Hydrothermal Scheelite Associated with Upper Cretaceous Intrusions in Romania: A Mineralogical Insight to the W Metallogeny
Abstract
1. Introduction
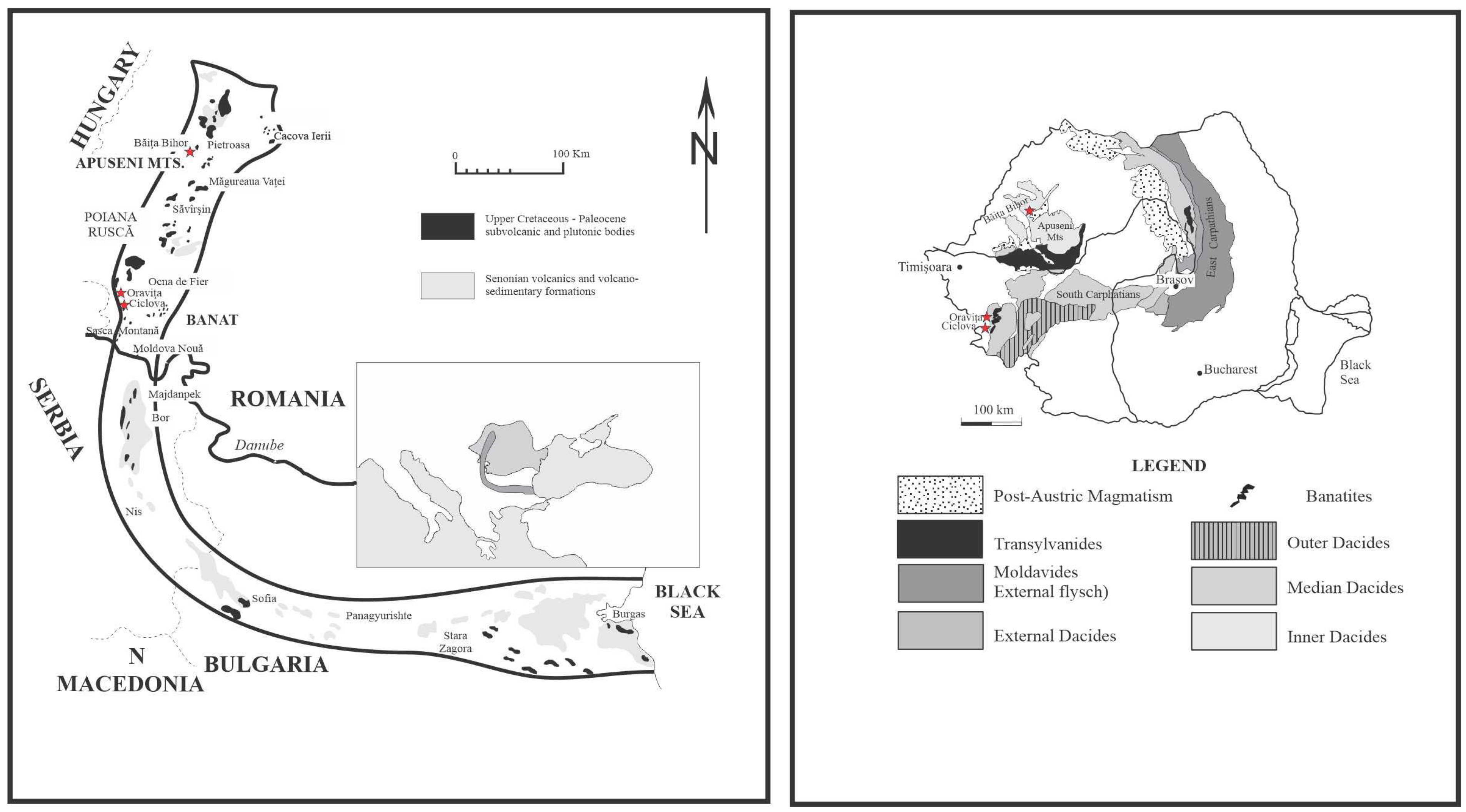
2. Geological Setting
3. Materials and Methods
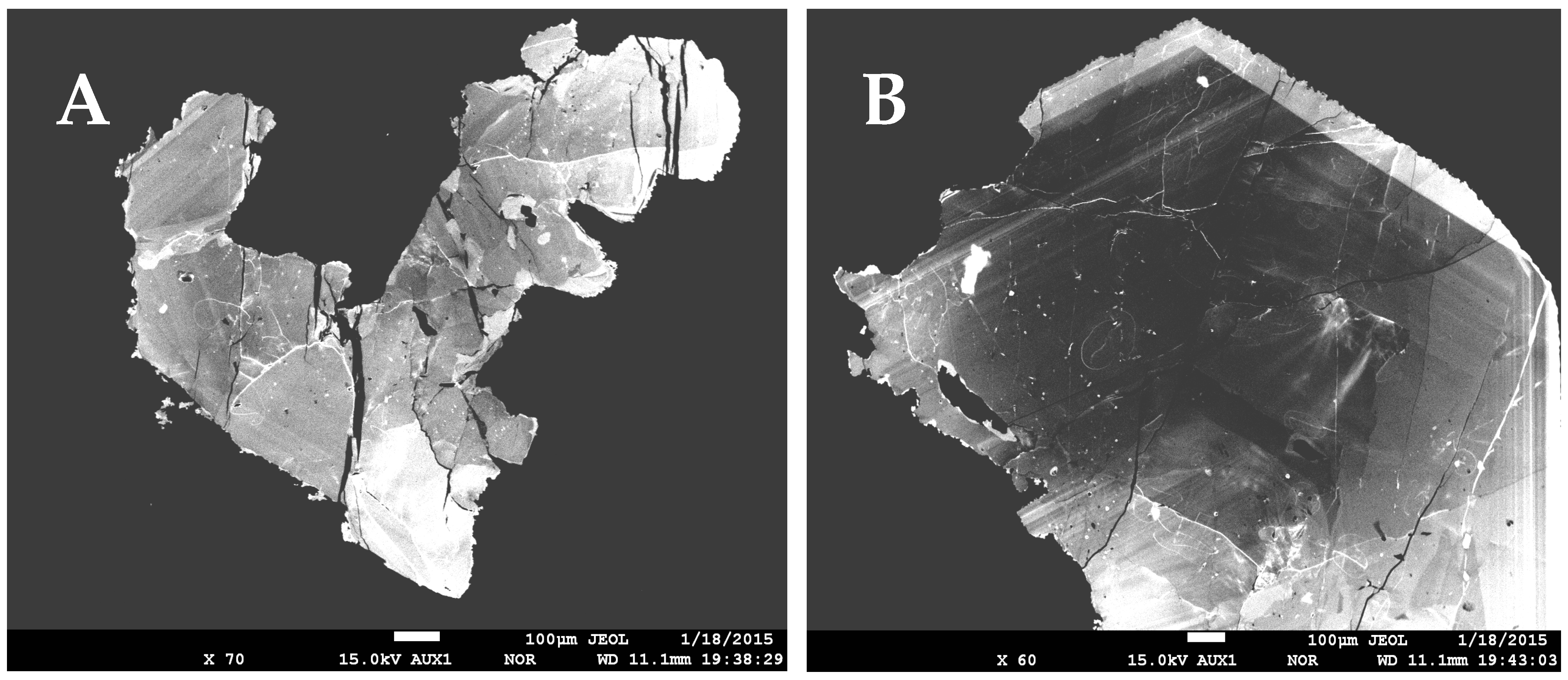
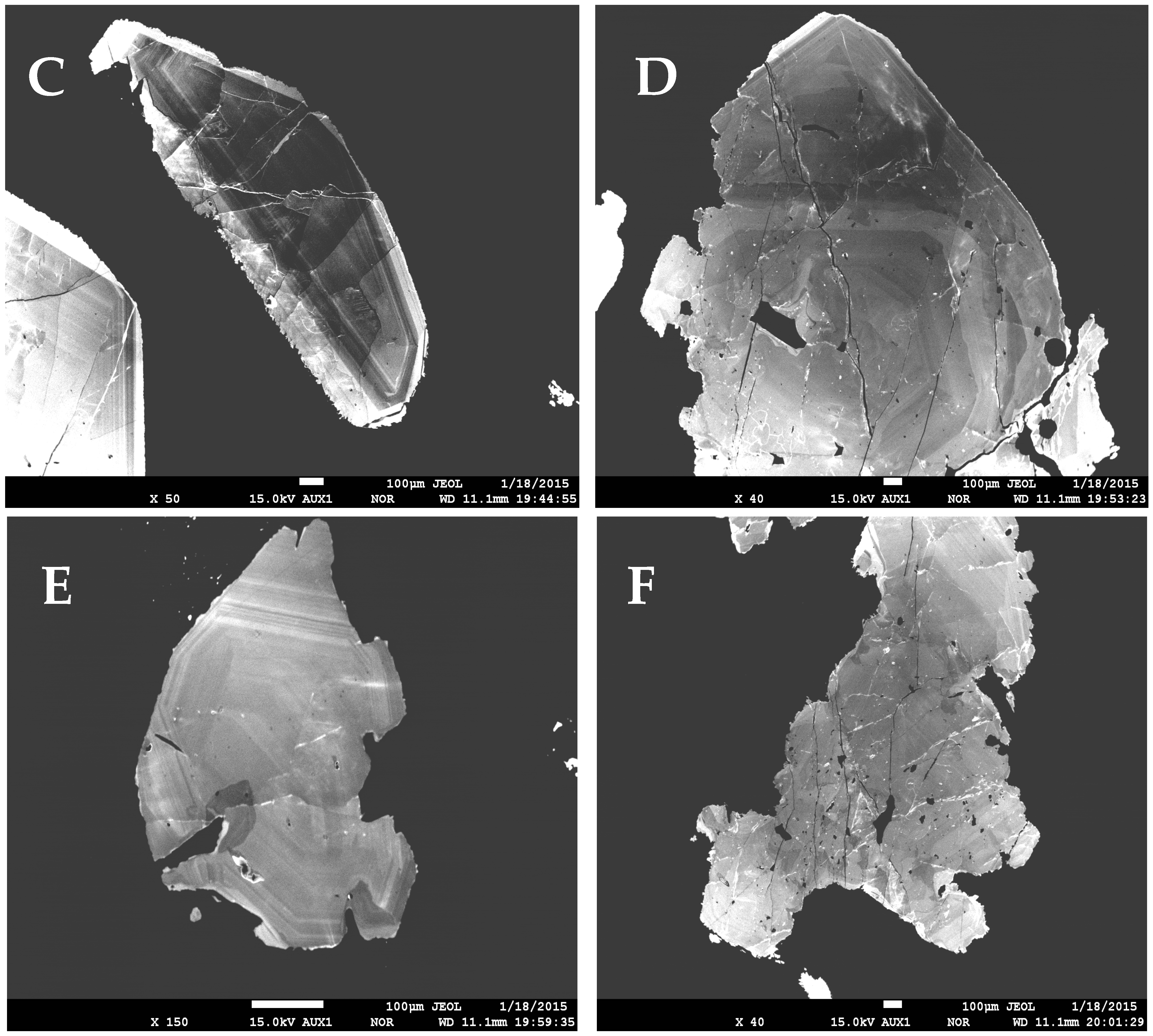
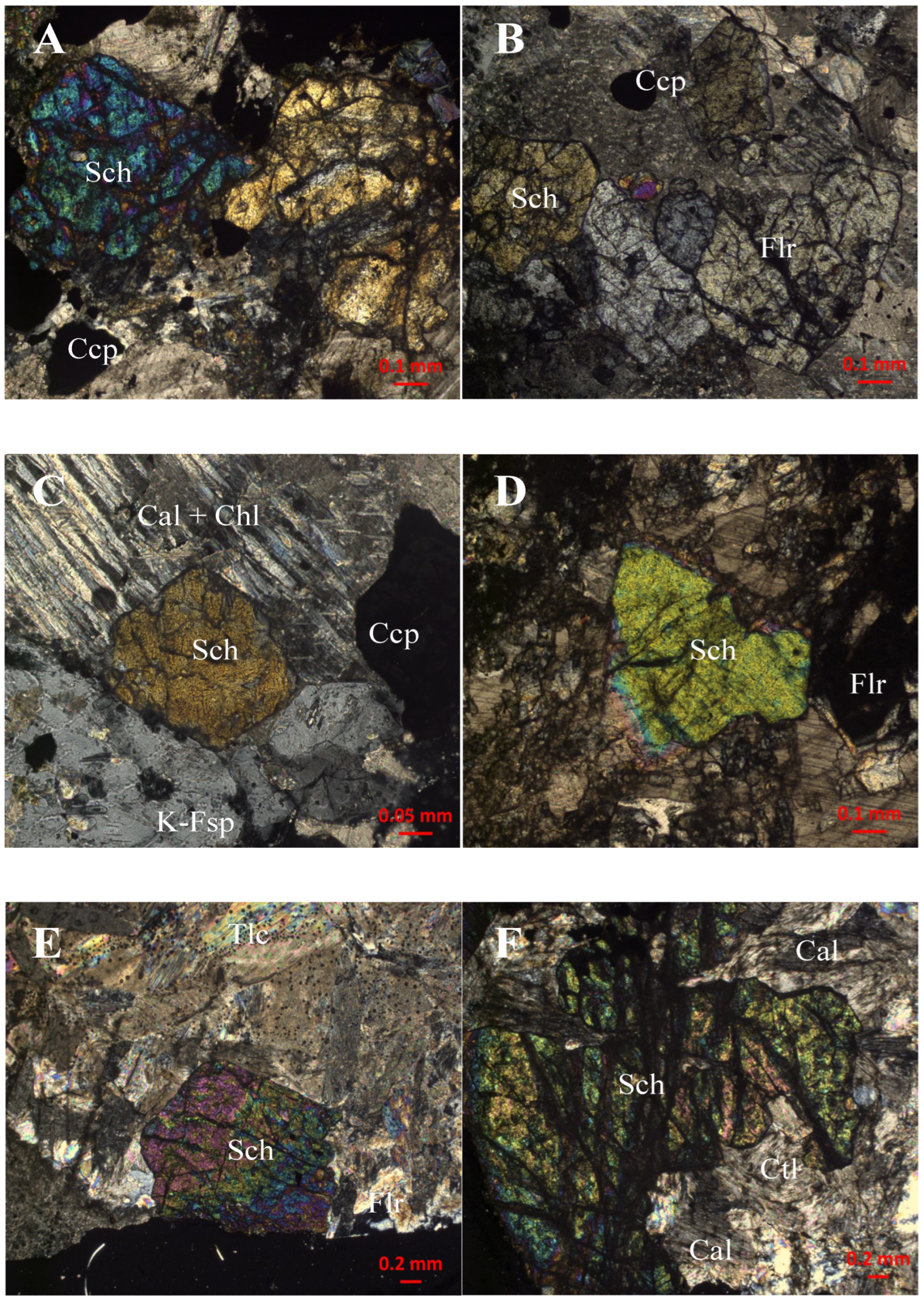
4. Mode of Occurrence and Morphology
5. Chemical Data
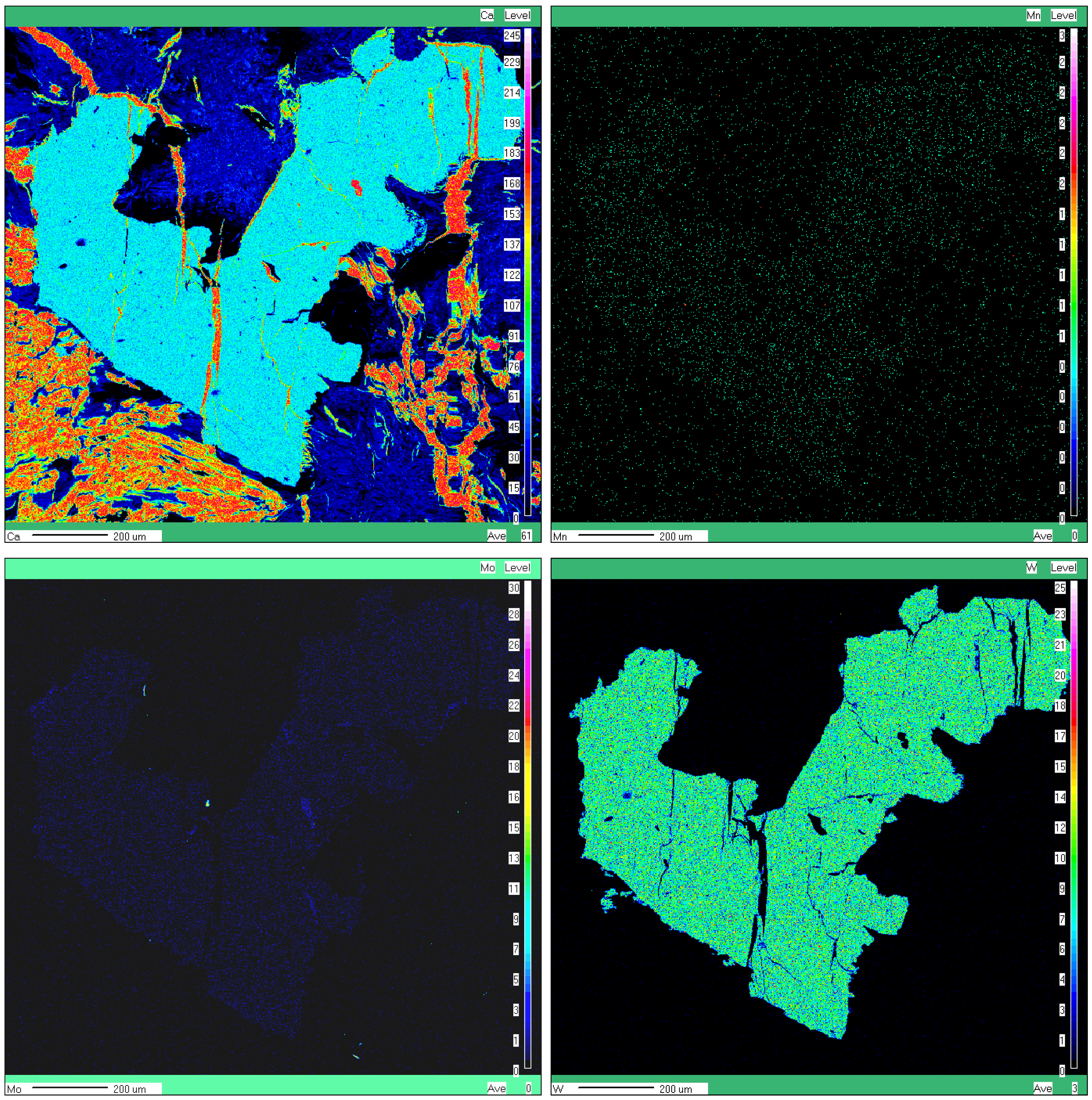
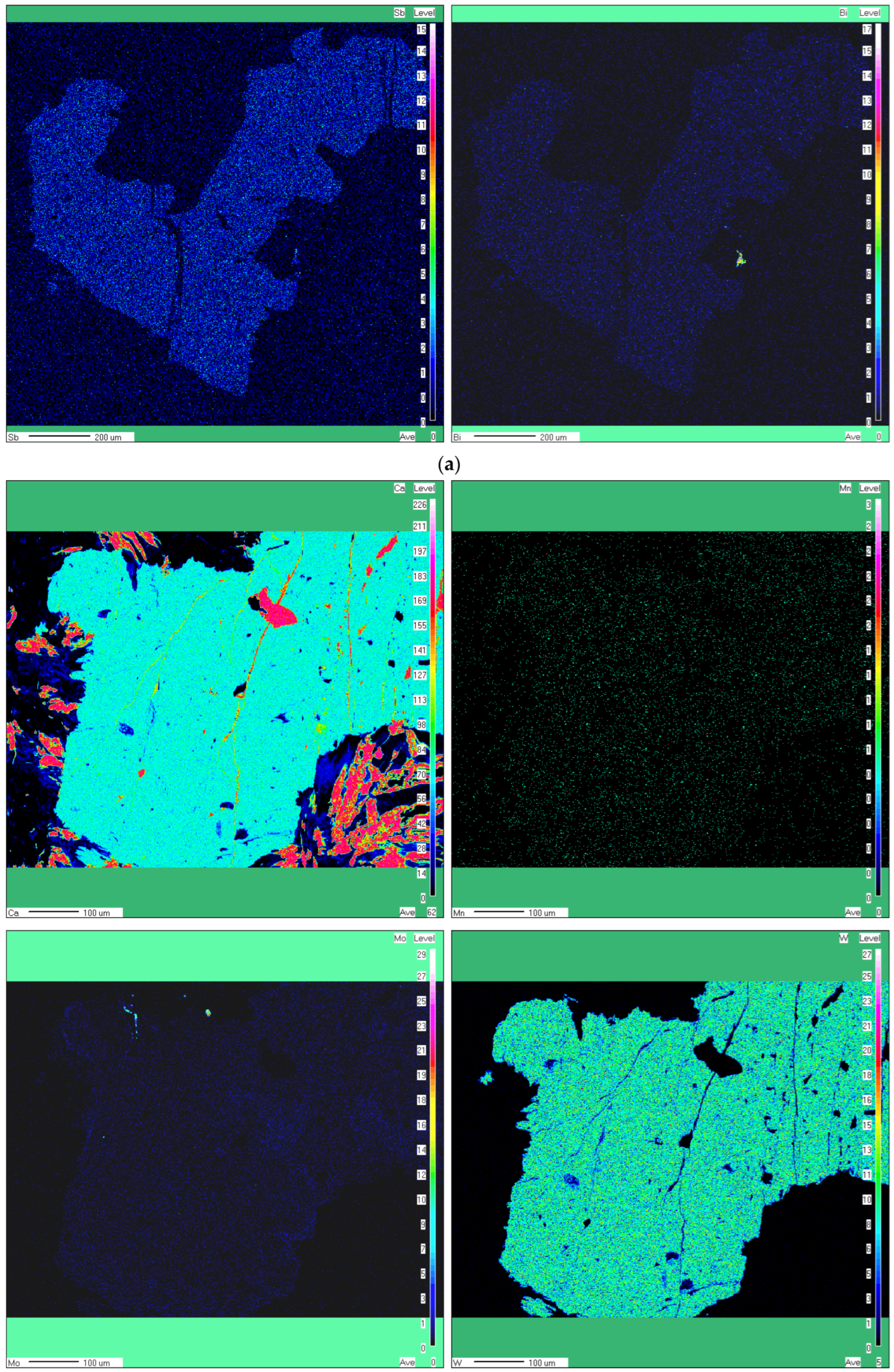
- No obvious chemical zoning was observed at the level of individual crystals, in spite of the CL zoning described below. Qualitative elemental X-ray maps exemplified in Figure 4a,b don’t show any zoning.
- A slight overcompensation in the four-fold coordinated cations (i.e., W + Mo > 1) could be observed in some samples, particularly at Oraviţa (Table 3). This phenomenon was already observed in some other cases (e.g., [12,42,43]) and is probably due to the EMPA interference effect of tungstite (ideally WO3·H2O) or rather hydrotungstite (ideally H2WO4·H2O), whose presence in all three occurrences was already mentioned [44].
- The isomorphism in the scheelite–powellite solid solution series is very restricted, reaching up to 2.4 mol.% powellite at Ciclova, up to 5.6 mol.% at Oravița, and up to 2.6 mol.% at Băița Bihor. The X-ray elemental maps failed to identify a chemical zoning at the crystal level, in spite of the general opinion that the dark CL oscillatory bands described above are enriched in Mo (i.e., [45,46]).
- Ba was sought but not detected, so the isomorphism toward ronpetersonite can’t be considered.
- The contents of stolzite (ideally PbWO4) in the solid solution are insignificant, reaching up to 0.1 mol.%.
- The small contents of Cu recorded in part of the samples could be rather due to submicrometer-sized cuprotungstite—ideally Cu2(WO4)(OH)2—inclusions, probably intruded on the cleavage directions; these inclusions are too small to be seized by the X-ray imaging.
- The collection of qualitative elemental X-ray maps (Figure 4a,b) together with SEM-EDS analyses confirmed that Sb and Bi are present in minor amounts in some scheelite grains. Apparently, both trivalent Sb and Bi are substitutes for Ca2+ through a mechanism similar to those reported by [47] and referred to in the case of REE and Y, i.e., 2Bi3+(Sb3+) + □ Ca = 3Ca2+, where □ Ca represents a vacancy site.
6. X-Ray Powder Diffraction Data
7. Physical Properties
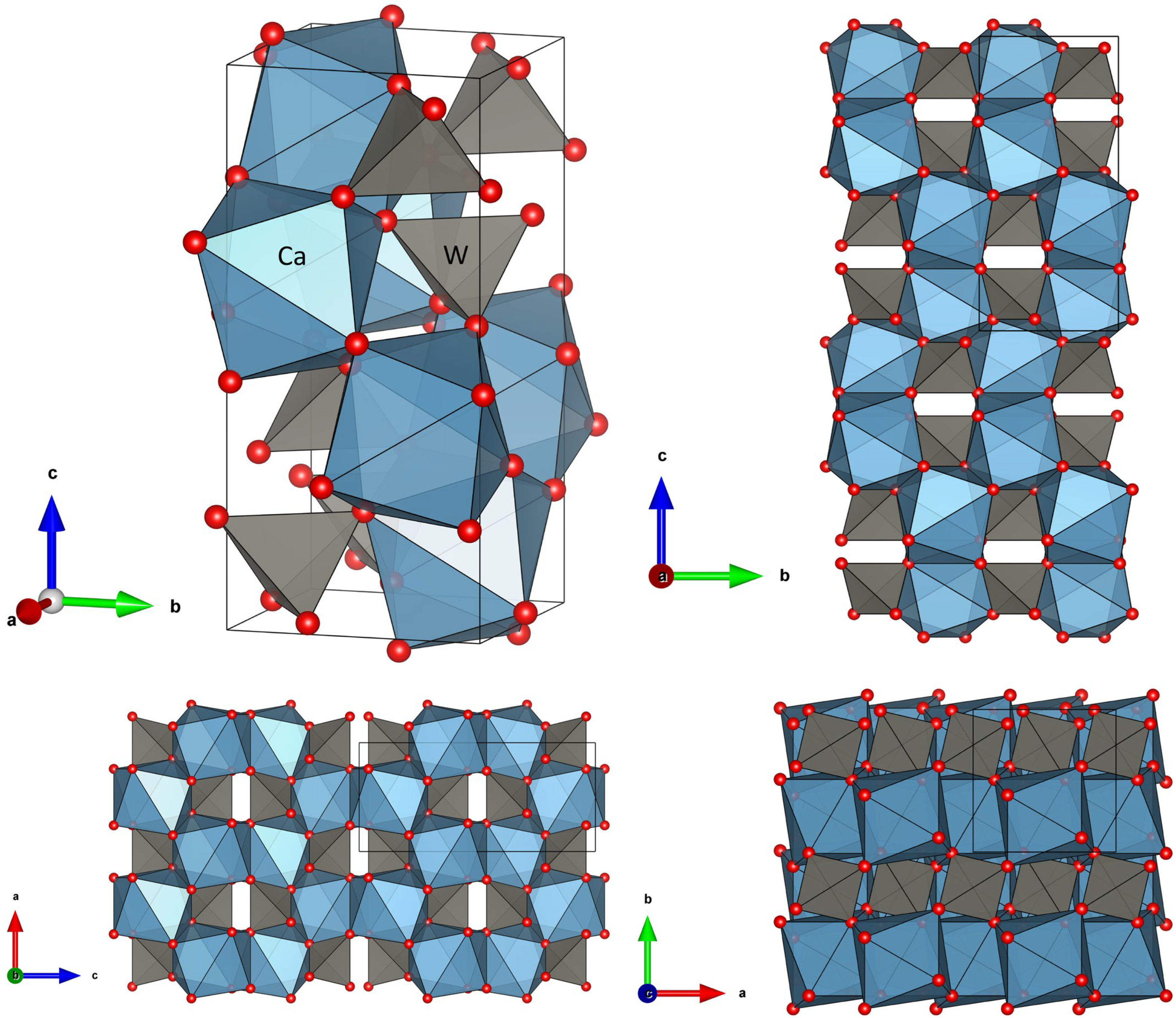
8. Structure
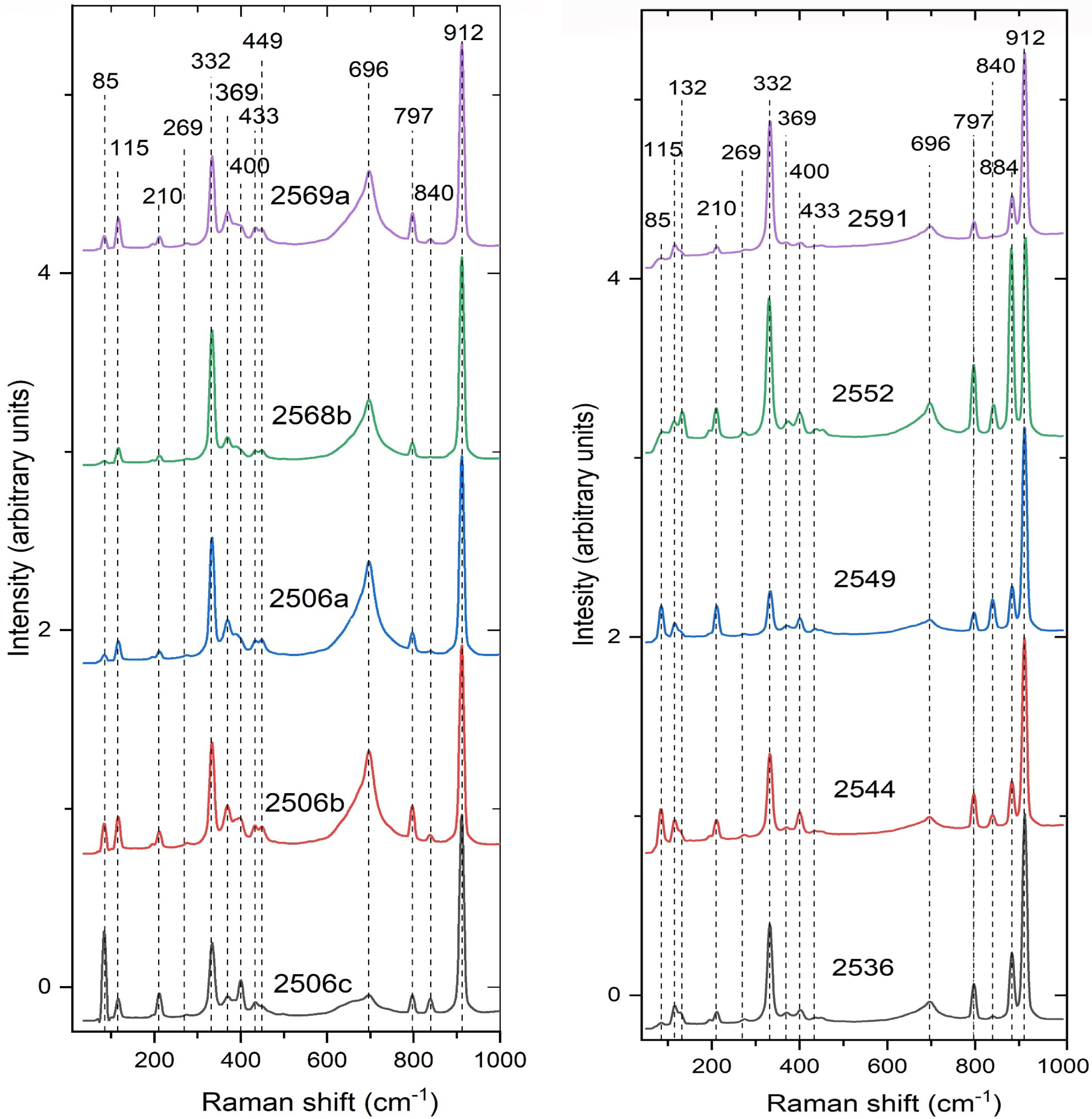
9. Infrared and Raman Behavior
| Structural Group | Vibrational Mode | Wavenumber (cm−1) | Character, Intensity (4) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Calculated | Ciclova | Oravița | Băița Bihor | |||||||
| IR (2) | Raman (3) | FTIR | Raman | IR | Raman | FTIR | Raman | |||
| WO4 | ν1 (Ag) symmetric stretching | 903 | 912 | 908 | 911 | 910 | 911 | 908 | 913 | shd, s |
| WO4 (?) | ν’1 (Bg) (?) | - | 893 | - | - | - | - | - | 883 | - |
| WO4 | ν3 (Bg) antisymmetric stretching | 808 | 838 | 810 | 838 | 811 | 839 | 810 | 841 | sh, vs |
| WO4 | ν’3 (Eg) antisymmetric stretching | 795 | 780 | 797 | 797 | 795 | 797 | 797 | 797 | shd, s |
| WO4 | ν2 (Ag) out-of-plane bending | 418 | 439 | 440 | 434 | 441 | 434 | 440 | 437 | sh, m |
| WO4 | ν’2 (Eg + Bg) out-of-plane bending | - | 403 | - | 401 | - | 400 | - | 402 | - |
| WO4 | ν4 (Bg) in-plane bending | 359 | - | - | 369 | 358 | 369 | - | 370 | sh, w |
| WO4 | ν’4 (Eg) in-plane bending | 348 | 330 | - | 333 | 326 | 333 | - | 332 | sh, m |
| WO4 | rotation R‖ (Ag) | 286 | 280 | - | 275 | 285 | - | - | 274 | sh, w |
| WO4 | rotation R⊥ (Eg) | - | 207 | - | 211 | - | 211 | - | 210 | |
| CaO8, WO4 | translation (Eg) Ca/Ca | - | 196 | - | 193 | - | 193 | - | 193 | |
| CaO8, WO4 | translation (Bg) W/W | - | 115 | - | 116 | - | 116 | - | 115 | |
| CaO8, WO4 | translation (Eg + Bg) W/W | - | 83 | - | 84 | - | 84 | - | 85 | |
10. Genetic Considerations
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- British Geological Survey. Tungsten. In British Geological Survey; BGS: Keyworth, UK, 2011; pp. 1–34. [Google Scholar]
- Yang, X. Beneficiation studies of tungsten ores—A review. Min. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Han, Z.; Golev, A.; Edraki, M.A. Review of tungsten resources and potential extraction from mine waste. Minerals 2021, 11, 701. [Google Scholar] [CrossRef]
- Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023; DG Grow, European Commission, Luxembourg Publication Office of the EU: Luxembourg, 2023; pp. 1–152. [Google Scholar]
- Rowan, L.R. Critical Mineral Resources: National Policy and Critical Minerals List; Congressional Research Service Report: CRS Report R47982 2025; Library of Congress: Washington, DC, USA, 2025; pp. 1–22. [Google Scholar]
- Hocquard, C. Les nouveaux métaux stratégiques: Métaux high-tech, «métaux verts», métaux stratégiques, vers une convergence. Mag’Mat 2008, 26, 1–30. [Google Scholar]
- Liu, Y.; Jia, D.; Zhou, Y.; Zhou, Y.; Zhao, J.; Li, Q.; Liu, B. Discovery of ABO4 scheelites with the extra low thermal conductivity through high-throughput calculations. J. Mater. 2020, 6, 702–711. [Google Scholar] [CrossRef]
- Li, S.; Bychkov, K.L.; Butenko, D.S.; Terebilenko, K.V.; Zhu, Y.; Han, W.; Baumer, V.N.; Slobodyanik, M.S.; Ji, H.; Klyui, N.I. Scheelite-related MIIxBi1−xV1−xMoxO4 (MII—Ca, Sr) solid solution-based photoanodes for enhanced photoelectrochemical water oxidation. Dalton Trans. 2020, 49, 2345–2355. [Google Scholar] [CrossRef]
- Shivakumar, C.; Saraf, R.; Behera, S.; Dhananjaya, N.; Nagabhushana, H. Scheelite-type MWO4 (M = Ca, Sr, and Ba) nanophosphors: Facile synthesis, structural characterization, photoluminescence, and photocatalytic properties. Mater. Res. Bull. 2015, 61, 422–432. [Google Scholar] [CrossRef]
- Potanina, E.A.; Orlova, A.I.; Mikhailov, D.A.; Nokhrin, A.V.; Chuvil’deev, V.N.; Boldin, M.S.; Sakharov, N.V.; Lantcev, E.A.; Tokarev, M.G.; Murashov, A.A. Spark Plasma Sintering of fine-grained SrWO4 and NaNd(WO4)2 tungstates ceramics with the scheelite structure for nuclear waste immobilization. J. Alloys Compd. 2019, 774, 182–190. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Chen, Q. Study on spectral characteristics and color origin of scheelite from Xuebaoding, Pingwu County, Sichuan Province, P.R. China. Minerals 2022, 12, 1344. [Google Scholar] [CrossRef]
- Cao, Q.; Shi, M.; Yuan, Y.; Ma, S.; Lu, H. Mineralogy and geochemical characteristics of scheelite deposit at Xuebaoding in Pingwu, Sichuan Province, China. Minerals 2024, 14, 38. [Google Scholar] [CrossRef]
- Koch, A. Űber den Vesuvian und Scheelit von Csiklova. Földtany Közlöny 1924, 54, 85–90. [Google Scholar]
- Superceanu, C. New occurrences of scheelite in contact deposits from the Banatitic geochemical province. Rev. Min. 1956, 4–5, 230–234. (In Romanian) [Google Scholar]
- Constantinescu, E.; Ilinca, G.; Ilinca, A. Laramian hydrothermal alteration and ore deposition inthe Oravița—Ciclova area, South–western Banat. D. S.—Inst. Geol. Geofiz. 1988, 72–73, 13–26. [Google Scholar]
- Cioflică, G.; Vlad, Ş.; Iosof, A.; Panican, A. Scheelite occurrences in the Bihor Massif. Rev. Roum. Géol. Géoph. Géogr. 1976, 20, 169–177. [Google Scholar]
- Berza, T.; Constantinescu, E.; Vlad, Ş.N. Upper Cretaceous magmatic series and associated mineralization in the Carpatho-Balkan Orogen. Res. Geol. 1998, 48, 291–306. [Google Scholar] [CrossRef]
- Cioflică, G.; Vlad, Ş. The correlation of the Laramian metallogenetic events belonging to the Carpatho-Balkan area. Rev. Roum. Géol. Géophys. Géogr. Sér. Géol. 1973, 17, 217–224. [Google Scholar]
- Ciobanu, C.L.; Cook, N.J.; Stein, H. Regional setting and geochronology of the Late Cretaceous Banatitic Magmatic and Metallogenetic Belt. Miner. Depos. 2002, 37, 541–567. [Google Scholar] [CrossRef]
- Zimmermann, A.; Stein, H.; Hannah, J.; Koželj, D.; Bogdanov, K.; Berza, T. Tectonic configuration of the Apuseni—Banat—Timok—Srednogorie belt, Balkans–Southern Carpathians, constrained by high precision Re–Os molybdenite ages. Miner. Depos. 2008, 43, 1–21. [Google Scholar] [CrossRef]
- Ilinca, G. Classic skarn localities of Romania: Contact metamorphism and mineralization related to Late Cretaceous magmatism. Acta Min.-Petr. 2010, 23, 1–50. [Google Scholar]
- Ilinca, G. Upper Cretaceous contact metamorphism and related mineralizations in Romania. Acta Min.-Petr. Abstr. Ser. 2012, 7, 59–64. [Google Scholar]
- Vlad, Ş.N. Banatite metallogeny of North Poiana Ruscă Mts. Revisited. Geol. Anali Balkanskoga Poluostrva 2020, 81, 67–77. [Google Scholar] [CrossRef]
- Săndulescu, M.; Kräutner, H.; Borcoş, M.; Năstăseanu, S.; Patrulius, D.; Ştefănescu, M.; Ghenea, C.; Lupu, M.; Savu, H.; Bercia, I.; et al. Geological Map of Romania, scale 1:1,000,000; Institute of Geology and Geophysics: Bucharest, Romania, 1978. [Google Scholar]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, Ş. World skarn deposits. In Economic Geology 100th Anniversary Volume; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 299–336. [Google Scholar]
- Soroiu, M.; Catilina, R.; Strutinski, C. K-Ar ages on some igneous rocks from the southwestern end of the South Carpathians (Banat Hills). Rev. Roum. Phys. 1986, 31, 849–854. [Google Scholar]
- Gallhofer, D. Magmatic Geochemistry and Geochronology in Relation to the Geodynamic and Metallogenetic Evolution of the Banat Region and Apuseni Mountains of Romania. Ph.D. Thesis, ETH Zürich, Zürich, Switzerland, 2015. Dissertation ETH no 22888. pp. 1–45. [Google Scholar]
- Bleahu, M.; Soroiu, M.; Catilina, R. On the Cretaceous tectonic-magmatic evolution of the Apuseni Mountains as revealed by K-Ar dating. Rev. Roum. Phys. 1984, 29, 123–130. [Google Scholar]
- Pavelescu, L.; Pop, G.O.; Weisz, E.; Popescu, G. La nature et l’âge du batholite banatitique de Bihor. Rep. XII-th Congress K.B.G.A. Cracow 1985, 98–101. [Google Scholar]
- Cioflică, G.; Jude, R.; Lupulescu, M. Cupriferous metallization processes associated with Upper Cretaceous–Eocene magmatites from Romania. Rom. J. Mineral. 1992, 76, 1–16. [Google Scholar]
- Vlad, Ş.N. Calcic skarns and transversal zoning in the Banat Mountains, Romania: Indicators of an Andean–type setting. Miner. Depos. 1997, 32, 446–471. [Google Scholar] [CrossRef]
- Marincea, Ş.; Dumitraş, D.-G. Contrasting types of boron-bearing deposits in magnesian skarns from Romania. Ore Geol. Rev. 2019, 112, 102952. [Google Scholar] [CrossRef]
- Cioflică, G.; Vlad, Ş.; Stoici, S. Repartition de la mineralization dans le skarn de Băița Bihorului. Rev. Roum. Géol. Géoph. Géogr. 1971, 15, 43–58. [Google Scholar]
- Tămaş, C.G.; Andrii, M.-P. Mineralogy of skarn ores from Băița Bihor, Northern Apuseni Mountains, Romania: A case study of Cu-, Bi- and Sn-minerals. Minerals 2020, 10, 436. [Google Scholar] [CrossRef]
- Armstrong, J.T. Quantitative analysis of silicates and oxide minerals: Comparison of Monte Carlo, ZAF and Phi-Rho-Z procedures. In Microbeam Analysis; Newbury, D.E., Ed.; San Francisco Press: San Francisco, CA, USA, 1988; pp. 239–246. [Google Scholar]
- Appleman, D.E.; Evans, H.T., Jr. Indexing and least-squares refinement of powder diffraction data. U.S. Geol. Surv. Comput. Contrib. 1973, 20, 60. [Google Scholar]
- Benoit, P.H. Adaptation to microcomputer of the Appleman-Evans program for indexing and least-squares refinement of powder-diffraction data for unit-cell dimensions. Am. Mineral. 1987, 72, 1018–1019. [Google Scholar]
- Kalinkina, E.V.; Kalinkin, A.M.; Forsling, W.; Makarov, A.M. Sorption of atmospheric carbon dioxide and structural changes of Ca and Mg silicate minerals during grinding. II. Enstatite, åkermanite and wollastonite. Int. J. Miner. Process. 2001, 61, 289–299. [Google Scholar] [CrossRef]
- Agilent Technologies. Xcalibur CCD System, CrysAlis Software System; Agilent Technologies: Oxfordshire, UK, 2012. [Google Scholar]
- Palatinus, L.; Chapuis, G. Superflip—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system Jana2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Xu, J.; Ciobanu, C.L.; Cook, N.; Slattery, A. Crystals from the powellite-scheelite series at the nanoscale: A case study from the Zhibula Cu skarn, Gangdese Belt, Tibet. Minerals 2019, 9, 340. [Google Scholar] [CrossRef]
- Wen, J.; Zheng, S.; Chu, X.; Jiang, H.; Zhao, Y. Mineralogical characteristics of scheelite in the Xi’an tungsten deposit, western Hunan. IOP Conf. Ser. Earth Env. Sci. 2021, 658, 012044. [Google Scholar] [CrossRef]
- Ilinca, G.; Marincea, Ş. Hydrotungstite from Oraviţa-Ciclova and Băiţa Bihor: The first occurrences in Romania. Rom. J. Mineral. 1993, 76 (Suppl. S1), 24–25. [Google Scholar]
- Poulin, R.S.; McDonald, A.M.; Kontak, D.J.; McClenaghan, M.B. On the relationship between cathodoluminescence and the chemical composition of scheelite from geologically diverse ore-deposit environments. Can. Mineral. 2016, 54, 1147–1173. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, T.; Shi, K.; White, N.C.; Fan, Y.; Wang, F.; Chen, X. Trace elements and textures of scheelite in porphyry-skarn Cu-Au systems: The example of Dongguashan deposit, eastern China. Ore Geol. Rev. 2022, 149, 105069. [Google Scholar] [CrossRef]
- Miranda, A.C.R. Chemical Composition of Scheelite and its Application as An Indicator Mineral. Ph.D. Thesis, Laval University, Québec, QC, Canada, 2023; pp. 1–459. [Google Scholar]
- Kay, M.I.; Frazer, B.C.; Almodovar, I. Neutron diffraction refinement of CaWO4. J. Chem. Phys. 1964, 40, 504–506. [Google Scholar] [CrossRef]
- Blanchard, F. X-ray powder data for CaWO4, synthetic scheelite. Powder Diffr. 1989, 4, 220–222. [Google Scholar] [CrossRef]
- Sleight, A.W. Accurate cell dimensions for ABO4 molybdates and tungstates. Acta Cryst. 1972, 28, 2899–2902. [Google Scholar] [CrossRef]
- Senyshyn, A.; Kraus, H.; Mikhailik, V.B.; Yakovyna, V. Lattice dynamics and thermal properties of CaWO4. Phys. Rev. 2004, 70, 214306. [Google Scholar] [CrossRef]
- Hazen, R.M.; Finger, L.W.; Mariathasan, J.W. High-pressure crystal chemistry of scheelite-type tungstates and molybdates. J. Phys. Chem Solids 1985, 46, 253–263. [Google Scholar] [CrossRef]
- Rabuffetti, F.A.; Culver, S.P.; Suescun, L.; Brutchey, R.L. Structural disorder in AMoO4 (A = Ca, Sr, Ba) scheelite nanocrystals. Inorg. Chem. 2014, 53, 1056–1061. [Google Scholar] [CrossRef]
- Shoji, T.; Sasaki, N. Fluorescent color and X-ray powder data of synthesized scheelite- powellite series as guides to determine its composition. Min. Geol. 1978, 28, 397–404. [Google Scholar]
- Palmer, M.C. Geochemical Characterisation of Scheelite from New Zealand. Ph.D. Thesis, Otago University, Dunedin, New Zealand, 2021; pp. 1–220. [Google Scholar]
- Lu, D.; Mao, J.; Ye, H.; Wang, P.; Chao, W.; Yu, M. Geochemistry of scheelite from Jiangligou skarn W-(Cu-Mo) deposit in the West Qinling orogenic belt, Northwest China: Implication on the multistage ore-forming processes. Ore Geol. Rev. 2023, 159, 105525. [Google Scholar] [CrossRef]
- Palache, C.; Berman, H.; Frondel, C. The System of Mineralogy. II. Halides, Nitrates, Borates, Carbonates, Sulfates, Phosphates, Arsenates, Tungstates, Molybdates, etc.; John Wiley and Sons: New York, NY, USA, 1951; pp. 1–1124. [Google Scholar]
- Mandarino, J.A. The Gladstone-Dale relationship—Part I: Derivation of new constants. Can. Mineral. 1976, 14, 498–502. [Google Scholar]
- Zalkin, A.; Templeton, D.H. X-ray diffraction refinement of the calcium tungstate structure. J. Chem. Phys. 1964, 40, 501–504. [Google Scholar] [CrossRef]
- Mandarino, J.A. The Gladstone-Dale relationship. IV. The compatibility concept and its application. Can. Mineral. 1981, 19, 441–450. [Google Scholar]
- Larsen, E.S. The microscopic determination of the nonopaque minerals. U.S. Geol. Surv. Bull. 1921, 679, 1–289. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Cryst. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Barker, A.S. Infrared lattice vibrations in calcium tungstate and calcium molybdate. Phys. Rev. A 1964, 135, 742–747. [Google Scholar] [CrossRef]
- Clark, G.M.; Doyle, W.P. IR spectra of anhydrous molybdates and tungstates. Spectrochim. Acta 1966, 22, 1441–1447. [Google Scholar] [CrossRef]
- Khanna, R.K.; Lippincott, E.R. Infrared spectra of some scheelite structures. Spectrochim. Acta A 1968, 24, 905–908. [Google Scholar] [CrossRef]
- Zorina, M.L.; Syritso, L.F. IR spectra and structures of tungstates. J. Appl. Crystallogr. 1972, 16, 774–776. [Google Scholar] [CrossRef]
- Tarte, P.; Liegeois-Duyckaerts, M. Vibrational studies of molybdates, tungstates and related compounds—I. New infrared data and assignments for the scheelite-type compounds XIIMoO4 and XIIWO4. Spectrochim. Acta A 1972, 28, 2029–2036. [Google Scholar] [CrossRef]
- Iishi, K. Study of the force field of scheelite. Z. Kristallogr. 1975, 141, 31–58. [Google Scholar] [CrossRef]
- Zhou, P.L. Infrared spectra of wolframite and scheelite from tungsten ore deposits in Southern Ganzhou, Jiangxi Province. Acta Mineral. Sinca 1984, 4, 319–322. [Google Scholar]
- Akimov, A.N.; Nikanovich, M.V.; Popov, V.G.; Umreiko, D.S. Calculation and investigation of the vibrational spectra of scheelite structures MeWO4 (Me = Ca, Sr, Ba, Pb). Zh. Prikladn. Spektr. 1986, 45, 225–232. (In Russian) [Google Scholar] [CrossRef]
- Chukanov, N.V. Infrared Spectra of Mineral Species. Extended Library; Springer Geochemistry/Mineralogy: Berlin/Heidelberg, Germany, 2014; pp. 1–1726. [Google Scholar]
- Russell, J.P.; Loudon, R. The first-order Raman spectrum of calcium tungstate. Proc. Phys. Soc. 1965, 85, 1029–1033. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Scott, J.F. Raman Spectra of CaWO4, SrWO4, CaMoO4, and SrMoO4. Phys. Rev. 1967, 157, 716–721. [Google Scholar] [CrossRef]
- Griffith, W.P. Raman studies on rock-forming minerals. Part II. Minerals containing MO3, MO4, and MO6 groups. J. Chem. Soc. A 1970, 2, 286–291. [Google Scholar] [CrossRef]
- Liegeois-Duyckaerts, M.; Tarte, P. Vibrational studies of molybdates, tungstates and related compounds—II. New Raman data and assignments for the scheelite-type compounds. Spectrochim. Acta A 1972, 28, 2037–2051. [Google Scholar] [CrossRef]
- Basiev, T.T.; Sobol, A.A.; Voronko, Y.K.; Zverev, P.G. Spontaneous Raman spectroscopy of tungstate and molybdate crystals for Raman lasers. Opt. Mater. 2000, 15, 205–216. [Google Scholar] [CrossRef]
- Crane, M.; Frost, R.L.; Williams, P.A.; Kloprogge, J.T. Raman spectroscopy of the molybdate minerals chillagite (tungstenian wulfenite-I4), stolzite, scheelite, wolframite and wulfenite. J. Raman Spectrosc. 2002, 33, 62–66. [Google Scholar] [CrossRef]
- Frost, R.L.; Duong, L.; Weier, M. Raman microscopy of selected tungstate minerals. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 1853–1859. [Google Scholar] [CrossRef]
- Müller, A.; Krebs, B. Normal coordinate treatment of XY4-type molecules and ions with Td symmetry. Part I. Force constants of a modified valence force field and of the Urey-Bradley force field. J. Molec. Spectr. 1967, 24, 180–197. [Google Scholar] [CrossRef]
- Khanna, R.K.; Brower, W.S.; Guscott, B.R.; Lippincott, E.R. Laser induced Raman spectra of some tungstates and molybdates. J. Res. Nat. Inst. Stand. Techn. Phys. Chem. A 1968, 72, 81–84. [Google Scholar] [CrossRef]
- Kanamori, H.; Hayashi, S.; Ikeda, Y.Y. External lattice vibration modes in scheelites. J. Phys. Soc. Jap. 1974, 36, 511–516. [Google Scholar] [CrossRef]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C.; Gerand, B.; Figlarz, M. Infrared and Raman study of WO3⋅xH2O tungsten trioxide hydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectr. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Newberry, R.; Swanson, S. Scheelite skarn granitoids: An evaluation of the roles of magmatic source and process. Ore Geol. Rev. 1986, 1, 57–81. [Google Scholar] [CrossRef]
- Hsu, L.C.; Galli, P. E, Origin of the scheelite-powellite series of minerals. Econ. Geol. 1973, 68, 681–696. [Google Scholar] [CrossRef]
- Hsu, L.C. Effects of oxygen and sulfur fugacities on the scheelite-tungstenite and powellite-molybdenite stability relations. Econ. Geol. 1977, 72, 664–670. [Google Scholar] [CrossRef]
- Ishihara, S. The magnetite-series and ilmenite-series granitic rocks. Mining Geol. 1977, 27, 293–305. [Google Scholar]
- Ishihara, S. The granitoid series and mineralization. In Economic Geology 75th Anniversary Volume; The Economic Geology Publishing Company: Littleton, CO, USA, 1981; pp. 458–484. [Google Scholar]
- Sato, K. Tungsten skarn deposit of the Fujigatani Mine, Southwest Japan. Econ. Geol. 1980, 75, 1066–1082. [Google Scholar] [CrossRef]
- Song, G.X.; Qin, K.Z.; Li, G.; Evans, N.J.; Chen, J. Scheelite elemental and isotopic signatures: Implication for the genesis of skarn-type W-Mo deposits in the Chizhou area, Anhui Province, eastern China. Amer. Miner. 2014, 99, 303–317. [Google Scholar] [CrossRef]
- Warr, L.N. IMA-CNMNC approved mineral symbols. Min. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Wang, X.-S.; Williams-Jones, A.E.; Hu, R.Z.; Lin-Bo, S.; Xian-Wu, B. The role of fluorine in granite-related hydrothermal tungsten ore genesis: Results of experiments and modelling. Geochim. Cosmochim. Acta 2021, 292, 170–187. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, R.; Chou, I.-M.; Wang, X.; Hu, W.; Zhang, W.; Lu, J.; Li, G.; Li, Z. Boron-rich ore-forming fluids in hydrothermal W-Sn deposits from South China: Insights from in situ Raman spectroscopic characterization of fluid inclusions. Ore Geol. Rev. 2021, 132, 104048. [Google Scholar] [CrossRef]



| Occurrence | Intrusion | Age of Intrusion | Protolith | Age of Protolith | Structural Unit |
|---|---|---|---|---|---|
| Ciclova Oraviţa | Ciclova-Oraviţa pluton | 79 ± 3 to 74 ± 3 Ma [26] (1) 73.9 ± 3.2 to 71.2 ± 4.1 Ma [27] (2) 86.77 ± 0.5 to 87.70 ± 0.5 [20] (3) | carbonated veins in endoskarn | Mesozoic | Locva unit |
| Băiţa Bihor | Bihor batholith | 77 ± 3 to 67 ± 3 Ma [28] (4) 70 ± 5 Ma [29] (5) 80.63 ± 0.3 to 78.69 ± 0.4 Ma [20] (3) 80.3 ± 1.6 Ma [27] (2) | dolostones | Anisian-Carnian; Carnian-Norian | Vălani unit; Vetre unit |
| Sample | 2506a | 2506b | 2506c | 2509a | 2509b | 2509c | 2534a | 2534b | 2575a | 2575b | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (1) | 12 | 15 | 16 | 11 | 13 | 13 | 14 | 11 | 12 | 11 | 128 |
| WO3 | 79.81 | 79.41 | 78.31 | 78.91 | 79.24 | 79.14 | 79.40 | 79.16 | 79.24 | 78.49 | 79.10 |
| MoO3 | 0.63 | 0.63 | 1.21 | 1.21 | 1.14 | 1.05 | 0.77 | 1.12 | 1.12 | 0.87 | 0.97 |
| Bi2O3 | 0.03 | 0.02 | 0.03 | 0.01 | 0.04 | 0.02 | 0.01 | 0.03 | 0.03 | 0.04 | 0.03 |
| CaO | 19.44 | 19.40 | 19.45 | 19.49 | 19.38 | 19.37 | 19.31 | 19.31 | 19.32 | 19.25 | 19.37 |
| MgO | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MnO | 0.02 | 0.03 | 0.04 | 0.03 | 0.02 | 0.03 | 0.06 | 0.04 | 0.03 | 0.02 | 0.03 |
| FeO (2) | 0.03 | 0.06 | 0.03 | 0.02 | 0.04 | 0.05 | 0.02 | 0.02 | 0.04 | 0.04 | 0.04 |
| PbO | 0.03 | 0.05 | 0.03 | 0.03 | 0.05 | 0.07 | 0.07 | 0.02 | 0.05 | 0.00 | 0.04 |
| CuO | 0.01 | 0.01 | 0.03 | 0.03 | 0.02 | 0.02 | 0.05 | 0.01 | 0.03 | 0.02 | 0.02 |
| Total | 100.00 | 99.63 | 99.13 | 99.73 | 99.93 | 99.75 | 99.67 | 99.71 | 99.86 | 98.73 | 99.60 |
| Number of Cations on the Basis of 4(O) | |||||||||||
| W | 0.988 | 0.986 | 0.974 | 0.976 | 0.979 | 0.980 | 0.986 | 0.980 | 0.980 | 0.982 | 0.981 |
| Mo | 0.013 | 0.013 | 0.024 | 0.024 | 0.023 | 0.021 | 0.015 | 0.022 | 0.022 | 0.018 | 0.019 |
| Bi | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ca | 0.995 | 0.996 | 1.000 | 0.996 | 0.990 | 0.992 | 0.991 | 0.989 | 0.988 | 0.996 | 0.994 |
| Mg | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mn | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 |
| Fe2+ | 0.001 | 0.002 | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 |
| Pb | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 |
| Cu | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.000 | 0.001 | 0.001 | 0.001 |
| Composition in End Members (mol.%) | |||||||||||
| scheelite | 98.70 | 98.70 | 97.60 | 97.60 | 97.70 | 97.90 | 98.50 | 97.80 | 97.80 | 98.20 | 98.10 |
| powellite | 1.30 | 1.30 | 2.40 | 2.40 | 2.30 | 2.10 | 1.50 | 2.20 | 2.20 | 1.80 | 1.90 |
| Sample | 2537a | 2537b | 2537c | 2538a | 2538b | 2538c | 2539 | 2568 | 2569a | 2569b | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (1) | 5 | 6 | 7 | 9 | 7 | 6 | 11 | 16 | 12 | 11 | 90 |
| WO3 | 79.86 | 79.30 | 79.87 | 80.38 | 80.36 | 80.48 | 79.42 | 77.79 | 77.95 | 77.82 | 79.03 |
| MoO3 | 0.14 | 0.33 | 0.25 | 0.37 | 0.32 | 0.27 | 1.32 | 2.85 | 1.92 | 1.97 | 1.29 |
| Bi2O3 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.02 | 0.02 | 0.00 | 0.01 | 0.01 |
| CaO | 19.29 | 19.53 | 19.55 | 19.42 | 19.36 | 19.44 | 19.56 | 19.90 | 19.46 | 19.42 | 19.53 |
| MgO | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 |
| MnO | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 |
| FeO (2) | 0.05 | 0.04 | 0.03 | 0.03 | 0.05 | 0.17 | 0.02 | 0.02 | 0.02 | 0.02 | 0.04 |
| PbO | 0.04 | 0.00 | 0.03 | 0.01 | 0.05 | 0.02 | 0.02 | 0.02 | 0.04 | 0.01 | 0.02 |
| CuO | 0.02 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 |
| Total | 99.41 | 99.22 | 99.76 | 100.22 | 100.17 | 100.39 | 100.38 | 100.61 | 99.40 | 99.26 | 99.94 |
| Number of Cations on the Basis of 4(O) | |||||||||||
| W | 0.997 | 0.990 | 0.993 | 0.994 | 0.995 | 0.994 | 0.975 | 0.944 | 0.963 | 0.963 | 0.975 |
| Mo | 0.003 | 0.007 | 0.005 | 0.007 | 0.006 | 0.005 | 0.026 | 0.056 | 0.038 | 0.039 | 0.025 |
| Bi | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ca | 0.996 | 1.008 | 1.004 | 0.993 | 0.991 | 0.993 | 0.993 | 0.998 | 0.994 | 0.993 | 0.996 |
| Mg | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 |
| Mn | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Fe2+ | 0.002 | 0.002 | 0.001 | 0.001 | 0.002 | 0.007 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 |
| Pb | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 |
| Cu | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Composition in End Members (mol.%) | |||||||||||
| scheelite | 99.70 | 99.30 | 99.50 | 99.30 | 99.40 | 99.50 | 97.40 | 94.40 | 96.20 | 96.11 | 97.50 |
| powellite | 0.30 | 0.70 | 0.50 | 0.70 | 0.60 | 0.50 | 2.60 | 5.60 | 3.80 | 3.89 | 2.50 |
| Sample | 2536 | 2544 | 2549 | 2552 | 2576 | 2577 | 2590 | 2591 | 2592 | 2593 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (1) | 11 | 10 | 8 | 9 | 12 | 10 | 10 | 13 | 11 | 11 | 105 |
| WO3 | 79.70 | 79.48 | 79.56 | 78.41 | 78.81 | 78.93 | 78.60 | 79.28 | 79.49 | 78.97 | 79.13 |
| MoO3 | 0.63 | 0.73 | 0.61 | 1.13 | 1.29 | 0.88 | 1.16 | 1.17 | 0.84 | 0.88 | 0.95 |
| Bi2O3 | 0.03 | 0.03 | 0.00 | 0.04 | 0.04 | 0.01 | 0.01 | 0.00 | 0.02 | 0.05 | 0.02 |
| CaO | 19.48 | 19.4 | 19.37 | 19.42 | 19.36 | 19.26 | 19.43 | 19.5 | 19.26 | 19.23 | 19.37 |
| MgO | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MnO | 0.02 | 0.07 | 0.01 | 0.04 | 0.03 | 0.02 | 0.03 | 0.02 | 0.01 | 0.03 | 0.03 |
| FeO (2) | 0.04 | 0.03 | 0.09 | 0.03 | 0.07 | 0.03 | 0.01 | 0.03 | 0.04 | 0.03 | 0.04 |
| PbO | 0.04 | 0.02 | 0.08 | 0.03 | 0.04 | 0.01 | 0.03 | 0.05 | 0.04 | 0.00 | 0.03 |
| CuO | 0.01 | 0.00 | 0.01 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.01 | 0.02 |
| Total | 99.95 | 99.76 | 99.78 | 99.12 | 99.67 | 99.17 | 99.30 | 100.08 | 99.73 | 99.20 | 99.59 |
| Number of Cations on the Basis of 4(O) | |||||||||||
| W | 0.987 | 0.986 | 0.987 | 0.976 | 0.975 | 0.984 | 0.976 | 0.978 | 0.986 | 0.984 | 0.982 |
| Mo | 0.013 | 0.015 | 0.012 | 0.023 | 0.026 | 0.018 | 0.023 | 0.023 | 0.017 | 0.018 | 0.019 |
| Bi | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 |
| Ca | 0.997 | 0.995 | 0.993 | 0.999 | 0.991 | 0.992 | 0.998 | 0.994 | 0.988 | 0.991 | 0.994 |
| Mg | 0.000 | 0.000 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mn | 0.001 | 0.003 | 0.000 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 |
| Fe2+ | 0.002 | 0.001 | 0.004 | 0.001 | 0.003 | 0.001 | 0.000 | 0.001 | 0.002 | 0.001 | 0.002 |
| Pb | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 |
| Cu | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 |
| Composition in End Members (mol.%) | |||||||||||
| scheelite | 98.70 | 98.50 | 98.80 | 97.70 | 97.40 | 98.20 | 97.70 | 97.70 | 98.31 | 98.20 | 98.10 |
| powellite | 1.30 | 1.50 | 1.20 | 2.30 | 2.60 | 1.80 | 2.30 | 2.30 | 1.69 | 1.80 | 1.90 |
| Sample | a (Å) | c (Å) | V (Å3) |
|---|---|---|---|
| Ciclova | |||
| 1189 (1) | 5.230(2) | 11.356(7) | 310.67(8) |
| 2492 (2) | 5.2431(1) | 11.3743(5) | 312.68(2) |
| 2506 | 5.243(4) | 11.366(14) | 312.46(53) |
| 2509 | 5.234(2) | 11.393 (15) | 312.06(37) |
| Oraviţa | |||
| 535 (1) | 5.231(1) | 11.343(3) | 310.41(14) |
| 2491 | 5.239(4) | 11.402 (9) | 313.02(53) |
| 2537 | 5.229(4) | 11.372(14) | 310.92(57) |
| 2569 (2) | 5.2425(2) | 11.3703(7) | 312.499(2) |
| Băiţa Bihor | |||
| Sch 01 (3) | 5.240(2) | 11.373(5) | 312.25(20) |
| Sch 02 (3) | 5.2384(9) | 11.370(4) | 312.00(12) |
| Sch 03 (3) | 5.2377(8) | 11.366(2) | 311.82(10) |
| 1050 (1) | 5.232(2) | 11.375(8) | 311.34(25) |
| 2536 | 5.243(1) | 11.380(4) | 312.85(16) |
| 2576 (2) | 5.2409(2) | 11.3705(6) | 312.314(2) |
| 2593 (2) | 5.2388(2) | 11.3667(9) | 311.959(1) |
| Sample | 2509b | 2539 | 2544 |
|---|---|---|---|
| Occurrence | Ciclova | Oraviţa | Băiţa Bihor |
| ω | 1.928 | 1.929 | 1.931 |
| ε | 1.945 | 1.946 | 1.948 |
| n | 1.934 | 1.935 | 1.937 |
| Mmol (1) | 286.298 | 285.614 | 286.801 |
| V (Å3) | 313.11 | 311.90 | 312.31 |
| Dm (g/cm3) | 6.08(1) | 6.08(1) | 6.10(1) |
| Dx (g/cm3) | 6.071 | 6.080 | 6.098 |
| KP (2) | 0.1536 | 0.1538 | 0.1536 |
| KC | 0.1492 | 0.1494 | 0.1488 |
| 1 − KP/KC | −0.0298 | −0.0294 | −0.0323 |
| KP′ (3) | 0.1538 | 0.1538 | 0.1537 |
| 1 − KP′/KC | −0.0313 | −0.0293 | −0.0327 |
| Sample | 2509 | 2538 | 2544 |
|---|---|---|---|
| Occurrence | Ciclova | Oravița | Băița Bihor |
| a (Å) | 5.2459(10) | 5.2380(2) | 5.2409(2) |
| c (Å) | 11.3777(5) | 11.3679(8) | 11.3705(6) |
| V (Å3) | 313.108(18) | 311.90(3) | 312.31(3) |
| Z | 4 | 4 | 4 |
| Dx (g/cm3) | 6.108 | 6.132 | 6.124 |
| Crystal size (mm3) | 0.177 × 0.124 × 0.091 | 0.35 × 0.22 × 0.1 | 0.283 × 0.21 × 0.195 |
| Absorption coefficient (mm−1) | 38.351 | 38.502 | 38.449 |
| F(000) | 504 | 504 | 504 |
| Max. 2ө (°) | 56.994 | 57.766 | 57.74 |
| Range of indices | −6 ≤ h ≤ 6 | −5 ≤ h ≤ 6 | −7 ≤ h ≤ 6 |
| −5 ≤ k ≤ 6 | −4 ≤ k ≤ 6 | −7 ≤ k ≤ 5 | |
| −13 ≤ l ≤ 15 | −14 ≤ l ≤ 14 | −12 ≤ l ≤ 15 | |
| Number of measured reflections | 1031 | 1011 | 1051 |
| Number of unique reflections | 188 | 191 | 194 |
| Independent non-zero reflections | 176 | 180 | 180 |
| Criterion for observed reflections | I > 2σ(I) | I > 2σ(I) | I > 2σ(I) |
| Number of refined parameters | 16 | 16 | 16 |
| R int | 0.0212 | 0.0261 | 0.0327 |
| R sigma | 0.0158 | 0.0181 | 0.0216 |
| R1 (F) with F0 > 4 σ(F0) * | 0.0144 | 0.0189 | 0.0216 |
| R1 (F) for all the unique reflections * | 0.0165 | 0.0204 | 0.0237 |
| wR2 (F2) * | 0.0348 | 0.0477 | 0.0539 |
| S (“goodness of fit”) | 1.227 | 1.267 | 1.206 |
| Min./max. residual e density, (eÅ−3) | −0.88/0.61 | −2.46/0.77 | −1.71/1.00 |
| Weighing scheme | 1/(σ2(I)2+ 0.0025(I)2 | ||
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|
| Sample 2509 Ciclova | ||||||
| Ca | 3.4(9) | 3.4(9) | 1.8(11) | 0 | 0 | 0 |
| W | 3.96(17) | 3.96(17) | 4.9(2) | 0 | 0 | 0 |
| O | 14.0(19) | 14.7(18) | 12.0(15) | −2.0(14) | −1.1(13) | −2.7(14) |
| Sample 2538 Oravița | ||||||
| Ca | 2.2(12) | 2.2(12) | 2.8(15) | 0 | 0 | 0 |
| W | 2.6(2) | 2.6(2) | 6.2(3) | 0 | 0 | 0 |
| O | 11(2) | 11(2) | 10(2) | 1.9(17) | −2.0(17) | 0.3(15) |
| Sample 2544 Băița Bihor | ||||||
| Ca | 7.7(13) | 7.7(13) | 4.2(16) | 0 | 0 | 0 |
| W | 7.6(3) | 7.6(3) | 8.4(3) | 0 | 0 | 0 |
| O | 16(2) | 18(2) | 15(2) | −1(2) | −3(2) | 1.9(18) |
| Atom | x | y | z | U (eq) |
|---|---|---|---|---|
| Sample 2509 Ciclova | ||||
| Ca | 0 | 7500 | 8750 | 2.9(8) |
| W | 0 | 2500 | 6250 | 4.27(15) |
| O | 1515(6) | 4909(6) | 7108(3) | 13.6(10) |
| Sample 2538 Oravița | ||||
| Ca | 0 | 7500 | 3750 | 2.4(10) |
| W | 5000 | 7500 | 6250 | 3.8(2) |
| O | 2594(7) | 5980(7) | 5392(4) | 10.7(12) |
| Sample 2544 Băița Bihor | ||||
| Ca | 0 | 7500 | 3750 | 6.5(11) |
| W | 5000 | 7500 | 6250 | 7.8(2) |
| O | 2592(9) | 6000(8) | 5393(4) | 16.4(14) |
| Sample | 2509 | 2538 | 2544 |
|---|---|---|---|
| Occurrence | Ciclova | Oravița | Băița Bihor |
| Bond distances (Å) | |||
| Ca-O x 4 | 2.443(4) | 2.442(5) | 2.440(5) |
| Ca-O x 4 | 2.478(4) | 2.474(4) | 2.482(5) |
| W-O x 4 | 1.784(4) | 1.781(4) | 1.778(5) |
| Occupancy | |||
| Ca | 0.83(15) | 0.84(2) | 0.83(2) |
| W | 0.91(10) | 0.91(14) | 0.87(15) |
| Sample 2509 Ciclova | Sample 2538 Oravița | Sample 2544 Băița Bihor | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | W | Σ | Ca | W | Σ | Ca | W | Σ | |
| O | 0.276 × 4 ↓ | 1.436 × 4 ↓ | 1.963 | 0.277 × 4 ↓ | 1.444 × 4 ↓ | 1.98 | 0.278 × 4 ↓ | 1.456 × 4 ↓ | 1.98 |
| 0.251 × 4 ↓ | 0.254 × 4 ↓ | 0.249 × 4 ↓ | |||||||
| Σ | 2.11 | 5.73 | 2.12 | 5.77 | 2.11 | 5.82 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marincea, Ş.; Dumitraş, D.-G.; Sava Ghineț, C.; Dincă, G.; Iancu, A.-M.; Hatert, F.; Depret, M.; Costin, G. Hydrothermal Scheelite Associated with Upper Cretaceous Intrusions in Romania: A Mineralogical Insight to the W Metallogeny. Minerals 2025, 15, 854. https://doi.org/10.3390/min15080854
Marincea Ş, Dumitraş D-G, Sava Ghineț C, Dincă G, Iancu A-M, Hatert F, Depret M, Costin G. Hydrothermal Scheelite Associated with Upper Cretaceous Intrusions in Romania: A Mineralogical Insight to the W Metallogeny. Minerals. 2025; 15(8):854. https://doi.org/10.3390/min15080854
Chicago/Turabian StyleMarincea, Ştefan, Delia-Georgeta Dumitraş, Cristina Sava Ghineț, George Dincă, Aurora-Măruța Iancu, Frédéric Hatert, Martin Depret, and Gelu Costin. 2025. "Hydrothermal Scheelite Associated with Upper Cretaceous Intrusions in Romania: A Mineralogical Insight to the W Metallogeny" Minerals 15, no. 8: 854. https://doi.org/10.3390/min15080854
APA StyleMarincea, Ş., Dumitraş, D.-G., Sava Ghineț, C., Dincă, G., Iancu, A.-M., Hatert, F., Depret, M., & Costin, G. (2025). Hydrothermal Scheelite Associated with Upper Cretaceous Intrusions in Romania: A Mineralogical Insight to the W Metallogeny. Minerals, 15(8), 854. https://doi.org/10.3390/min15080854








