Traces of Cadmium Modulate the Morphology of Silver Crystals Produced from the Controlled Cooling of a Primary Lead Melt
Abstract
1. Introduction
- Optimized stirring: The Ag crystals produced during cooling showed a sensitive response to the stirring rpm and the stirrer type and position.
- Vacuum sample impregnation: The as-solidified Ag crystal structure was found to be very fragile and difficult to handle for subsequent X-ray imaging. The as-solidified Ag crystal structure was preserved by prompt lead drainage followed by impregnation with acrylic resin under vacuum to in-fill the open pore structure of the sample.
2. Experimental Methods
2.1. Synthesis of Silver Crystals
2.2. Assay of Impurities and Silver Content
2.3. XRT Characterization
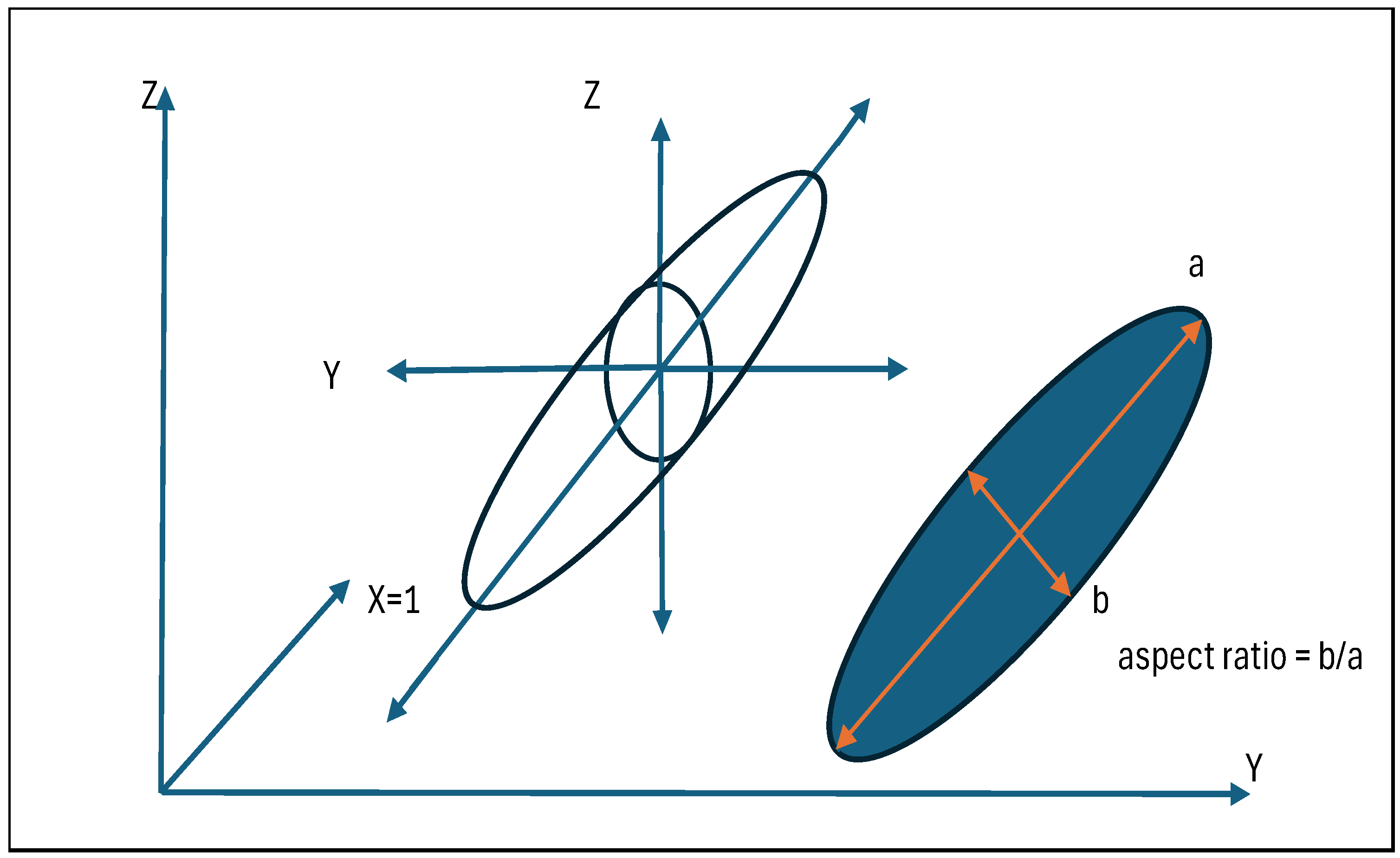
- Length3d: The maximum Feret diameter of the crystal in mm.
- Breadth3d: The minimum Feret diameter of the crystal in mm.
- Aspect Ratio: Breadth3d divided by Length3d.
- ShapeVA_3D: Value describing shape. Values of 1 indicate a perfect sphere while higher values indicate less compact, complex volumes.
- Volume: Volume of the crystal in mm3.
2.4. Particle Size Distribution via Dynamic Picture Analysis
- Particle size standard distribution;
- Aspect ratio;
- Sphericity;
- Convexity.
- Dispersing pressure: 0.5 bar;
- Dispersing Nozzle: 4 mm;
- Measurement Range M9 (17–4000 µm);
- Frame Rate: 175 Hz (FPS);
- Approx. sample mass = 500 mg per measurement;
- 30,000 + particles examined (1 measurement);
- Data treatment: The used diameter definition was the EQPC (equivalent diameter of a circle with the same projected area) with no data filter treatment applied. As a volume model (Q3), a sphere was applied.
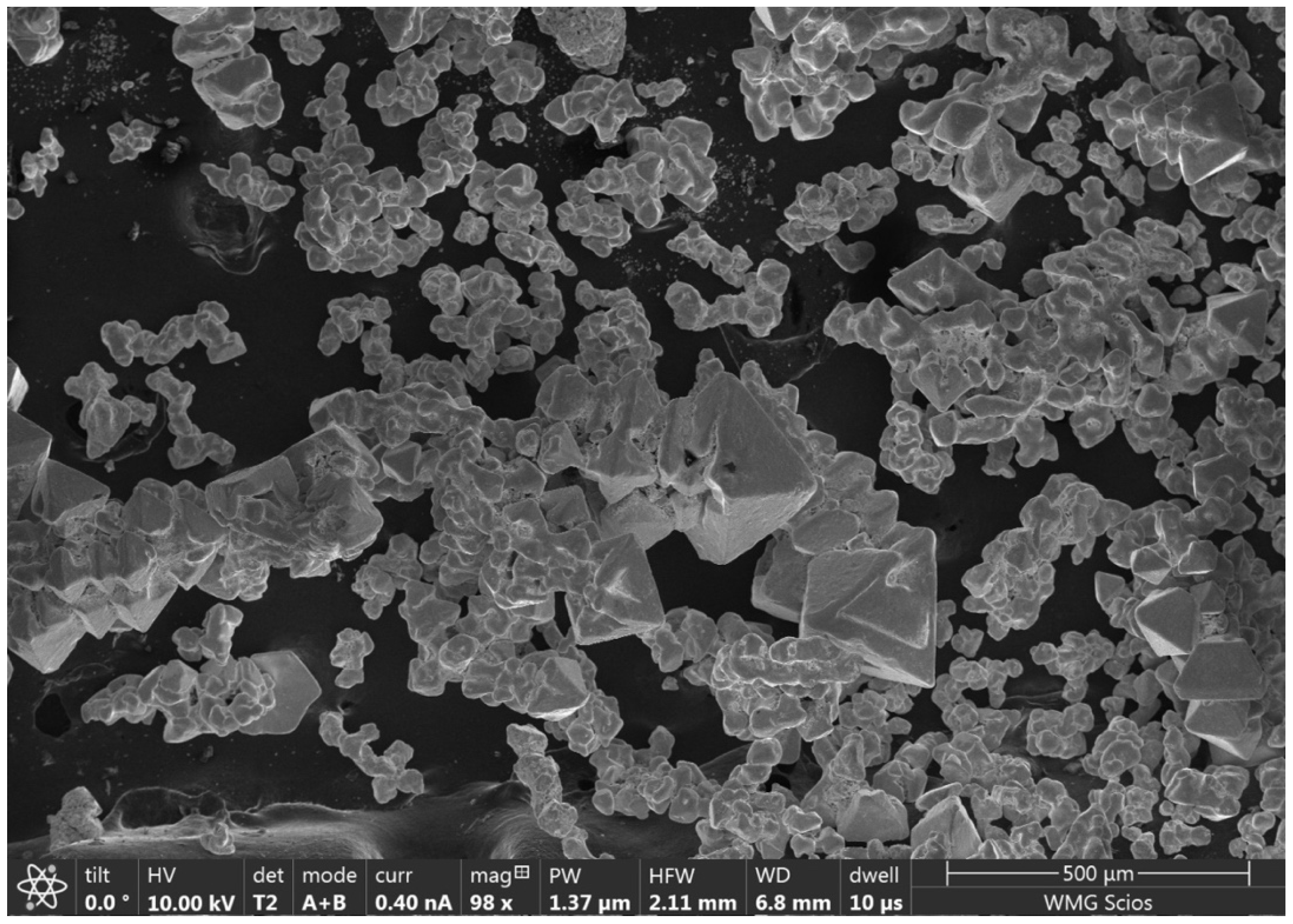
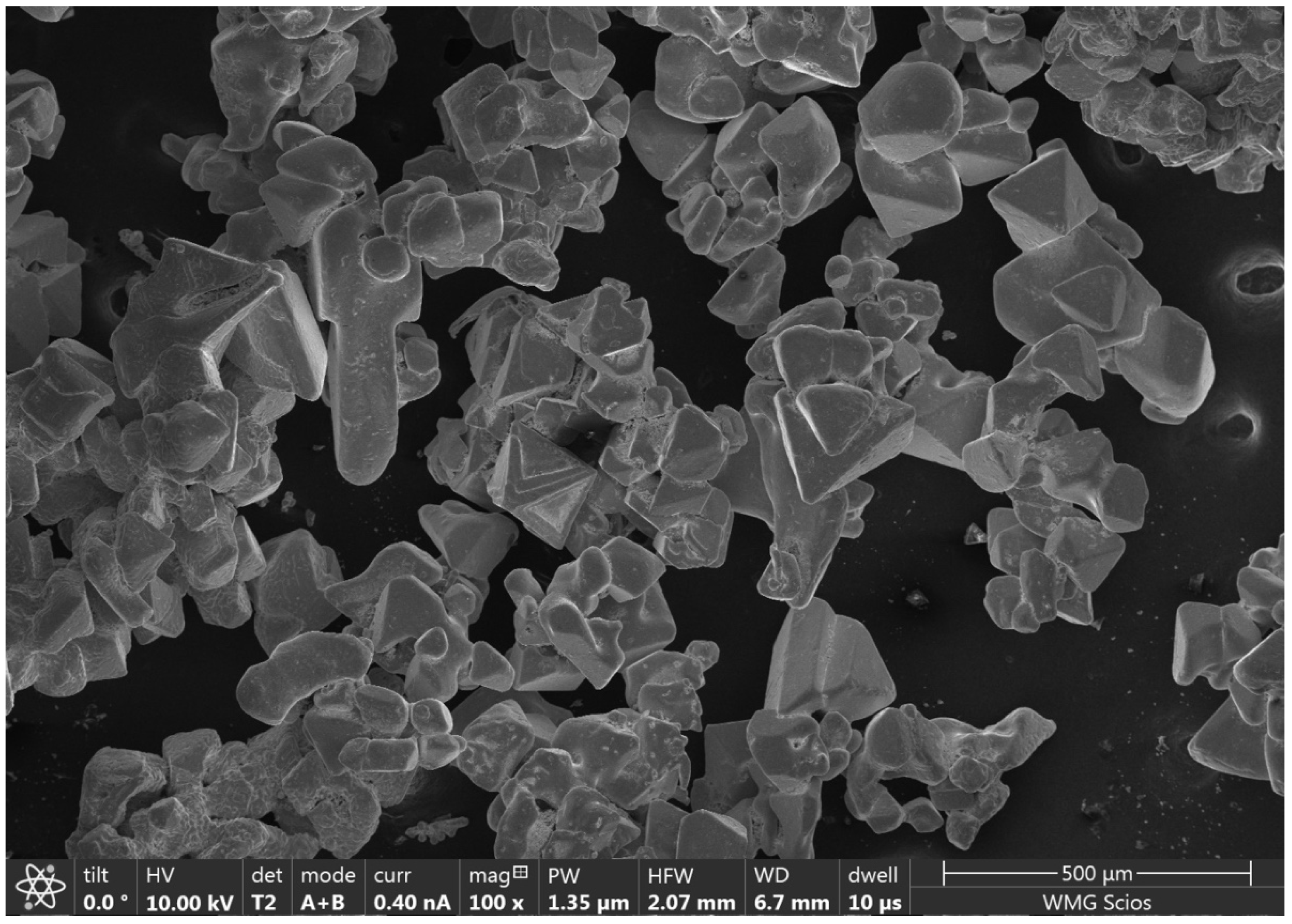
2.5. Scanning Electron Microscopy
3. Results and Discussion
3.1. Assay Impurities and Inoculant Species
3.2. Dimension and Shape Characterization of Silver Crystals
3.3. Silver Crystals Aspect Ratios
3.4. Dynamic Picture Analysis of Unconsolidated Crystals and Shape Characterization
3.5. SEM Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Process Flowsheet for Sample Preparation and Characterization

Appendix B. Definition of Characteristic Dimensions for Ag Crystals
References
- Butts, A.; Coxe, C. Silver Economics Metallurgy and Use; Van Nostrand: Princeton, NJ, USA, 1967. [Google Scholar]
- 2024 Interim Silver Marker Review; The Silver Institute: Washington, DC, USA, 2024.
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Evanoff, D.D., Jr.; Chumanov, G. Synthesis and Optical Properties of Silver Nanoparticles and Arrays. Chemphyschem 2005, 6, 1221–1231. [Google Scholar] [CrossRef]
- González, A.L.; Noguez, C. Optical properties of silver nanoparticles. Phys. Status Solidi. C 2007, 4, 4118–4126. [Google Scholar] [CrossRef]
- Jiang, Z.-J.; Liu, C.-Y.; Sun, L.-W. Catalytic Properties of Silver Nanoparticles Supported on Silica Spheres. J. Phys. Chem. B 2005, 109, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Shaker Ardakani, L.; Surendar, A.; Thangavelu, L.; Mandal, T. Silver nanoparticles (Ag NPs) as catalyst in chemical reactions. Synth. Commun. 2021, 51, 1516–1536. [Google Scholar] [CrossRef]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Pagliaro, M.; Della Pina, C.; Mauriello, F.; Ciriminna, R. Catalysis with silver: From complexes and nanoparticles to morals and single-atom catalysts. Catalysts 2020, 10, 1343. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, D.; Xie, S.; Xia, X.; Huang, C.Z.; Xia, Y. Synthesis of Silver Octahedra with Controlled Sizes and Optical Properties via Seed-Mediated Growth. ACS Nano 2013, 7, 4586–4594. [Google Scholar] [CrossRef]
- Kim, D.Y.; Li, W.; Ma, Y.; Yu, T.; Li, Z.-Y.; Park, O.O.; Xia, Y. Seed-Mediated Synthesis of Gold Octahedra in High Purity and with Well-Controlled Sizes and Optical Properties. Chem. A Eur. J. 2011, 17, 4759–4764. [Google Scholar] [CrossRef]
- Chiang, C.; Huang, M.H. Synthesis of Small Au-Ag Core-Shell Cubes, Cuboctahedra, and Octahedra with Size Tunability and Their Optical and Photothermal Properties. Small 2015, 11, 6018–6025. [Google Scholar] [CrossRef] [PubMed]
- Gugua, E.C.; Ujah, C.O.; Asadu, C.O.; Von Kallon, D.V.; Ekwueme, B.N. Electroplating in the modern era, improvements and challenges: A review. Hybrid Adv. 2024, 7, 100286. [Google Scholar] [CrossRef]
- Zhou, J.; Hao, B.; Meng, Y.; Yang, H.; Chen, W.; Zhang, L.; Liu, J.; Yan, C.; Qian, T. Levelling the Zn Anode by Crystallographic Orientation Manipulation. Nano Lett. 2023, 23, 10148–10156. [Google Scholar] [CrossRef] [PubMed]
- Ashiru, O.A. Influence of bath additives on the composition of electrodeposited silver coatings. Surf. Interface Anal. 1995, 23, 618–622. [Google Scholar] [CrossRef]
- King, S.; Striolo, A. Correction: Performance Metrics of the Newly Introduced Britannia Silver Process: Significant Reductions in Operating Costs, Energy Usage, and Scope 1 Carbon Emissions in the Industrial-Scale Refining of Silver. J. Sustain. Metall. 2024, 10, 2115. [Google Scholar] [CrossRef]
- King, S.; Rajoo, D.; Norori-McCormac, A.; Striolo, A. Characterization of Kinetics-Controlled Morphologies in the Growth of Silver Crystals from a Primary Lead Melt. Minerals 2024, 14, 56. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Ambrusi, R.E.; García, S.G.; Pronsato, M.E. DFT study of the formation of Cd–Ag surface alloys on Ag surfaces. Comput. Mater. Sci. 2016, 118, 316–324. [Google Scholar] [CrossRef]
- Kozlov, S.A.; Potolokov, N.A.; Gusev, A.V.; Fedorov, V.A. Effect of Impurities on Crystal Growth of Gallium. Inorg. Mater. 2003, 39, 1267–1270. [Google Scholar] [CrossRef]
- Tiller, W.A. Preferred growth direction of metals. JOM 1957, 9, 847–855. [Google Scholar] [CrossRef]
- Karakaya, I.; Thompson, W.T. The Ag-Pb (Silver-Lead) system. Bull. Alloy Phase Diagr. 1987, 8, 326–334. [Google Scholar] [CrossRef]
- NIST: Phase Diagram and Computational Thermodynamics Ag-Pb System. Available online: https://www.metallurgy.nist.gov/phase/solder/agpb.html (accessed on 15 January 2024).
- Kallmann, S.; Hobart, E.W. Determination of silver, gold and palladium by a combined fire-assay atomic-absorption procedure. Talanta 1970, 17, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Sun, Y.; Zhu, W.; Zeng, Z.; Ma, G.; He, H.; He, M. Determination of Silver by Fire Assay Gravimetry Combined with Mathematic Correction. Int. J. Miner. Process. Extr. Metall. 2018, 3, 65–75. [Google Scholar] [CrossRef]
- Marie-Pierre, G.; Téreygeol, F.; Peyrat, F. Initial experiments on silver refining: How does a cupellation furnace work in the 16th century? Hist. Metall. 2010, 44, 126–135. [Google Scholar]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Avizo Software User’s Guide 2019 Thermo-Fisher Scientific. Available online: https://assets.thermofisher.com/TFS-Assets/MSD/Product-Guides/users-guide-avizo-software-2019.pdf (accessed on 29 March 2024).
- Holmes, J.F. LEAD AND LEAD ALLOYS: Corrosion Resistance—Part 3. Anti-Corros. Methods Mater. 1963, 10, 69–71. [Google Scholar] [CrossRef]
- Coles, E.L.; Gibson, J.G.; Hinde, R.M. The corrosion of lead by dilute aqueous organic acids. J. Appl. Chem. 1958, 8, 341–348. [Google Scholar] [CrossRef]
- Arya, L.M.; Heitman, J.L.; Thapa, B.B.; Bowman, D.C. Predicting Saturated Hydraulic Conductivity of Golf Course Sands from Particle-Size Distribution. Soil Sci. Soc. Am. J. 2010, 74, 33–37. [Google Scholar] [CrossRef]
- Wang, J.-P.; Francois, B.; Lambert, P. Equations for hydraulic conductivity estimation from particle size distribution—A dimensional analysis. Water Resour. Res. 2017, 53, 8127–8134. [Google Scholar] [CrossRef]
- Xiong, Y.; Dong, L.; Long, X.; Chen, M.; Huang, G. Pore-network model to quantify internal structure and hydraulic characteristics of randomly packed grains with different morphologies. Granul. Matter. 2022, 24, 10. [Google Scholar] [CrossRef]
- Robinson, D.A.; Friedman, S.P. Observations of the effects of particle shape and particle size distribution on avalanching of granular media. Phys. A 2002, 311, 97–110. [Google Scholar] [CrossRef]
- Peng, J.; Shen, Z.; Zhang, J. Measuring the Effect of Pack Shape on Gravel’s Pore Characteristics and Permeability Using X-ray Diffraction Computed Tomography. Materials 2022, 15, 6173. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Kudrolli, A. Maximum and minimum stable random packings of Platonic solids. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2010, 82 Pt 1, 061304. [Google Scholar] [CrossRef]
- Slepian, Z. The Average Projected Area Theorem—Generalization to Higher Dimensions. arXiv 2011, arXiv:1109.0595. [Google Scholar] [CrossRef]
- Marks, L.D.; Peng, L. Nanoparticle shape, thermodynamics and kinetics. J. Phys. Condens. Matter 2016, 28, 053001. [Google Scholar] [CrossRef]
- Nelson, S.A. Twinning, Polymorphism, Polytypism, Pseudomorphism; Tulane University: New Orleans, LA, USA, 2013. [Google Scholar]
- Nesse, W.D. Introduction to Mineralogy; Oxford University Press: New York, NY, USA, 2000; pp. 87–91. ISBN 9780195106916. [Google Scholar]
- Yasnikov, I.S.; Tsybuskina, I.I. Morphology of silver single crystals obtained by electrodeposition. Tech. Phys. 2008, 53, 1515–1518. [Google Scholar] [CrossRef]
- Sympatec Particle Measurement Glossary 2021. Available online: https://www.sympatec.com/en/particle-measurement/glossary/ (accessed on 29 March 2024).







| Sample Name | Ag% | Inoculant Species | XRT Resin | PSD Unconsolidated | SEM Unconsolidated |
|---|---|---|---|---|---|
| CAD A1 | 62.2 | Cd | |||
| CAD B1 | 73.0 | Cd | |||
| CAD A2 | 69.6 | Cd | |||
| CAD B2 | 69.8 | Cd | ✓ S5 | ✓ | ✓ |
| CAD A3 | 62.6 | Cd | |||
| CAD B3 | 61.8 | Cd | |||
| AL A1 | 68.4 | Al | |||
| AL B1 | 67.2 | Al | |||
| AL A2 | 69.2 | Al | |||
| AL B2 | 66.2 | Al | |||
| AL A3 | 63.4 | Al | |||
| AL B3 | 69.0 | Al | |||
| MG A1 | 75.0 | Mg | ✓ S4 | ✓ | ✓ |
| MG B1 | 74.2 | Mg | |||
| MG A2 | 40.2 | Mg | ✓ S3 | ✓ | ✓ ✓ P |
| MG B2 | 47.0 | Mg | |||
| MG A3 | 49.2 | Mg | |||
| MG B3 | 73.0 | Mg | |||
| LI A1 | 70.6 | Li | |||
| LI B1 | 72.0 | Li | |||
| LI A2 | 66.6 | Li | |||
| LI B2 | 72.4 | Li | |||
| LI A3 | 69.2 | Li | |||
| LI B3 | 71.2 | Li | |||
| Benchmark A1 | 75.0 | None | |||
| Benchmark B1 | 68.8 | None | |||
| Benchmark A2 | 77.8 | None | |||
| Benchmark B2 | 77.6 | None | ✓ | ✓ | |
| Benchmark A3 | 75.2 | None | |||
| Benchmark B3 | 79.0 | None | ✓ S6 | ✓ | ✓ |
| Pb-Mg 1 A | 68.3 | Mg | ✓ | ||
| Pb-Mg 1 B | 70.4 | Mg | ✓ | ||
| Pb-Mg 2 A | 62.4 | Mg | ✓ | ||
| Pb-Mg 2 B | 74.6 | Mg | ✓ |
| Species | Mass% |
|---|---|
| Ag | 77.80 |
| Zn | 0.04 |
| Pb | 21.14 |
| Cu | 0.08 |
| Sum | 100.00 |
| Sample | Threshold Value Used |
|---|---|
| Mg 2a (S3) | 28,000 |
| Mg 1a (S4) | 24,123 |
| Cd 2b(S5) | 27,033 |
| Benchmark 3b (S6) | 27,400 |
| Metals Analysed by ICP-Ms (%) | A | B | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Al | As | Bi | Cd | Mg | Ni | Pb | Sb | Sn | Zn | Sum | 100-Sum | Ag | Ag |
| Benchmark 1 | <0.01 | 0.01 | 0.01 | <0.01 | 0.01 | <0.01 | 22.54 | <0.01 | <0.01 | 0.05 | 22.76 | 77.24 | 75.0 | 68.8 |
| Benchmark 2 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 21.14 | <0.01 | <0.01 | 0.04 | 21.34 | 78.66 | 77.8 | 77.6 |
| Mg 1 | 0.03 | <0.01 | <0.01 | <0.01 | 0.49 | <0.01 | 53.05 | <0.01 | <0.01 | 0.05 | 53.69 | 46.31 | 40.0 | 47.0 |
| Mg 2 | 0.01 | <0.01 | <0.01 | <0.01 | 0.43 | <0.01 | 35.84 | <0.01 | <0.01 | 0.04 | 36.39 | 63.69 | 75.0 | 74.2 |
| Al 1 | <0.01 | <0.01 | <0.01 | 0.01 | 0.16 | <0.01 | 31.57 | <0.01 | <0.01 | 0.01 | 31.82 | 68.18 | 68.4 | 67.2 |
| Al 2 | <0.01 | <0.01 | <0.01 | 0.01 | 0.17 | <0.01 | 33.50 | <0.01 | <0.01 | 0.04 | 33.79 | 66.21 | 69.2 | 66.2 |
| Cd 1 | <0.01 | <0.01 | <0.01 | 0.57 | <0.01 | <0.01 | 29.04 | <0.01 | <0.01 | 0.01 | 29.72 | 70.28 | 62.2 | 73.0 |
| Cd 2 | <0.01 | <0.01 | <0.01 | 0.55 | <0.01 | <0.01 | 29.25 | <0.01 | <0.01 | 0.02 | 29.92 | 70.08 | 69.6 | 69.8 |
| Li 1 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | <0.01 | 29.62 | <0.01 | <0.01 | 0.02 | 26.74 | 73.26 | 70.6 | 72.0 |
| Li 2 | 0.01 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | 25.03 | <0.01 | <0.01 | 0.01 | 25.13 | 74.87 | 66.6 | 72.4 |
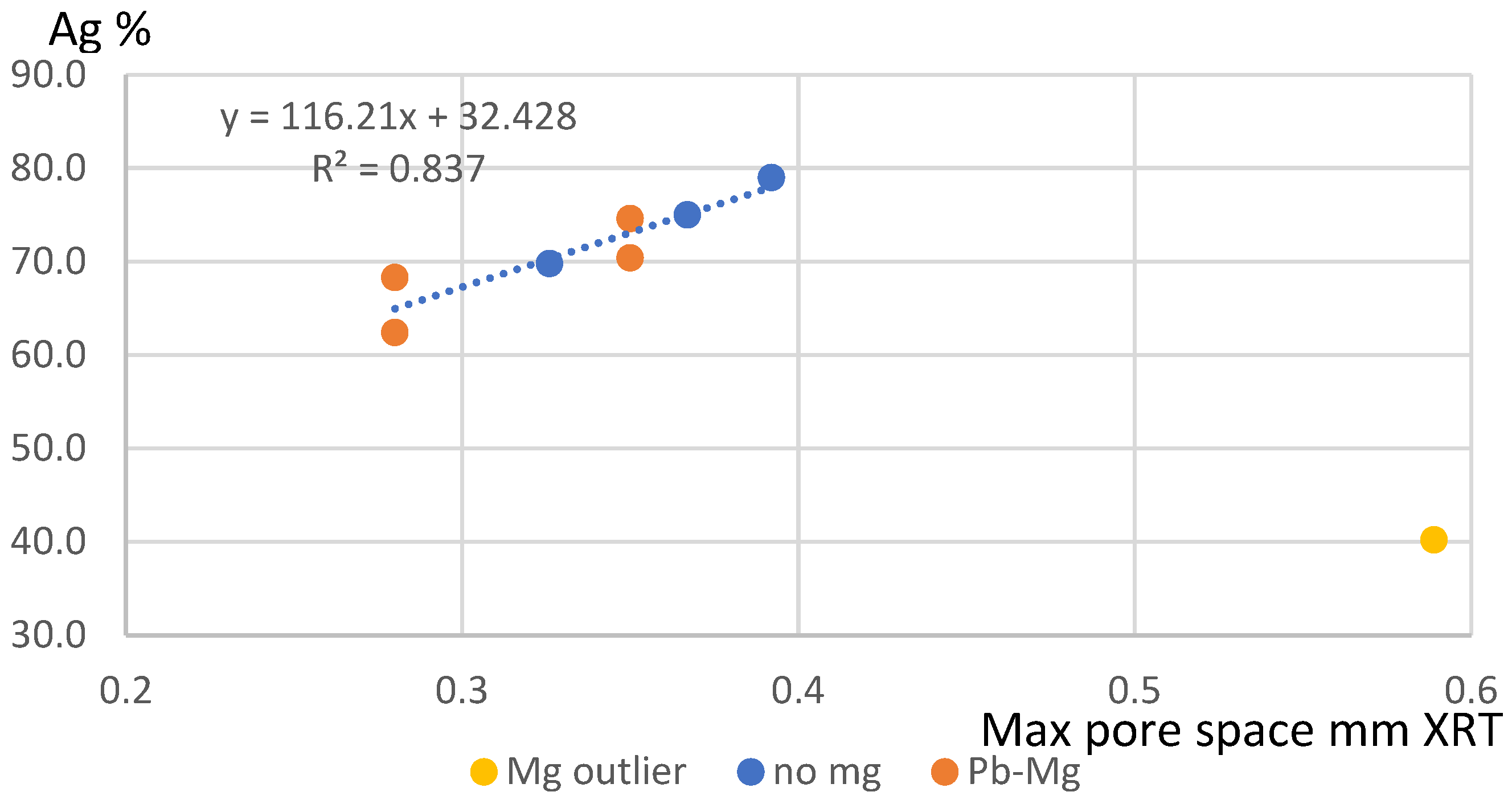
| Series | Sample No | Sample | Ag% | Length 3d mm | Breadth 3d mm | vol mm3 | Max Pore Space mm | Diam From Vol mm | PSD RCPE MM |
|---|---|---|---|---|---|---|---|---|---|
| initial | 4 | mg A2 | 40.2 | 0.2812 | 0.1849 | 0.002206 | 0.589 | 0.1618 | 0.132 |
| 1 | mg A1 | 75.0 | 0.2358 | 0.1448 | 0.001386 | 0.367 | 0.1386 | 0.112 | |
| 3 | cad B2 | 69.8 | 0.257 | 0.159 | 0.001549 | 0.326 | 0.1438 | 0.294 | |
| 2 | benchmark B3 | 79.0 | 0.2667 | 0.1845 | 0.002363 | 0.392 | 0.1656 | 0.308 | |
| recent | pb mg A2 | Pb-Mg A2 | 62.4 | 0.3149 | 0.2181 | 0.0031 | 0.28 | 0.1812 | |
| pb mg B2 | Pb-Mg B2 | 74.6 | 0.4172 | 0.2787 | 0.0052 | 0.35 | 0.2153 | ||
| recent | pb mg A1 | Pb-Mg A1 | 68.3 | 0.3149 | 0.2181 | 0.0031 | 0.28 | 0.1812 | |
| pb mg B1 | Pb-Mg B1 | 70.4 | 0.4172 | 0.2787 | 0.0052 | 0.35 | 0.2153 |
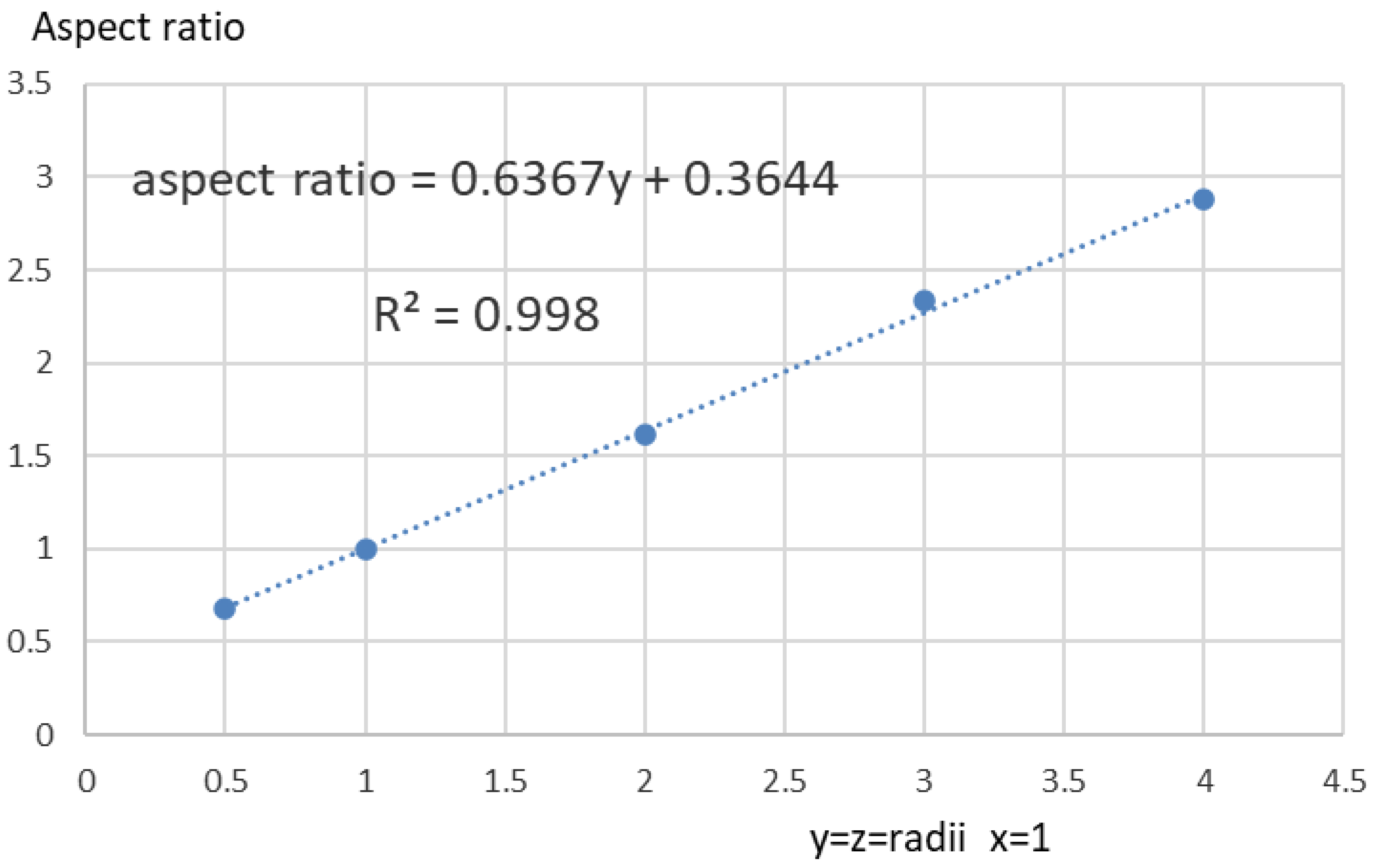
| Series | Sample No | Sample | Ag% | Length 3d mm | Breadth 3d mm | XRT Aspect | PSD Aspect |
|---|---|---|---|---|---|---|---|
| initial | 4 | mg A2 | 40.2 | 0.28 | 0.18 | 0.66 | 0.67 |
| 1 | mg A1 | 75.0 | 0.24 | 0.14 | 0.61 | 0.65 | |
| 3 | cad B2 | 69.8 | 0.26 | 0.16 | 0.62 | 0.59 | |
| 2 | benchmark B3 | 79.0 | 0.27 | 0.18 | 0.69 | 0.66 | |
| recent | pb mg in 2 | Pb-Mg A2 | 62.4 | 0.31 | 0.22 | 0.69 | |
| pb mg out 2 | Pb-Mg B2 | 74.6 | 0.42 | 0.28 | 0.67 | ||
| recent | pb mg in 1 | Pb-Mg A1 | 68.3 | 0.31 | 0.22 | 0.69 | |
| pb mg out 1 | Pb-Mg B1 | 70.4 | 0.42 | 0.28 | 0.67 | ||
| mean | 0.66 | 0.64 | |||||
| SD | 0.03 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, S.; Striolo, A.; Wilson, P.F.; West, G.; Williams, M.A.; Piller, M. Traces of Cadmium Modulate the Morphology of Silver Crystals Produced from the Controlled Cooling of a Primary Lead Melt. Minerals 2025, 15, 853. https://doi.org/10.3390/min15080853
King S, Striolo A, Wilson PF, West G, Williams MA, Piller M. Traces of Cadmium Modulate the Morphology of Silver Crystals Produced from the Controlled Cooling of a Primary Lead Melt. Minerals. 2025; 15(8):853. https://doi.org/10.3390/min15080853
Chicago/Turabian StyleKing, Steven, Alberto Striolo, Paul F. Wilson, Geoff West, Mark A. Williams, and Michael Piller. 2025. "Traces of Cadmium Modulate the Morphology of Silver Crystals Produced from the Controlled Cooling of a Primary Lead Melt" Minerals 15, no. 8: 853. https://doi.org/10.3390/min15080853
APA StyleKing, S., Striolo, A., Wilson, P. F., West, G., Williams, M. A., & Piller, M. (2025). Traces of Cadmium Modulate the Morphology of Silver Crystals Produced from the Controlled Cooling of a Primary Lead Melt. Minerals, 15(8), 853. https://doi.org/10.3390/min15080853







