Analysis of Occurrence States of Rare Earth Elements in the Carbonatite Deposits in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. REE Concentration
2.3. Concentration Coefficient (CC)
2.4. Mineralogical Techniques
2.5. Roasting Experiment
3. Results and Discussion
3.1. REE Analysis
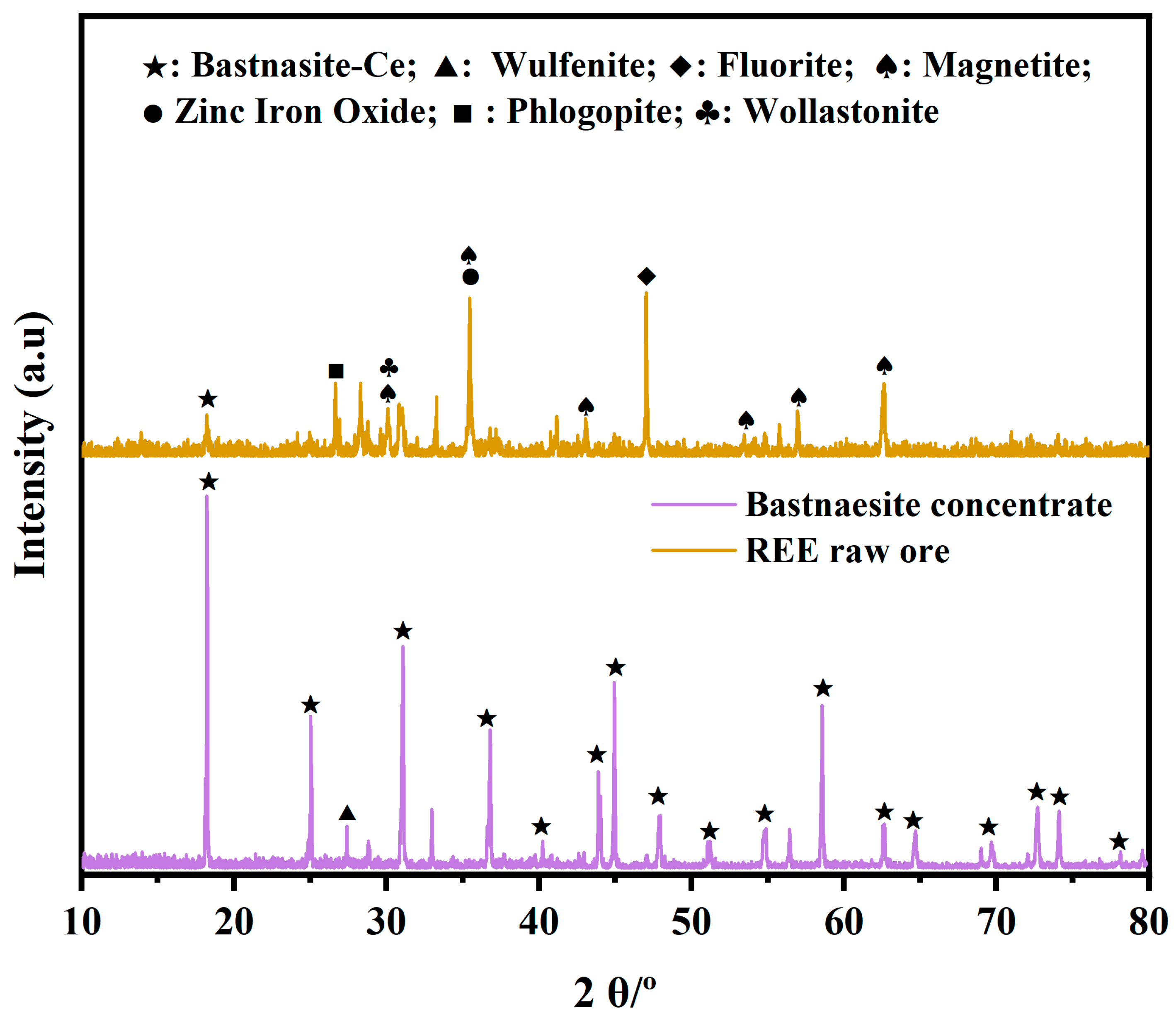
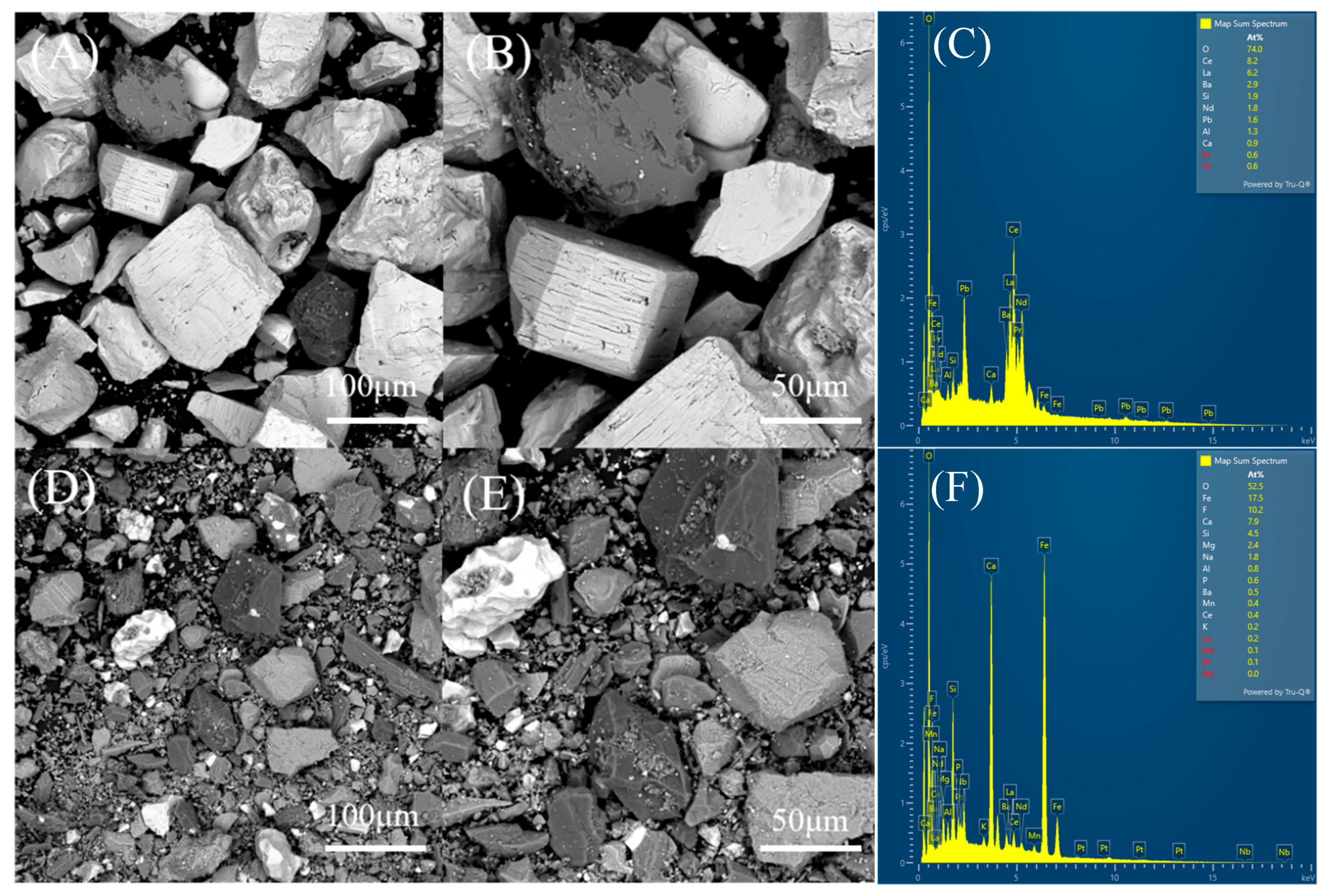
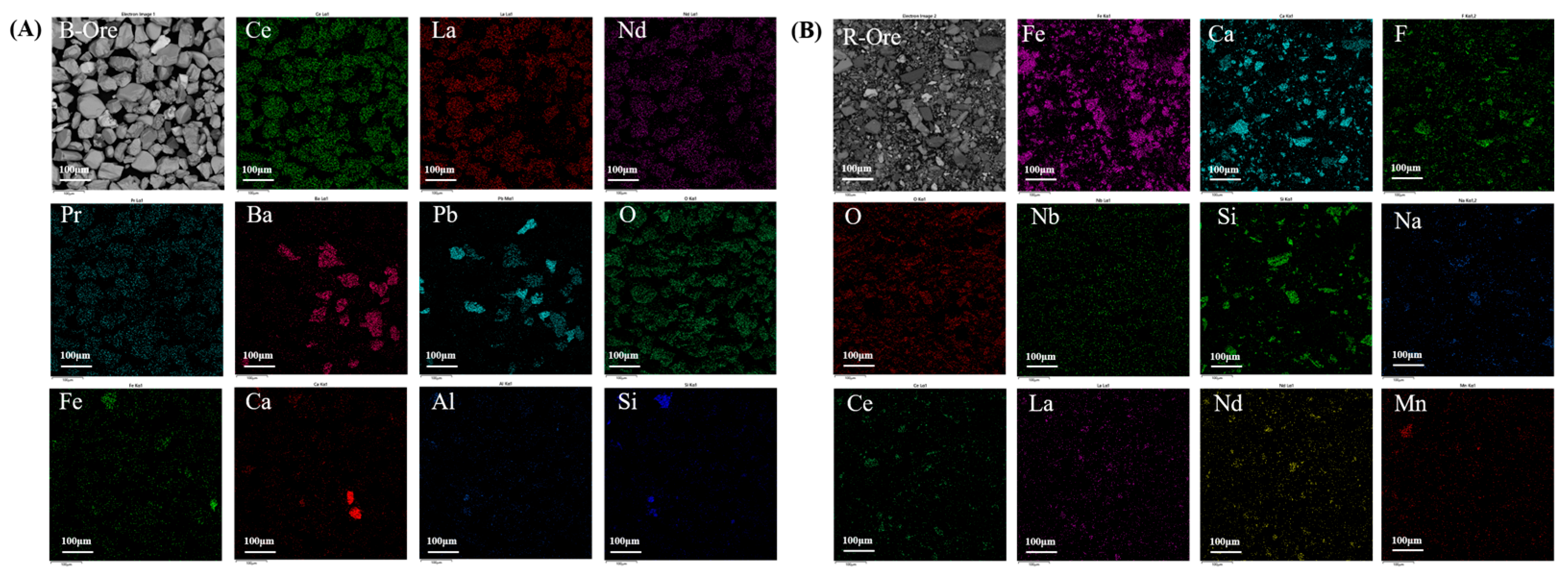
3.2. REE Ore Composition
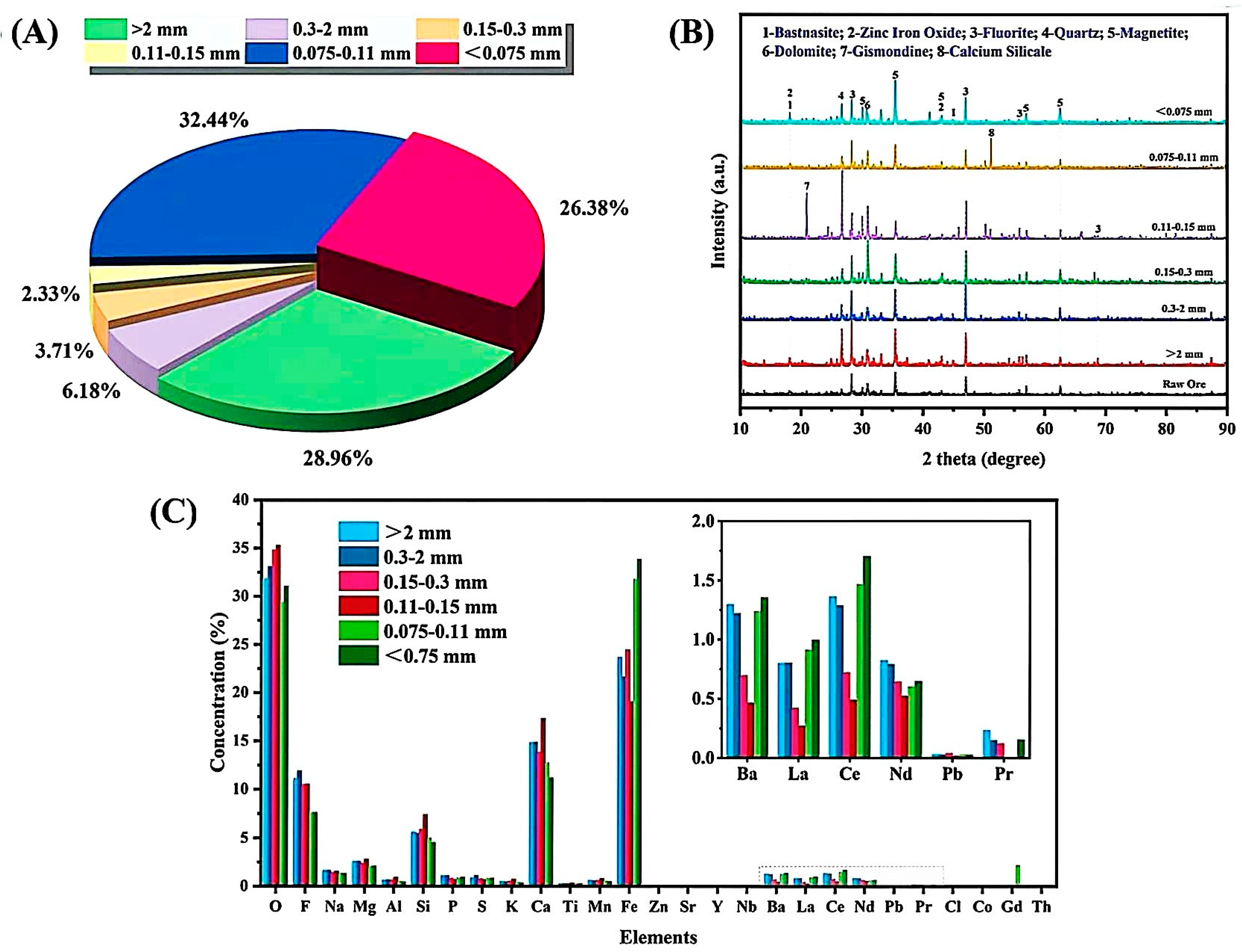
3.3. Analysis of REE Raw Ores in Different Particles Sizes
3.4. Thermal Decomposition Behavior of REE Ores
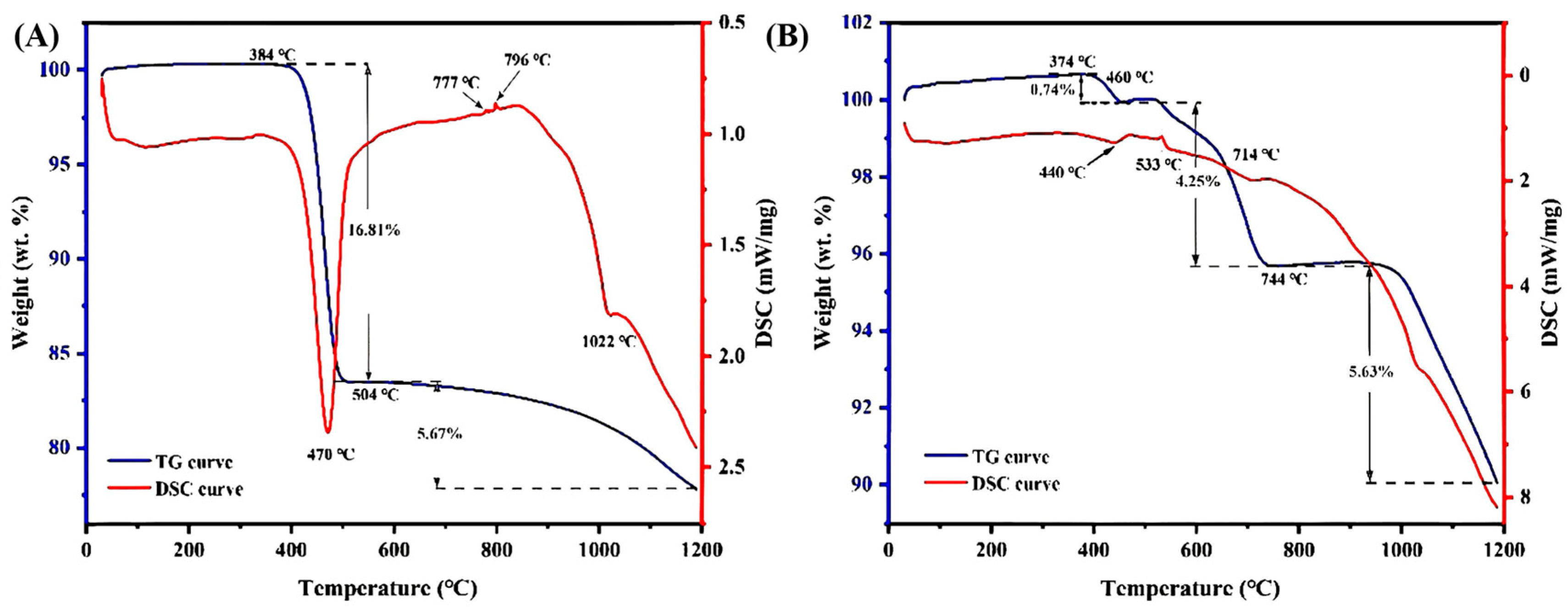
3.4.1. TG-DSC Analysis
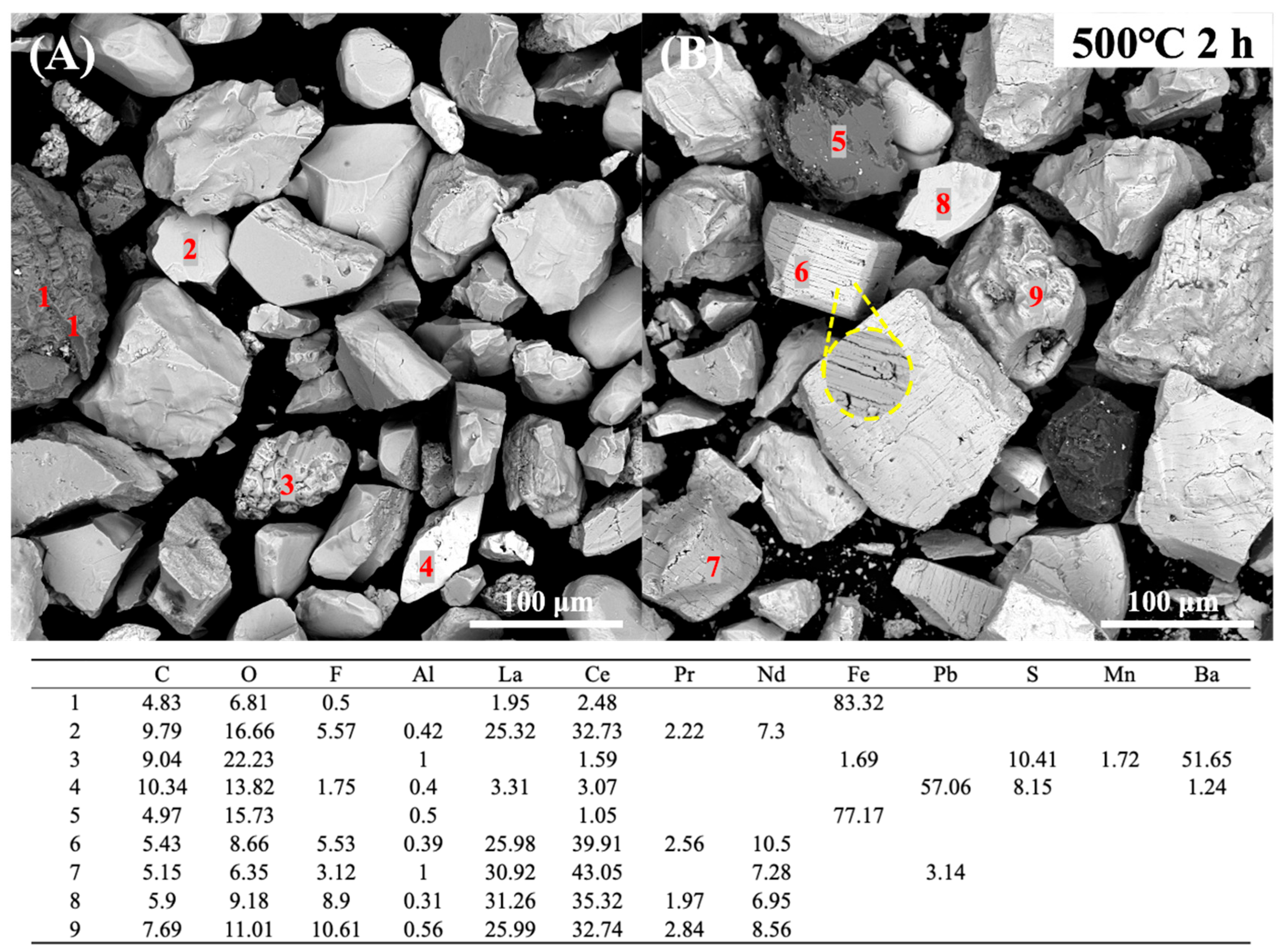
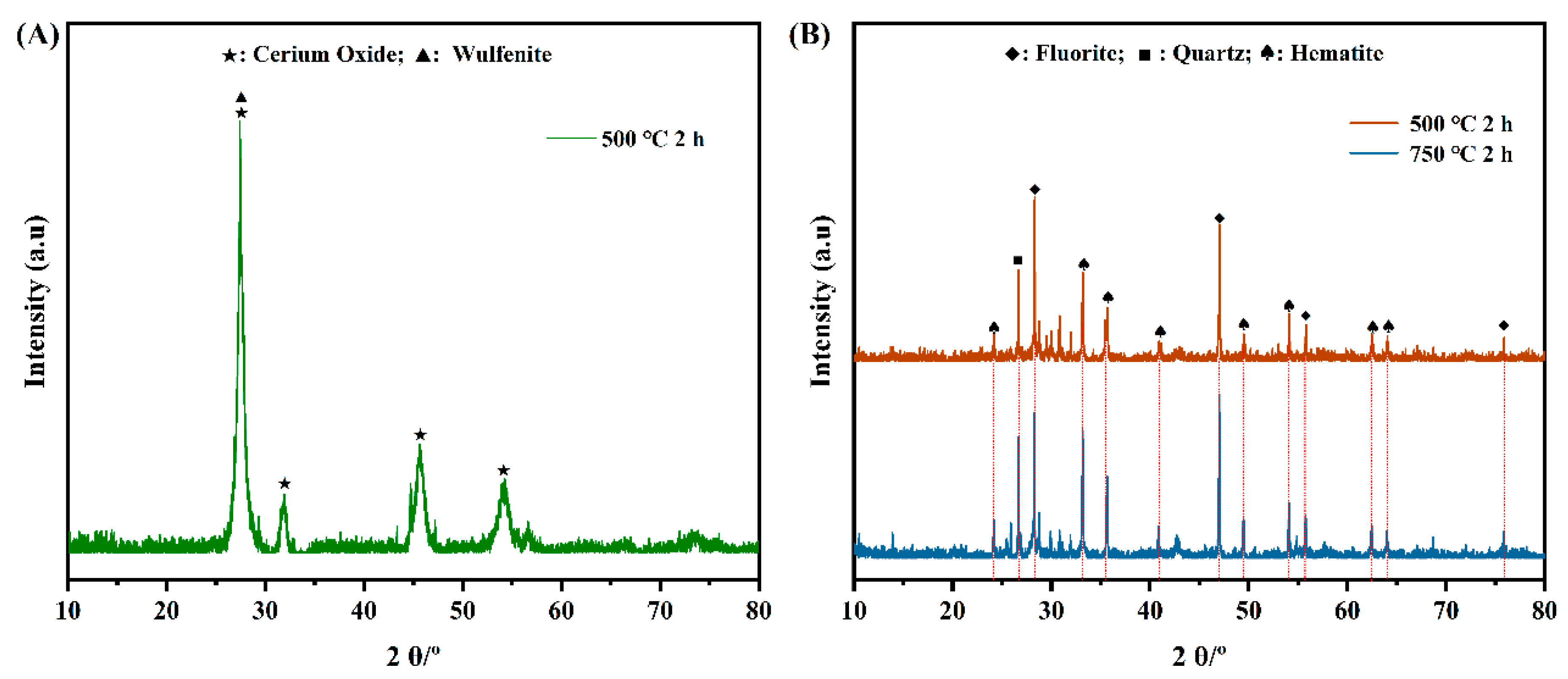
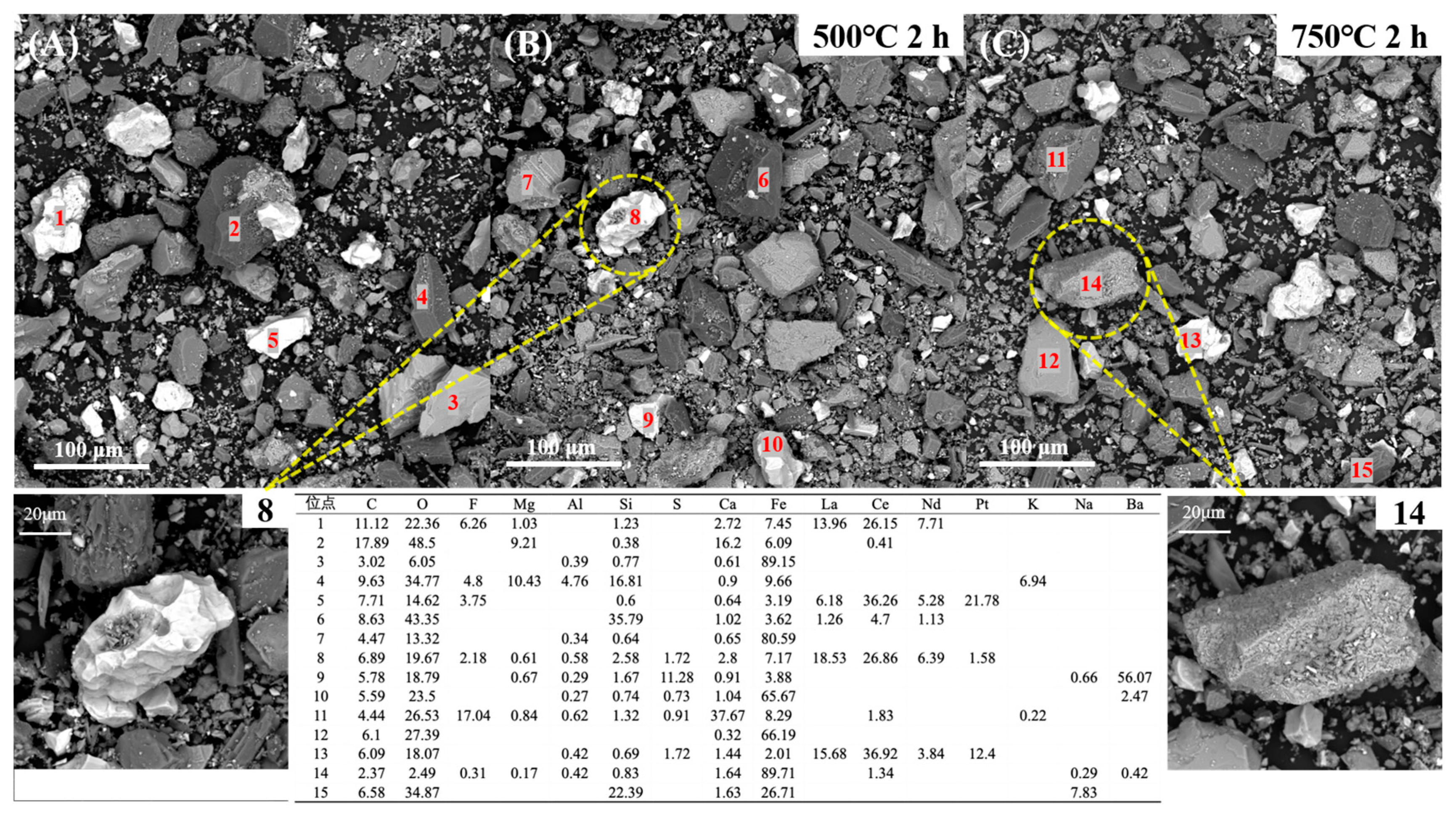
3.4.2. Variation in REE Ores After Roasting
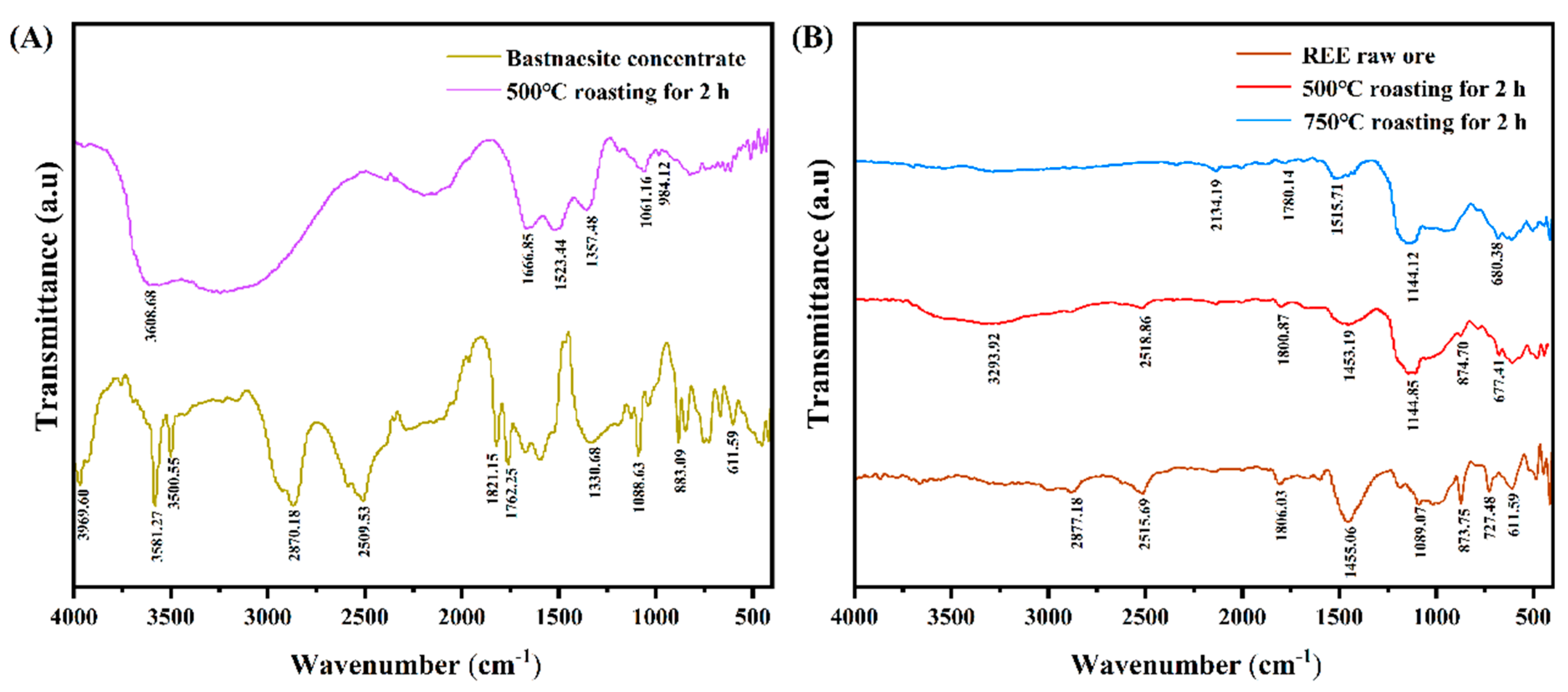
3.4.3. FTIR Analysis
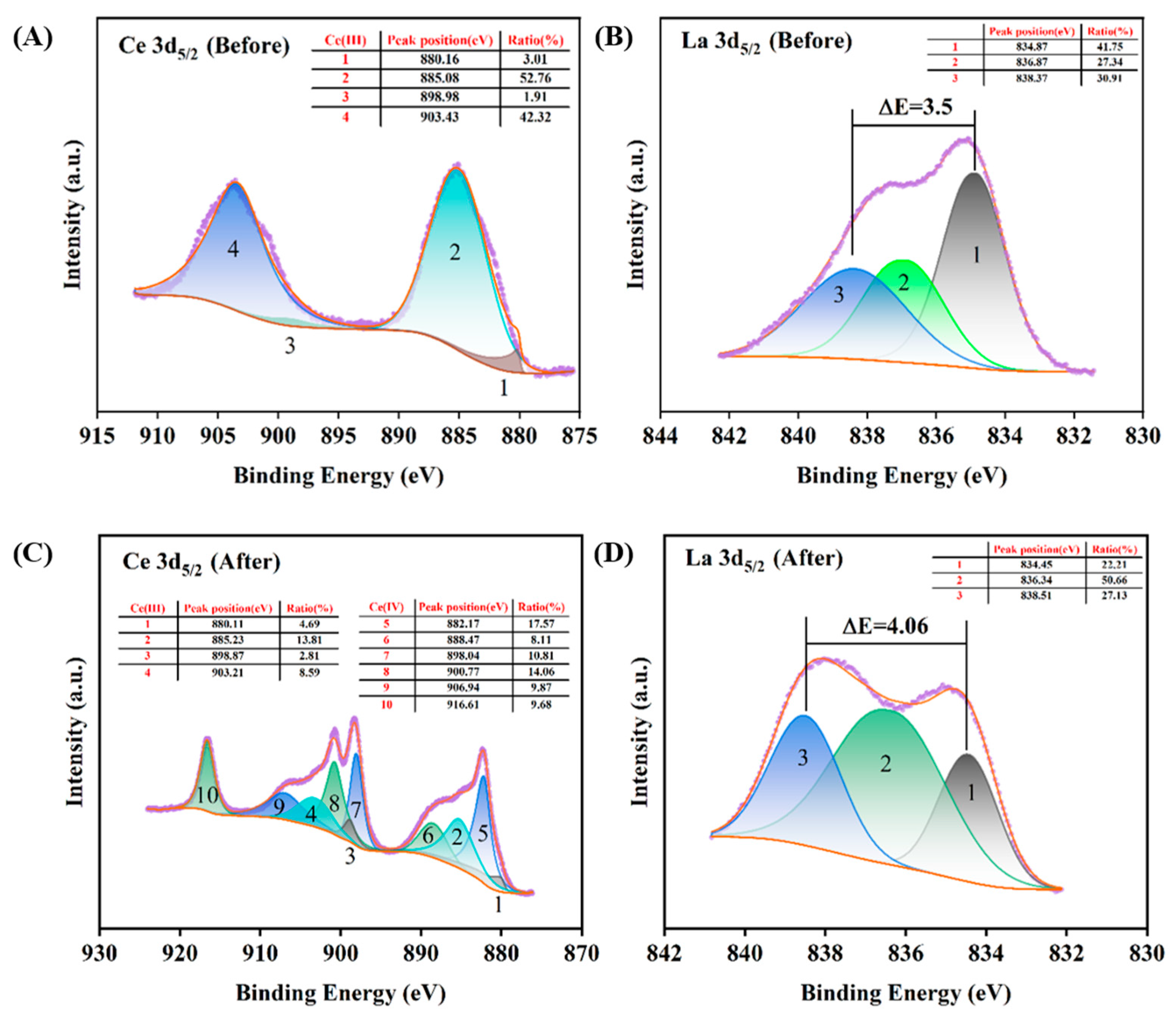
3.4.4. XPS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Erust, C.; Karacahan, M.K.; Uysal, T. Hydrometallurgical Roadmaps and Future Strategies for Recovery of Rare Earth Elements. Miner. Process. Extr. Metall. Rev. 2023, 44, 436–450. [Google Scholar] [CrossRef]
- Neil, G.C.; Ture, D.; Richard, M.H.; Alan, T.H. Nomenclature of Inorganic Chemistry-IUPAC Recommendations 2005. Chemistry International-Newsmagazine for IUPAC. Chem. Int. 2005, 27, 25–26. [Google Scholar]
- Chen, Z.Y.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H.D. Recent advances in selective separation technologies of rare earth elements: A review. J. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y. Mechanisms of element precipitation in carbonatite-related rare-earth element deposits: Evidence from fluid inclusions in the Maoniuping deposit, Sichuan Province, southwestern China. Ore Geol. Rev. 2019, 107, 218–238. [Google Scholar] [CrossRef]
- Yin, J.N.; Song, X. A review of major rare earth element and yttrium deposits in China. Aust. J. Earth Sci. 2021, 69, 1–25. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S.; Sun, Y.; Chekryzhov, I.Y. Coal deposits as promising sources of rare metals for alternative power and energy-efficient technologies. Appl. Geochem. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Hower, J.; Granite, E.; Mayfield, D.; Lewis, A.; Finkelman, R. Notes on Contributions to the Science of Rare Earth Element Enrichment in Coal and Coal Combustion Byproducts. Minerals 2016, 6, 32. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Chen, C. Global Potential of Rare Earth Resources and Rare Earth Demand from Clean Technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Chen, P.; Ilton, E.S.; Wang, Z.; Rosso, K.M.; Zhang, X. Global rare earth element resources: A concise review. Appl. Geochem. 2024, 175, 106158. [Google Scholar] [CrossRef]
- Wang, X.; Yao, M.; Li, J.; Zhang, K.; Zhu, H.; Zheng, M. China’s Rare Earths Production Forecasting and Sustainable Development Policy Implications. Sustainability 2017, 9, 1003. [Google Scholar] [CrossRef]
- Fan, H.; Yang, K.; Hu, F.; Liu, S.; Wang, K. The giant Bayan Obo REE-Nb-Fe deposit, China: Controversy and ore genesis. Geosci. Front. 2016, 7, 335–344. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Z. A synthesis of mineralization styles with an integrated genetic model of carbonatite-syenite-hosted REE deposits in the Cenozoic Mianning-Dechang REE metallogenic belt, the eastern Tibetan Plateau, southwestern China. J. Asian Earth Sci. 2017, 137, 35–79. [Google Scholar] [CrossRef]
- Qiao, S.; Bian, X.; Cui, J.; Cen, P.; Wu, W. Clean metallurgy and technical progress of light rare earth minerals. Can. Metall. Q. 2023, 63, 538–550. [Google Scholar]
- Yuheng, J.; Yan, L. Factors controlling the generation and diversity of giant carbonatite-related rare earth element deposits: Insights from the Mianning–Dechang belt. Ore Geol. Rev. 2020, 121, 103472. [Google Scholar] [CrossRef]
- Wang, K.; Fang, A.; Zhang, J.; Yu, L.; Dong, C.; Zan, J.; Hao, M.; Hu, F. Genetic relationship between fenitized ores and hosting dolomite carbonatite of the Bayan Obo REE deposit, Inner Mongolia, China. J. Asian Earth Sci. 2019, 174, 189–204. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, J.; Deng, Q.; Men, P.; Zhang, Y.; Shi, X. Study on REE occurrence in a Svanbergite and basic ore characteristics. Physicochem. Probl. Miner. Process. 2022, 58, 147377. [Google Scholar] [CrossRef]
- Hou, X.; Yang, Z.; Wang, Z.; Wang, W. The occurrences and geochemical characteristics of thorium in iron ore in the Bayan Obo deposit, Northern China. Acta Geochim. 2019, 39, 139–154. [Google Scholar] [CrossRef]
- Xiong, C.; Xie, H.; Wang, Y.; Wang, C.; Li, Z.; Yang, C. Microdistribution and Mode of Rare Earth Element Occurrence in the Zhijin Rare Earth Element-Bearing Phosphate Deposit, Guizhou, China. Minerals 2024, 14, 223. [Google Scholar] [CrossRef]
- Li, W.; Chen, J.; Cheng, S.; Sun, J.; Zhang, X. Thermal decomposition mechanism and kinetics of bastnaesite in suspension roasting process: A comparative study in N2 and air atmospheres. J. Rare Earths 2024, 42, 1809–1816. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Shuai, G.; Long, Z.; Cui, D.; Huang, X. Thermal decomposition and oxidation of bastnaesite concentrate in inert and oxidative atmosphere. J. Rare Earths 2018, 36, 758–764. [Google Scholar] [CrossRef]
- Taylor, S.R. Abundance of chemical elements in the continental crust a new table.pdf. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Dai, S.f.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Xing, Y.; Zhang, W.; Song, W.; Wang, P. Enrichment of U-Se-Mo-Re-V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Miner. Depos. 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Shuai, Z.; Zhu, Y.; Gao, P.; Han, Y. Rare earth elements resources and beneficiation: A review. Miner. Eng. 2024, 218, 109011. [Google Scholar] [CrossRef]
- Hou, X.; Yang, Z.; Wang, Z. The occurrence characteristics and recovery potential of middle-heavy rare earth elements in the Bayan Obo deposit, Northern China. Ore Geol. Rev. 2020, 126, 103737. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Xue, X.; Ru, H.; Huang, X.; Yang, H. Leaching of fluorine and rare earths from bastnaesite calcined with aluminum hydroxide and the recovery of fluorine as cryolite. Rsc Adv. 2017, 7, 14053–14059. [Google Scholar] [CrossRef]
- Liu, P.; Yang, L.; Wang, Q.; Wan, B.; Ma, Q.; Chen, H.; Tang, Y. Speciation transformation of rare earth elements (REEs) during heating and implications for REE behaviors during coal combustion. Int. J. Coal Geol. 2020, 219, 103371. [Google Scholar] [CrossRef]
- Salameh, Y.; Albadarin, A.B.; Allen, S.; Walker, G.; Ahmad, M.N.M. Arsenic(III,V) adsorption onto charred dolomite: Charring optimization and batch studies. Chem. Eng. J. 2015, 259, 663–671. [Google Scholar] [CrossRef]
- García, A.C.; Latifi, M.; Chaouki, J. Kinetics of calcination of natural carbonate minerals. Miner. Eng. 2020, 150, 106279. [Google Scholar] [CrossRef]
- Khan, U.S.; Amanullah; Manan, A.; Khan, N.; Mahmood, A.; Rahim, A. Transformation mechanism of magnetite nanoparticles. Mater. Sci. 2015, 33, 278–285. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Zhang, D.; Gao, K.; Li, J.; Weng, Z.; Xu, W. Phase change, micro-structure and reaction mechanism during high temperature roasting of high grade rare earth concentrate. J. Rare Earths 2020, 38, 1140–1150. [Google Scholar] [CrossRef]
- Tong, Z.; Hu, X.; Wen, H. Effect of roasting activation of rare earth molten salt slag on extraction of rare earth, lithium and fluorine. J. Rare Earths 2023, 41, 300–308. [Google Scholar] [CrossRef]
- Fukuhara, M.; Sampei, A. Effects on high-temperature-elastic properties on Æ-=â-quartz phase. J. Mater Ials Sc Ience Lett. 1999, 18, 751–753. [Google Scholar] [CrossRef]
- Duan, H.; Liu, W.; Wang, X.; Zhao, L.; Fang, P.; Gu, X. Preparation of a novel bis hydroxamic collector and its impact on bastnaesite flotation. Miner. Eng. 2020, 156, 106496. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, X. Practical Infrared Spectroscopy, 1st ed.; Science Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Huseynov, E.; Garibov, A.; Mehdiyeva, R.; Huseynova, E. Fourier transform infrared spectroscopic study of gamma irradiated SiO2 nanoparticles. Int. J. Mod. Phys. B 2018, 32, 1850074. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Xu, L.; Xue, K.; Zhang, X.; Shi, X.; Liu, C.; Meng, J. Synthesis and utilization of a novel oleate hydroxamic acid collector for the flotation separation of bastnaesite from barite. Miner. Eng. 2023, 204, 108405. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Zhang, X.; Wu, J.; Dong, Z.; Liu, Z.; Hao, X. Decomposition Model of Bastnaesite and Its Fluorine Oxygen Coupling Escape Mechanism. ACS Omega 2024, 9, 38437–38451. [Google Scholar] [CrossRef]
| B-Ore | R-Ore | B-Ore | R-Ore | B-Ore | R-Ore | |||
|---|---|---|---|---|---|---|---|---|
| O | 24.23 | 30.62 | Ca | 0.40 | 10.86 | Nb | - | 0.06 |
| F | 9.13 | 7.68 | Ti | 0.12 | 0.27 | Mo | 0.86 | - |
| Na | 0.29 | 1.28 | Mn | - | 0.44 | Ba | 2.42 | - |
| Mg | 0.04 | 2.02 | Fe | 1.88 | 34.51 | La | 18.05 | 1.08 |
| Al | 0.43 | 0.49 | Co | - | 0.04 | Ce | 27.22 | 1.78 |
| Si | 1.09 | 4.27 | Zn | - | 0.02 | Pr | 1.67 | 0.14 |
| P | 0.10 | 0.91 | Sr | 0.08 | - | Nd | 6.58 | 0.71 |
| S | 1.44 | 0.87 | Y | 0.07 | - | Pb | 3.51 | 0.04 |
| K | 0.10 | 0.35 | Zr | 0.09 | 1.46 | Th | 0.21 | 0.03 |
| B-Ore | R-Ore | |
|---|---|---|
| La | 218,233.57 ± 78.18 | 801.15 ± 1.56 |
| Ce | 281,005.70 ± 238.47 | 1771.01 ± 0.38 |
| Pr | 27,800.43 ± 21.72 | 254.86 ± 1.75 |
| Nd | 65,439.73 ± 79.43 | 749.55 ± 5.50 |
| Element | R-Ore (%) | Crustal Average (ppm) | CC |
|---|---|---|---|
| Ca | 10.86 | 4.15 × 104 | 2.6 |
| P | 0.91 | 1050 | 8.7 |
| Fe | 34.51 | 5.63 × 104 | 6 |
| Y | 0.02 | 33 | 6 |
| Nb | 0.06 | 20 | 30 |
| Ba | 1.46 | 425 | 34 |
| Th | 0.03 | 9.6 | 33 |
| F | 7.68 | 625 | 123 |
| La | 1.08 | 30 | 361 |
| Ce | 1.78 | 60 | 296 |
| Pr | 0.14 | 8.2 | 167 |
| Nd | 0.71 | 28 | 254 |
| Slight Enrichment | Enrichment | High Enrichment | Abnormal Enrichment |
|---|---|---|---|
| 2 ≤ CC < 5 | 5 ≤ CC < 10 | 10 ≤ CC < 100 | CC ≥ 100 |
| Ca | P, Fe, Y | Nb, Ba, Th | F, La, Ce, Pr, Nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; He, N.; Hu, L.; Liu, Y.; Gong, J.; Zhao, H. Analysis of Occurrence States of Rare Earth Elements in the Carbonatite Deposits in China. Minerals 2025, 15, 866. https://doi.org/10.3390/min15080866
Jiang Z, He N, Hu L, Liu Y, Gong J, Zhao H. Analysis of Occurrence States of Rare Earth Elements in the Carbonatite Deposits in China. Minerals. 2025; 15(8):866. https://doi.org/10.3390/min15080866
Chicago/Turabian StyleJiang, Zuopei, Ni He, Liang Hu, Yayuan Liu, Jingyi Gong, and Hongbo Zhao. 2025. "Analysis of Occurrence States of Rare Earth Elements in the Carbonatite Deposits in China" Minerals 15, no. 8: 866. https://doi.org/10.3390/min15080866
APA StyleJiang, Z., He, N., Hu, L., Liu, Y., Gong, J., & Zhao, H. (2025). Analysis of Occurrence States of Rare Earth Elements in the Carbonatite Deposits in China. Minerals, 15(8), 866. https://doi.org/10.3390/min15080866








