Table Olive Wastewater Treatment Using the Clay Mineral Palygorskite as Adsorbent
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. TOW Characteristics
2.3. Pal Sample Characterization
2.4. Batch Kinetic Experiments
2.5. Analytical Methods
2.6. Adsorption Isotherm Models
2.7. Adsorption Kinetic Models
3. Results and Discussion
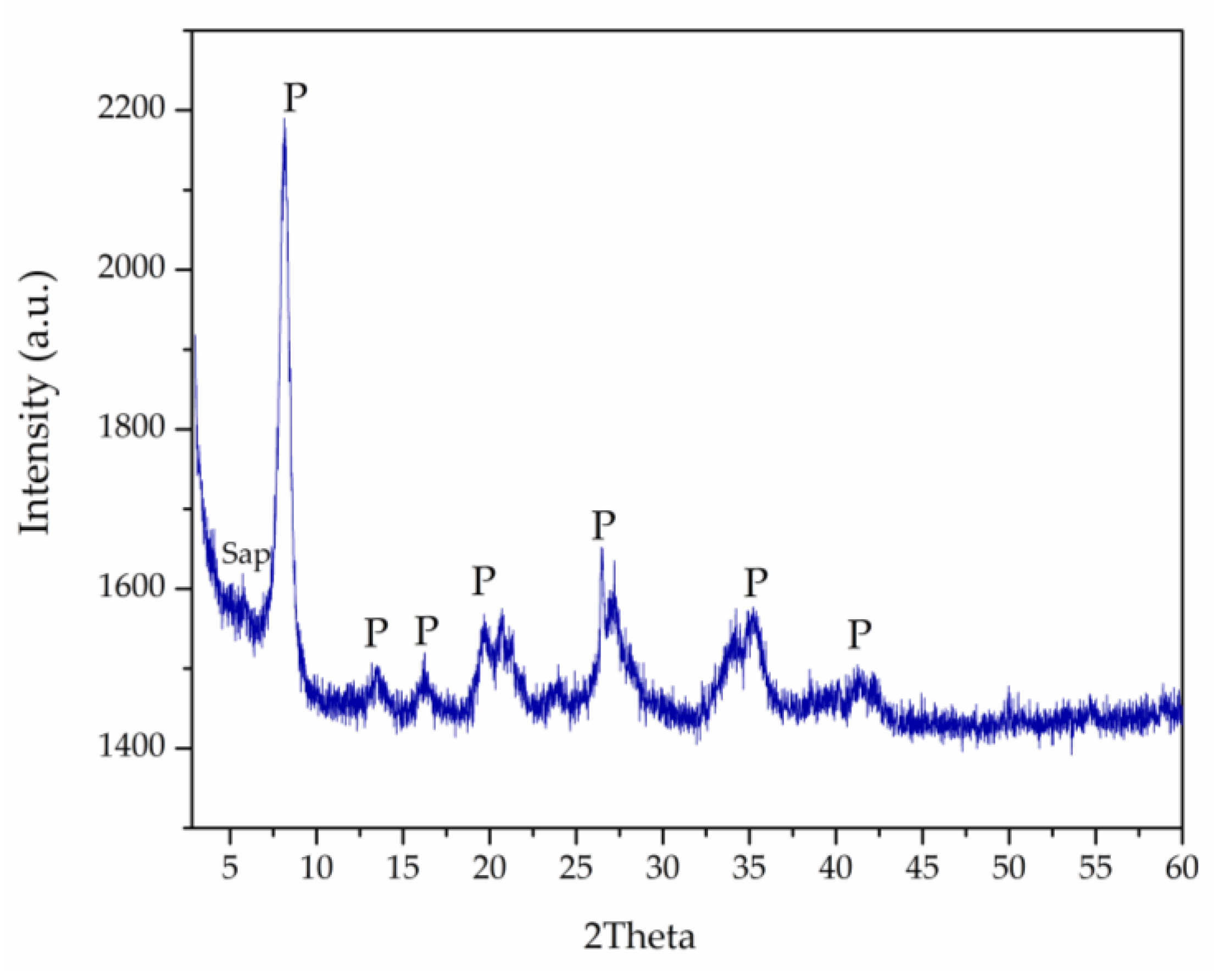
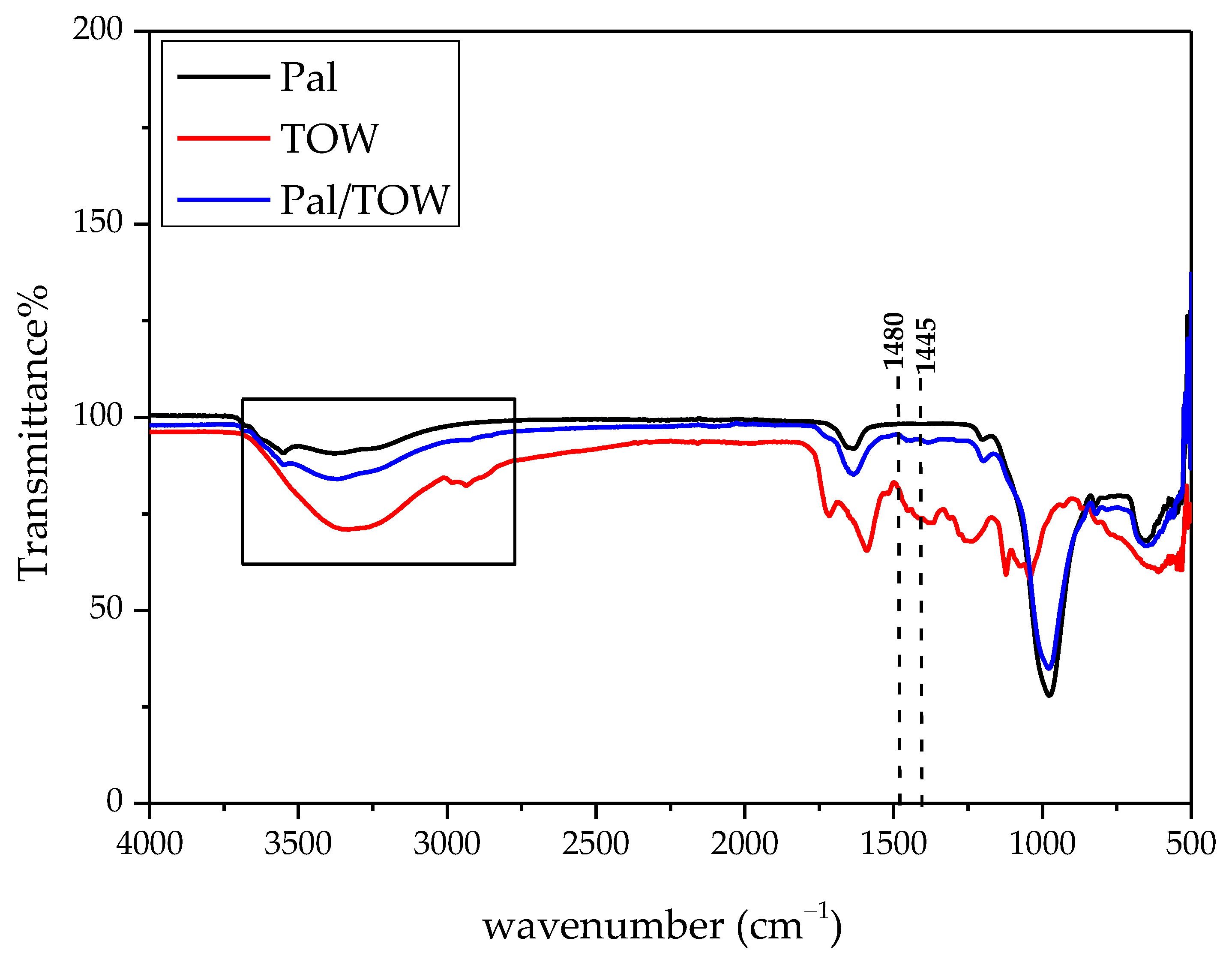
3.1. Pal Characterization
3.2. Adsorption Batch Kinetic Experiments
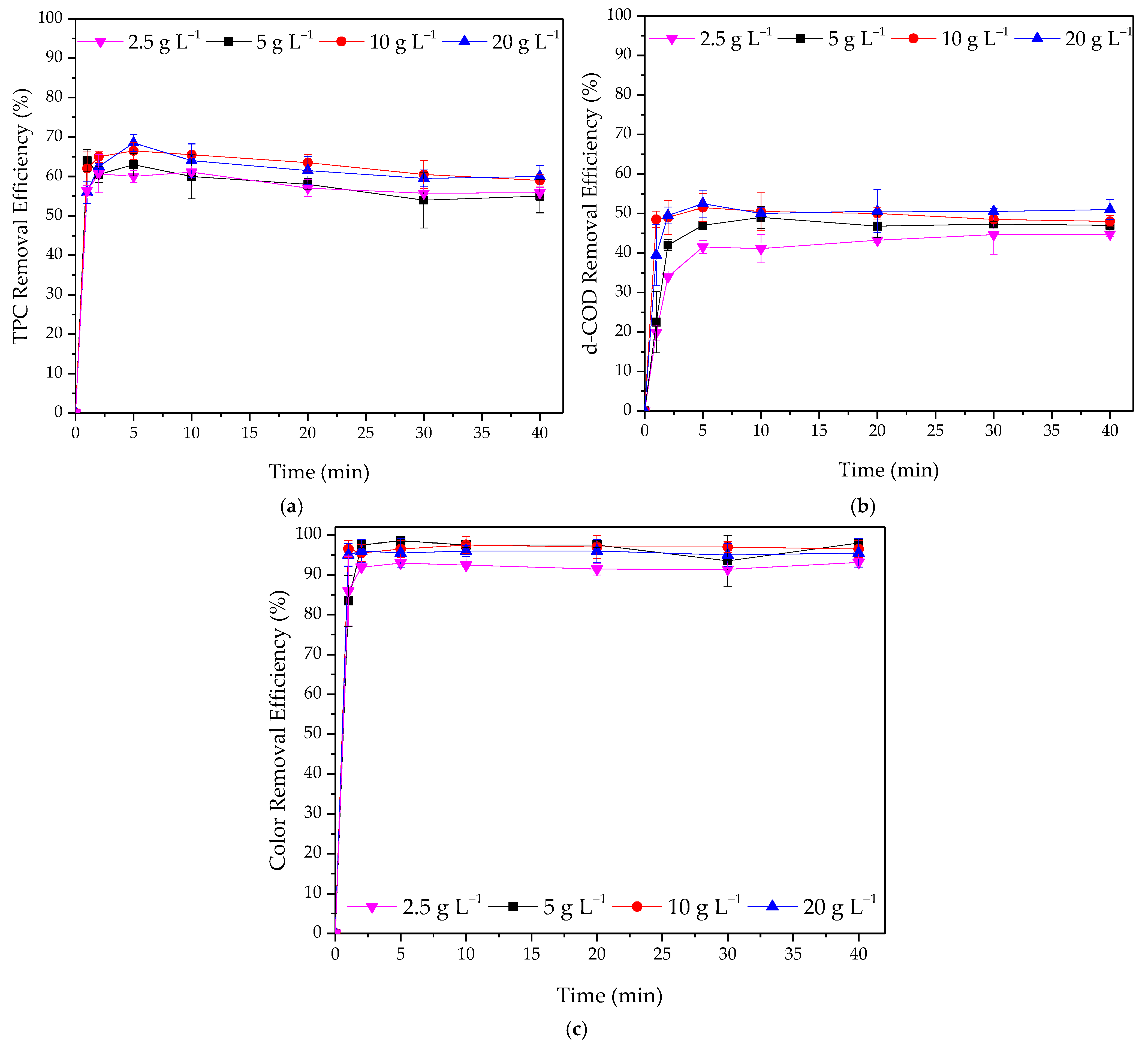
3.2.1. Effect of Adsorbent Dosage
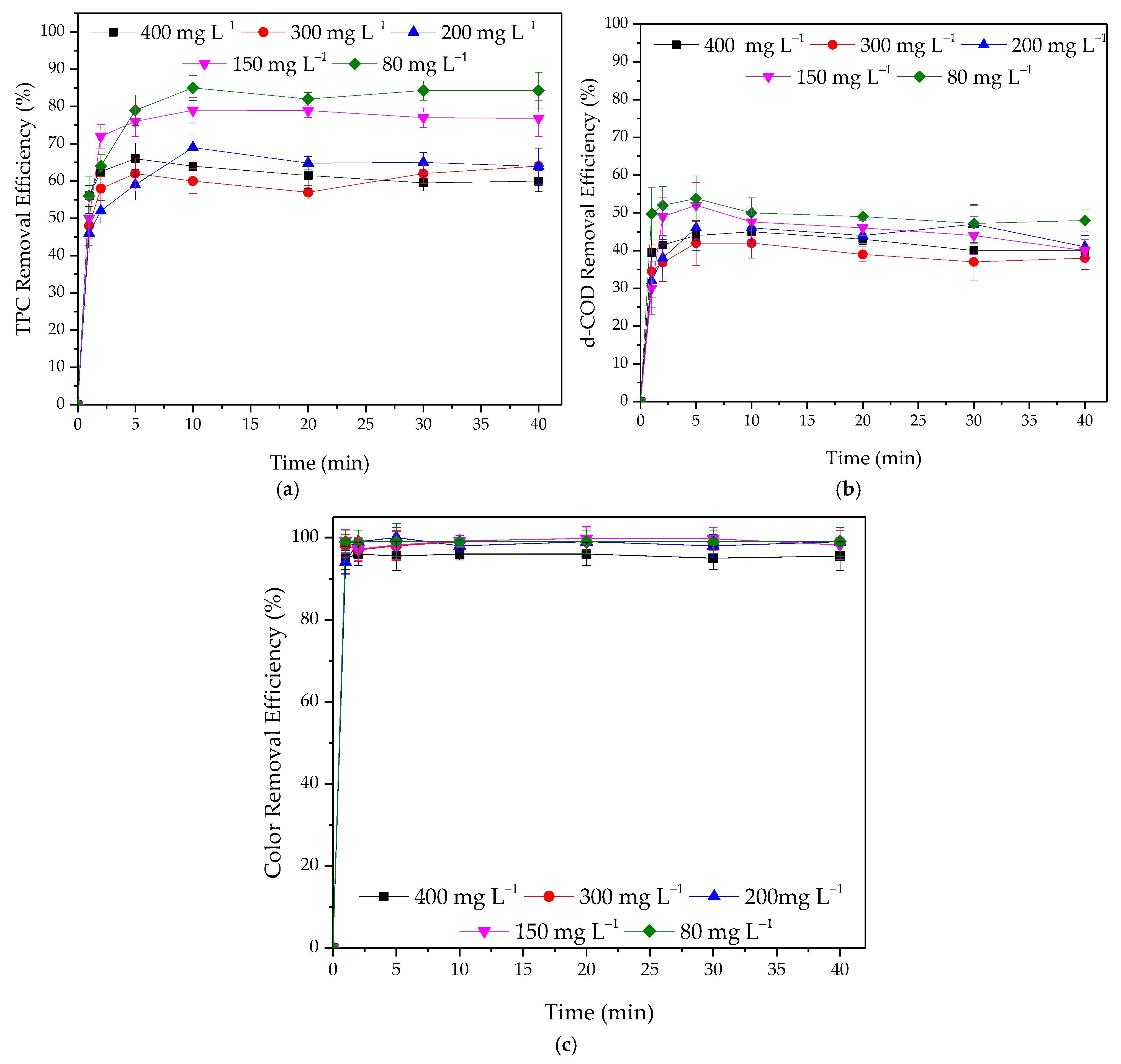
3.2.2. Effect of Initial Concentration
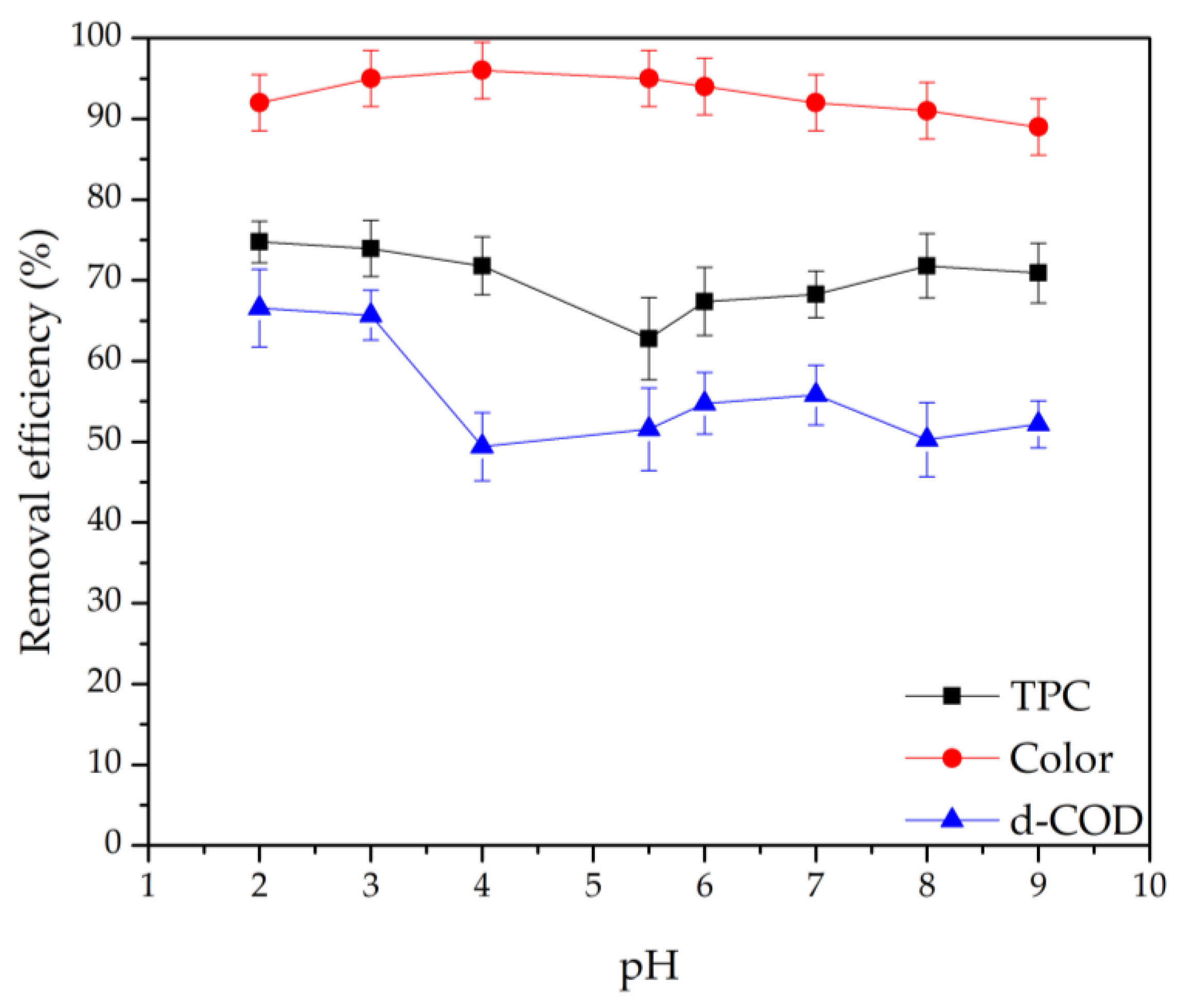
3.2.3. Effect of pH
3.3. Adsorption Isotherm Models
3.4. Adsorption Kinetic Models
3.5. Suggested Mechanism for TOW Treatment by Pal
3.6. Practical Implementations for Potential Pilot Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huertas-Alonso, A.J.; Gonzalez-Serrano, D.J.; Hadidi, M.; Salgado-Ramos, M.; Orellana-Palacios, J.C.; Sánchez-Verdú, M.P.; Xia, Q.; Simirgiotis, M.J.; Barba, F.J.; Dar, B.N.; et al. Table Olive Wastewater as a Potential Source of Biophenols for Valorization: A Mini Review. Fermentation 2022, 8, 215. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Mastralexi, A.; Filippidou, M.; Tsimidou, M.Z. Challenges in the Processing Line of Spanish Style Cv. Chalkidiki Green Table Olives Spontaneously Fermented in Reduced NaCl Content Brines. Eur. J. Lipid Sci. Technol. 2020, 122, 1900453. [Google Scholar] [CrossRef]
- Rincón-Llorente, B.; De la Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Borja-Padilla, R. Table Olive Wastewater: Problem, Treatments and Future Strategy. A Review. Front. Microbiol. 2018, 9, 1641. [Google Scholar] [CrossRef] [PubMed]
- Aldana, J.C.; Acero, J.L.; Álvarez, P.M. Membrane Filtration, Activated Sludge and Solar Photocatalytic Technologies for the Effective Treatment of Table Olive Processing Wastewater. J. Environ. Chem. Eng. 2021, 9, 105743. [Google Scholar] [CrossRef]
- Carbonell-Alcaina, C.; Álvarez-Blanco, S.; Bes-Piá, M.A.; Mendoza-Roca, J.A.; Pastor-Alcañiz, L. Ultrafiltration of Residual Fermentation Brines from the Production of Table Olives at Different Operating Conditions. J. Clean. Prod. 2018, 189, 662–672. [Google Scholar] [CrossRef]
- Bizerea Spiridon, O.; Preda, E.; Botez, A.; Pitulice, L. Phenol Removal from Wastewater by Adsorption on Zeolitic Composite. Environ. Sci. Pollut. Res. 2013, 20, 6367–6381. [Google Scholar] [CrossRef]
- Solomakou, N.; Goula, A.M. Treatment of Olive Mill Wastewater by Adsorption of Phenolic Compounds. Rev. Environ. Sci. Biotechnol. 2021, 20, 839–863. [Google Scholar] [CrossRef]
- Tatoulis, T.; Stefanakis, A.; Frontistis, Z.; Akratos, C.S.; Tekerlekopoulou, A.G.; Mantzavinos, D.; Vayenas, D.V. Treatment of Table Olive Washing Water Using Trickling Filters, Constructed Wetlands and Electrooxidation. Environ. Sci. Pollut. Res. 2017, 24, 1085–1092. [Google Scholar] [CrossRef]
- Aziz, K.; Haydari, I.; Kaya, S.; Mandi, L.; Ouazzani, N.; Aziz, F. Phenolic Compounds Removal in Table Olive Processing Wastewater by Column Adsorption: Conditions’ Optimization. Environ. Sci. Pollut. Res. 2024, 31, 38835–38845. [Google Scholar] [CrossRef]
- El Abbadi, S.; El Moustansiri, H.; Douma, M.; Bouazizi, A.; Arfoy, B.; Calvo, J.I.; Tijani, N. Enhancing the Performance of Alumina-Pillared Clay for Phenol Removal from Water Solutions and Polyphenol Removal from Olive Mill Wastewater: Characterization, Kinetics, Adsorption Performance, and Mechanism. J. Water Process Eng. 2024, 63, 105432. [Google Scholar] [CrossRef]
- Zango, Z.U.; Rozaini, M.N.; Bakar, N.H.H.A.; Zango, M.U.; Haruna, M.A.; Dennis, J.O.; Alsadig, A.; Ibnaouf, K.H.; Aldaghri, O.A.; Wadi, I.A. Advancements in Clay Materials for Trace Level Determination and Remediation of Phenols from Wastewater: A Review. Separations 2023, 10, 125. [Google Scholar] [CrossRef]
- Aly, A.A.; Hasan, Y.N.Y.; Al-Farraj, A.S. Olive Mill Wastewater Treatment Using a Simple Zeolite-Based Low-Cost Method. J. Environ. Manag. 2014, 145, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Yousef, R.; Qiblawey, H.; El-Naas, M.H. Adsorption as a Process for Produced Water Treatment: A Review. Processes 2020, 8, 1657. [Google Scholar] [CrossRef]
- Amirhassani, S.; Askari, S.; Piravi Vanak, Z.; Honarvar, M.; Rashidi Nodeh, H. Kinetic Modeling of the Removal of Phenolic Compounds from Olive Mill Wastewater by Hierarchical Zeolite/Carbon Nanotubes: Isotherm, Kinetics, and Thermodynamic. Sep. Sci. Technol. 2024, 60, 31–48. [Google Scholar] [CrossRef]
- Ait-Hmane, A.; Mandi, L.; Ouazzani, N.; Ait Hammou, H.; Hejjaj, A.; Alahiane, S.; Assabbane, A. Combined Treatment of Olive Mill Wastewater by Multi-Soil-Layering Ecotechnology and Adsorption on Activated Carbon/Lime. Desalin. Water Treat. 2021, 233, 253–260. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Al-Rawashdeh, N.A.F.; Almurabi, M.; Abdelnabi, J.; Al Bawab, A. Phenolic Compounds Removal from Olive Mill Wastewater Using the Composite of Activated Carbon and Copper-Based Metal-Organic Framework. Materials 2023, 16, 1159. [Google Scholar] [CrossRef]
- Djebbar, M.; Djafri, F.; Bouchekara, M.; Djafri, A. Adsorption of Phenol on Natural Clay. Appl. Water Sci. 2012, 2, 77–86. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption Properties and Mechanisms of Palygorskite for Removal of Various Ionic Dyes from Water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Ahmat, A.M.; Thiebault, T.; Guégan, R. Phenolic Acids Interactions with Clay Minerals: A Spotlight on the Adsorption Mechanisms of Gallic Acid onto Montmorillonite. Appl. Clay Sci. 2019, 180, 105188. [Google Scholar] [CrossRef]
- Cunha, R.; Trigueiro, P.; Orta Cuevas, M.d.M.; Medina-Carrasco, S.; Duarte, T.M.; Honório, L.M.d.C.; Damacena, D.H.L.; Fonseca, M.G.; da Silva-Filho, E.C.; Osajima, J.A. The Stability of Anthocyanins and Their Derivatives through Clay Minerals: Revising the Current Literature. Minerals 2023, 13, 268. [Google Scholar] [CrossRef]
- Trendafilova, I.; Popova, M. Porous Silica Nanomaterials as Carriers of Biologically Active Natural Polyphenols: Effect of Structure and Surface Modification. Pharmaceutics 2024, 16, 1004. [Google Scholar] [CrossRef] [PubMed]
- Lazaratou, C.V.; Papoulis, D.; Vayenas, D.V.; Pospíšil, M. Molecular Simulation Approach for NO3−-N and NH4+-N Sorption and Desorption in the Pores of Palygorskite and Sepiolite Clay Minerals. Appl. Clay Sci. 2024, 254, 107371. [Google Scholar] [CrossRef]
- Suárez, M.; García-Rivas, J.; Morales, J.; Lorenzo, A.; García-Vicente, A.; García-Romero, E. Review and New Data on the Surface Properties of Palygorskite: A Comparative Study. Appl. Clay Sci. 2022, 216, 106311. [Google Scholar] [CrossRef]
- Zhuang, G.; Li, L.; Li, M.; Yuan, P. Influences of Micropores and Water Molecules in the Palygorskite Structure on the Color and Stability of Maya Blue Pigment. Microporous Mesoporous Mater. 2022, 330, 111615. [Google Scholar] [CrossRef]
- Zhang, Z.; Gui, W.; Wei, J.; Cui, Y.; Li, P.; Jia, Z.; Kong, P. Functionalized Attapulgite for the Adsorption of Methylene Blue: Synthesis, Characterization, and Adsorption Mechanism. ACS Omega 2021, 6, 19586–19595. [Google Scholar] [CrossRef]
- Xu, C.; Feng, Y.; Li, H.; Li, Y.; Yao, Y. Purification of Natural Palygorskite Clay: Process Optimization, Cleaner Production, Mineral Characterization, and Decolorization Performance. Appl. Clay Sci. 2024, 250, 107268. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Zhao, Y.; Xie, M. Cationic Surfactant Modified Attapulgite for Removal of Phenol from Wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128479. [Google Scholar] [CrossRef]
- Guo, X.; He, C.; Sun, X.; Liang, X.; Chen, X.; Liu, X.Y. Adsorption of Phenol from Aqueous Solution by Four Types of Modified Attapulgites. Int. J. Environ. Sci. Technol. 2019, 16, 793–800. [Google Scholar] [CrossRef]
- Zhao, Z.; Fu, D.; Ma, Q. Adsorption Characteristics of Bisphenol A from Aqueous Solution onto HDTMAB-Modified Palygorskite. Sep. Sci. Technol. 2014, 49, 81–89. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1919, 40, 1361–1403. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Aydın Temel, F.; Kuleyin, A. Ammonium Removal from Landfill Leachate Using Natural Zeolite: Kinetic, Equilibrium, and Thermodynamic Studies. Desalin. Water Treat. 2016, 57, 23873–23892. [Google Scholar] [CrossRef]
- Karri, R.R.; Jayakumar, N.S.; Sahu, J.N. Modelling of Fluidised-Bed Reactor by Differential Evolution Optimization for Phenol Removal Using Coconut Shells Based Activated Carbon. J. Mol. Liq. 2017, 231, 249–262. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The Sorption of Lead(II) Ions on Peat. Water Res. 1999, 33, 578–584. [Google Scholar] [CrossRef]
- Cifuentes-Cabezas, M.; María Sanchez-Arévalo, C.; Antonio Mendoza-Roca, J.; Cinta Vincent-Vela, M.; Álvarez-Blanco, S. Recovery of Phenolic Compounds from Olive Oil Washing Wastewater by Adsorption/Desorption Process. Sep. Purif. Technol. 2022, 298, 121562. [Google Scholar] [CrossRef]
- Genethliou, C.; Triantaphyllidou, I.E.; Giannakis, D.; Papayianni, M.; Sygellou, L.; Tekerlekopoulou, A.G.; Koutsoukos, P.; Vayenas, D.V. Simultaneous Removal of Ammonium Nitrogen, Dissolved Chemical Oxygen Demand and Color from Sanitary Landfill Leachate Using Natural Zeolite. J. Hazard. Mater. 2021, 406, 124679. [Google Scholar] [CrossRef]
- Vythoulkas, K.; Stamatakis, M.; Pozo, M.; Argyraki, A. Factors Affecting the Bleaching Performance of Thermally Activated Palygorskite and Palygorskite-Smectite Clays from the Ventzia Basin, West Macedonia, Greece. Clays Clay Miner. 2025, 73, e5. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems-with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, W.; Tian, G.; Wang, D.; Zhang, Z.; Kang, Y.; Zong, L.; Wang, A. All-into-One Strategy to Synthesize Mesoporous Hybrid Silicate Microspheres from Naturally Rich Red Palygorskite Clay as High-Efficient Adsorbents. Sci. Rep. 2016, 6, 39599. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, D.; Tan, D.; Yuan, P.; Chen, M. FTIR Spectroscopy Study of the Structure Changes of Palygorskite under Heating. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1052–1057. [Google Scholar] [CrossRef]
- Meirelles, L.M.A.; Barbosa, R.d.M.; Sanchez-Espejo, R.; García-Villén, F.; Perioli, L.; Viseras, C.; Moura, T.F.A.d.L.e.; Raffin, F.N. Investigation into Brazilian Palygorskite for Its Potential Use as Pharmaceutical Excipient: Perspectives and Applications. Materials 2023, 16, 4962. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.L.; Yuan, J.J. Thermal Decomposition Behavior of Hydroxytyrosol (HT) in Nitrogen Atmosphere Based on TG-FTIR Methods. Molecules 2018, 23, 404. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Maddheshiaya, D.N.; Rawat, S.; Singh, J. Adsorption and Equilibrium Studies of Phenol and Para-Nitrophenol by Magnetic Activated Carbon Synthesised from Cauliflower Waste. Environ. Eng. Res. 2020, 25, 742–752. [Google Scholar] [CrossRef]
- Dehmani, Y.; Franco, D.S.P.; Georgin, J.; Lamhasni, T.; Brahmi, Y.; Oukhrib, R.; Mustapha, B.; Moussout, H.; Ouallal, H.; Sadik, A. Comparison of Phenol Adsorption Property and Mechanism onto Different Moroccan Clays. Water 2023, 15, 1881. [Google Scholar] [CrossRef]
- Ouallal, H.; Dehmani, Y.; Moussout, H.; Messaoudi, L.; Azrour, M. Kinetic, Isotherm and Mechanism Investigations of the Removal of Phenols from Water by Raw and Calcined Clays. Heliyon 2019, 5, e01616. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Petrovich, D.S.; Ilinichna, K.T.; Morton, D.W. HPTLC and FTIR Fingerprinting of Olive Leaves Extracts and ATR-FTIR Characterisation of Major Flavonoids and Polyphenolics. Molecules 2021, 26, 6892. [Google Scholar] [CrossRef]
- Song, C.; Wu, S.; Cheng, M.; Tao, P.; Shao, M.; Gao, G. Adsorption Studies of Coconut Shell Carbons Prepared by KOH Activation for Removal of Lead(Ii) from Aqueous Solutions. Sustainibility 2014, 6, 86–98. [Google Scholar] [CrossRef]
- Giustetto, R.; Wahyudi, O. Sorption of Red Dyes on Palygorskite: Synthesis and Stability of Red/Purple Mayan Nanocomposites. Microporous Mesoporous Mater. 2011, 142, 221–235. [Google Scholar] [CrossRef]
- Pan, Z.; Zeng, B.; Shen, L.; Teng, J.; Lai, T.; Zhao, L.; Yu, G.; Lin, H. Innovative Treatment of Industrial Effluents through Combining Ferric Iron and Attapulgite Application. Chemosphere 2024, 358, 142132. [Google Scholar] [CrossRef]
- Genethliou, C.; Lazaratou, C.V.; Triantaphyllidou, I.E.; Xanthaki, E.; Mourgkogiannis, N.; Sygellou, L.; Tekerlekopoulou, A.G.; Koutsoukos, P.; Vayenas, D.V. Adsorption Studies Using Natural Palygorskite for the Treatment of Real Sanitary Landfill Leachate. J. Environ. Chem. Eng. 2022, 10, 108545. [Google Scholar] [CrossRef]
- Zhai, P.; Liu, H.; Sun, F.; Chen, T.; Zou, X.; Wang, H.; Chu, Z.; Wang, C.; Liu, M.; Chen, D. Carbonization of Methylene Blue Adsorbed on Palygorskite for Activating Peroxydisulfate to Degrade Bisphenol A: An Electron Transfer Mechanism. Appl. Clay Sci. 2022, 216, 106327. [Google Scholar] [CrossRef]
- Cui, X.; Liao, J.; Liu, H.; Tang, W.; Tie, C.; Tian, S.; Li, Y. Adsorption of Phenols from Aqueous Solution with A PH-Sensitive Surfactant-Modified Bentonite. Separations 2023, 10, 523. [Google Scholar] [CrossRef]
- Ba Mohammed, B.; Yamni, K.; Tijani, N.; Alrashdi, A.A.; Zouihri, H.; Dehmani, Y.; Chung, I.M.; Kim, S.H.; Lgaz, H. Adsorptive Removal of Phenol Using Faujasite-Type Y Zeolite: Adsorption Isotherms, Kinetics and Grand Canonical Monte Carlo Simulation Studies. J. Mol. Liq. 2019, 296, 111997. [Google Scholar] [CrossRef]
- Nikolic, V.; Ilic, D.; Nikolic, L.; Stanojevic, L.; Cakic, M.; Tacic, A.; Ilic-Stojanovic, S. The Synthesis and Characterization of Iron(II): Gluconate. Savrem. Tehnol. 2014, 3, 16–24. [Google Scholar] [CrossRef]
- Rocha, J.; Borges, N.; Pinho, O. Table Olives and Health: A Review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; Gao, H.; Yang, H.; Wang, F.; Chen, X.; Fang, L.; Tang, S.; Yi, Z.; Li, D. A Simple Polyacrylamide Gel Route for the Synthesis of MgAl2O4 Nanoparticles with Different Metal Sources as an Efficient Adsorbent: Neural Network Algorithm Simulation, Equilibrium, Kinetics and Thermodynamic Studies. Sep. Purif. Technol. 2022, 281, 119855. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Wang, X. Selective Adsorption of Tannin from Flavonoids by Organically Modified Attapulgite Clay. J. Hazard. Mater. 2008, 160, 382–387. [Google Scholar] [CrossRef]
- Khachay, A.; Yous, R.; Khalladi, R.; Cherifi, H.; Belaid, B.; Alharthi, M.N.; Salvestrini, S.; Mouni, L. Understanding the Adsorption Mechanism of Phenol and Para-Chlorophenol onto Sepiolite Clay: A Combined DFT Calculations, Molecular Dynamics Simulations, and Isotherm Analysis. Water 2025, 17, 1335. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Papageorgiou, C.S.; Paraskeva, C.A. Technoeconomic Analysis of the Recovery of Phenols from Olive Mill Wastewater through Membrane Filtration and Resin Adsorption/Desorption. Sustainibility 2021, 13, 2376. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Paraskeva, C.A. Isolation of Organic Compounds with High Added Values from Agro-Industrial Solid Wastes. J. Environ. Manag. 2018, 216, 183–191. [Google Scholar] [CrossRef]
- Kodjapashis, M.P.; Zentelis, A.D.; Zagklis, D.P.; Sygouni, V.; Paraskeva, C.A. Resin Adsorption of Phenolic Compounds from Olive Leaf and Coffee Residue Extracts: Batch and Packed Column Adsorption Experimental Investigation and Mathematical Modeling. Separations 2023, 10, 313. [Google Scholar] [CrossRef]
- Tri, N.L.M.; Thang, P.Q.; Van Tan, L.; Huong, P.T.; Kim, J.; Viet, N.M.; Phuong, N.M.; Al Tahtamouni, T.M. Removal of Phenolic Compounds from Wastewaters by Using Synthesized Fe-Nano Zeolite. J. Water Process Eng. 2020, 33, 101070. [Google Scholar] [CrossRef]
- Lazaratou, C.V.; Triantaphyllidou, I.E.; Pantelidis, I.; Chalkias, D.A.; Kakogiannis, G.; Vayenas, D.V.; Papoulis, D. Using Raw and Thermally Modified Fibrous Clay Minerals as Low Concentration NH4+–N Adsorbents. Environ. Sci. Pollut. Res. 2022, 29, 17737–17756. [Google Scholar] [CrossRef] [PubMed]
- Lazaratou, C.V.; Panagopoulos, S.D.; Vayenas, D.V.; Panagiotaras, D.; Papoulis, D. Thermally Modified Palygorskite Usage as Adsorbent in Fixed-Bed Reactor for High Concentration NH4+-N Removal and Further Application as N—Fertilizer in Hydroponic Cultivation. Materials 2022, 15, 6541. [Google Scholar] [CrossRef]


| Physicochemical Properties | Pal Sample |
|---|---|
| Specific surface area (m2 g−1) | 185 |
| Total pore volume (cm3 g−1) | 0.307 |
| Micropore volume (cm3 g−1) | 0.022 |
| Parameters | Langmuir | Parameters | Freundlich |
|---|---|---|---|
| Linear Fitting | |||
| R2 | 0.982 | R2 | 0.992 |
| qmax (mg g−1) | 0.068 | 1/n | 1.931 |
| KL (L mg−1) | 0.634 | KF | 1.244 |
| RSS | 1.054 | RSS | 0.035 |
| Nonlinear Fitting | |||
| R2 | 0.883 | R2 | 0.892 |
| qmax (mg g−1) | 0.051 | 1/n | 0.757 |
| KL (L mg−1) | 0.053 | KF | 1.848 |
| RSS | 5.737 | RSS | 5.293 |
| Parameters | PFO | Parameters | PSO |
|---|---|---|---|
| Linear Fitting | |||
| R2 | 0.690 | R2 | 0.999 |
| qecal (mg g−1) | 0.867 | qecal (mg g−1) | 0.085 |
| qeexp (mg g−1) | 12.48 | qeexp (mg g−1) | 12.48 |
| k1 (min−1) | 0.048 | k2 (g mg−1 min−1) | 0.008 |
| RSS | 0.680 | RSS | 0.004 |
| Nonlinear Fitting | |||
| R2 | 0.846 | R2 | 0.983 |
| qe (mg g−1) | 12.27 | qecal (mg g−1) | 12.36 |
| qeexp (mg g−1) | 12.48 | qeexpl (mg g−1) | 12.48 |
| k1 (min−1) | 3.226 | k2 (g mg−1 min−1) | 1.705 |
| RSS | 0.035 | RSS | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazaratou, C.V.; Rosoglou, J. Table Olive Wastewater Treatment Using the Clay Mineral Palygorskite as Adsorbent. Minerals 2025, 15, 861. https://doi.org/10.3390/min15080861
Lazaratou CV, Rosoglou J. Table Olive Wastewater Treatment Using the Clay Mineral Palygorskite as Adsorbent. Minerals. 2025; 15(8):861. https://doi.org/10.3390/min15080861
Chicago/Turabian StyleLazaratou, Christina Vasiliki, and John Rosoglou. 2025. "Table Olive Wastewater Treatment Using the Clay Mineral Palygorskite as Adsorbent" Minerals 15, no. 8: 861. https://doi.org/10.3390/min15080861
APA StyleLazaratou, C. V., & Rosoglou, J. (2025). Table Olive Wastewater Treatment Using the Clay Mineral Palygorskite as Adsorbent. Minerals, 15(8), 861. https://doi.org/10.3390/min15080861








