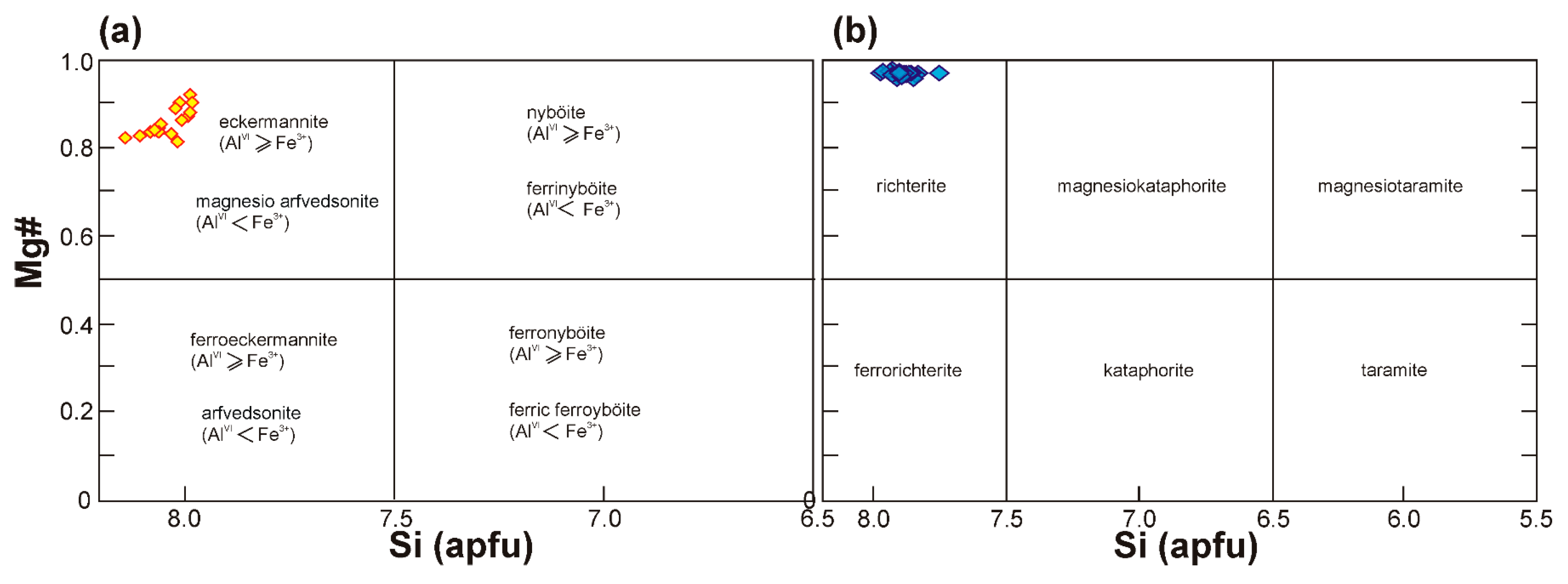
4.3.2. Amphibole
4.3.3. Mica
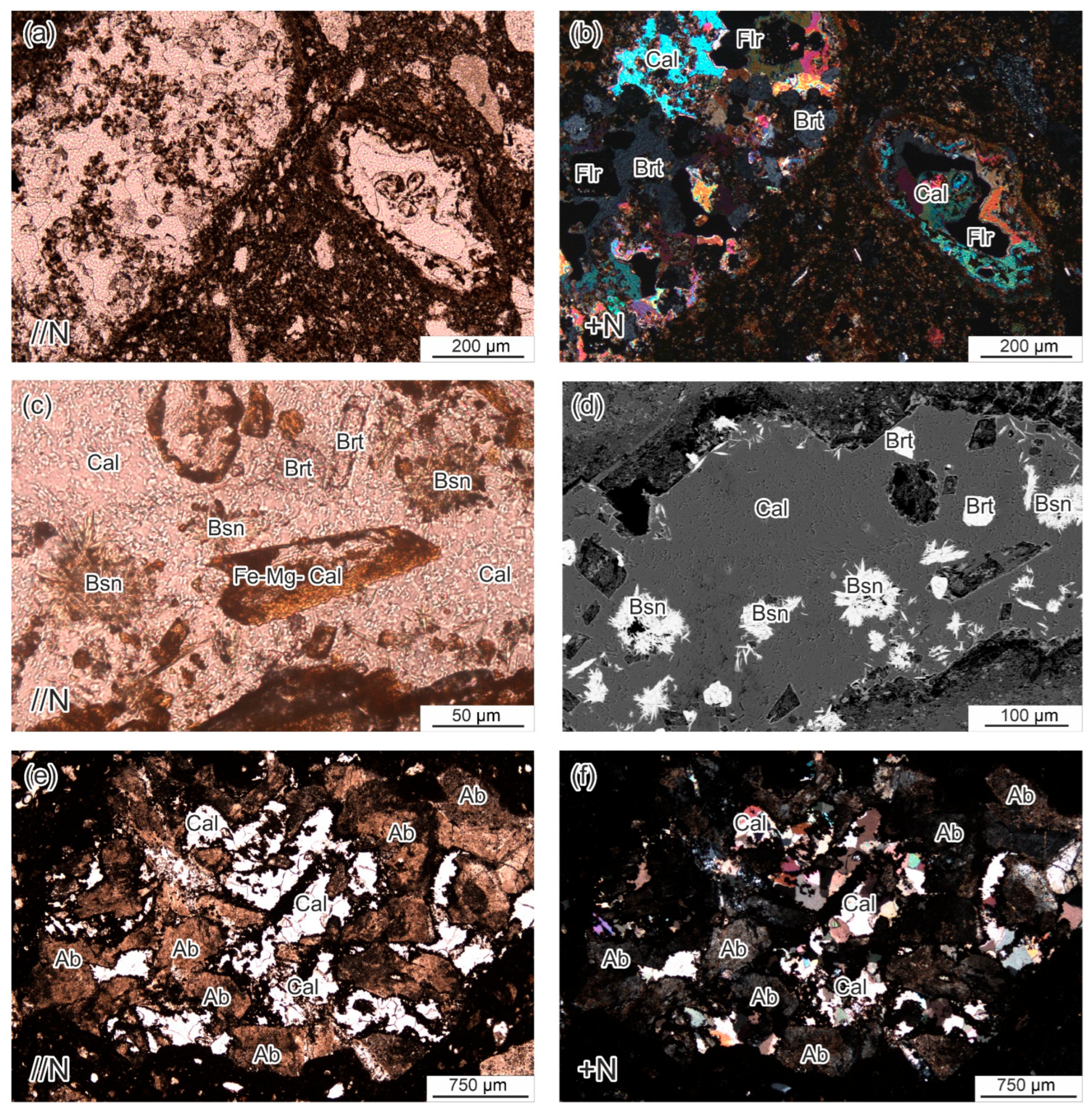
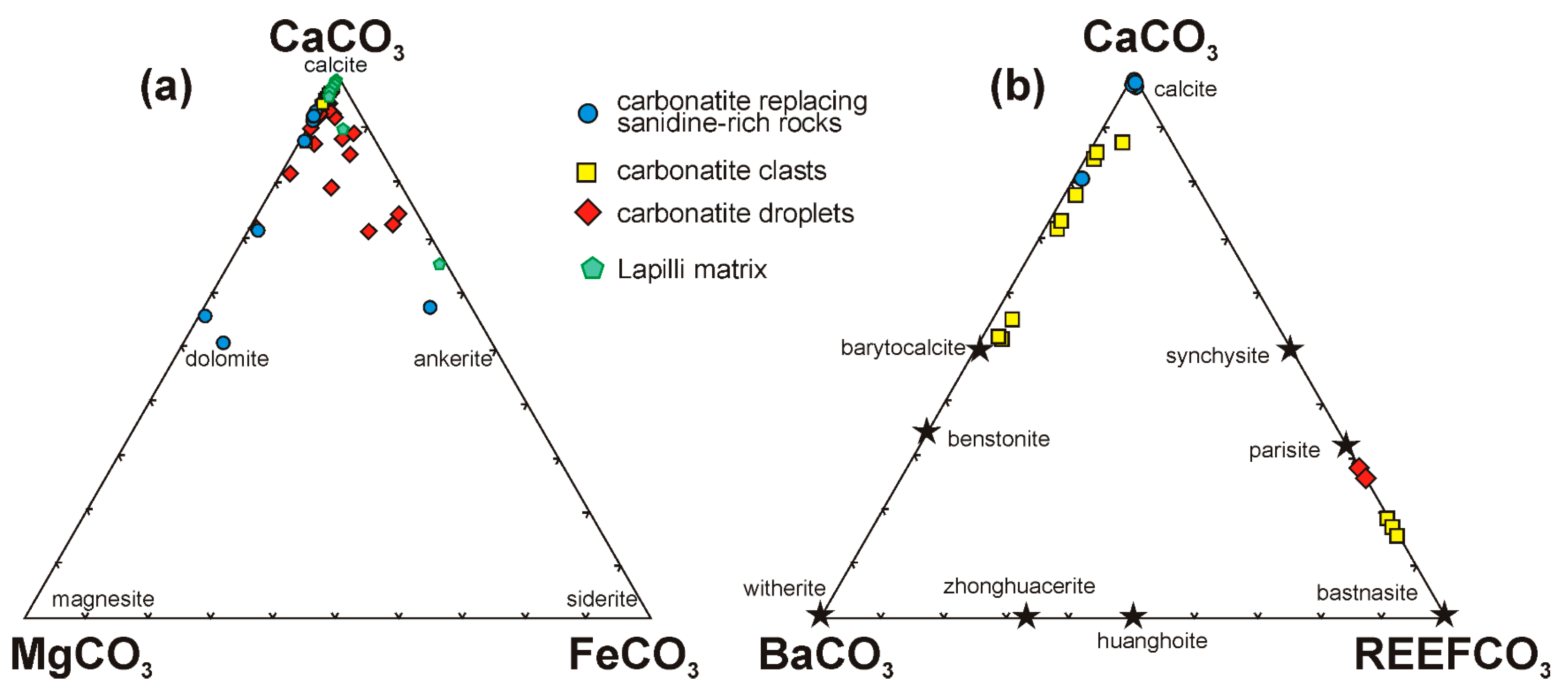
4.3.4. Carbonates and Fluorocarbonates
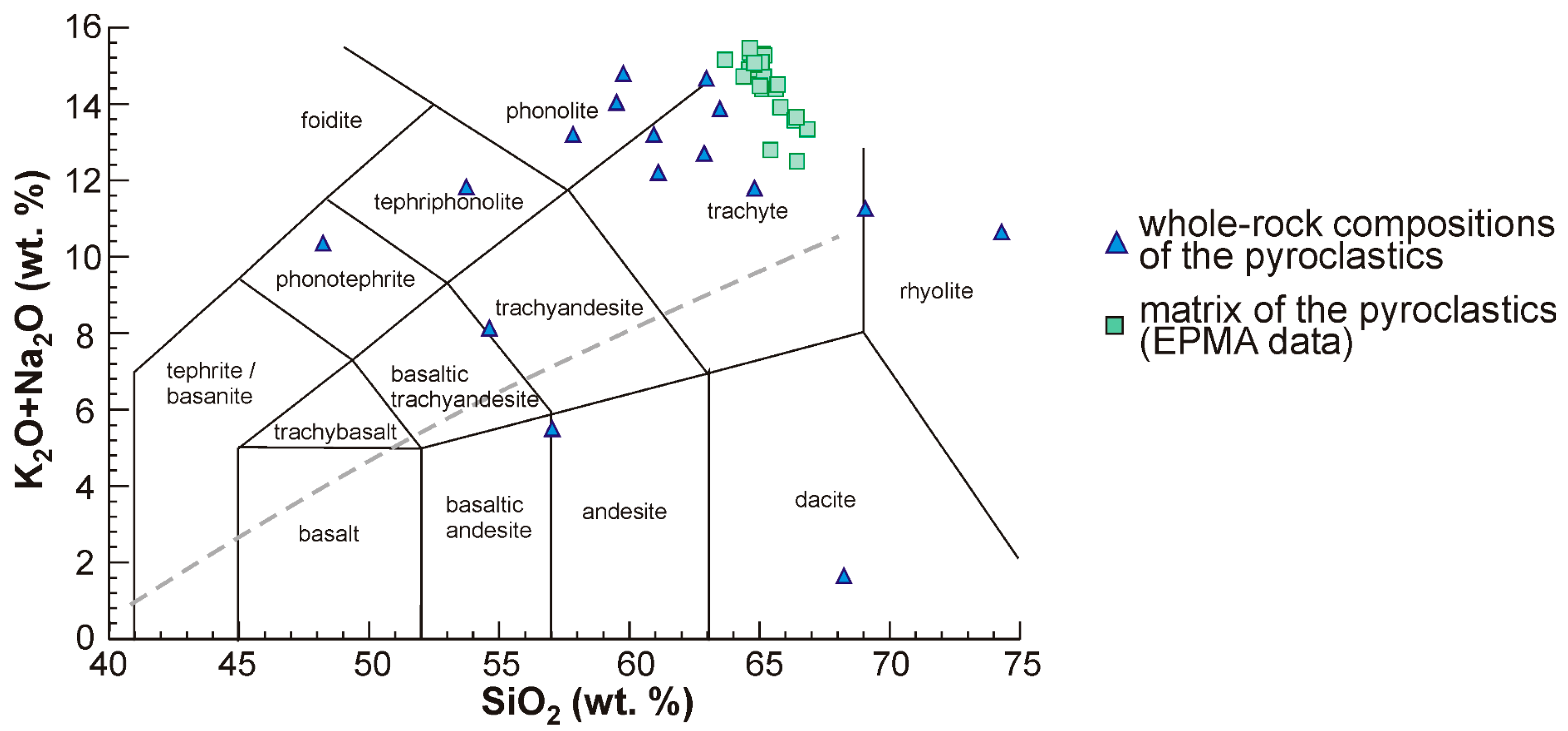
4.3.5. Matrix Composition
5. Discussion
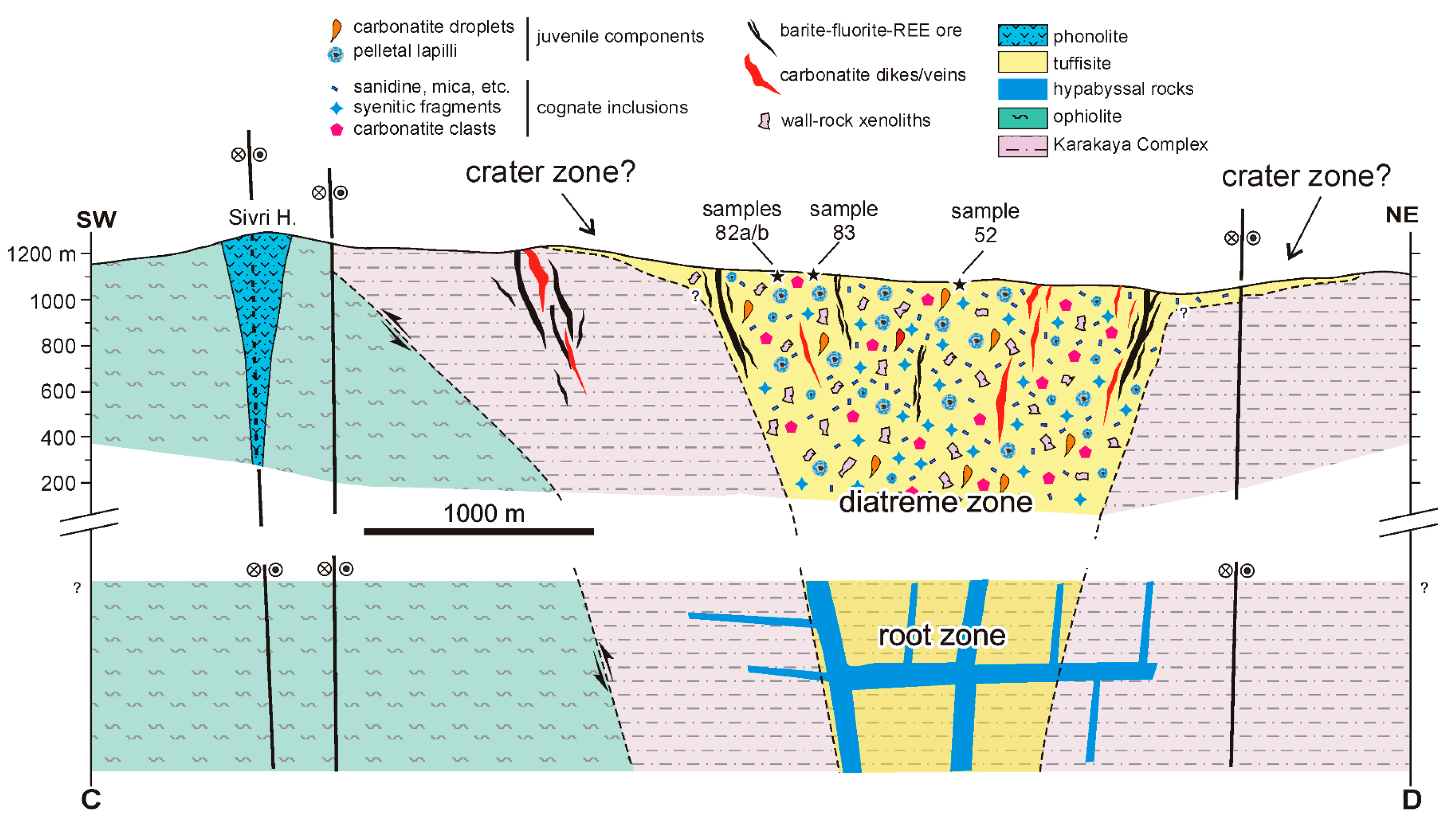
5.1. Implications for a Diatreme Structure
5.2. Regional Implications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPMA | Electron probe microanalyzer |
Appendix A
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | 51a-c4 | 51a-c4 | 82b-c6 | 82b-c6 | 51a-c9 | 51a-c9 | 82a-c7 | 82a-c7 | 51a-c5 | 51a-c5 | 82a-c4 | 82a-c4 | 51a-c7 | 51a-c7 |
| SiO2 | 63.72 | 65.60 | 63.32 | 66.22 | 62.43 | 63.95 | 65.09 | 62.53 | 61.58 | 63.56 | 68.30 | 67.58 | 62.47 | 62.25 |
| Al2O3 | 19.88 | 19.52 | 19.00 | 19.57 | 19.02 | 19.03 | 18.30 | 18.06 | 19.38 | 19.46 | 20.86 | 20.51 | 19.39 | 18.46 |
| FeO | 0.25 | 0.01 | 1.73 | 0.81 | 0.18 | 0.21 | 0.12 | 0.50 | 0.28 | 0.42 | - | 1.18 | 0.57 | 0.52 |
| CaO | 0.07 | 0.04 | - | 0.04 | - | 0.08 | 0.02 | 0.01 | 0.03 | 0.06 | 0.17 | - | 0.06 | 0.04 |
| Na2O | 0.38 | 0.38 | 0.83 | 5.93 | 1.47 | 1.39 | 0.76 | 0.83 | 1.76 | 1.98 | 11.06 | 10.49 | 1.82 | 1.73 |
| K2O | 14.62 | 15.45 | 13.20 | 6.96 | 14.03 | 13.72 | 15.13 | 14.22 | 12.59 | 12.40 | 0.50 | 0.76 | 12.70 | 13.06 |
| BaO | 2.68 | 1.15 | 0.04 | 0.03 | 0.96 | 1.90 | 0.38 | 1.74 | 3.27 | 3.14 | 0.03 | 0.02 | 3.19 | 2.74 |
| Total | 101.69 | 102.14 | 99.11 | 99.58 | 98.21 | 100.58 | 99.92 | 99.73 | 99.16 | 101.21 | 100.96 | 100.78 | 100.49 | 99.12 |
| Si | 2.94 | 2.97 | 2.94 | 2.98 | 2.95 | 2.96 | 3.01 | 2.94 | 2.92 | 2.94 | 2.96 | 2.95 | 2.92 | 2.95 |
| Al | 1.08 | 1.04 | 1.04 | 1.04 | 1.06 | 1.04 | 1.00 | 1.00 | 1.08 | 1.06 | 1.06 | 1.05 | 1.07 | 1.03 |
| Fe2+ | 0.01 | 0.00 | 0.07 | 0.03 | 0.01 | 0.01 | 0.00 | 0.02 | 0.01 | 0.02 | - | 0.04 | 0.02 | 0.02 |
| Ca | 0.00 | 0.00 | - | 0.00 | - | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | - | 0.00 | 0.00 |
| Na | 0.03 | 0.03 | 0.07 | 0.52 | 0.13 | 0.12 | 0.07 | 0.08 | 0.16 | 0.18 | 0.93 | 0.89 | 0.16 | 0.16 |
| K | 0.86 | 0.89 | 0.78 | 0.40 | 0.85 | 0.81 | 0.89 | 0.85 | 0.76 | 0.73 | 0.03 | 0.04 | 0.76 | 0.79 |
| Ba | 0.05 | 0.02 | 0.00 | 0.00 | 0.02 | 0.03 | 0.01 | 0.03 | 0.06 | 0.06 | 0.00 | 0.00 | 0.06 | 0.05 |
| Total | 4.97 | 4.97 | 4.95 | 4.96 | 5.01 | 4.99 | 4.98 | 5.02 | 5.00 | 4.98 | 4.99 | 4.99 | 5.00 | 5.01 |
| End-member compositions (%) | ||||||||||||||
| KAl2Si3O8 | 91.3 | 94.4 | 91.2 | 43.6 | 84.7 | 83.6 | 92.2 | 88.8 | 77.4 | 75.7 | 2.9 | 4.5 | 77.2 | 79.0 |
| NaAlSi3O8 | 3.6 | 3.5 | 8.7 | 56.4 | 13.5 | 12.8 | 7.1 | 7.9 | 16.4 | 18.4 | 97.1 | 95.4 | 16.8 | 15.9 |
| BaAl2Si2O8 | 5.1 | 2.2 | 0.1 | 0.1 | 1.8 | 3.6 | 0.7 | 3.3 | 6.2 | 5.9 | 0.1 | 0.0 | 6.0 | 5.1 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 57.25 | 56.25 | 55.82 | 56.30 | 56.30 | 56.47 | 57.63 | 57.03 | 56.19 | 57.38 | 56.83 | 56.19 | 56.75 |
| TiO2 | 0.54 | 1.37 | 0.29 | 0.81 | 0.80 | 1.62 | 0.23 | 0.28 | 0.31 | 0.26 | 0.16 | 0.22 | 0.18 |
| Al2O3 | 0.39 | 0.61 | 0.41 | 0.36 | 0.28 | 0.33 | 0.06 | 0.05 | 0.07 | 0.02 | 0.06 | 0.07 | 0.03 |
| FeO | 9.65 | 9.77 | 10.36 | 9.39 | 9.16 | 7.89 | 1.45 | 1.45 | 1.63 | 1.39 | 1.60 | 1.92 | 1.64 |
| MnO | 1.93 | 2.03 | 1.14 | 1.62 | 0.81 | 1.02 | 0.19 | 0.29 | 0.28 | 0.33 | 0.25 | 0.31 | 0.29 |
| MgO | 16.45 | 15.64 | 16.21 | 16.80 | 16.59 | 17.15 | 24.65 | 24.52 | 24.19 | 24.23 | 24.13 | 23.74 | 23.78 |
| CaO | 0.90 | 0.60 | 0.61 | 0.74 | 0.35 | 0.32 | 6.44 | 7.04 | 6.85 | 7.17 | 6.71 | 6.93 | 6.38 |
| Na2O | 9.43 | 9.50 | 9.65 | 9.54 | 9.76 | 10.03 | 5.39 | 5.14 | 4.30 | 4.81 | 5.40 | 5.29 | 5.26 |
| K2O | 1.91 | 1.93 | 1.93 | 1.85 | 1.94 | 1.50 | 3.33 | 3.61 | 3.93 | 3.47 | 2.94 | 2.93 | 3.43 |
| F | 3.02 | 3.12 | 3.70 | 3.78 | 3.63 | 3.76 | 1.73 | 1.29 | 1.20 | 1.25 | 1.59 | 1.38 | 1.49 |
| --O=F | 1.27 | 1.31 | 1.56 | 1.59 | 1.53 | 1.58 | 0.73 | 0.54 | 0.51 | 0.53 | 0.67 | 0.58 | 0.63 |
| Total | 100.29 | 99.54 | 98.67 | 99.67 | 98.24 | 98.62 | 100.36 | 100.16 | 98.46 | 99.79 | 99.00 | 98.40 | 98.61 |
| Si | 8.06 | 8.02 | 8.03 | 7.99 | 8.10 | 8.06 | 7.89 | 7.83 | 7.85 | 7.89 | 7.88 | 7.86 | 7.91 |
| Ti | 0.06 | 0.15 | 0.03 | 0.09 | 0.09 | 0.17 | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 |
| Al (IV) | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 |
| Al (VI) | 0.07 | 0.10 | 0.07 | 0.05 | 0.05 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fe2+ | 0.64 | 0.76 | 0.71 | 0.53 | 0.75 | 0.63 | 0.17 | 0.17 | 0.19 | 0.16 | 0.19 | 0.22 | 0.19 |
| Fe3+ | 0.50 | 0.40 | 0.54 | 0.58 | 0.35 | 0.31 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.03 | 0.03 | 0.04 | 0.03 | 0.04 | 0.03 |
| Mg | 3.45 | 3.32 | 3.48 | 3.55 | 3.56 | 3.65 | 5.03 | 5.02 | 5.04 | 4.97 | 4.99 | 4.95 | 4.94 |
| Ca | 0.14 | 0.09 | 0.09 | 0.11 | 0.05 | 0.05 | 0.94 | 1.04 | 1.02 | 1.06 | 1.00 | 1.04 | 0.95 |
| Na | 2.57 | 2.63 | 2.69 | 2.63 | 2.72 | 2.77 | 1.43 | 1.37 | 1.16 | 1.28 | 1.45 | 1.44 | 1.42 |
| K | 0.34 | 0.35 | 0.35 | 0.34 | 0.36 | 0.27 | 0.58 | 0.63 | 0.70 | 0.61 | 0.52 | 0.52 | 0.61 |
| F | 1.36 | 1.42 | 1.70 | 1.72 | 1.67 | 1.71 | 0.75 | 0.56 | 0.53 | 0.54 | 0.70 | 0.61 | 0.66 |
| OH | 0.64 | 0.58 | 0.30 | 0.28 | 0.33 | 0.29 | 1.25 | 1.44 | 1.47 | 1.46 | 1.30 | 1.39 | 1.34 |
| Total | 15.82 | 15.82 | 16.00 | 15.88 | 16.03 | 15.97 | 16.09 | 16.13 | 16.04 | 16.03 | 16.08 | 16.09 | 16.08 |
| Mg# | 0.84 | 0.81 | 0.83 | 0.87 | 0.83 | 0.85 | 0.97 | 0.97 | 0.96 | 0.97 | 0.96 | 0.96 | 0.96 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 42.88 | 44.03 | 44.04 | 44.00 | 43.45 | 43.58 | 43.96 | 42.66 | 44.46 |
| TiO2 | 0.72 | 0.85 | 0.92 | 1.02 | 1.09 | 0.80 | 0.77 | 0.82 | 0.55 |
| Al2O3 | 10.37 | 9.70 | 9.39 | 9.40 | 9.20 | 9.67 | 9.90 | 10.35 | 9.45 |
| FeO | 6.61 | 5.57 | 5.62 | 6.05 | 6.09 | 5.32 | 5.24 | 5.81 | 9.48 |
| MnO | 0.65 | 0.60 | 0.72 | 0.67 | 0.67 | 0.64 | 0.60 | 0.76 | 0.50 |
| MgO | 21.73 | 22.11 | 22.18 | 21.83 | 21.90 | 21.30 | 22.62 | 22.35 | 19.37 |
| BaO | 0.09 | 0.14 | 0.03 | 0.10 | 0.07 | 0.18 | 0.11 | 0.07 | 0.06 |
| CaO | 0.04 | 0.03 | 0.04 | 0.05 | 0.02 | 0.04 | 0.08 | 0.06 | 0.11 |
| Na2O | 0.36 | 0.42 | 0.44 | 0.41 | 0.47 | 0.37 | 0.36 | 0.34 | 0.33 |
| K2O | 10.03 | 10.05 | 10.19 | 9.99 | 10.17 | 10.26 | 10.17 | 10.22 | 8.48 |
| F | 4.30 | 4.92 | 4.96 | 4.91 | 4.72 | 4.98 | 4.93 | 4.56 | 3.71 |
| SrO | - | - | - | - | - | - | - | - | - |
| Total | 97.83 | 98.43 | 98.54 | 98.46 | 97.86 | 97.15 | 98.73 | 98.01 | 96.50 |
| T.Si | 3.13 | 3.20 | 3.21 | 3.21 | 3.19 | 3.21 | 3.19 | 3.12 | 3.24 |
| T.Al | 0.76 | 0.69 | 0.69 | 0.68 | 0.69 | 0.69 | 0.73 | 0.80 | 0.56 |
| T.Fe3+ | 0.11 | 0.10 | 0.10 | 0.11 | 0.12 | 0.10 | 0.09 | 0.08 | 0.20 |
| sumTet | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| M.Al | 0.13 | 0.14 | 0.12 | 0.13 | 0.11 | 0.15 | 0.12 | 0.09 | 0.25 |
| M.Mg | 2.36 | 2.40 | 2.41 | 2.37 | 2.40 | 2.34 | 2.44 | 2.43 | 2.07 |
| M.Fe2+ | 0.28 | 0.24 | 0.25 | 0.27 | 0.27 | 0.29 | 0.24 | 0.27 | 0.30 |
| M.Fe3+ | 0.01 | - | - | - | - | - | - | 0.01 | 0.08 |
| M.Ti | 0.05 | 0.06 | 0.07 | 0.07 | 0.08 | 0.07 | 0.06 | 0.06 | 0.05 |
| M.Mn | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.03 |
| Sumoct | 2.89 | 2.88 | 2.90 | 2.88 | 2.89 | 2.89 | 2.89 | 2.90 | 2.78 |
| Oct.Vac. | 0.11 | 0.12 | 0.10 | 0.12 | 0.11 | 0.11 | 0.11 | 0.10 | 0.22 |
| A.K | 0.95 | 0.94 | 0.96 | 0.94 | 0.96 | 0.98 | 0.95 | 0.97 | 0.83 |
| A.Na | 0.05 | 0.06 | 0.06 | 0.06 | 0.07 | 0.05 | 0.05 | 0.05 | 0.05 |
| A.Ba | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| A.Ca | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 |
| Suminter | 1.00 | 1.01 | 1.02 | 1.01 | 1.03 | 1.04 | 1.01 | 1.02 | 0.88 |
| InterVac. | - | - | - | - | - | - | - | - | 0.12 |
| W.F | 0.98 | 1.12 | 1.13 | 1.12 | 1.09 | 1.15 | 1.11 | 1.04 | 0.81 |
| W.OH | 0.77 | 0.60 | 0.58 | 0.58 | 0.62 | 0.60 | 0.62 | 0.71 | 0.81 |
| W.O2- | 0.25 | 0.27 | 0.29 | 0.30 | 0.30 | 0.25 | 0.27 | 0.25 | 0.39 |
| Fe3+/Fetot | 0.30 | 0.30 | 0.29 | 0.29 | 0.31 | 0.26 | 0.27 | 0.25 | 0.48 |
| Mg# | 0.89 | 0.91 | 0.90 | 0.90 | 0.90 | 0.89 | 0.91 | 0.90 | 0.87 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FeO | 0.01 | 0.04 | 23.10 | 0.54 | 15.66 | 0.73 | 0.00 | 0.64 | 1.29 | 1.12 | 0.00 | 0.00 | 0.16 | 0.02 | 13.35 | 15.07 | 0.52 | 0.00 | 0.15 | 0.12 |
| MnO | 0.00 | 0.01 | 0.22 | 0.04 | 0.16 | 0.09 | 0.08 | 0.02 | 0.19 | 1.27 | 0.00 | 0.00 | 0.03 | 0.00 | 0.06 | 0.03 | 0.00 | 0.00 | 0.13 | 0.03 |
| MgO | 2.62 | 2.20 | 2.47 | 10.39 | 10.77 | 18.39 | 1.71 | 4.57 | 0.72 | 0.74 | 0.03 | 0.03 | 1.13 | 2.50 | 3.55 | 1.06 | 0.37 | 0.08 | 0.85 | 0.36 |
| CaO | 53.47 | 54.17 | 29.93 | 39.39 | 14.47 | 33.69 | 52.06 | 48.80 | 15.37 | 14.76 | 4.82 | 4.35 | 55.24 | 58.62 | 39.20 | 39.68 | 7.63 | 8.57 | 55.97 | 54.59 |
| BaO | 0.00 | 0.00 | 0.11 | 0.10 | 8.09 | 0.00 | 0.10 | 0.00 | 30.79 | 27.47 | 0.15 | 0.11 | 0.04 | 0.00 | 0.00 | 0.06 | 0.20 | 0.30 | 0.00 | 0.03 |
| SrO | 0.34 | 0.38 | 0.05 | 0.10 | 0.05 | 0.11 | 0.02 | 0.06 | 0.06 | 0.00 | 2.67 | 2.44 | 0.02 | 0.24 | 0.04 | 0.02 | 2.61 | 2.42 | 0.34 | 0.45 |
| La2O3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.06 | n.a. | n.a. | n.a. | 24.14 | 23.72 | n.a. | n.a. | n.a. | n.a. | 22.85 | 22.17 | n.a. | n.a. |
| Ce2O3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 2.31 | 1.76 | 31.48 | 32.10 | 0.00 | 0.00 | 0.00 | 0.00 | 27.28 | 29.75 | 0.10 | 0.08 |
| Pr2O3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.24 | n.a. | n.a. | n.a. | 2.85 | 2.91 | n.a. | n.a. | n.a. | n.a. | 2.57 | 2.61 | n.a. | n.a. |
| Nd2O3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 8.40 | 8.89 | n.a. | n.a. | n.a. | n.a. | 7.40 | 8.21 | n.a. | n.a. |
| Sm2O3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.57 | 0.72 | n.a. | n.a. | n.a. | n.a. | 0.61 | 0.81 | n.a. | n.a. |
| ThO2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 4.33 | 4.45 | n.a. | n.a. | n.a. | n.a. | 5.60 | 5.73 | n.a. | n.a. |
| F | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 4.05 | 4.12 | n.a. | n.a. | n.a. | n.a. | 5.70 | 3.99 | n.a. | n.a. |
| --O=F | 1.70 | 1.73 | 2.40 | 1.68 | ||||||||||||||||
| CO2 | 44.97 | 45.10 | 40.53 | 42.67 | 35.15 | 47.08 | 42.81 | 43.72 | 23.23 | 22.22 | 22.91 | 22.63 | 44.72 | 48.85 | 42.88 | 41.58 | 23.98 | 24.74 | 45.19 | 43.55 |
| Total | 101.40 | 101.92 | 97.51 | 93.66 | 84.58 | 100.25 | 96.90 | 97.82 | 74.14 | 69.56 | 100.44 | 100.34 | 101.36 | 110.27 | 99.18 | 97.56 | 98.81 | 101.25 | 102.87 | 99.41 |
| CaCO3 | 93.31 | 94.25 | 57.96 | 72.43 | 32.31 | 56.17 | 95.44 | 87.60 | 51.91 | 52.12 | 16.51 | 15.10 | 96.94 | 94.17 | 71.75 | 74.89 | 24.96 | 27.17 | 97.20 | 98.36 |
| End-member compositions (%) | ||||||||||||||||||||
| MgCO3 | 6.37 | 5.31 | 6.66 | 26.58 | 33.45 | 42.67 | 4.36 | 11.42 | 3.37 | 3.64 | 0.14 | 0.13 | 2.76 | 5.59 | 9.04 | 2.79 | 1.66 | 0.37 | 2.04 | 0.91 |
| FeCO3 | 0.01 | 0.06 | 34.92 | 0.77 | 27.29 | 0.95 | 0.00 | 0.89 | 3.39 | 3.10 | 0.00 | 0.00 | 0.22 | 0.02 | 19.08 | 22.21 | 1.33 | 0.00 | 0.20 | 0.17 |
| MnCO3 | 0.00 | 0.01 | 0.34 | 0.05 | 0.28 | 0.12 | 0.11 | 0.03 | 0.50 | 3.53 | 0.00 | 0.00 | 0.04 | 0.00 | 0.09 | 0.05 | 0.00 | 0.00 | 0.17 | 0.05 |
| SrCO3 | 0.32 | 0.36 | 0.05 | 0.10 | 0.06 | 0.10 | 0.02 | 0.06 | 0.11 | 0.00 | 4.95 | 4.58 | 0.02 | 0.21 | 0.04 | 0.03 | 4.63 | 4.16 | 0.32 | 0.44 |
| BaCO3 | 0.00 | 0.00 | 0.08 | 0.07 | 6.61 | 0.00 | 0.07 | 0.00 | 38.05 | 35.48 | 0.19 | 0.13 | 0.02 | 0.00 | 0.00 | 0.04 | 0.24 | 0.35 | 0.00 | 0.02 |
| LaCO3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 28.46 | 28.31 | n.a. | n.a. | n.a. | n.a. | 25.74 | 24.21 | n.a. | n.a. |
| CeCO3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2.67 | 2.13 | 36.84 | 38.04 | n.a. | n.a. | n.a. | n.a. | 30.51 | 32.24 | 0.06 | 0.05 |
| PrCO3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 3.32 | 3.43 | n.a. | n.a. | n.a. | n.a. | 2.87 | 2.81 | n.a. | n.a. |
| NdCO3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 9.58 | 10.28 | n.a. | n.a. | n.a. | n.a. | 8.07 | 8.68 | n.a. | n.a. |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 63.49 | 62.76 | 62.83 | 63.82 | 62.80 | 63.67 | 63.86 | 63.59 | 66.06 | 64.67 | 64.55 | 65.13 | 65.28 | 65.53 | 64.53 |
| TiO2 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.05 | 0.04 | 0.04 | 0.03 | 0.01 | 0.02 | 0.04 | 0.04 | 0.01 | 0.05 |
| Al2O3 | 18.91 | 18.33 | 18.91 | 18.68 | 19.14 | 19.01 | 19.42 | 19.18 | 19.43 | 19.82 | 18.83 | 19.45 | 19.10 | 20.16 | 19.63 |
| FeO | 0.49 | 0.63 | 0.57 | 0.56 | 0.54 | 0.52 | 0.50 | 0.50 | 0.38 | 0.26 | 0.92 | 0.51 | 0.44 | 0.52 | 0.56 |
| MnO | - | 0.03 | 0.01 | 0.04 | 0.00 | - | 0.01 | 0.03 | - | - | - | 0.01 | - | - | - |
| MgO | - | 0.53 | 0.00 | 0.01 | - | 0.00 | 0.02 | 0.00 | 0.23 | 0.02 | - | - | - | 0.00 | - |
| CaO | 0.12 | 1.29 | 0.07 | 1.91 | 0.18 | 0.05 | 0.10 | 0.04 | 0.06 | 0.63 | 0.19 | 0.08 | 0.07 | 0.08 | 0.08 |
| Na2O | 1.25 | 0.68 | 1.83 | 1.30 | 1.48 | 1.60 | 1.67 | 1.59 | 0.12 | 0.15 | 1.41 | 1.55 | 1.60 | 1.49 | 1.59 |
| K2O | 12.82 | 14.22 | 12.81 | 11.17 | 12.96 | 12.75 | 12.42 | 13.13 | 14.34 | 14.60 | 13.88 | 12.20 | 13.72 | 10.83 | 12.74 |
| P2O5 | 0.01 | 0.01 | - | - | 0.01 | 0.01 | - | - | 0.00 | 0.26 | 0.02 | - | - | - | - |
| BaO | 2.39 | 1.27 | 2.65 | 2.48 | 2.92 | 2.49 | 2.66 | 2.62 | 0.41 | 0.75 | 1.62 | 2.40 | 1.24 | 2.82 | 2.52 |
| Ce2O3 | 0.13 | 0.12 | 0.20 | 0.16 | 0.12 | 0.19 | 0.14 | 0.18 | 0.03 | 0.17 | 0.03 | 0.15 | 0.04 | 0.15 | 0.08 |
| Total | 99.64 | 99.90 | 99.90 | 100.16 | 100.19 | 100.35 | 100.85 | 100.91 | 101.09 | 101.42 | 101.46 | 101.52 | 101.54 | 101.57 | 101.78 |
References
- Mitchell, R.H. Kimberlites: Mineralogy, Geochemistry, and Petrology, 2nd ed.; Plenum Press: New York, NY, USA, 1986; pp. 1–453. [Google Scholar] [CrossRef]
- Rakovan, J. Diatreme. Rocks Miner. 2006, 81, 153–154. [Google Scholar] [CrossRef]
- Kjarsgaard, B. Kimberlite pipe models: Significance for exploration. In Proceedings of the International Kimberlite Conference, Bangalore, India, 6 February 2012. [Google Scholar] [CrossRef]
- Lorenz, V.; Suhr, P.; Shur, S. Phreatomagmatic maar-diatreme volcanoes and their incremental growth: A model. In Monogenetic Volcanism; Nemeth, K., Carrasco-Nunez, G., Aranda-Gomez, J.J., Smith, I.E.M., Eds.; Geological Society, London, Special Publications: London, UK, 2017; Volume 446. [Google Scholar] [CrossRef]
- Mitchell, R.H. Kimberlites, Orangeites, and Related Rocks; Plenum Press: New York, NY, USA, 1995; pp. 1–410. [Google Scholar] [CrossRef]
- Mitchell, R.; Scott Smith, B.H. Mineralogy of kimberley-type pyroclastic kimberlite and the transition to hypabyssal kimberlite. In Proceedings of the International Kimberlite Conference, Gaborone, Botswana, 18–22 September 2017. [Google Scholar] [CrossRef]
- Kjarsgaard, B. Volcanology of Kimberlite. In Diamonds Short Course Notes; Tosdal, R., Ed.; Cordilleran Round-Up: Vancouver, BC, Canada, 2003. [Google Scholar]
- Sparks, R.S.J.; Baker, L.; Brown, R.J.; Field, M.; Schumacher, J.; Stripp, G.; Walters, A.L. Dynamics of Kimberlite Volcanism. J. Volcanol. Geotherm. Res. 2006, 155, 18–48. [Google Scholar] [CrossRef]
- Cloos, H. Bau und Tatigkeit von Tuffschloten. Untersuchungen an dem Schwäbischen Vulkan. Geol. Rundsch. 1941, 32, 705–800. [Google Scholar]
- Francis, E.H.; Forsyth, I.H.; Chisholm, J.I. The geology of East Fife. H.M. Stationery Off.: London, UK, 1977; pp. 171–198. [Google Scholar]
- Francis, E.H. Tuffisite. In Encyclopedia of Earth Science; Springer: Boston, MA, USA, 1989; pp. 574–575. [Google Scholar] [CrossRef]
- Lloyd, F.E.; Stoppa, F. Pelletal lapilli in diatremes-some inspiration from the old masters. Geolines 2003, 15, 65–71. Available online: https://geolines.gli.cas.cz/fileadmin/volumes/volume15/G15-065.pdf (accessed on 25 June 2025).
- Masun, K.M.; Scott Smith, B.H. The Pimenta Bueno kimberlite field, Rondonia, Brazil: Tuffisitic kimberlite and transitional textures. J. Volcanol. Geotherm. Res. 2008, 174, 81–89. [Google Scholar] [CrossRef]
- Mitchell, R.M.; Skinner, E.M.W.; Scott Smith, B.H. Tuffisitic kimberlites from the Wesselton Mine, South Africa: Mineralogical characteristics relevant to their formation. Lithos 2009, 112, 452–464. [Google Scholar] [CrossRef]
- Stoppa, F.; Lloyd, F.; Rosatelli, G. CO2 as the virtual propellant of carbonatite kamafugite conjugate pairs and the eruption of diatremic tuffisite. Per. Mineral. 2003, 72, 205–222. Available online: https://www.scirp.org/%28S%28lz5mqp453edsnp55rrgjct55%29%29/reference/referencespapers?referenceid=781814 (accessed on 25 June 2025).
- Stoppa, F.; Schiazza, M.; Rosatelli, G.; Castorina, F.; Sharygin, V.V.; Ambrosio, F.A.; Vicentini, N. Italian carbonatite system: From mantle to ore-deposit. Ore Geol. Rev. 2019, 114, 103041. [Google Scholar] [CrossRef]
- Scott Smith, B.H.; Nowicki, T.E.; Russell, J.K.; Webb, K.J.; Mitchell, R.H.; Hetman, C.M.; Harder, M.; Skinner, E.M.W.; Robey, J.A. Kimberlite Terminology and Classification. In Proceedings of the 10th International Kimberlite Conference, Bangalore, India, 5–11 February 2012; Pearson, D., Ed.; Springer: New Delhi, India, 2013; pp. 1–17. [Google Scholar] [CrossRef]
- Mitchell, R.H. Kimberlites, Orangeites, Lamproites, Melilitites, and Minettes: A Petrographic Atlas, 1st ed.; Almaz Press Inc.: Thunder Bay, ON, Canada, 1997; pp. 1–243. [Google Scholar]
- Clement, C.R. Kimberlites from the Kao Pipe, Lesotho. In Lesotho Kimberlites; Nixon, P.H., Ed.; Lesotho National Development Corporation: Maseru, Lesotho, 1973; pp. 110–121. [Google Scholar]
- Gernon, T.M.; Brown, R.J.; Tait, M.A.; Hincks, T.K. The origin of pelletal lapilli in explosive kimberlite eruptions. Nat. Commun. 2012, 3, 832. [Google Scholar] [CrossRef]
- Lorenz, V. Phreatomagmatic origin of olivine melilite diatremes in Swabian Alb, Germany. In Proceedings of the Second International Kimberlite Conference; Boyd, F.R., Meyer, H.O.A., Eds.; American Geophysical Union: Washington, DC, USA, 1979; pp. 354–363. [Google Scholar]
- Carracedo Sánchez, M.; Sarrionandia, F.; Arostegui, J.; Ibarguchi, J.I.G. Silicate glass micro and nanospherules generated in explosive eruptions of ultrabasic magmas: Implications for the origin of pelletal lapilli. J. Volcanol. Geotherm. Res. 2015, 293, 13–24. [Google Scholar] [CrossRef]
- Webb, K.; Hetman, C. Magmaclasts in kimberlite. Lithos 2021, 396–397, 106197. [Google Scholar] [CrossRef]
- Carnevale, G.; Zanon, V. Characterisation of Pelletal Lapilli in Explosive Melilitite–Carbonatite Eruptions: An Example from Mt. Vulture Volcano (Southern Italy). Geosciences 2024, 14, 349. [Google Scholar] [CrossRef]
- Kopylova, M.G.; Sismondo, C.; Gaudet, M. Metasomatic textural changes in hypabyssal transitional kimberlites: Inferences for pyroclastic kimberlites. Mineral. Petrol. 2025, in press. [CrossRef]
- Nasir, S. Petrology of Late Jurassic allochthonous ultramafic lamprophyres within the Batain Nappes, Northeastern Oman. Int. Geol. Rev. 2016, 58, 913–928. [Google Scholar] [CrossRef]
- Prokopyev, I.; Doroshkevich, A.; Starikova, A.; Kovalev, S.; Nugumanova, Y.; Izokh, A. Petrogenesis of juvenile pelletal lapilli in ultramafic lamprophyres. Sci. Rep. 2023, 13, 5841. [Google Scholar] [CrossRef]
- Schmincke, H.U.; Sumita, M.; Chakraborty, S.; Hansteen, T.H. Origin of maar clusters at the type locality Eifel (Germany): H2O or CO2? Bull. Volcanol. 2025, 87, 14. [Google Scholar] [CrossRef]
- Cooper, A.F.; Reid, D.L. Textural evidence for calcite carbonatite magmas, Dicker Willem, southwest Namibia. Geology 1991, 19, 1193–1196. [Google Scholar] [CrossRef]
- Riley, T.R.; Bailey, D.K.; Lloyd, F.E. Extrusive carbonatite from the Quaternary Rockeskyll complex, West Eifel, Germany. Canad. Mineral. 1996, 34, 389–401. Available online: https://rruff.info/doclib/cm/vol34/CM34_389.pdf (accessed on 25 June 2025).
- Junqueira-Brod, T.C.; Gaspar, J.C.; Brod, J.A.; Kafino, C.V. Kamafugitic diatremes: Their textures and field relationships with examples from the Goiás Alkaline Province, Brazil. J. S. Am. Earth Sci. 2005, 18, 337–353. [Google Scholar] [CrossRef]
- Hay, R.L. Melilitite-carbonatite tuffs in the Laetolil Beds of Tanzania. Contrib. Mineral. Petrol. 1978, 67, 357–367. [Google Scholar] [CrossRef]
- Paone, A. A Review of Carbonatite Occurrences in Italy and Evaluation of Origins. Open J. Geol. 2013, 3, 66–82. [Google Scholar] [CrossRef]
- Genç, Y. Geological Properties of Kizilcaören (Beylikova-Eskişehir) Carbonatite-Hosted Rare Earth Elements (REE)-Thorium-Barite-Fluorite Deposit and its Importance for Turkey. In Proceedings of the 73rd Geological Congress of Türkiye, Ankara, Türkiye, 24–28 May 2021; pp. 818–819. [Google Scholar]
- Hatzl, T. Die Genese Der Karbonatit-und Alkalivulkanit-Assoziierten Fluorit-Baryt-Bastnasit-Vererzung Bei Kızılcaören (Turkei). Ph.D. Thesis, Technical University of Munich, Munich, Germany, 1992. [Google Scholar]
- Kaplan, H. Rare earth elements and thorium complex deposit of Kizilcaören village, Sivrihisar-Eskişehir, Turkey. Bull. Geol. Eng. Turk. 1977, 2, 69–76. [Google Scholar]
- Nikiforov, A.V.; Öztürk, H.; Altuncu, S.; Lebedev, V.A. Kizilcaören Ore-bearing Complex with Carbonatites (Northwestern Anatolia, Turkey): Formation Time and Mineralogy of Rocks. Geol. Ore Depos. 2014, 56, 35–60. [Google Scholar] [CrossRef]
- Özgenç, İ. Geology and REE-geochemistry of carbothermal bastnaesite–fluorite–barite deposit of Kızılcaören (Sivrihisar, Eskişehir). Geol. Bull. Turk. 1993, 36, 1–11. [Google Scholar]
- Öztürk, H.; Altuncu, S.; Hanilçi, N.; Kasapçı, C.; Goodenough, K.M. Rare Earth Element-Bearing Fluorite Deposits of Turkey: An Overview. Ore Geol. Rev. 2019, 105, 423–444. [Google Scholar] [CrossRef]
- Stumpfl, E.F.; Kırıkoğlu, M.S. Fluorite-Barite-Rare Earths Deposit at Kizilcaören, Turkey. Mitt. Osterr. Ges. 1986, 78, 193–200. Available online: https://www.zobodat.at/pdf/MittGeolGes_78_0193-0200.pdf (accessed on 1 July 2025).
- Çimen, O.; Corcoran, L.; Kuebler, C.; Simonetti, S.; Simonetti, A. Geochemical, stable (O, C, and B) and radiogenic (Sr, Nd, Pb) isotopic data from theEskişehir-Kızılcaören (NW-Anatolia) and the Malatya-Kuluncak (E-central Anatolia) F-REE-Th deposits, Turkey: Implications for nature of carbonate-hosted mineralization. Turk. J. Earth Sci. 2020, 29, 798–814. [Google Scholar] [CrossRef]
- Delaloye, M.; Özgenç, İ. Petrography and age determinations of the alkaline volcanic rocks and carbonatite of Kιzılcaören district, Beylikahır-Eskişehir, Turkey. Schweiz. Mineral. Petrogr. Mitteilungen 1983, 63, 289–294. [Google Scholar] [CrossRef]
- Gültekin, A.H.; Örgün, Y.; Suner, F. Geology, mineralogy and fluid inclusion data of the Kizilcaoren fluorite- barite- REE deposit, Eskisehir, Turkey. J. Asian Earth Sci. 2003, 21, 365–376. [Google Scholar] [CrossRef]
- Okay, A.I.; Tüysüz, O. Tethyan sutures of northern Turkey. J. Geol. Soc. 1999, 156, 475–515. [Google Scholar] [CrossRef]
- Göncüoğlu, M.C.; Turhan, N.; Şentürk, K.; Özcan, A.; Uysal, S.; Yalınız, M.K. A geotraverse across northwestern Turkey: Tectonic units of the Central Sakarya region and their tectonic evolution. J. Geol. Soc. 2000, 173, 139–162. [Google Scholar] [CrossRef]
- Önen, A.P. Neotethyan ophiolitic rocks of the Anatolides of NW Turkey and comparison with Tauride ophiolites. J. Geol. Soc. 2003, 160, 947–962. [Google Scholar] [CrossRef]
- Fornash, K.F.; Cosca, M.A.; Whitney, D.L. Tracking the timing of subduction and exhumation using 40Ar/39 phengite ages in blueschist- and eclogite-facies rocks (Sivrihisar, Turkey). Contrib. Min. Pet. 2016, 171, 67. [Google Scholar] [CrossRef]
- Sarıfakıoğlu, E.; Özen, H.; Hall, C. Petrogenesis of extension-related alkaline volcanism in Karaburhan (Sivrihisar–Eskisehir), NW Anatolia, Turkey. J. Asian Earth Sci. 2009, 35, 502–515. [Google Scholar] [CrossRef]
- Leake, B.E.; Woolley, A.R.; Arps, C.E.; Birch, W.D.; Gilbert, M.C.; Grice, J.D.; Hawthorne, F.C.; Kato, A.; Kisch, H.J.; Krivovichev, V.G.; et al. Nomenclature of Amphiboles; Report of the Subcommittee on Amphiboles of the International Mineralogical Association Commission on New Minerals and Mineral Names. Mineral. Mag. 1997, 61, 295–310. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Behrens, H.; Holtz, F. Calculating biotite formula from electron microprobe analysis data using a machine learning method based on principal components regression. Lithos 2020, 356–357, 105371. [Google Scholar] [CrossRef]
- Stoppa, F. The San Venanzo maar and tuff ring, Umbria, Italy: Eruptive behaviour of a carbonatite–melilitite volcano. Bull. Volcanol. 1996, 57, 563–577. [Google Scholar] [CrossRef]
- Stoppa, F.; Lloyd, F.E.; Tranquilli, A.; Schiazza, M. Comment on: Development of spheroid “composite” bombs by welding of juvenile spinning and isotropic droplets inside a mafic “eruption” column by Carracedo Sánchez et al. (2009). J. Volcanol. Geotherm. Res. 2011, 204, 107–116. [Google Scholar] [CrossRef]
- Keller, J. Carbonatitic volcanism in the Kaiserstuhl alkaline complex: Evidence for highly fluid carbonatitic melts at the earth’s surface. J. Volcanol. Geotherm. Res. 1981, 9, 423–431. [Google Scholar] [CrossRef]
- Carracedo Sánchez, M.; Sarrionandia, F.; Arostegui, J.; Larrondo, E.; Ibarguchi, J.I.G. Development of spheroidal composite bombs by welding of juvenile spinning and isotropic droplets inside a mafic eruption column. J. Volcanol. Geotherm. Res. 2009, 186, 265–279. [Google Scholar] [CrossRef]
- Junqueira-Brod, T.C.; Brod, J.A.; Thompson, R.N.; Gibson, S.A. Spinning droplets: A conspicuous lapilli-size structure in kamafugitic diatremes of southern Goiás, Brazil. Rev. Brasil. Geosci. 1999, 29, 437–440. [Google Scholar] [CrossRef]
- Stoppa, F.; Cirilli, S.; Sorci, A.; Broom-Fendley, S.; Principe, C.; Perna, M.G.; Rosatelli, G. Igneous and sedimentary ‘limestones’: The puzzling challenge of a converging classification. Geol. Soc. 2023, 520, 327–352. [Google Scholar] [CrossRef]
- Lefebvre, N.; Kopylova, M.; Kivi, K. Archean calc-alkaline lamprophyres of Wawa, Ontario, Canada: Unconventional diamondiferous volcaniclastic rocks. Precambr. Res. 2005, 138, 57–87. [Google Scholar] [CrossRef]
- Stoppa, F.; Principe, C. Eruption style and petrology of a new carbonatitic suite from the Mt. Vulture Southern Italy: The Monticchio Lakes Formation. J. Volcanol. Geotherm. Res. 1997, 78, 251–265. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Lane, S.J. The origin of accretionary lapilli. Bull. Volcanol. 1994, 56, 398–411. [Google Scholar] [CrossRef]
- Schumacher, R.; Schmincke, H.U. Internal structure and occurrence of accretionary lapilli—A case study at Laacher See Volcano. Bull. Volcanol. 1991, 53, 612–634. [Google Scholar] [CrossRef]
- Dawson, J.B. Kimberlites and Their Xenoliths, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1980. [Google Scholar] [CrossRef]
- Junqueira-Brod, T.C.; Brod, J.A.; Gaspar, J.C.; Jost, H. Kamafugitic diatremes: Facies characterisation and genesis—Examples from the Goia’s Alkaline Province, Brazil. Lithos 2004, 76, 261–282. [Google Scholar] [CrossRef]
- Ersoy, E.Y.; Palmer, M.R. Eocene-Quaternary magmatic activity in the Aegean: Implications for mantle metasomatism and magma genesis in an evolving orogeny. Lithos 2013, 180–181, 5–24. [Google Scholar] [CrossRef]
- Morteani, G.; Satır, M. The Bastnaesite-Fluorite-Barite Deposit of the Kizilcaören District, Eskisehir, Turkey. In Lanthanides, Tantalum and Niobium Mineralogy, Geochemistry, Characteristics of Primary Ore Deposits, Prospecting, Processing and Applications Proceedings of a Workshop in Berlin, November 1986; Springer: Berlin/Heidelberg, Germany, 1989; pp. 189–194. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Deady, E.A.; Beard, C.D.; Broom-Fendley, S.; Elliott, H.A.L.; van den Berg, F.; Öztürk, H. Carbonatites and Alkaline Igneous Rocks in Post-Collisional Settings: Storehouses of Rare Earth Elements. J. Earth Sci. 2021, 32, 1332–1358. [Google Scholar] [CrossRef]
- Naydenov, K.; Peytcheva, I.; von Quadt, A.; Sarov, S.; Kolcheva, K.; Dimov, D. The Maritsa strike-slip shear zone between Kostenets and Krichim towns, South Bulgaria—Structural, petrographic and isotope geochronology study. Tectonophysics 2013, 595–596, 69–89. [Google Scholar] [CrossRef]
- Ersoy, E.Y.; Akal, C.; Genç, Ş.C.; Candan, O.; Palmer, M.R.; Prelević, D.; Uysal, İ.; Mertz-Kraus, R. U-Pb zircon Geochronology of the Paleogene—Neogene Volcanism in the NW Anatolia: Its implications for the Late Mesozoic-Cenozoic Geodynamic Evolution of the Aegean. Tectonophysics 2017, 717, 284–301. [Google Scholar] [CrossRef]
- Türkoğlu, E.; Zulauf, G.; Linckens, J.; Ustaömer, T. Dextral strike-slip along the Kapıdağ shear zone (NW Turkey): Evidence for Eocene westward translation of the Anatolian plate. Int. J. Earth Sci. 2016, 105, 2061–2073. [Google Scholar] [CrossRef]
- Arık, T.; Ünal, A.; Altunkaynak, Ş. An Eocene transtensional shear zone driven by the strain localization along slab break-off: Implications from the sheared syn-kinematic northern Kapıdağ Pluton (NW Anatolia, Turkey). J. Asian Earth Sci. 2025, 279, 106444. [Google Scholar] [CrossRef]
- Okay, A.I.; Satır, M.; Zattin, M.; Cavazza, W.; Topuz, G. An Oligocene ductile strike-slip shear zone: The Uludağ Massif, Northwest Turkey-implications for the westward translation of Anatolia. Geol. Soc. Am. Bull. 2008, 120, 893–911. [Google Scholar] [CrossRef]
- Yaltırak, C. Tectonic evolution of the Marmara Sea and its surroundings. Mar. Geol. 2002, 190, 493–529. [Google Scholar] [CrossRef]
- Hou, Z.; Tian, S.; Xie, Y.; Yang, Z.; Yuan, Z.; Yin, S.; Yi, L.; Fei, H.; Zou, T.; Bai, G.; et al. The Himalayan Mianning–Dechang REE belt associated with carbonatite–alkaline complexes, eastern Indo-Asian collision zone, SW China. Ore Geol. Rev. 2009, 36, 65–89. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ersoy, Y.E.; Yavuz, H.; Uysal, İ.; Palmer, M.R.; Müller, D. The Petrology of Tuffisite in a Trachytic Diatreme from the Kızılcaören Alkaline Silicate–Carbonatite Complex, NW Anatolia. Minerals 2025, 15, 867. https://doi.org/10.3390/min15080867
Ersoy YE, Yavuz H, Uysal İ, Palmer MR, Müller D. The Petrology of Tuffisite in a Trachytic Diatreme from the Kızılcaören Alkaline Silicate–Carbonatite Complex, NW Anatolia. Minerals. 2025; 15(8):867. https://doi.org/10.3390/min15080867
Chicago/Turabian StyleErsoy, Yalçın E., Hikmet Yavuz, İbrahim Uysal, Martin R. Palmer, and Dirk Müller. 2025. "The Petrology of Tuffisite in a Trachytic Diatreme from the Kızılcaören Alkaline Silicate–Carbonatite Complex, NW Anatolia" Minerals 15, no. 8: 867. https://doi.org/10.3390/min15080867
APA StyleErsoy, Y. E., Yavuz, H., Uysal, İ., Palmer, M. R., & Müller, D. (2025). The Petrology of Tuffisite in a Trachytic Diatreme from the Kızılcaören Alkaline Silicate–Carbonatite Complex, NW Anatolia. Minerals, 15(8), 867. https://doi.org/10.3390/min15080867











