Abstract
A small, strongly schistose Ni-laterite occurrence at the Vermion ophiolite (40°26′ Ν, 22°10′ Ε), Northen Greece, along a strong shear zone, is characterized by relatively high Ni, Co and Mn contents, magnetite as the dominant mineral, garnet (grossularite), theophrastite [β-Ni(OH)2], otwayite-like phase (ideally Ni2CO3(OH)2.H2O), (Ni, Co, Mn)-hydroxides, and Ni-phyllosilicates. New analytical data, including black-white and color back-scattered electron images (BSEIs), elemental mapping and scanning, and Raman Spectroscopy, alongside silicates and hydroxides revealed the presence of varying silica content (less than 1 to 29 wt.%) in theophrastite and in (Ni, Co, Mn ± Fe)-hydroxides, although the X-ray powder diffraction data correspond to those of pure hydroxides. The gradual stacking of fine fibrous otwayite-like crystals to the boundaries of successive thin layers and within layers themselves, results in porous mineral phases of varying density shifting towards more compact mineral with increasing residence time. The presented data suggest that a potential explanation of the presence of Si in theophrastite may be the precipitation of Si after initial Ni-hydroxyl-carbonate fine crystals deposition. A potential sequence of the stability of Ni-minerals at Vermion may be as follows: Hydroxyl-carbonates < [β-Ni(OH)2] (theophrastite) < (Ni, Co, Mn)(OH)2 < Ni-phyllosilicates; this may be a significant factor for Ni-exploration in Ni-larerite deposits.
Keywords:
Ni-minerals; theophrastite; (Ni; Co; Mn ± Fe)-hydroxides; Raman spectroscopy; ophiolites; Greece; Vermion 1. Introduction
Two known pseudopolymorphs of Ni(OH)2 are included in the layered double hydroxides (LDHs) denoted as α-Ni(OH)2 and β-Ni(OH)2 [1]. The α-Ni(OH)2 is characterized by an increased interlayer distance: 7.6 Å instead of 4.6 Å observed in β-nickel hydroxide [2]. Stabilization of α-nickel hydroxide is achieved by increasing isomorphous substitution of Ni2+ with a suitable trivalent cation M3+ to yield a layer composition of [Ni2+1−xM3+x(OH)2]x+. It has been shown that mixed Ni–Mn–Co hydroxides are mixed-valence compounds; only Ni ions are in the 2+ oxidation state, while Mn and Co ions are in the 3+/4+ and 2+/3+ state, respectively [3].
The β-phase material, which is isostructural with brucite, Mg(OH)2 [4] has been described as mineral in nature [5]. Also, among natural mineral of the group of hydroxides [X(OH)2] pyrochroite [Mn(OH)2] has been discovered in the Pajsberg mine, Sweden [6], theophrastite in metamorphosed Fe-Ni-laterites at the Vermion ophiolite, Greece [7], whilst Co(OH)2 is known only as a synthetic compound. Theophrastite (approved as a new mineral from IMA (No 1980–59) is named after Theophrastus (ca. 371–286 BCE), a Greek philosopher, mineralogist, and author (in 315 B.C.) of the first mineralogy textbook [7]. Theophrastite from Vermion is also associated with (Co, Mn, Ni)(OH)-hydroxides, Ni-rich serpentine, garnet, vesuvianite and magnetite, all crosscutting earlier deformed assemblages, while they may be intersected by serpentine and Ni-serpentine [8,9]. Theophrastite, from Unst (U.K.), has been described as Mg-bearing, occurs at the periphery of the chromitite body, and is associated with a very poorly crystalline Ni-containing mixed hydroxide of the pyroaurite type and/or zaratite [5]. Recently, theophrastite was described in association with aggregates of hydroxylated nickel carbonates, such as zaratite [Ni3CO3(OH)4.4H2O], otwayite [Ni2CO3(OH)2.H2O], nullaginite [Ni2CO3(OH)2], and hellyerite [NiCO3.6H2O] aggregates in partially serpentinized dunite at Fujiwara, in the Sanbagawa metamorphic belt of a high-pressure intermediate type, Japan [10]. Nickel carbonate hexahydrate (hellyerite) is known as a synthetic compound [11]. This assemblage was described in a composite grain composed of millerite, heazlewoodite, awaruite, and magnetite, suggesting that the constituent minerals probably formed together, during low- temperature alteration or the weathering of serpentinites and related nickel-rich ores [10]. Various types of disorder, including hydration, stacking fault disorder, mechanical stresses and the incorporation of ionic impurities, which are frequently present in nickel hydroxide materials, have been discussed [12]. Among the layered double hydroxides (LDHs), the carbonate intercalated phases are most ubiquitous, due to the affinity of metal hydroxides to CO2 in the associated rocks and dissolved carbonates in water [13].
In the present study, black-white and color back-scattered electron images (BSEIs), element mapping and Raman spectroscopy for theophrastite (Ni, Co, Mn ± Fe)-hydroxides, and silicate minerals, from the Vermion Fe-Ni-laterites revealed wide compositional variations, and micro-texture relationships, due to recurrent changes in the conditions during their precipitation. Besides pure β-Ni(OH)2, the presented data revealed the presence of theophrastite in close association with otwayite-like and Ni-phyllosilicates, while significant silica content was determined in thin layers characterized as theophrastite by X-ray diffraction (XRD) providing new insights into their genesis.
2. Geological Outline
Dismembered Upper Jurassic–Lower Cretaceous ophiolite masses outcrop in the Vermion mountain along the eastern margin of the Pelagonian massif. They are considered to have been derived from the Almopias subzone of the Vardar zone and were overthrust westward onto the Pelagonian massif [14]. They are mainly composed of mantle peridotites consisting of harzburgite, dunite, and orthopyroxene-bearing dunite, and, to a lesser extent, of crustal magmatic rocks (pyroxenites and gabbros).
Small (1–3 m in length, 0.3–1 m in thickness) chromitite occurrences of high Cr type, the Cr/(Cr + Al) ratio ranging between 0.67 and 0.70, and Mg/(Mg + Fe2+) from 0.63 to 0.65 at Vermion, are similar to other chromitites in Eastern Thessaly, all located in the eastern part of the Almopias (Axios) geotectonic zone [15]. Small allochthonous Fe-Ni laterite occurrences have been described in northern Greece, including Vermion. Laterite crusts have been exhumed, transported, and re-deposited on peridotites. Later, they have been buried by sedimentary rocks, forming pseudo-autochthonous Fe–Ni deposits, and finally, metamorphosed in the amphibolite facies [16,17]. Nickel-bearing minerals at Vermion are found within small, strongly schistosed Ni-laterite occurrences, dominated by magnetite, located along a strong shear zone, which define a trend parallel to the general NW-SE displacement direction of ophiolites in the Balkan peninsula [18].
A peculiarity of the Vermion Fe-Ni laterite occurrences (Figure 1) is the relatively high Mn, Ni, and Co contents as suggested by the bulk rock analyses and the presence of chromite and (Ni, Co, Mn)-hydroxides. Specifically, at the top of the Vermion Mountain (Stournari, 40°26′ Ν, 22°10′ Ε) is found a relatively thin mixed zone near the contact between a laterite occurrence and the overlying Cretaceous limestone containing abundant Ca-Si mineral, such as vesuvianite and garnet, theophtastite [(Ni(OH)2], and other Ni-bearing minerals [8,16,19].
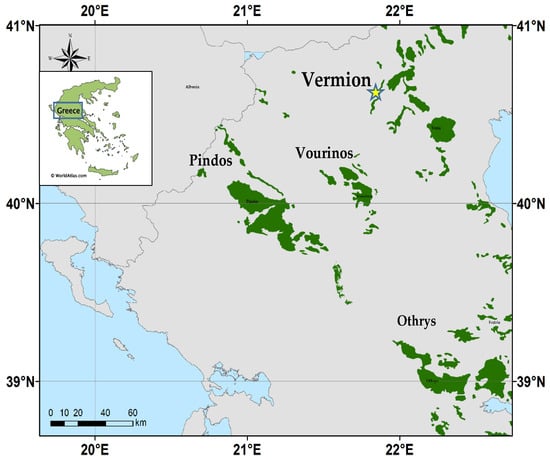
Figure 1.
Location of the major ophiolites in central-north Greece, including the Vermion complex, associated with Fe-Ni-laterite occurrences, where the mineral theophrastite is found (yellow star).
3. Materials and Methods
Polished thin sections of metamorphosed Ni laterite were investigated using a reflected light microscope and a scanning electron microscope (SEM), at the University of Göttingen, Germany, using an ARL-SEMQ electron microprobe. Analytical conditions were 15 kV accelerating voltage and a 150 nA beam current. X-ray diffraction data for theophrastite were obtained using a polycrystalline sample scanned with a Philips diffractometer used with a monochromator line 104 CuKa radiation, at the University of Göttingen, Germany, Ni filter, Si internal standard. Details of the physical and optical properties, chemistry, and X-ray powder diffraction data of theophrastite are available in the publication dealing with its discovery [7].
Furthermore, examination of gold-coated polished thin sections from the theophrastite-bearing metamorphosed Fe-Ni laterite occurrences from the Vermion Mountain, was carried out at the Hellenic Survey of Geology & Mineral Exploration, using a Jeol JSM-IT500 SEM instrument (JEOL USA, Inc., Peabody, MA, USA) equipped with an Oxford 100 Ultramax analytical device (Oxford Instruments, Abingdon, UK), with the following operating conditions: accelerating voltage (V) 15 kV, beam current (I) 20 nA, and beam diameter <1 μm.
X-ray powder diffraction (XRD) data for recently discovered (Co, Mn, Ni) (OH)2 hydroxides were obtained, using a Siemens Model 5005 X-ray diffractometer (Bruker AXS GmbH., Karlsruhe, Germany), Cu Ka radiation at 40 kV, 40 nA, 0.020° step, size and 1.0 s step time. The XRD patterns (Supplementary S1) were evaluated using the EVA 10.0 program of the Siemens DIFFRAC (Bruker AXS GmbH) and a D5005 software package (NKUA). Although single-crystal X-ray studies for (Ni, Co, Mn)-hydroxides could not be carried out, because of the small crystal size, the XRD data have shown similarity to those of theophrastite (Supplementary S1); [20,21].
Raman spectroscopy analyses were conducted with the use of a Renishaw in Via confocal Raman microscope, with a CCD detector, equipped with a green and a red laser line, emitting at 532 and 785 nm, respectively. Spectra were obtained with the 785 nm laser, due to the significantly better signal-to-noise ratio, compared to the green line. The laser was set at a power range of 0.5 to 1 mW, coupled with a monochromator grating of 1200 groves per mm2, and with a slit opening of 65 μm. Scattered radiation was collected through a pinhole positioned in front of the spectrometer. The sample was exposed to the laser for an acquisition time of 20 s, through a 50X long-working distance objective lens with 0.55 numerical aperture, using extended scans and reaching a spectral range from 100 to 4000 cm−1. An edge filter was utilized to minimize Rayleigh scattering below 100 cm−1 in the Raman spectrum. The spectrometer was calibrated for lateral shift using a Si 520 cm−1 Raman peak before analyses. The same analytical conditions were applied for Raman mapping as well. Data processing was performed with WiRETM software, version 5.6 for Windows (www.renishaw.com, accessed on 20 June 2025). Spectra were corrected for background noise with the use of a polynomial baseline and a cosmic ray removal was performed. A preliminary evaluation and interpretation of the obtained spectra was performed in WiRETM through a spectrum search in the built-in RRUFF database.
4. Mineralogical Characteristics
Theophrastite may be associated with (Ni, Co, Mn)-hydroxides, chlorite, serpentine, Ni–serpentine (garnierite), garnet (grossularite), and magnetite, all having a common origin, related to the strong late tectonic evolution, overprinting earlier deformation events (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). The compact Fe-Ni-laterite ore is characterized by the lack of a pisolitic–oolitic texture. Magnetite, being the major mineral, may occur in intergrowths with theophrastite (Figure 6e) whereas chromite, (Ni, Co, Mn)-hydroxides, Ni-silicates (garnets, vesuvianite, chlorite serpentine and talc), calcite, and sulfides (mostly millerite, pentlandite, chalcopyrite, and pyrite) are found in lesser amounts.
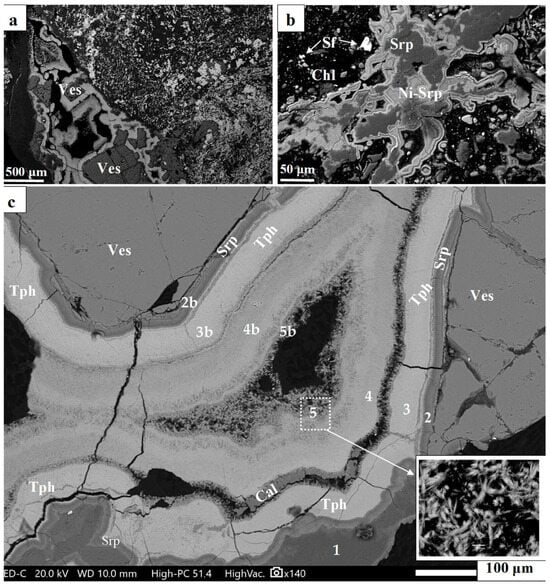
Figure 2.
Back-scattered electron images (BSEIs) showing the multistage redeposition of laterite ore and the concentric structural growth of Ni-minerals (a–c). The sites of the analyzed thin layers covering fragmented crystal of vesuvianite are provided (c); Table 1. Abbreviations: Tph = theophrastite; Ves = Vesuvianite; Srp = serpentine; Cal = calcite. Numbers = Spot analyses (Table 1).

Table 1.
Representative electron microprobe analyses of Ni-minerals with concentric development. Abbreviations and numbers as those in Figure 2. Abbreviation: n.d. = no detected.
Table 1.
Representative electron microprobe analyses of Ni-minerals with concentric development. Abbreviations and numbers as those in Figure 2. Abbreviation: n.d. = no detected.
| Figure 2c | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ves | Srp | Ni-Srp | Tph | |||||||||||||
| wt.% | 1 | 2 | 2b | 3 | 3 | 3b | 3b | 3b | 4 | 4b | 4b | 4b | 4b | 5b | 5b | |
| SiO2 | 37.2 | 40.4 | 36.3 | 31.2 | 20.2 | 18.5 | 13.0 | 15.5 | 0.8 | 23.8 | 27.6 | 28.4 | 25.2 | 27.5 | n.d. | n.d. |
| MgO | 2.2 | 36.1 | 21.0 | 22.1 | n.d. | n.d. | n.d | n.d. | n.d. | n.d. | n.d. | n.d. | n.d | n.d | 1.2 | n.d. |
| NiO | n.d. | 3.2 | 23.9 | 17.2 | 59.7 | 61.0 | 61.5 | 61.3 | 66.2 | 55.9 | 51.0 | 53.1 | 50.5 | 51.9 | 47.7 | 46.9 |
| CaO | 35.2 | n.d | n.d | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | n.d. | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | n.d. | 0.1 |
| Fe2O3 | 3.6 | 1.6 | n.d | n.d | n.d. | n.d. | n.d | n.d. | 1.5 | n.d. | n.d. | 0.2 | n.d | n.d | n.d. | 0.2 |
| Cr2O3 | 0.4 | n.d | 0.11 | n.d | 0.1 | 0.3 | 0.1 | 0.2 | n.d. | 0.2 | 0.2 | n.d. | 0.2 | 0.2 | n.d. | n.d. |
| Al2O3 | 17.2 | 1.6 | n.d. | n.d | n.d. | n.d. | n.d | n.d. | n.d. | n.d. | n.d. | n.d. | n.d | n.d | n.d. | n.d. |
| Total | 95.8 | 82.9 | 81.3 | 70.7 | 80.2 | 80.1 | 74.8 | 77.2 | 68.5 | 80.2 | 79.1 | 82.0 | 76.1 | 79.9 | 48.9 | 47.2 |
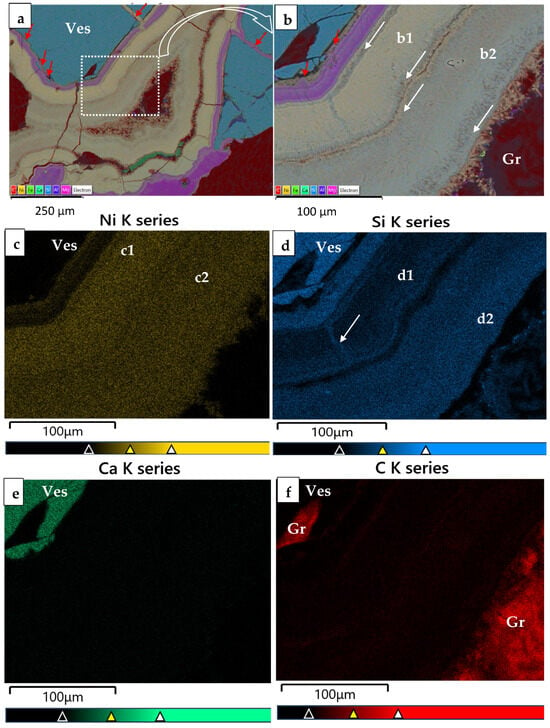
Figure 3.
Color back-scattered electron images (BSEI) of concentric structural growth of Ni-minerals along a fracture of laterite ore. (a,b): Repeated small layers of Ni-Si minerals are separated by a very fine zone composed of very fine fibrous minerals (white arrows); (c–f): X-ray elemental maps, showing difference in the intensity of color/composition of successive layers; cracks in vesuvianite are filled by material of varying composition, similar to that of neighboring layers (red arrows); carbon content (a,b,f, red color) is probably because incomplete cleaning of carbon coating for previous microprobe analysis.
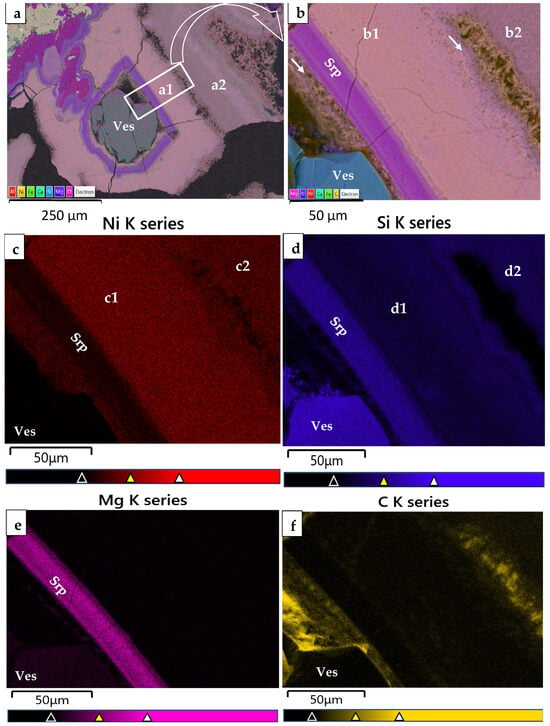
Figure 4.
Color back-scattered electron images (BSEI) of the mapping for Ni-minerals from Vermion (high magnification from Figure (a), displaying X-ray elemental maps showing the distribution of Ni, Si, Mg and C. It is obvious that that the Ni and Si distribution is not homogeneous within a1 and a2 layers, as there is a difference in terms of their content (a–f); there is a mixed material between vesuvianite and serpentine and between theophrastite thin layers (white arrows).
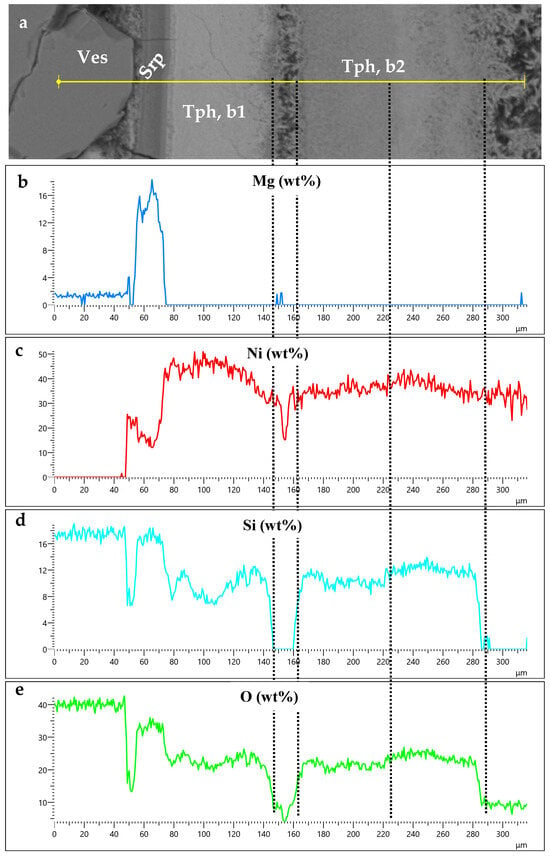
Figure 5.
Scanning along a back-scattered electron image of concentric thin layers covering a vesuvianite crystal (a) showing the variation in Mg, Ni, Si, and O (b–e) highlights the presence of Si within theophrastite layers (b).
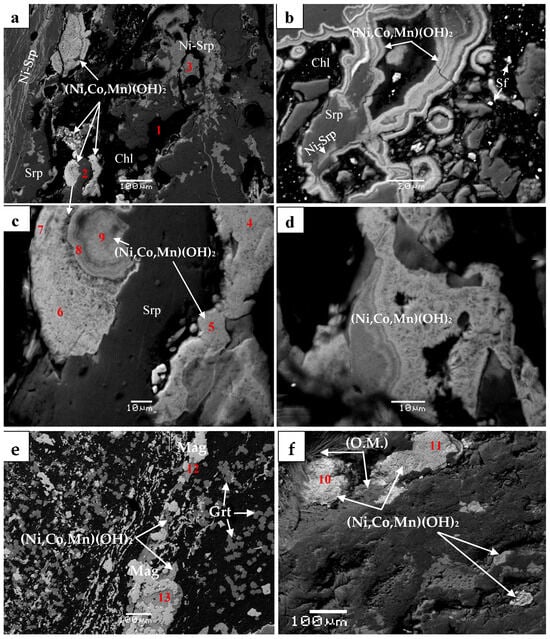
Figure 6.
Back-scattered electron images showing textural relationship of (Co, Mn, Ni)-hydroxides exhibiting concentric structural growth and silicate minerals cutting all previous formations (a); covering phyllosilicates (b). The high-resolution images (c,d) show the porous structure; (Ni, Fe)-hydroxides associated with magnetite and garnet (e); pseudomorphic (Co, Mn, Ni)-hydroxides covering organic matter (white) as well as a subsequent state of hydroxide mineralization (dark gray) overlying partly the previous one (f). Abbreviations: Chl = chlorite; Srp = serpentine; Ni-srp = Ni-bearing serpentine; Sf = sulfides; Mag = magnetite; O.M. = organic matter (micro-textures resembling fossilized micro-organisms). Numbers 1−13 correspond to spot analyses in Table 2. Data source: Present study [8].

Table 2.
Representative electron microprobe analyses of Ni-minerals. Abbreviations and numbers as those on Figure 6. Abbreviation: n.d. = no detected.
Table 2.
Representative electron microprobe analyses of Ni-minerals. Abbreviations and numbers as those on Figure 6. Abbreviation: n.d. = no detected.
| Figure 6e | Figure 6a | Figure 6c | Figure 6f | Figure 6e | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
| wt.% | Grt | Chl | Srp | Ni-Srp | (Ni, Co, Mn ± Fe)-hydroxides | |||||||||
| SiO2 | 37.0 | 30.1 | 36.2 | 42.3 | 1.6 | 1.7 | 2.8 | 1.4 | 11.2 | 19.4 | 7.4 | 3.0 | 0.6 | 1.0 |
| MgO | n.d. | 29.1 | 37.0 | 1.6 | 2.7 | 2.5 | 2.2 | 1.5 | 2.9 | 2.5 | 4.3 | 1.6 | 0.4 | 0.4 |
| NiO | n.d. | 1.2 | 0.3 | 43.3 | 14.9 | 15.8 | 15.4 | 15.1 | 18.3 | 27.2 | 13.8 | 15.2 | 65.8 | 69.1 |
| CoO | n.d. | n.d. | n.d | n.d. | 7.8 | 7.9 | 7.5 | 6.7 | 10.2 | 5.8 | 12.3 | 8.2 | 7.8 | 0.6 |
| MnO | n.d. | n.d. | 0.4 | n.d. | 43.3 | 42.8 | 38.6 | 41.3 | 22.0 | 13.9 | 29.5 | 39.2 | n.d. | n.d. |
| CaO | 32.9 | n.d. | n.d | n.d. | 2.3 | 2.2 | 1.9 | 2.1 | 1.1 | 0.8 | 2.5 | 3.3 | n.d. | n.d. |
| FeO | 26.6 | 6.6 | 3.0 | n.d. | n.d. | n.d. | 0.5 | n.d. | 0.7 | 0.5 | 0.4 | 0.4 | 5.1 | 1.5 |
| Cr2O3 | 1.6 | n.d. | n.d | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Al2O3 | 0.9 | 19.7 | 8.9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total | 98.0 | 86.7 | 85.8 | 87.2 | 72.6 | 72.9 | 68.9 | 68.1 | 66.4 | 70.1 | 70.2 | 70.9 | 79.7 | 72.6 |
A salient feature of chromite in the metamorphosed Fe-Ni-laterites from Vermion is the presence of zoned grains with a gradual increase in Mn, Co and Zn outwards of the chromite. These elements attain the greatest values at the periphery of chromite cores and in the ferrian-chromite, reaching values up to 13.0, 4.1 and 2.1 wt.%, respectively, while they drop off to negligible values in magnetite [17]. Nickel is mainly hosted in chlorite, serpentine, theophrastite, and (Ni, Co, Mn)-hydroxides.
4.1. Mineralogy and Texture
The back-scattered electron images (BSEIs) from Fe-Ni-laterites at Vermion showed a multistage evolution accompanied by a multistage redistribution of the analyzed elements (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). Structural features of the ore indicate that hydroxylated Ni-minerals appear covering vesuvianite crystals in a repeated sequence from vesuvianite towards a fracture in the highly schistose laterite ore (Figure 2, Figure 3 and Figure 4).
The back-scattered electron images (BSEIs) indicate that serpentine has been transformed into Ni-serpentine (garnierite), but the composition of hydroxylated Ni-bearing minerals from Vermion covering vesuvianite crystals is not clear (Figure 2). Furthermore, color BSEI showed that the very fine fibrous crystals aligned perpendicular to the boundaries of successive thin layers are Ni and carbon (C)-bearing (Figure 3 and Figure 4 and Supplementary S2 and S3). However, high carbon (C) contents (Figure 3a,b,f; Figure 4, red color) have been recorded, due to incomplete cleaning of carbon coating from previous microprobe analyses. Therefore, the identification of C in such tiny phases is uncertain. Nevertheless, the absence of Si, the Ni content and the low total of fine fibrous crystals (analyses No 5, Table 1) may point to the presence of a hydroxyl-carbonate mineral. There is a similarity in the color of the layer (a2) and the contact of the layer (a1) as well as within fractures of the latter (Figure 3b,d, white arrows), suggesting probably more Si in the circulated fluids during evolution of the whole system.
In addition, theophrastite has been found to be associated with (Co, Mn, Ni) (OH)2 hydroxides, of varying composition occurring as successive thin (a few to tens of μm) layers, composed of fine fibrous and porous crystals, the whole formation being fragmented and dismembered (Figure 5 and Figure 6).
Although the total for most electron microprobe analyses for theophrastite is low (Table 1), due probably to the porous nature of hydroxides (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6), assuming the empirical formula (based on Co + Mn + Ni = 1 atomic proportion per formula unit (a.p.f.u.), there is a wide compositional range from Co-dominated: (Co0.59Mn0.24Ni0.17)1.00(OH)2 in the central parts to Mn-nominated (Mn0.51Ni0.26Co0.23)Σ1.00(OH)2 towards the periphery showing a good negative correlation [8].
4.2. Raman Spectroscopic Characterization of Ni-Hydroxides and Ni-Hydrated Carbonates
Raman analyses were conducted on a metallographic specimen from the studied site, containing Ni-minerals, observed in the optical microscope. This specimen has not been, previously, coated with carbon, and therefore no graphite interference was expected in the Raman spectra obtained.
In situ Raman spectroscopic analyses, of the botryoidal, green bands containing Ni-phases, indicate the presence of fine-grained mineral intergrowths mixed at a micron-scale. This is indicated by the complex nature of the Raman spectra, in which more than one mineral phase was identified. The mineral phase components in all spectra are theophrastite, otwayite-like phase and Ni-phyllosilicates such as Ni-serpentine and Ni-talc. The presence of theophrastite is indicated by (a) a visible peak at ~3581 cm−1 corresponding to—internal OH symmetric stretching vibrations, and (b) prominent peaks at 313 and 448 cm−1, resulting from Ni-OH rotational lattice vibrations [22,23]. A broad peak at ~865–870 cm−1 may be attributed to the rotational Eg (R) lattice mode vibrations [24,25]. An otwayite-like phase was identified by the combined occurrence of peaks at 217, 445, 530, 617, 1067, 1075, 1350, and 2935 cm−1. The 217 peak is attributed to lattice modes, whereas the 445 and 617 peaks have been associated with the v4 and v2 bending modes of SO42− in the otwayite structure [26]. The presence of the 1068 and 1075 peaks is attributed to symmetric stretching vibrations of CO32−, as well as the 1350 peak whereas the peak at 2935 cm−1 has been associated with the presence of organic impurities [26]. There is, however, a notable absence of the main v1 (symmetric in-plane stretching) vibrational mode of SO42− at ~980 cm−1 that is indicative of otwayite [26]. Several factors affecting this mode could be a low S concentration (as indicated by EDS analyses), temperature and pressure of crystallization, as well as crystal structure defects (lowering of symmetry) resulting from cation substitution, and/or hydration [27]. Considering, therefore, a) the collective spectral similarities of the obtained Raman spectra to that of otwayite, b) the striking low totals of their respective EDS analyses (Table 1, analyses 5b) which could indicate the presence of a hydrated carbonate Ni-rich mineral, and (c) the increased concentration of carbon in areas (Figure 4b,f) of very fine fibrous minerals similar to those of otwayite-like spectra, we interpret this phase as an otwayite-like phase. Garnierite phyllosilicates such as Ni-serpentine and talc have been identified by the presence of Raman peaks at 211, 360, and 620 cm−1 for the former, and at 368 and 681 cm−1, for the latter. The detected peaks of Ni-serpentine at ~210 cm−1 are attributed to the presence of either chrysotile or lizardite [28].
The obtained, composite Raman spectra fall into three main categories based on the relative spectral dominance of each mineral component. The first spectral group includes mineral mixtures of predominantly theophrastite with a minor otwayite-like phase (Figure 7a), the second group comprises spectra with a dominant otwayite-like phase, subordinate theophrastite and minor Ni-serpentine and talc (Figure 7b), whereas the third group represents mixtures of Ni-phyllosilicates with a minor otwayite-like phase (Figure 7c).
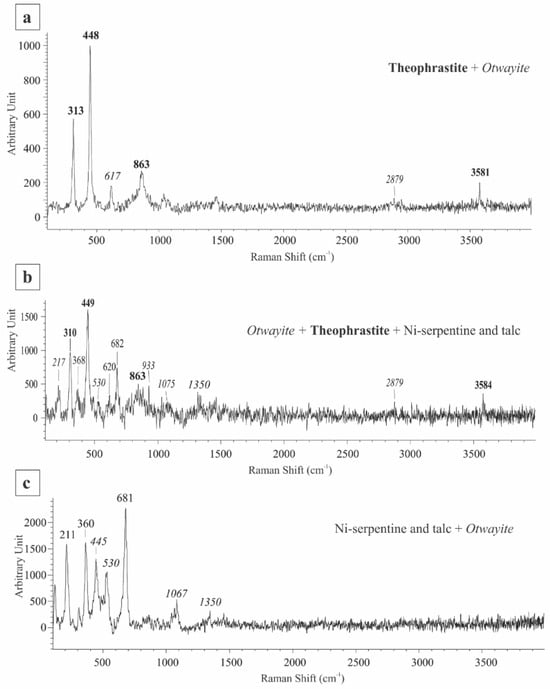
Figure 7.
Representative Raman spectra of mineral mixtures from the garnierite in Vermion Fe-Ni laterites, representing (a) dominant theophrastite and otwayite-like phase, (b) dominant otwayite-like phase, theophrastite and Ni-phyllosilicates and (c) dominant Ni-phyllosilicates and otwayite-like phase. Numbers in bold, normal and italics indicate peaks positions of theophrastite, Ni-phyllosilicates, and otwayite-like phase, respectively.
In order to investigate the spatial distribution of dominant mineral components in the garnierite mixture, Raman maps of indicative peak intensities were produced along a traverse in the botryoidal structure, encompassing optically different layers (Figure 8a). Higher intensities of the ~210 cm−1 peak indicate the presence of Ni-serpentine and spatially coincide with the distribution of the 366 cm−1, an indicative peak of talc (Figure 8b,c), forming phyllosilicate layers in the outermost part of the structure. Spatial dominance of theophrastite is demonstrated by the distribution of the 448 and 313 cm−1 peaks, the most intense of which are in the innermost and intermediate layers of the botryoidal (Figure 8d,e). Otwayite-like phase dominance in the mineral mixtures, on the other hand, is spatially confined to the intermediate zone between the theophrastite and Ni-phyllosilicate zones, where the 617 and 2879 cm−1 peaks are the most intense (Figure 8f,h).
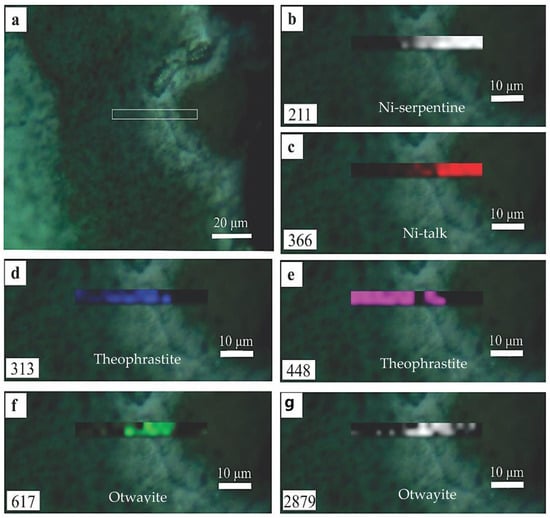
Figure 8.
Raman maps of peak intensity demonstrating the spatial distribution of dominant Ni-minerals within a defined area of the sample (a) using the intensities for selected indicative peaks for Ni-serpentine (b), Ni-talc (c), theophrastite (d,e), and otwayite-like phase (f,g). The position number of each mapped peak is indicated in each map.
5. Discussion
5.1. Source of Ni, Co and Mn and Their Distribution in Secondary Phases
In general, Ni-bearing minerals at Vermion, are found within small, strongly schistose Ni-laterite occurrences, dominated by magnetite. In addition to magnetite in the form of rims surrounding chromite cores, magnetite occurs as intergrowths with theophrastite and is associated with garnet (grossularite), sulfides, and (Ni, Co, Mn)-hydroxides (Figure 2b and Figure 6e,f). They have been affected by brittle and plastic deformation, subsequent to their formation, as is revealed by the presence of the dismembered aggregates and their orientation parallel to the direction of schistosity of the Ni-laterite ore (Figure 2 and Figure 6). Moreover, the presence of fragmented (Ni, Co, Mn)-hydroxides cemented by silicates and the replacement of serpentine by Ni-serpentine (Figure 6) suggest that a Ni-enrichment event of serpentine may post-date that of the deposition and fragmentation of (Ni, Co, Mn)-hydroxides.
Detailed studies have shown that the relatively high Mn, Co, Ni, and Zn in bulk laterite analyses are partly hosted in zoned chromite grains, exhibiting a ferrian-chromite zone with significant Mn, Co and Zn contents, due to the substitution for Mg2+ and Fe2+ by Mn, Zn and Co in the chromite lattice [17,19], in Ni(OH)2 (theophrastite) [7] and (Mn, Co, Ni) hydroxides [8]. The high degree of mobility of Mn and Co particularly through redox processes ensures that they can change phase during weathering, of ophiolitic rocks (mostly olivine and sulfides like pentlandite), (Mn, Co)-rich siliceous sediments and cherts inter-bedded with mafic rocks [29,30,31], transport, deposition, and diagenesis [29,30,31].
5.2. Diversity in the Mineral Theophrastite and (Ni,Co,Mn)-Hydroxides
The structure of Ni(OH)2 comprises a hexagonal close packing of (OH) ions with an alternate layer of octahedral sites occupied by Ni2+ ions resulting in a stacking of charge-neutral layers having the composition Ni(OH)2 [12]. An investigation of the crystal structure and electronic states of ions in mixed (Ni, Mn, Co)-hydroxides has shown that their structure is like that of Ni(OH)2, Co(OH)3 and Mn(OH)2, in the interlayer spaces, and they are mixed-valence compounds. Only Ni ions are in a 2+ oxidation state, while Mn and Co ions may be in a 3+/4+ and 2+/3+ state, respectively [3]. Theophrastite, the rare in nature β-Ni(OH)2, which has been described at Vermion, is isostructural with brucite, Mg(OH)2 and several other metals M(OH)2, including the (Ni, Co, Mn)-hydroxides [4,7,8]. Theophrastite in chromitite, Unst, Shetland Islands, Scotland, is Mg-bearing and is associated with a very poorly crystalline Ni-phase containing mixed hydroxide of the pyroaurite type or zaratite [5]. Theophrastite in nickel ores associated with ophiolites, Tasmania, Australia, is mixed with poorly crystalline pyroaurite and zaratite [32], while theophrastite is associated with zaratite, otwayite, nullaginite, and hellyerite aggregates in serpentinized dunite from Fujiwara, the Sanbagawa metamorphic belt, Japan [10]. These authors have focused on the formation of theophrastite–zaratite aggregates through the desulfurization of heazlewoodite (Ni3S2). Specifically, the process of desulfurization of heazlewoodite (decomposition to millerite, theophrastite, and zaratite) is illustrated schematically, enabling us to better understand the behavior of sulfur in shallow subduction zones [10].
Theophrastite from Vermion differs compared to the above ophiolites in terms of its wide variation in morphology and mineral chemistry. Besides pure Ni(OH)2, theophrastite [7], although the X-ray powder diffraction data corresponds to those of pure hydroxides [Supplementary S1], the studied samples exhibit varying silica content, ranging from less than 1 wt.% to up 28.9 wt.%, (Table 1 and Table 2; Figure 6). The growth of hydroxide on phyllosilicates may be a process where hydroxide species are accumulated on the surface of phyllosilicate minerals. In general, chemical and physical processes showing that amorphous or colloidal silicates can adsorb onto the surface of layered double hydroxides (LDHs) through electrostatic interactions have been well established: adsorption is facilitated by the negatively charged surface of the silicates and the positively charged layers of the LDH structure. [2,3,12,13]. Additionally, an epitaxial relationship between the precipitate and the mineral substrate has been shown [33,34,35,36]. These authors proposed that Ni(OH)2 may interact with phyllosilicates in various ways (adsorption, coprecipitation) and deposited on a substrate of layer silicates from circulated fluids, and that the (001) planes of the Ni(OH)2 aligned parallel to the basal (001) plane of the layer silicates, without any change in the XRD data.
Although the use of higher-resolution techniques is required to identify possible silicate phases or amorphous content within theophrastite thin layers, elevated Si content (Table 1) may be explained through geochemical processes. Silica can be adsorbed onto the surface of Ni(OH)2 and/or co-precipitate during the transformation of Ni-bearing hydroxyl carbonates into Ni(OH)2, and evolution of the circulated fluids to Si-rich and low CO2 fluids [12,13]. Nickel-rich phyllosilicates, having both Ni and Si, may be found as fine intergrowths [28]. In addition, color back-scattered electron images (BSEIs), element mapping (Figure 3 and Figure 4), scanning along a BSEI of thin layers covering a vesuvianite crystal (Figure 5) and Raman spectroscopy (Figure 7 and Figure 8) highlighted the presence of Si within layers, and the presence of a mixed fine fibrous material resembling otwayite, within thin zones along both sides of the thin layers (Figure 3 and Figure 4, white and red arrows).
In the Vermion allochthonous Ni-laterites, the occurrence of (Ni, Co, Mn)-hydroxides displaying micro-textures, similar to fossilized marine algae (Figure 4f), suggests a potential role of biomineralization in their genesis. Although, the morphology of fossilized organic matter alone is not conclusive, because abiotic processes can create life-like shape, the Raman spectroscopy revealing the presence of organic impurities in the otwayite-like phase may indicate the direct involvement of organic matter and existence of appropriate conditions for metal bio-mineralization.
5.3. Potential Conditions Controlling the Metal Mobility and Their Stability
During a long time, evolution of repeated mobilization and re-deposition of elements, associated with Ni-laterite ores from Vermion may indicate that depending on the reaction conditions, either a metal hydroxide, a mixed layered double hydroxide (LDHs), or a phyllosilicate mineral may be formed at the mineral surface [33,34,35,36]. A significant mutual substitution of Mn, Ni, and Co is facilitated in minerals. The reactivity sequence for Mg, Ni, Co, and Mn (Mg < Mn < Co ≈ Ni) suggests that Mg in LDHs is less stable than Mn, while Co and Ni in LDHs have comparable stability and are more stable than Mn [12]; Figure 6. Also, the elevated Ni content in secondary silicate minerals may be related to the crystal field stabilization energy (CFSE) as exemplified by the preferable distribution of Ni in garnierite with the highest CFSE [37]. Moreover, the repeated mobilization and re-deposition of elements may be explained in terms of the equilibrium solubility of minerals. The most important exchange reaction in the serpentine and Ni-serpentine (garnierite) paragenesis is that of Ni for Mg [28]:
Mg3Si2O5(OH)4 + 3Ni2+aq = Ni3Si2O5(OH)4 + 3Mg2+aq
At equilibrium in this reaction, the calculated Ni/Mg ratios involved in the exchange reaction, thermodynamic data have shown that Ni is much more stable in the formation mechanisms [38]. In addition, Scheckel et al. [36], have shown that the stability of the surface precipitates increases with increasing aging time, due probably to the transformation of the metal hydroxide or LDHs into a more stable phyllosilicate phase.
Calculations of Ce and Eu anomalies based on the trace element content in separated magnetite from Fe–Ni-laterites in Northern Greece [39] and appropriate equations [40] have shown negative Ce and Eu anomalies: Ce/Ce* = 0.41 and Eu/Eu* = 0.32 [Ce/Ce* = (2Ce/Ce chondrite)/(La/La chondrite + Pr/Pr chondrite) and Eu/Eu* = (2Eu/Eu chondrite)/(Sm/Sm chondrite + Gd/Gd chondrite)]; [39,40]. If the presence of the Eu anomaly, caused by the ability of Eu to exist either Eu2+ or Eu3+ states, the Eu/Eu* anomalies seem to be affected by physico/chemical conditions, such as a redox potential [41,42,43]. Diagenetic re-mobilization of Eu is possible under conditions of reduction to Eu2+ at low oxidation potential, as is suggested by the presence of organic matter (Figure 6f); [40]. The texture relationships between magnetite, theophastite [Ni(OH)2], and (Ni,Co,Mn)(OH)2 (Figure 6e) suggest a common mineral-forming system, along shear zones, probably at low temperature (80 °C to 115 °C) [44], in an alkaline environment, as has been suggested [45] and confirms the presence of calcite, vesuvianite and grossularite [Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6].
Thus, considering the stability of Ni-phyllosilicates, (Ni, Co, Mn)(OH)2, and Ni-hydroxyl-carbonates, a potential sequence may be as follows: Hydroxyl-carbonates (otwayite-like), occurring as fine fibrous crystals along the boundaries of successive thin layers (analyses No 5, Table 1; Figure 5) are favored in a carbonate ions (CO32−) environment, such as the shearing mixed zone at the contact of the Vermion Ni-laterite with overlying limestone. Their gradual stacking in successive thin layers results in porous mineral phases of varying density (Figure 2c, Figure 3b and Figure 4b), which are characterized by elevated Si content. Thus, theophastite [β-Ni(OH)2] seems to be more stable under alkaline, but lower CO2, compared to that for hydroxyl-carbonates, and higher Si conditions [44,45]. The [β-Ni(OH)2] (theophrastite) is less stable than (Ni, Co, Mn)(OH)2 [37,38] while Ni-phyllosilicates are generally considered more stable than (Ni, Co, Mn)-hydroxides, due to the layered structure of phyllosilicates and their ability to incorporate Ni [12,13,35,36,37]. Such a sequence of Ni minerals is consistent with their abundance in the Vermion laterite samples (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6).
5.4. Applications
Since Ni-laterites represent a major part of the Ni resources worldwide, the Ni-bearing minerals have been studied extensively by many authors, and it has been concluded that the phyllosilicates of the garnierite-group are the dominant Ni-type ([28] and references therein). Present data showing low stability for Ni (OH)2, specifically less stable than (Ni, Co, Mn ± Fe)-hydroxides and Ni-phyllosilicates, may be applied to the interpretation of the rare occurrence of theophrastite and the Ni-exploration. The low stability of Ni(OH)2 may be a significant factor in nickel exploration from laterite deposits, because the precipitation behavior of Ni(OH)2 at relatively high pH [46,47] may provide evidence of orientation in laterite deposits (profile) to potential areas for Ni concentration.
Contamination of soil and groundwater by Ni may be a significant environmental problem, particularly in areas surrounding Ni-laterite deposits and other Ni-bearing raw materials related to ophiolites [48]. During erosion and mining of laterites soil contamination, due to the transport of Ni-rich particulates, and leaching of Ni (depending on redox conditions) and transport into groundwater are topics of extensive study, aiming to limit exposures and minimize the public health risks [12,28,34,35,49].
6. Conclusions
The presented new analytical data, including black-white and color back-scattered electron images (BSEI), element mapping, scanning along BSEI and Raman spectroscopy of minerals from the Vermion Fe-Ni-laterites, led us to the following conclusions:
- Dominant minerals in the studied samples were garnet (grossularite), vesuvianite, serpentine, and magnetite while Ni-minerals are theophrastite [β-Ni(OH)2], otwayite-like phase [ideally Ni2CO3(OH)2.H2O] (Ni, Co, Mn)-hydroxides, and Ni-phyllosilicates.
- Although the X-ray powder diffraction data for Ni-bearing minerals correspond to those of pure hydroxides, silica content ranging from less than 1 to 29 wt.% SiO2 is exhibited in theophrastite and in (Ni, Co, Mn ± Fe)-hydroxides.
- The interpretation of the Si content in theophrastite from Vermion is uncertain. A potential explanation could be the initial precipitation of fine fibrous Ni-hydrated carbonate crystals, followed by gradual stacking, silica adsorption onto the surface of Ni(OH)2, and/or co- precipitation of Si and fine Ni-phyllosilicate intergrowths.
- The low stability of Ni(OH)2 compared to that for (Ni, Co, Mn) (OH)2 and Ni- phyllosilicates, may provide evidence of potential areas for Ni concentration in laterite deposits, being a factor for Ni-exploration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min15080857/s1, for XRD pattern, Supplementary S1 XRD pattern; Supplementary S2 and S3—Selected black-white and colar BSE-images, probe analyses, X-ray elemental maps and X-ray spectra of minerals from area1 and area2 of laterite sample with Ni-minerals, Area 1 of the studied samples: Back-scattered electron images (BSEI), spectra and SEM/EDS analyses; Area 2 of the studied samples: BSEI, spectra and SEM/EDS analyses.
Author Contributions
Conceptualization, M.E.-E., C.K., A.P., T.M., F.Z. and M.P.; Investigation, C.K. and M.P.; Methodology, M.E.-E., C.K., A.P., T.M. and F.Z.; Software, M.E.-E., C.K. and A.P.; Validation, M.E.-E., A.P. and M.P.; Writing—original draft, M.E.-E. and A.P.; Writing—review and editing, M.E.-E., C.K., T.M., F.Z. and M.P. All authors will be updated at each stage of manuscript processing, including submission, revision, and revision reminder, via emails from our system or the assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
Many thanks are expressed to the University of Athens for the financial support and Evaggelos Michaelidis for his assistance with the SEM/EDS analysis. The consideration of this work and constructive suggestions by the anonymous reviewers are very much appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bode, H.; Dehmelt, K.; Witte, J. Zur Kenntnis der Nickelhydroxidelektrode-I. Uber das Nickel(II)-Hydroxidhydrat. Electrochim. Acta 1966, 11, 1079–1087. [Google Scholar] [CrossRef]
- Oliva, P.; Leonarki, J.; Laurent, J.F.; Delmas, C.; Braconnier, J.J.; Figlarz, M.; Fievet, F.; de Guibert, A. Review of the Structure and the Electrochemistry of Nickel Hydroxides and Oxy-Hydroxides. J. Power Sources 1982, 8, 229–255. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T.; Kaichev, V.V. Mixed layered Ni–Mn–Co hydroxides: Crystal structure, electronic state of ions, and thermal decomposition. Short Commun. J. Power Sources 2007, 174, 735–740. [Google Scholar] [CrossRef]
- McEwen, M.; Reche, R.; Rosenberg, F.F. Genesis of nickel laterites and bauxites in Greece during the Jurassic and the Cretaceous and their relation to ultrabasic rocks. Ore Geol. Rev. 1987, 2, 359–404. [Google Scholar] [CrossRef]
- Livingstone, A.; Bish, D.L. On the new mineral thoephrastite, a nickel hydroxide, from Unst, Shetland, Scotland. Mineral. Mag. 1982, 46, 1–5. [Google Scholar] [CrossRef]
- Igelström, L.J. Pyrochroït, ein neues Mineral. Ann. Phys. Chem. 1864, 198, 181–182. [Google Scholar] [CrossRef]
- Marcopoulos, T.; Economou, M. Theophrastite, Ni(OH)2, a new mineral from northern Greece. Am. Mineral. 1981, 66, 1020–1021. [Google Scholar]
- Economou-Eliopoulos, M.; Eliopoulos, D. A new solid solution [(Co, Mn, Ni)(OH)2], in the Vermion Mt (Greece) and its genetic significance for the mineral group of hydroxides. In Chemical Mineralogy, Smelting and Metallization; McLaughlin, E.D., Braux, L.A., Eds.; Nova Science Publishers: New York, NY, USA, 2009; pp. 2–19. [Google Scholar]
- Economou-Eliopoulos, M.; Zaccarini, F. On the Origin of New and Rare Minerals Discovered in the Othrys and Vermion Ophiolites, Greece: An Overview. Minerals 2022, 12, 1214. [Google Scholar] [CrossRef]
- Arai, S.; Ishimaru, S.; Miura, M.; Akizawa, N.; Mizukami, T. Post-serpentinization formation of theophrastite-zaratite by heazlewoodite desulfurization: An implication for shallow behavior of sulfur in a subduction complex. Minerals 2020, 10, 806. [Google Scholar] [CrossRef]
- Williams, K.L. Nickel Mineralisation in Western Tasmania; Proceed; Australian Institute of Mining and Metallurgy: Carlton, Australia, 1958; pp. 263–302. [Google Scholar]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. R. Soc. A 2015, 471, 20140792. [Google Scholar] [CrossRef]
- Jayanthi, K.; Kamath, P.V. A crystal chemical approach to a cation-ordered structure model for carbonate-intercalated layered. Cryst. Growth Des. 2016, 16, 4450–4456. [Google Scholar] [CrossRef]
- Mercer, J. Etude geologique des zones internes des Hellenides en Macedoine centrale (Greece). Ann. Geol. Pays Hellen 1966, 20, 596. [Google Scholar]
- Economou, G.; Economou, M. Some chromite occurrences from the areas of Vermio and Veria, Macedonia, Greece. In Proceedings of the Conference Metallogeny of Basic and Ultrabasic Rocks, Edinburgh, UK, 9–12 April 1985; pp. 351–354. [Google Scholar]
- Paraskevopoulos, G.; Economou, M. Genesis of magnetite ore occurrences by metasomatism of chromite ores in Greece. Neues Jahrb. Mineral. Abh. 1980, 140, 29–53. [Google Scholar]
- Paraskevopoulos, G.; Economou, M. Mn-rich chromite from podiform type chromite ore in serpentinites of northern Greece. Amer. Mineral. 1981, 66, 1013–1019. [Google Scholar]
- Eliopoulos, D.; Economou-Eliopoulos, M. Geochemical and mineralogical characteristics of Fe–Ni and bauxitic–laterite deposits of Greece. Ore Geol. Rev. 2000, 16, 41–58. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M. Apatite and Mn, Zn, Co-enriched chromite in Ni-laterites of northern Greece and their genetic significance. J. Geoch. Explor. 2003, 80, 41–54. [Google Scholar] [CrossRef]
- Li, L.X.; Liu, J.F.; Li, Y.D. Low-temperature conversion synthesis of M(OH)2 (M = Ni,Co, Fe) nanoflakes and nanorods. Mater. Chem. Phys. 2003, 80, 222–227. [Google Scholar] [CrossRef]
- Vidotti, M.; Renan, P.; Salvador, R.P.; Ponzio, E.A. Córdoba de Torresi, S.I. Mixed Ni/Co hydroxide nanoparticles synthesized by sonochemical method. J. Nanosci. Nanotechnol. 2007, 7, 3221–3226. [Google Scholar] [CrossRef]
- Murli, C.; Sharma, S.M.; Kulshreshtha, S.K.; Sikka, S.K. High-pressure behavior of b-Ni (OH)2—A Raman scattering study. Physica 2001, 307, 111–116. [Google Scholar] [CrossRef]
- Bantignies, J.-L.; Deabate, S.; Righi, A.; Rols, S.; Hermet, P.; Sauvajol, J.-L.; Henn, F. New insight into the vibrational behavior of Nickel Hydroxide and Oxyhydroxide using inelastic neutron scattering, far/mid-infrared, and Raman spectroscopies. J. Phys. Chem. C 2008, 112, 2193–2201. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Poirier, S.; Bock, C.; MacDougall, B.R. Raman and Infrared spectroscopy of α and β phases of thin nickel hydroxide films electrochemically formed on nickel. J. Phys. Chem. A 2012, 116, 6771–6784. [Google Scholar] [CrossRef]
- Johnston, C.; Graves, P.R. In situ Raman spectroscopy study of the nickel oxyhydroxide electrode (NOE) system. Appl. Spectr. 1990, 44, 105–115. [Google Scholar] [CrossRef]
- Frost, R.L.; Weier, M.L.; Martens, W.N.; Mills, S.J. The hydroxylated nickel carbonates otwayite and paraotwayite—A SEM, EDX and vibrational spectroscopic study. N. Jb. Miner. Abh. 2006, 183, 107–116. [Google Scholar] [CrossRef]
- Mabrouk, K.B.; Kauffmann, T.H.; Aroui, H.; Fontana, M.D. Raman study of cation effect on sulphate vibration modes in solid state and aqueous solutions. J. Raman Spectr. 2013, 44, 1603–1608. [Google Scholar] [CrossRef]
- Villanova-De-Benavent, C.; Aiglsperger, T.; Jawhari, T.; Proenza, J.A.; Galí, S. Micro-Raman spectroscopy of garnierite minerals: A useful method for phase identification. Macla 2012, 16, 180–181. [Google Scholar]
- Robertson, A.H.F.; Varnavas, S. The origin of hydrothermal metalliferous sediments associated with the Early Mesozoic Othrys and Vourinos ophiolites, mainland Greece. Sed. Geol. 1993, 83, 87–113. [Google Scholar] [CrossRef]
- Golightly, J.P. Progress in understanding the evolution of nickel lateritics. In The Challenge of Finding New Mineral Resources—Global Metallogeny, Innovative Exploration, and New Discoveries; Goldfarb, R.J., Marsh, E.E., Monecke, T., Eds.; Economic Geologogy Special Publication 15; Society of Economic Geologists, Inc.: Littleton, CO, USA, 2010; Volume 15, pp. 451–486. [Google Scholar]
- Frakes, L.; Bolton, B. Effects of ocean chemistry, sea level and climate on the formation of primary sedimentary manganese ore deposits. Econ. Geol. 1992, 87, 1207–1217. [Google Scholar] [CrossRef]
- Henry, D.A.; Birch, W.D. Otwayite and theophrastite from the Lord Brassey Mine, Tasmania. Mineral. Mag. 1992, 56, 252–255. [Google Scholar] [CrossRef][Green Version]
- Ohtsuka, K.; Koga, L.; Tsunoda, M.; Suda, M.; Mikiya Ono, M. Epitaxial Growth of Nickel(l1) Hydroxide on layer Silicate and Derived Nickel-(layer Silicate) Nanocomposite. J. Am. Cerom SOC 1990, 73, 1719–1725. [Google Scholar] [CrossRef]
- Scheckel, K.G.; Scheinost, A.C.; Ford, R.G.; Sparks, D.L. Stability of layered Ni hydroxide surface precipitates—A dissolution kinetics study. Geochim. Cosmochim. Acta 2000, 64, 2727–2735. [Google Scholar] [CrossRef]
- Scheckel, K.G.; Sparks, D.L. Dissolution kinetics of nickel surface precipitates on clay mineral and oxide surfaces. Soil Sci. Soc. Am. J. 2001, 65, 685–694. [Google Scholar] [CrossRef]
- Tan, X.; Fang, M.; Ren, X.; Mei, H.; Shao, D.; Wang, X. Effect of Silicate on the Formation and Stability of Ni–Al LDH at the γ-Al2O3 Surface. Environ. Sci. Technol. 2014, 48, 13138–13145. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Anceau, G.; Calas, G.; Decarreau, A. Nickel-bearing clay minerals: Optical spectroscopic study of nickel crystal chemistry. Clay Miner. 1985, 20, 367–387. [Google Scholar] [CrossRef]
- Senna, M.; Fujiwara, Y.; Isobe, T.; Tanaka, J. Molecular dynamic–molecular orbital combined study on the solid-state interfacial reaction under mechanical stressing. Solid State Ion. 2001, 141–142, 32–38. [Google Scholar] [CrossRef]
- Eliopoulos, D.G.; Economou-Eliopoulos, M. Trace Element Distribution in Magnetite Separates of Varying Origin: Genetic and Exploration Significance. Minerals 2020, 9, 759. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific: Oxford, UK, 1985. [Google Scholar]
- Norton, S. Laterite and bauxite formation. Econ. Geol. 1973, 63, 353–361. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Boyce, A.J.; Fallick, A.E. 100th Anniversary special paper: On hydrothermal convection systems and the emergence of life. Econ. Geol. 2005, 100, 419–438. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. The role of microorganisms on the mineralogical and geochemical characteristics of the Parnassos-Ghiona bauxite deposits, Greece. J. Geochem. Explor. 2007, 93, 67–77. [Google Scholar] [CrossRef]
- Glemser, O.; Einerhand, J. Uber cohere Nickelhydroxide. Z. Anorg. Chem. 1950, 261, 26–42. [Google Scholar] [CrossRef]
- Economou, M.; Marcopoulos, T. Origin of the new mineral theophrastite, Ni(OH)2. Chem. Erde 1983, 42, 53–66. [Google Scholar]
- Peltier, E.; Allada, R.; Navrotsky, A.; Sparks, D.L. Nickel Solubility and Precipitation in Soils: A Thermodynamic Study. Clays Clay Miner. 2006, 54, 153–164. [Google Scholar] [CrossRef]
- Ni, X.; Zhao, Q.; Zhang, Y.; Song, J.; Zheng, H.; Yang, K. Large scale synthesis and electrochemical characterization of hierarchical b-Ni(OH)2 flowers. Solid State Sci. 2006, 8, 1312–1317. [Google Scholar] [CrossRef]
- Villanova-de-Benavent, C.; Proenza, J.A.; Galí, S.; García-Casco, A.; Tauler, E.; Lewis, J.F.; Longo, F. Garnierites and garnierites: Textures, mineralogy and geochemistry of garnierites in the Falcondo Ni-laterite deposit, Dominican Republic. Ore Geol. Rev. 2014, 58, 91–109. [Google Scholar] [CrossRef]
- Bloise, A.; Ricchiuti, C.; Punturo, R.; Pereira, D. Potentially toxic elements (PTEs) associated with asbestos, chrysotile, tremolite and actinolite in the Calabria region (Italy). Chem. Geol. 2020, 558, 119896. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).