Advances in Graphite Recycling from Spent Lithium-Ion Batteries: Towards Sustainable Resource Utilization
Abstract
1. Introduction
2. Graphite: Properties and Applications
2.1. Properties
2.2. Applications
3. Graphite for LIBs: Primary Sources, Global Market and Production Processes
3.1. Primary Sources
3.2. Global Market: Supply and Demand
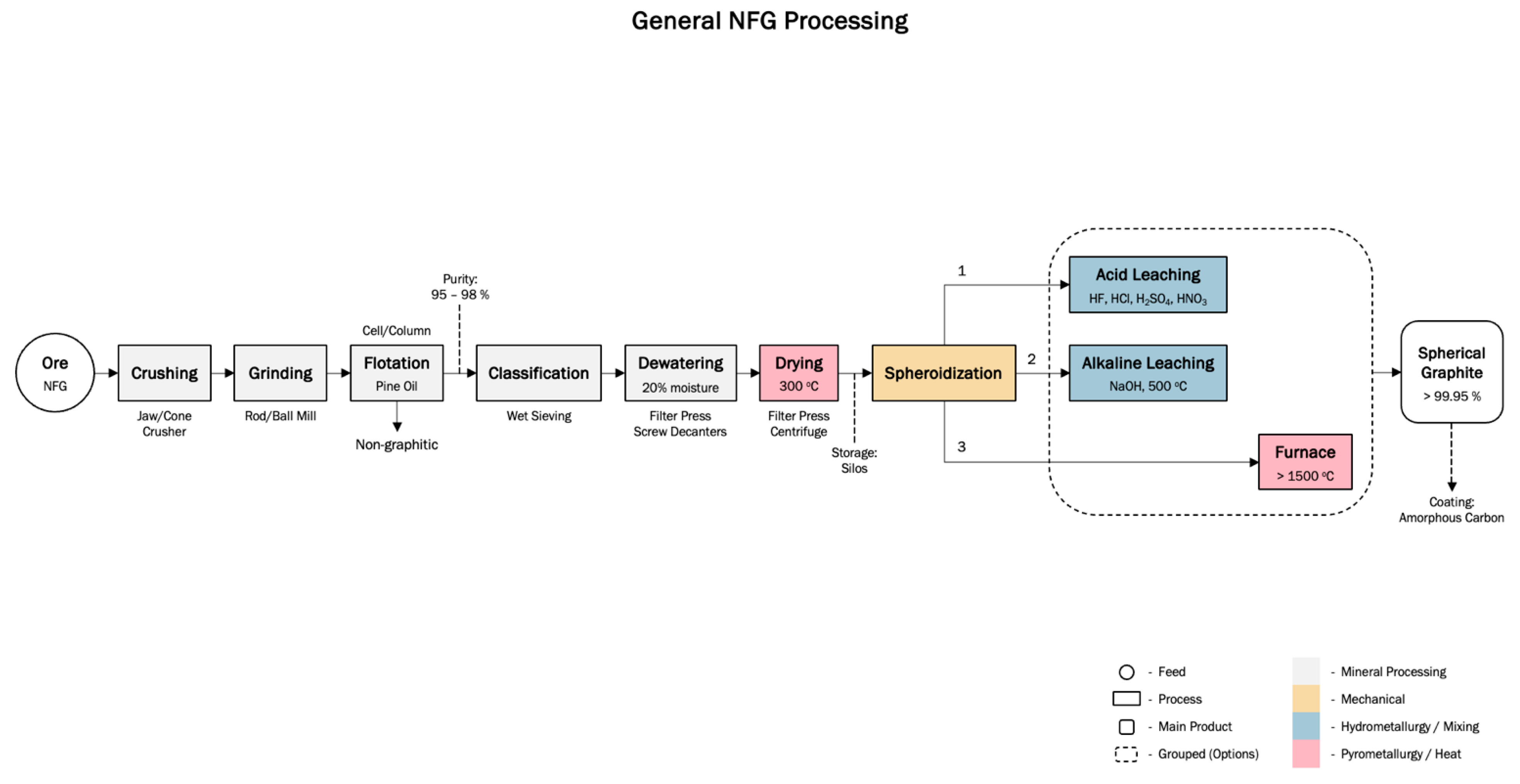
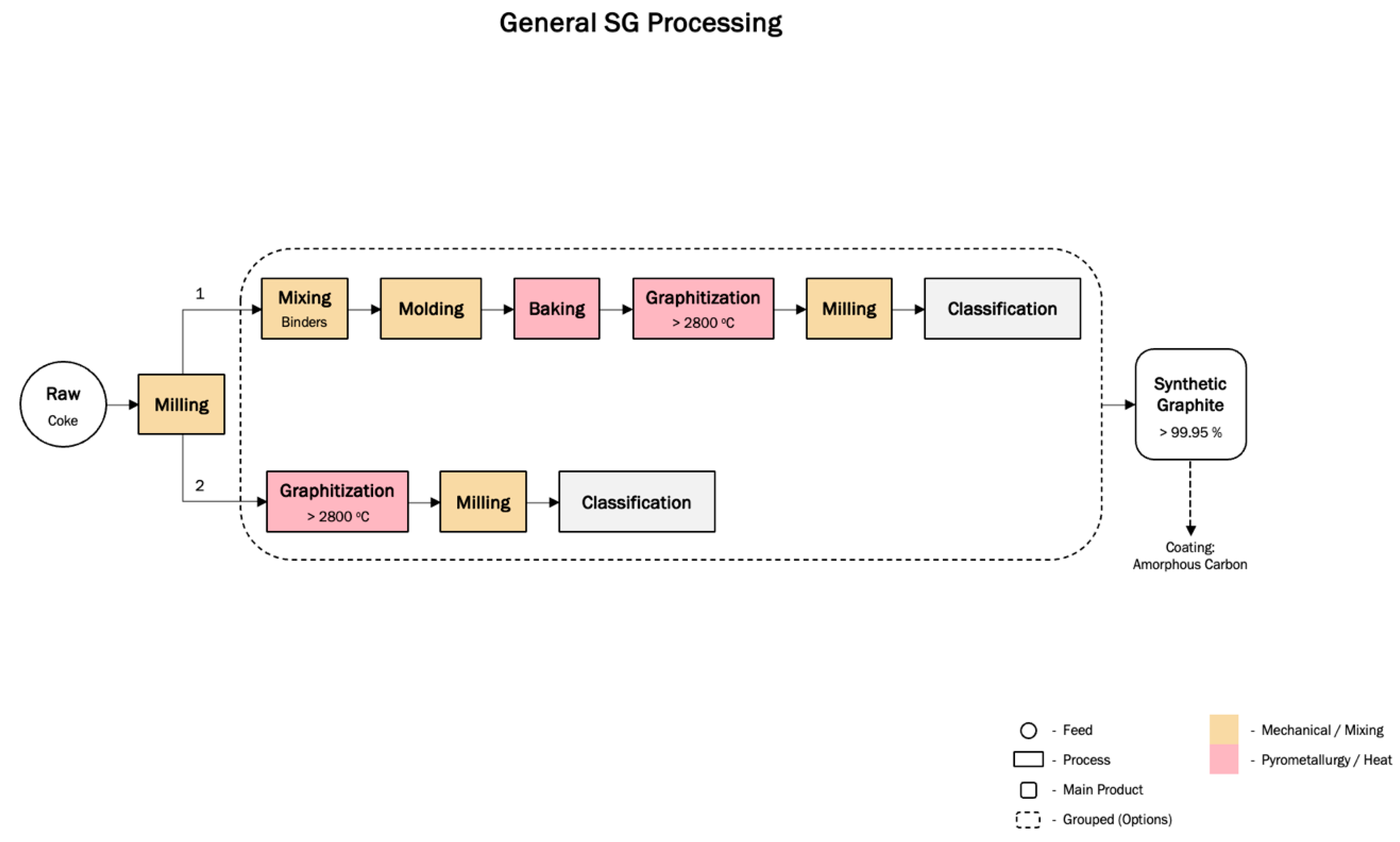
3.3. Production Processes
3.3.1. Natural Graphite Production
3.3.2. Synthetic Graphite Production
4. Graphite Recycling: Secondary Sources, Current Processing and Emerging Technologies
4.1. Spent LIBs
Metallurgical-Based Recycling Process
4.2. Graphite Recycling
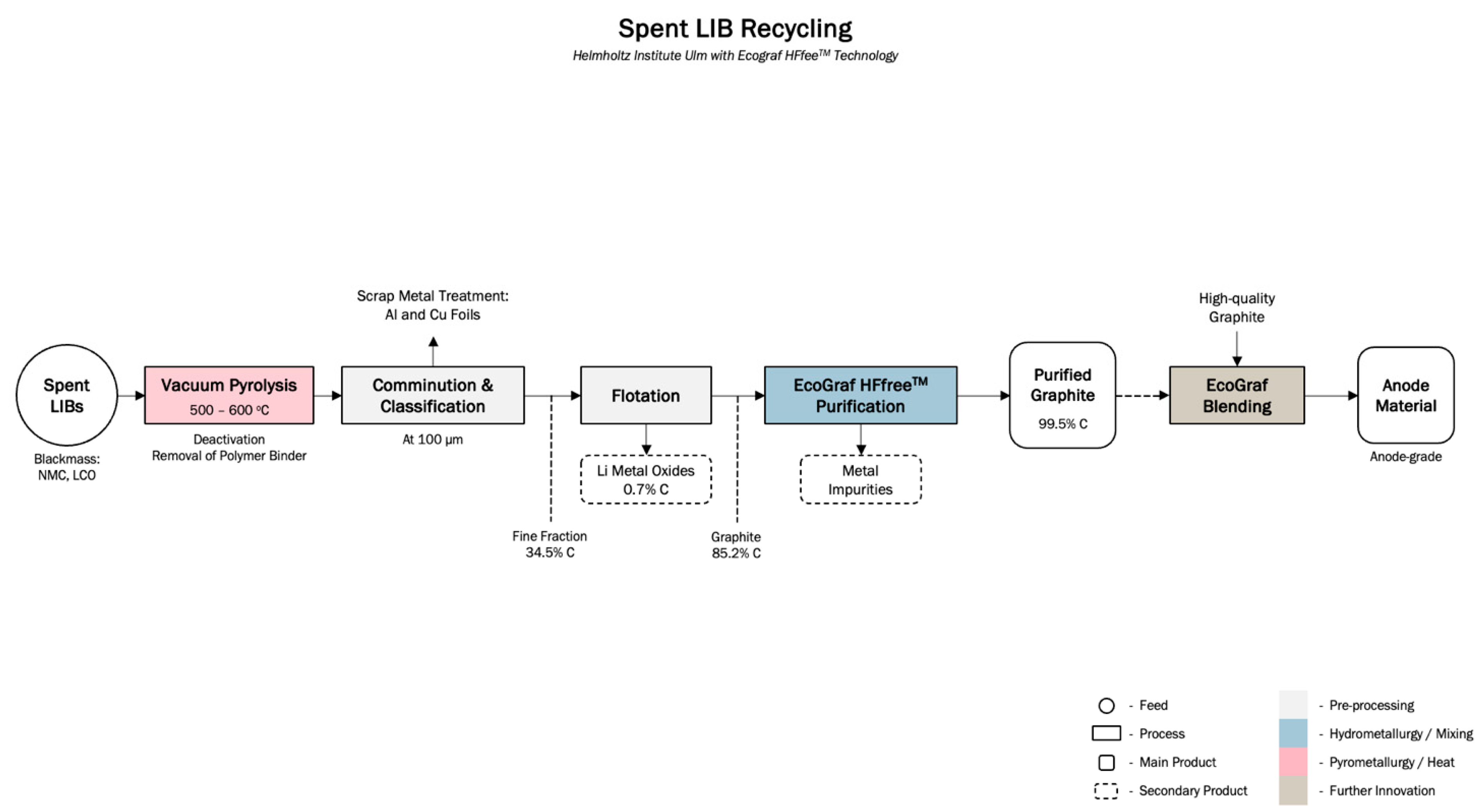
4.2.1. Graphite Recycling Program and Emerging Technologies
4.2.2. Graphite Recycling Studies
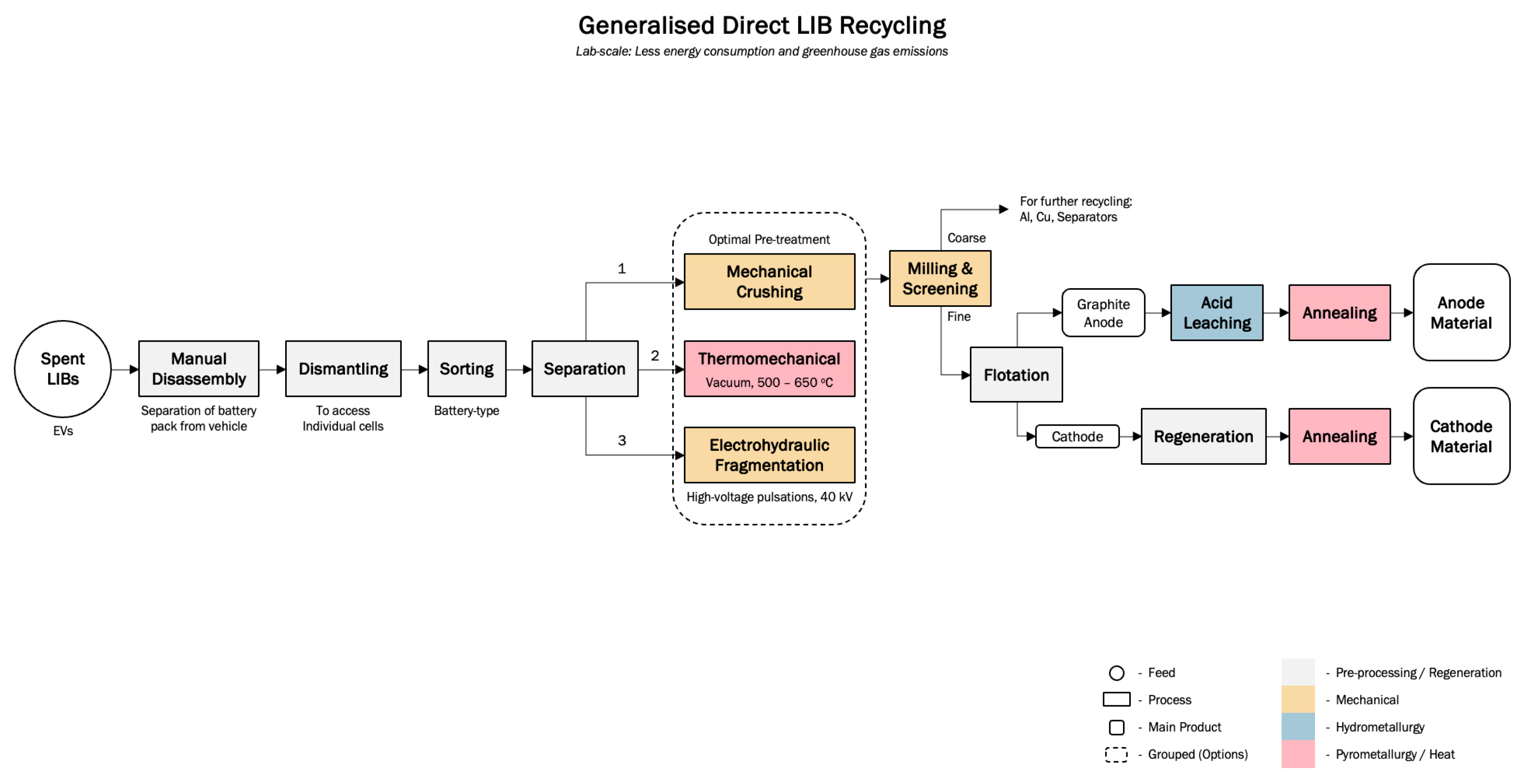
4.2.3. Direct Recycling of Spent LIBs
4.3. Other Secondary Sources
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Lu, Y.; Adelhelm, P.; Titirici, M.-M.; Hu, Y.-S. Intercalation chemistry of graphite: Alkali metal ions and beyond. Chem. Soc. Rev. 2019, 48, 4655–4687. [Google Scholar] [CrossRef]
- Frohs, W.; Jäger, H. Industrial Carbon and Graphite Materials: Raw Materials, Production and Applications; Wiley-VCH: Weinheim, Germany, 2021. [Google Scholar]
- Pierson, H.O. Handbook of Carbon, Graphite, Diamond, and Fullerenes: Properties, Processing, and Applications; Noyes Publications: Park Ridge, NJ, USA, 1993. [Google Scholar]
- International Energy Agency. The Role of Critical Minerals in Clean Energy Transitions; IEA: Paris, France, 2021. [Google Scholar]
- European Commission; Directorate-General for Internal Market, Industry Entrepreneurship; Smes; Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- U.S. Geological Survey. Mineral Commodity Summaries 2025; U.S. Geological Survey: Reston, VA, USA, 2025; p. 212. [Google Scholar]
- Bauer, D.; Khazdozian, H.; Mehta, J.; Nguyen, R.T.; Severson, M.H.; Vaagensmith, B.C.; Toba, L.; Zhang, B.; Hossain, T.; Sibal, A.P.; et al. 2023 Critical Materials Strategy; U.S. Department of Energy: Washington, DC, USA, 2023. [Google Scholar]
- Hughes, A.; Britt, A.; Pheeney, J.; Morfiadakis, A.; Kucka, C.; Colclough, H.; Munns, C.; Senior, A.; Cross, A.; Hitchman, A.; et al. Australia’s Identified Mineral Resources 2024; Geoscience Australia: Canberra, Australia, 2025. [Google Scholar]
- Critical Minerals Office. Critical Minetals Strategy 2023–2030; Critical Minerals Office, Department of Industry, Science and Resources: Canberra, Australia, 2023. [Google Scholar]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Gaines, L. Lithium-ion battery recycling processes: Research towards a sustainable course. Sustain. Mater. Technol. 2018, 17, e00068. [Google Scholar] [CrossRef]
- Jena, K.K.; AlFantazi, A.; Mayyas, A.T. Comprehensive Review on Concept and Recycling Evolution of Lithium-Ion Batteries (LIBs). Energy Fuels 2021, 35, 18257–18284. [Google Scholar] [CrossRef]
- Rezaei, M.; Nekahi, A.; Kumar, M.R.A.; Nizami, A.; Li, X.; Deng, S.; Nanda, J.; Zaghib, K. A review of lithium-ion battery recycling for enabling a circular economy. J. Power Sources 2025, 630, 236157. [Google Scholar] [CrossRef]
- Tembo, P.M.; Dyer, C.; Subramanian, V. Lithium-ion battery recycling—A review of the material supply and policy infrastructure. NPG Asia Mater. 2024, 16, 43. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Y.; Jiao, B.; Chang, N.; Wu, M.; Liu, G.; Lin, X.; Weng, X.; Chen, J.; Zhang, L.; et al. Direct recycling of spent Li-ion batteries: Challenges and opportunities toward practical applications. iScience 2023, 26, 107676. [Google Scholar] [CrossRef]
- Cornelio, A.; Zanoletti, A.; Bontempi, E. Recent progress in pyrometallurgy for the recovery of spent lithium-ion batteries: A review of state-of-the-art developments. Curr. Opin. Green Sustain. Chem. 2024, 46, 100881. [Google Scholar] [CrossRef]
- Reinhart, L.; Vrucak, D.; Woeste, R.; Lucas, H.; Rombach, E.; Friedrich, B.; Letmathe, P. Pyrometallurgical recycling of different lithium-ion battery cell systems: Economic and technical analysis. J. Clean. Prod. 2023, 416, 137834. [Google Scholar] [CrossRef]
- Davis, K.; Demopoulos, G.P. Hydrometallurgical recycling technologies for NMC Li-ion battery cathodes: Current industrial practice and new R&D trends. RSC Sustain. 2023, 1, 1932–1951. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Razeghi, M. The Mystery of Carbon: An Introduction to Carbon Materials; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-based nanomaterials/allotropes: A glimpse of their synthesis, properties and some applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef]
- Mudd, G.; Josso, P.; Shaw, R.; Luce, A.; Singh, N.; Horn, S.; Bide, T.; Currie, D.; Elliott, H.; Grant, H.; et al. UK 2024 Criticality Assessment; BGS Press: Nottingham, UK, 2024. [Google Scholar]
- Fetherston, J.M. Graphite in Western Australia; Department of Mines and Petroleum: East Perth, Australia, 2015; 84p. [Google Scholar]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Natarajan, S.; Divya, M.L.; Aravindan, V. Should we recycle the graphite from spent lithium-ion batteries? The untold story of graphite with the importance of recycling. J. Energy Chem. 2022, 71, 351–369. [Google Scholar] [CrossRef]
- Simandl, G.J.; Paradis, S.; Akam, C. Graphite deposit types, their origin, and economic significance. Br. Columbia Minist. Energy Mines Br. Columbia Geol. Surv. 2015, 3, 163–171. [Google Scholar]
- Chehreh Chelgani, S.; Rudolph, M.; Kratzsch, R.; Sandmann, D.; Gutzmer, J. A Review of Graphite Beneficiation Techniques. Miner. Process. Extr. Metall. Rev. 2016, 37, 58–68. [Google Scholar] [CrossRef]
- Scherba, C.; Montreuil, J.-F.; Barrie, C.T. Chapter 15: Geology and Economics of the Giant Molo Graphite Deposit, Southern Madagascar. In Metals, Minerals, and Society; Society of Economic Geologists: Littleton, CO, USA, 2018; pp. 347–363. [Google Scholar]
- Luque, F.J.; Huizenga, J.M.; Crespo-Feo, E.; Wada, H.; Ortega, L.; Barrenechea, J.F. Vein graphite deposits; geological settings, origin, and economic significance. Miner. Depos. 2014, 49, 261–277. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VA, USA, 2023; p. 210. [Google Scholar]
- Summerfield, D. Australian Resource Reviews: Graphite 2018; Geoscience Australia: Canberra, Australia, 2019. [Google Scholar] [CrossRef]
- Keeling, J. Uley graphite; a world-class resource. MESA J. 2000, 18, 6–11. [Google Scholar]
- Lincoln Minerals Limited. Kookaburra Graphite Project Delivers 99.97% TGC Purity. Available online: https://www.listcorp.com/asx/lml/lincoln-minerals-limited/news/kookaburra-graphite-project-delivers-99-97-percentage-tgc-purity-3192588.html (accessed on 5 June 2025).
- Xiaowei, L.; Jean-Charles, R.; Suyuan, Y. Effect of temperature on graphite oxidation behavior. Nucl. Eng. Des. 2004, 227, 273–280. [Google Scholar] [CrossRef]
- Adham, K.; Bowes, G. Natural Graphite Purification Through Chlorination in Fluidized Bed Reactor. In Proceedings of the First Global Conference on Extractive Metallurgy; Springer: Cham, Switzerland, 2018; pp. 2505–2512. [Google Scholar]
- Ding, Y.-S.; Li, W.-N.; Iaconetti, S.; Shen, X.-F.; DiCarlo, J.; Galasso, F.S.; Suib, S.L. Characteristics of graphite anode modified by CVD carbon coating. Surf. Coat. Technol. 2006, 200, 3041–3048. [Google Scholar] [CrossRef]
- Xiao, P.; Wang, Z.; Long, K.; Yang, J.; Liu, X.; Ling, C.; Chen, L.; Mei, L. Stable cycling and low-temperature operation utilizing amorphous carbon-coated graphite anodes for lithium-ion batteries. RSC Adv. 2024, 14, 13277–13285. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, S.; Lin, S.; Li, Z.; Chen, Y.; Wang, C. Opportunity and challenges in recovering and functionalizing anode graphite from spent lithium-ion batteries: A review. Environ. Res. 2024, 247, 118216. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; He, W.; Li, G.; Huang, J. A review on management of spent lithium ion batteries and strategy for resource recycling of all components from them. Waste Mana. Res. 2018, 36, 99–112. [Google Scholar] [CrossRef]
- Skeete, J.-P.; Wells, P.; Dong, X.; Heidrich, O.; Harper, G. Beyond the EVent horizon: Battery waste, recycling, and sustainability in the United Kingdom electric vehicle transition. Energy Res. Soc. Sci. 2020, 69, 101581. [Google Scholar] [CrossRef]
- Collis, G.E.; Dai, Q.; Loh, J.S.C.; Lipson, A.; Gaines, L.; Zhao, Y.; Spangenberger, J. Closing the Loop on LIB Waste: A Comparison of the Current Challenges and Opportunities for the U.S. and Australia towards a Sustainable Energy Future. Recycling 2023, 8, 78. [Google Scholar] [CrossRef]
- Chitre, A.; Freake, D.; Lander, L.; Edge, J.; Titirici, M.-M. Towards a More Sustainable Lithium-Ion Battery Future: Recycling LIBs from Electric Vehicles. Batter. Supercaps 2020, 3, 1125. [Google Scholar] [CrossRef]
- Anh Nguyen, T.-H.; Oh, S.-Y. Anode carbonaceous material recovered from spent lithium-ion batteries in electric vehicles for environmental application. Waste Manag. 2021, 120, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Aravindan, V. Recycling Strategies for Spent Li-Ion Battery Mixed Cathodes. ACS Energy Lett. 2018, 3, 2101–2103. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- King, S.; Boxall, N.J. Lithium battery recycling in Australia: Defining the status and identifying opportunities for the development of a new industry. J. Clean. Prod. 2019, 215, 1279–1287. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. An Urgent Call to Spent LIB Recycling: Whys and Wherefores for Graphite Recovery. Adv. Energy Mater. 2020, 10, 2002238. [Google Scholar] [CrossRef]
- Ruan, D.; Zou, K.; Du, K.; Wang, F.; Wu, L.; Zhang, Z.; Wu, X.; Hu, G. Recycling of Graphite Anode from Spent Lithium-ion Batteries for Preparing Fe-N-doped Carbon ORR Catalyst. ChemCatChem 2021, 13, 2025–2033. [Google Scholar] [CrossRef]
- Jung, J.; Sui, P.-C.; Zhang, J. Hydrometallurgical Recycling of Lithium-Ion Battery Materials; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Valio, J. Critical Review on Li Ion Battery Recycling Technologies. Master’s Thesis, Aalto University, Espoo, Finland, 2017. [Google Scholar]
- Akram, M.N.; Abdul-Kader, W. Sustainable Development Goals and End-of-Life Electric Vehicle Battery: Literature Review. Batteries 2023, 9, 353. [Google Scholar] [CrossRef]
- Chaudhary, V.; Lakhera, P.; Kim, K.-H.; Deep, A.; Kumar, P. Insights into the Eco-Friendly Recovery Process for Valuable Metals from Waste Lithium-ion Batteries by Organic Acids Leaching. Sep. Purif. Rev. 2023, 53, 82–99. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Retriev Technologies Inc. Renewing Resources for a Sustainable Future—Company Overview; Retriev Technologies Inc.: Anaheim, CA, USA, 2022. [Google Scholar]
- SK TES. Sustainable Battery Recycling Solutions. Available online: https://www.sktes.com/it-services/commercial-battery-recycling (accessed on 29 June 2025).
- GEM Co. Ltd. Full Life Cycle Value Chain Business of Power Batteries. Available online: https://en.gem.com.cn/Business/info.aspx (accessed on 29 June 2025).
- Metal Tech News. ABTC Awarded $150M for New Recycling Plant. Available online: https://www.metaltechnews.com/story/2024/10/02/tech-bytes/abtc-awarded-150m-for-new-recycling-plant/1965.html (accessed on 29 June 2025).
- Rho Motion. Fortum to Receive a €4.5 Million Grant for Its Recycling Facility in Ikaalinen, Finland. Available online: https://rhomotion.com/news/fortum-to-receive-a-e4-5-million-grant-for-its-recycling-facility-in-ikaalinen-finland/ (accessed on 29 June 2025).
- Northvolt. Europe’s Largest Electric Vehicle Battery Recycling Plant Begins Operations. Available online: https://northvolt.com/articles/hydrovolt (accessed on 29 June 2025).
- Gotion. Building a Greener Supply Chain—How China’s Gotion is Making Green Energy “Accessible and Sustainable”. Available online: https://en.gotion.com.cn/news/company-news-256.html (accessed on 29 June 2025).
- Fastmarkets. Green Li-ion Launches First US Battery-Grade pCAM-Producing Recycling Facility. Available online: https://www.fastmarkets.com/insights/green-li-ion-launches-first-us-battery-grade-pcam-producing-recycling-facility/ (accessed on 29 June 2025).
- Tesla Rati. Tesla Giga Nevada Displayed 100 Metric Tons per Week Recycling Capacity in 2022. Available online: https://www.teslarati.com/tesla-giga-nevada-displays-100-metric-tons-per-week-recycling/ (accessed on 29 June 2025).
- ChemAnalyst. News. Sumitomo Metal Mining Plans Construction of Recycling Facilities for Lithium ion. Available online: https://www.chemanalyst.com/NewsAndDeals/NewsDetails/sumitomo-metal-mining-plans-construction-of-recycling-facilities-for-lithium-ion-27107 (accessed on 29 June 2025).
- Ascend Elements. Battery Resourcers to Open North America’s Largest Lithium-ion Battery Recycling Facility by August. Available online: https://ascendelements.com/battery-resourcers-to-open-north-americas-largest-lithium-ion-battery-recycling-facility-by-august/ (accessed on 29 June 2025).
- Industry Intelligence Inc. Glencore, Li-Cycle Jointly Study Feasibility of a Recycling Hub, Using Existing Metallurgical Facility in Portovesme, Italy; Hub to be Europe’s Largest Source of Recycled Battery Grade Lithium, Nickel And Cobalt, with Capacity of 50,000-70,000 Tonnes/Year. Available online: https://www.industryintel.com/news/glencore-li-cycle-jointly-study-feasibility-of-a-recycling-hub-using-existing-metallurgical-facility-in-portovesme-italy-hub-to-be-europe-s-largest-source-of-recycled-battery-grade-lithium-nickel-and-cobalt-with-capacity-of-50-000-70-000-tonnes-or-year-158994657576 (accessed on 29 June 2025).
- Korea Joongang Daily. Posco Joint Venture Finishes EV Battery Recycling Plant. Available online: https://koreajoongangdaily.joins.com/2023/07/09/business/industry/Korea-Posco-Holdings-Posco-HY-Clean-Metal/20230709164641945.html (accessed on 29 January 2025).
- YT Finance. CATL Shares Hit Highest in Five Months on New Investment Projects. Available online: https://www.yuantalks.com/catl-hit-highest-in-nearly-five-months-on-new-project-in-waste-battery-recovery-lithium-material-production/ (accessed on 29 June 2025).
- Semco Carbon. Graphite Recycling. Available online: https://www.semcocarbon.com/blog/graphite-recycling (accessed on 29 June 2025).
- Semco Carbon. Recycling Graphite. Available online: https://www.semcocarbon.com/blog/recycling-graphite-waste-into-usable-graphite-material (accessed on 29 June 2025).
- Reuters. European EV Battery Material Startups Make Recycling Breakthroughs. Available online: https://www.reuters.com/sustainability/climate-energy/european-ev-battery-material-startups-make-recycling-breakthroughs-2025-02-13/ (accessed on 29 June 2025).
- EcoGraf. German Research Institute Confirms Recycled Graphite Performance. Available online: https://www.ecograf.com.au/wp-content/uploads/2024/11/04_EGR_ASX_German-Research-Confirms-Graphite-Performance_27-February-2024.pdf (accessed on 28 June 2025).
- Olutogun, M.; Vanderbruggen, A.; Frey, C.; Rudolph, M.; Bresser, D.; Passerini, S. Recycled graphite for more sustainable lithium-ion batteries. Carbon Energy 2024, 6, e483. [Google Scholar] [CrossRef]
- EcoGraf. Australian Patent Granted Second EcoGraf HFfree® Purification Patent. Available online: https://www.ecograf.com.au/wp-content/uploads/2025/05/27_EGR_ASX_Australian-Patent-Granted_7-May-2025-Final.pdf (accessed on 28 June 2025).
- Renascor Resources Limited. HF-Free Technology Achieves 99.99% Purity: Eco-Friendly Purification Process for Siviour Graphite; Renascor Resources Limited: South Australia, 2021/05/28 2021. Available online: https://renascor.com.au/wp-content/uploads/2021/06/Renascors-HF-Free-Technology-Achieves-99.99-Purity-2216606.pdf (accessed on 29 July 2025).
- Adham, K. Hydrofluoric Acid’s Safe Utilization for Natural Graphite Purification. In Proceedings of the 63rd Conference of Metallurgists, COM 2024; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Hydrogen Fluoride Panel, A.C.C. Emergency Preparedness and Response Guidelines for Anhydrous Hydrogen Fluoride (AHF) and Hydrofluoric Acid (HF); American Chemistry Council: Washington, DC, USA, 2018. [Google Scholar]
- Yang, J.; Fan, E.; Lin, J.; Arshad, F.; Zhang, X.; Wang, H.; Wu, F.; Chen, R.; Li, L. Recovery and Reuse of Anode Graphite from Spent Lithium-Ion Batteries via Citric Acid Leaching. ACS Appl. Energy Mater. 2021, 4, 6261–6268. [Google Scholar] [CrossRef]
- Kosenko, A.; Pushnitsa, K.; Chernyavsky, V.; Novikov, P.; Popovich, A.A. Graphite Regeneration and NCM Cathode Type Synthesis from Retired LIBs by Closed-Loop Cycle Recycling Technology of Lithium-Ion Batteries. Energies 2024, 17, 5570. [Google Scholar] [CrossRef]
- Zeng, G.; Zhou, R.; Hu, C.; Zhao, H.; Gao, H.; Huang, J.; Yu, J.; Luo, F.; Wang, Z.; Deng, C.; et al. Recycling of graphite from spent lithium–ion batteries via low-temperature polyvinyl chloride roasting-assisted leaching. Carbon 2025, 238, 120182. [Google Scholar] [CrossRef]
- Badenhorst, C.; Kuzniarska-Biernacka, I.; Guedes, A.; Mousa, E.; Ramos, V.; Rollinson, G.; Ye, G.; Valentim, B. Recovery of Graphite from Spent Lithium-Ion Batteries. Recycling 2023, 8, 79. [Google Scholar] [CrossRef]
- Chen, W.; Salvatierra, R.V.; Li, J.T.; Kittrell, C.; Beckham, J.L.; Wyss, K.M.; La, N.; Savas, P.E.; Ge, C.; Advincula, P.A.; et al. Flash Recycling of Graphite Anodes. Adv. Mater. 2023, 35, 2207303. [Google Scholar] [CrossRef]
- Gong, H.; Xiao, H.; Ye, L.; Ou, X. High-performance expanded graphite regenerated from spent lithium-ion batteries by integrated oxidation and purification method. Waste Manag. 2023, 171, 292–302. [Google Scholar] [CrossRef]
- Lai, Y.; Zhu, X.; Li, J.; Gou, Q.; Li, M.; Xia, A.; Huang, Y.; Zhu, X.; Liao, Q. Recovery and regeneration of anode graphite from spent lithium-ion batteries through deep eutectic solvent treatment: Structural characteristics, electrochemical performance and regeneration mechanism. Chem. Eng. J. 2023, 457, 141196. [Google Scholar] [CrossRef]
- Zhu, X.-d.; Xiao, J.; Mao, Q.-y.; Zhang, Z.-h.; Tang, L.; Zhong, Q.-f. Recycling of waste carbon residue from spent lithium-ion batteries via constant-pressure acid leaching. Trans. Nonferrous Met. Soc. China 2022, 32, 1691–1704. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y.; Chen, L.; Chu, W.; Huang, Y.; Jia, Y.; Wang, M. An innovative approach to recover anode from spent lithium-ion battery. J. Power Sources 2021, 483, 229163. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Fu, Y.; Xie, W.; Feng, Y.; Alejandro, K. Hydrometallurgical enhanced liberation and recovery of anode material from spent lithium-ion batteries. Waste Manag. 2021, 126, 517–526. [Google Scholar] [CrossRef]
- You, H.Y.; Hui, J.N.; Zhou, Y.L.; Vittore, K.; Zhang, J.R.; Chaney, L.E.; Chinta, S.; Zhao, Y.H.; Lim, G.; Lee, D.; et al. Sustainable Production of Biomass-Derived Graphite and Graphene Conductive Inks from Biochar. Small 2024, 20, 202406669. [Google Scholar] [CrossRef] [PubMed]
- Makowska, M.; Dziosa, K. Influence of different pyrolysis temperatures on chemical composition and graphite-like structure of biochar produced from biomass of green microalgae Chlorella sp. Environ. Technol. Innov. 2024, 35, 103667. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hanif, M.A.; Song, H.; Kim, S.; Cho, Y.; Ryu, S.-K.; Kim, H.G. Wood-Derived Graphite: A Sustainable and Cost-Effective Material for the Wide Range of Industrial Applications. Crystals 2024, 14, 309. [Google Scholar] [CrossRef]
- Hegde, S.S.; Bhat, B.R. Biomass waste-derived porous graphitic carbon for high-performance supercapacitors. J. Energy Storage 2024, 76, 109818. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Xie, K.; Chen, X.; Hu, Y.; Ma, W. Purification of Waste Graphite from Crucibles Used in Photovoltaic Crystallization by an Alkali-Acid Method. Metals 2023, 13, 1180. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, S.; Jin, Y.; Zhao, L.; Chen, S.; Yang, H.; Cui, Y.; Svanberg, R.; Tang, C.; Jiang, J.; et al. Establishment of green graphite industry: Graphite from biomass and its various applications. SusMat 2023, 3, 402–415. [Google Scholar] [CrossRef]
- Veldevi, T.; Raghu, S.; Kalaivani, R.A.; Shanmugharaj, A.M. Waste tire derived carbon as potential anode for lithium-ion batteries. Chemosphere 2022, 288, 132438. [Google Scholar] [CrossRef]
- Jabarullah, N.H.; Kamal, A.S.; Othman, R. A modification of palm waste lignocellulosic materials into biographite using iron and nickel catalyst. Processes 2021, 9, 1079. [Google Scholar] [CrossRef]
- Destyorini, F.; Irmawati, Y.; Hardiansyah, A.; Widodo, H.; Yahya, I.N.D.; Indayaningsih, N.; Yudianti, R.; Hsu, Y.I.; Uyama, H. Formation of nanostructured graphitic carbon from coconut waste via low-temperature catalytic graphitisation. Eng. Sci. Technol. Int. J. 2021, 24, 514–523. [Google Scholar] [CrossRef]
- Xing, B.; Zhang, C.; Cao, Y.; Huang, G.; Liu, Q.; Zhang, C.; Chen, Z.; Yi, G.; Chen, L.; Yu, J. Preparation of synthetic graphite from bituminous coal as anode materials for high performance lithium-ion batteries. Fuel Process. Technol. 2018, 172, 162–171. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Lee, K.H. Full graphitization of amorphous carbon by microwave heating. RSC Adv. 2016, 6, 24667–24674. [Google Scholar] [CrossRef]
| Property | General | Anisotropic * | Refs. | |
|---|---|---|---|---|
| ab | c | |||
| Appearance | Black, greyish black; shiny | – | – | [3,23] |
| Transparency | Opaque | – | – | [3,20,23] |
| Density (300 K, 1 atm, g/cm3) | 2.25–2.31 | – | – | [2,3,20,23] |
| Thermal Stress Resistance (Factor R, W/m) | 50 000 | – | – | [2] |
| Sublimation Point (1 atm, K) | ~4000 | – | – | [3] |
| Mohs Hardness | – | 0.5 | 9–10 | [2] |
| Elastic Modulus (GPa) | – | 1060 | 36–36.5 | [2,3] |
| Electrical Resistivity (Ω·m) | – | 2.5–5.0 × 10−6 | 3000 × 10−6 | [3] |
| Electrical Conductivity (S/m) | – | 2–3 × 105 | 3.3 × 102 | [2] |
| Thermal Conductivity (25 °C, W/m·K) | – | 398 | 2.2 | [3] |
| Stability | Resistant to acids and alkalis under most conditions; oxidation above 350–400 °C in air | – | – | [3] |
| Interaction | Description | Roles | Associated Properties |
|---|---|---|---|
| σ-bond | Covalent bonds formed by direct overlap of sp2 hybridized orbitals within the layers | Supports the planar hexagonal lattice with rigid framework | High in-plane mechanical strength and thermal conductivity |
| π-bond | Covalent bonds formed by sideways overlap of unhybridized p-orbitals within the layers | Creates delocalized π-electron cloud, allowing in-plane electron delocalization | High in-plane electrical conductivity |
| van der Waals forces | Weak intermolecular attractions between the layers | Maintains interlayer stacking | Anisotropic behavior; lubricity, softness, easy cleavage, low conductivity |
| Application | Source Type * | Purity | Size | General Use | Share * | Refs. |
|---|---|---|---|---|---|---|
| % | % | |||||
| Batteries | NG (F, A); SG | >99.95 | – | Anode material for LIBs—used in portable devices, energy storage systems (ESS) and EVs | 25 increasing | [2,4,5,6,7,8] |
| Refractories | NG | – | – | Linings | 35 | [2,5,6,8] |
| Foundry | NG (F) | >85 | – | Graphite crucibles and molds | 7 | [2] |
| Lubricants | NG | – | <1 mm | Graphite-based lubricants—used in hot metal formation, self-lubricating bearings | 10 | [2,6,8] |
| Fuel Cells | NG (F) | – | – | Proton exchange fuel cells (PEMFCs) and phosphoric acid fuel cell (PAFC)– use graphite as bipolar plates, and gas diffusion layers for PEMFCs | – | [2,6] |
| Carbon Nanomaterials | NG (F) | – | 1–100 nm | Used as additives or catalyst supports for high-temp PEMFC (140–160 °C operating temp) | – | [2,23] |
| Expanded Graphite (EG) | NG (F) | – | – | Graphite foils—used for typical sealing applications; used in manufacturing flexible graphite plates for fuel cells | 3 | [2] |
| Graphite Intercalation Compounds (GICs) | NG (F) | – | – | Used as precursors for the preparation of expanded graphite (EG) | – | [2] |
| Traditional | NG | – | – | Used as graphite pencils | 5 | [2,8] |
| Property | Flake | Vein | Amorphous | Refs. |
|---|---|---|---|---|
| Origin | Syngenetic—from sapropelitic, silica-bearing sediments via catazonal and mesozonal metamorphism | Epinenetic—possibly from carbonaceous hydrothermal or pneumatolytic fluids | Syngenetic—from coals via epizonal metamorphism | [2,3,26,27,28,29,30,31] |
| % Composition | ||||
| Carbon (C) | <60–95 | 90–99 Up to 100% (Sri Lanka) | 30–90 | [2,3,25,30,31] |
| Sulfur (S) | 0.1 | 0.7 | 0.1 | [3] |
| Density (g/cm3) | 2.29 | 2.26 | 2.31 | [3] |
| DoG * (%) | 99.9 | 100 | 28 | [3] |
| d-spacing (002) (nm) | 0.3355 | 0.3354 | 0.3361 | [3] |
| ACD (mm) | <0.1 | <0.01 | <0.001 | [2] |
| Morphology | Plate | Plate Needle | Granular | [3] |
| Mine Production | Reserves | ||||
|---|---|---|---|---|---|
| 2021 | 2022 | 2023 | 2024 | 2024 | |
| Brazil | 82,000 | 87,000 | 66,300 | 68,000 | 74,000,000 |
| China | 820,000 | 850,000 | 1,210,000 | 1270,000 | 81,000,000 |
| Madagascar | 70,000 | 110,000 | 63,000 | 89,000 | 27,000,000 |
| Mozambique | 72,000 | 170,000 | 98,000 | 75,000 | 25,000,000 |
| Sri Lanka | 3000 | 3000 | 3000 | 3300 | 1,500,000 |
| World Total * | 1,130,000 | 1,300,000 | 1,530,000 | 1,600,000 | 290,000,000 |
| No. | Company | Country | Status | Capacity | Process ** | Graphite | Losses | Refs. |
|---|---|---|---|---|---|---|---|---|
| ton/y | ||||||||
| 1 | Retriev Technologies Inc. (Toxco) | USA Canada | Operational | ~18,000 | Pre, M, H | Recovered as metal oxide—carbon cake | Plastic | *, [58] |
| 2 | Umicore ValÉas™ | USA Bruxelles, Belgium | Operational | 7000 | Pre, H, P | Used as reductant for metals | Graphite electrolyte, plastics | * |
| 3 | AkkuSer Oy | Finland | Operational | 4000 | Pre, M | Part of black mass | Plastic | * |
| 4 | TES France S.A.S. (Recupyl Valibat) | France | Operational | ~5000 | M, H | Separated in leaching stage | Graphite, Cu | *, [59] |
| 5 | BatRec Industrie AG | Switzerland | Operational | 200 | Pre, H, P | – | – | * |
| 6 | Inmetco | USA | Operational | 6000 | P | – | – | * |
| 7 | Glencore (Xstrata) | Switzerland Canada/Norway | Operational | 3000 7000 | H, P | – | – | * |
| 8 | Brunp Recycling Technology Co., Ltd. | China | Operational | 10,000 | H, P | – | – | * |
| 9 | JX Nippon Mining | Japan | Operational | 5000 | H, P | – | – | * |
| 10 | Quzhou Huayo | China | Operational | 40,000 | P | – | – | * |
| 11 | DOWA Eco-System | Japan | Operational | 6500 | P | – | – | * |
| 12 | Redux Recycling | Germany Austria | Operational | 50,000 | H | – | – | * |
| 13 | Green Eco-Manufacture, GEM Co., Ltd. | China | Operational | 200,000 | H | – | – | *, [60] |
| 14 | Li-Cycle | USA Canada | Operational | 5000 5000 | H | – | – | * |
| 15 | Taisen | China | Operational | 6000 | H | – | – | * |
| 16 | Envirostream | Australia | Operational | 3000 | Pre | – | – | * |
| 17 | Guanghua Sci-Tech | China | Operational | 12,000 | Pre | – | – | * |
| 18 | Accurec GmbH® | Krefeld, Germany | Operational | 4000–6000 | Pre, M, H, P | Partially burnt, used as a reductant, and slagged | Graphite, electrolyte, polymers | * |
| 19 | American Battery Technology Company (ABTC) | USA | Operational | 20,000 | – | – | – | *, [61] |
| 20 | Fortum | Finland | Operational | ~3000–5000 | – | – | – | *, [62] |
| 21 | Hydrovolt (Northvolt—Hydro) | Norway | Operational | 12,000 | – | – | – | *, [63] |
| 22 | Gotion High-Tech | China | Operational | 50,000 | – | – | – | *, [64] |
| 23 | Green Li-ion | USA | Operational | 730 | – | – | – | *, [65] |
| 24 | Tesla | USA | Operational | ~5000 | – | – | – | *, [66] |
| 25 | Aalto University | Finland | Emerging | – | M, H, P | Lost in the furnace | Graphite, binder plastic, Cu, water | * |
| 26 | Steven Loop: OnTo | USA | Emerging | – | Pre, M, H, P | Separated using DMS, recovered | Graphite, binder | * |
| 27 | LithoRec | Germany | Emerging | 2000 | Pre, M, H, P | Separated in leaching stage | Electrolyte | * |
| 28 | Sumitomo Metal Mining, SMM Co., Ltd. (Sumitomo—Sony) | Japan | Planned | 10,000 (150) | (Pre, H, P) | Calcined but not recovered | Graphite, electrolyte, plastics, Li, Ni | [67] (*) |
| 29 | Ascend Elements (Battery Resourcers) | USA | Planned | 30,000 | (Pre, M, H, P) | – | (Electrolyte) | [68] (*) |
| 30 | Glencore & Li-cyle | Switzerland & USA | Planned | ~50,000–70,000 | – | – | – | *, [69] |
| 31 | Posco Hy Clean Metal | South Korea | Planned | 12,000 | – | – | – | *, [70] |
| 32 | Bangpu Recycling Technology Co., Ltd. | China | Planned | 500,000 | H | – | – | *, [71] |
| No. | Company | Country | Status | Capacity | Process ** | Progress | Refs. |
|---|---|---|---|---|---|---|---|
| ton/y | |||||||
| 1 | Semco Carbon | USA | Operational | ~1800 | M, H, P | Recycles graphite from external companies and within operations, offering alternatives to pristine graphite | [72,73] |
| 2 | Tozero | Germany | Emerging | 2000 | H | ~ EUR 17 million for graphite recycling “Net zero” emissions with renewable energy | [74] |
| 3 | EcoGraf | Australia | Emerging | – | Pre, H, P | Helmholtz Institute research work confirmed effectivity of EcoGraf HFfree™ purification technology | [75,76] |
| No. | Author | Year | Remarks | Application |
|---|---|---|---|---|
| 1 | Zeng et al. [83] | 2025 | Graphite from spent LIBs was purified and regenerated using low-temperature spent polyvinyl chloride (PVC) roasting-assisted leaching. Purity level reached 99.9%, then regenerated at 1000 °C. Achieved a specific capacity of 111.5 mAh/g, 75% retention rate after 500 cycles at 1 C, and 99% coulombic efficiency (CE). | LIB anodes |
| 2 | Kosenko et al. [82] | 2024 | Spent graphite regenerated with organic acid leaching of 1.5 M malic acid and 3% H2O2, then annealed in Ar atmosphere. Regenerated graphite (RG) has achieved a specific discharge capacity of 340.4 mAh/g at 0.1 C, and 99.9% CE. | LIB anodes |
| 3 | Badenhorst et al. [84] | 2023 | Graphite from spent LIBs was recovered using a combination of mechanical and chemical treatment. Citric acid leaching was employed and graphite-rich products achieved purity range 74%–88%. | LIB anodes (potential) |
| 4 | Chen et al. [85] | 2023 | Anode waste from spent LIBs was treated with flash joule heating within seconds followed by 0.1 M HCl leaching achieved a specific capacity of 351 mAh/g at 0.2 C, 77.3% capacity retention after 400 cycles at 0.5 C using LiFePO4 as cathode. | LIB anodes |
| 5 | Gong et al. [86] | 2023 | Anode has been manually separated from spent LIBs and treated by water leaching, then recovered and prepared for atmospheric plasma jet printing, which achieved a specific capacity of 402 mAh/g, and 500 mAh/g after 1000 cycles. | LIB anodes |
| 6 | Lai et al. [87] | 2023 | Waste graphite from spent LIBs was recovered and regenerated using deep eutectic solvent (DES) leaching. Achieved a high specific capacity of 449.4 mAh/g at 0.1 C, 285.4 mAh/g after 500 cycles at 1 C, 96% retention rate, and 100% CE. | LIB anodes |
| 7 | Zhu et al. [88] | 2022 | Waste carbon residue (WCR) was purified to 99.5% using constant-pressure acid leaching at 60 °C, 12% HF concentration, 180 min, and 25:1 liquid-to-solid ratio. Achieved a 91.86% DoG, 19.205 μm D50, and high thermal stability. | LIB anodes |
| 8 | Cao et al. [89] | 2021 | Graphite was separated from Cu foil by electrolysis, with purity of about 95%, reused to prepare for LIB anodes. Achieved a discharge and charge specific capacity of 427.81 mAh/g and 350.47 mAh/g at 0.1 C, with about 98% CE after 2nd cycle. | LIB anodes |
| 9 | Li et al. [90] | 2021 | Spent anode material has been manually separated from spent LIBs, and exfoliated from Cu foil using deionized water bath with rotator. | LIB anodes (potential) |
| 10 | Yang et al. [81] | 2021 | Anode graphite regenerated with organic acid leaching of 0.2 M citric acid, at 90 °C, 1:50 g/mL S/L ratio, and 50 min reaction time. Achieved a high discharge capacity of 330 mAh/g at 0.5 C after 80 cycles, and about 99% CE. | LIB anodes |
| No. | Author | Year | Remarks | Application |
|---|---|---|---|---|
| 1 | Kim et al. [93] | 2024 | Investigated graphitization of wood at varying temperatures with and without catalyst. Graphitization without a catalyst occurred at higher temperature. | General industrial applications (including batteries) |
| 2 | Hegde et al. [94] | 2024 | Teak sawdust was used for porous graphitic carbon synthesis using FeCl3-assisted carbonization and KOH activation. | Supercapacitor electrodes |
| 3 | Makowska et al. [92] | 2024 | Chlorella sp. biochar pyrolyzed at 400–900 °C in CO2 atmosphere. Amorphous nature of biochar develops at higher temperature. | – |
| 4 | You et al. [91] | 2024 | Hardwood biochar was used for graphite synthesis through pre-heating carbonization, Fe-catalyzed graphitization (slow cooling) and acid wash using H2SO4. | Alternative to natural and synthetic graphite |
| 5 | Shi et al. [96] | 2023 | Green graphite from biomass using pyrolysis and catalytic graphitization. Optimized green graphite achieved a reversible capacity of 264 mAh/g, 97% capacity retention over 100 cycles in a half-cell, and 99.3% CE. | LIB anodes, printing, refractories |
| 6 | Zhang et al. [95] | 2023 | Used alkali-acid method with NaOH and HCl to purify waste graphite from crucibles. Achieved 98.45% fixed carbon from 93.09%. | LIB anodes (potential) |
| 7 | Veldevi et al. [97] | 2022 | Waste tire was used for graphite synthesis through aqua regia treatment and pyrolysis using N2/CO2 atmosphere. Achieved a reversible specific discharge capacity of 350 mAh/g, 81% capacity retention after 500 cycles at 300 mA/g, and 99% CE. | LIB anodes |
| 8 | Destyorini et al. [99] | 2021 | Coconut coir was used for graphite synthesis through low-temperature catalytic graphitization at 1300 °C using Ni-based catalyst. Achieved 84.88% DoG, and 25.75 S/cm conductivity from 14.97 S/cm. | Fuel cells |
| 9 | Jabarullah et al. [98] | 2021 | Palm kernel shell (PKS) was used for graphite synthesis through catalytic graphitization at 800–1300 °C using Fe/Ni-based catalyst. Achieved a highly ordered graphitic structure at 2θ = 26.5°, surface area of 202.932 m2/g, and 0.208 cm3/g. | – |
| 10 | Xing et al. [100] | 2018 | Bituminous coal was used for graphite synthesis through high-temperature graphitization at 2000–2800 °C. The synthetic graphite achieved reversible capacity of 310.3 mAh/g at 0.1 C current rate, 95.3% capacity retention after 100 cycles. | LIB anodes |
| 11 | Kim et al. [101] | 2016 | Amorphous carbon (activated carbon powder) was graphitized using catalytic microwave heating. Graphitization achieved in 5 min using microwave heating at a 1400 W. Similar results achieved using thermal graphitization after 1 h at 1000 °C. | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondoc, M.J.C.; Jorolan, J.H.; Eom, H.-S.; Lee, G.-G.; Alorro, R.D. Advances in Graphite Recycling from Spent Lithium-Ion Batteries: Towards Sustainable Resource Utilization. Minerals 2025, 15, 832. https://doi.org/10.3390/min15080832
Bondoc MJC, Jorolan JH, Eom H-S, Lee G-G, Alorro RD. Advances in Graphite Recycling from Spent Lithium-Ion Batteries: Towards Sustainable Resource Utilization. Minerals. 2025; 15(8):832. https://doi.org/10.3390/min15080832
Chicago/Turabian StyleBondoc, Maria Joriza Cañete, Joel Hao Jorolan, Hyung-Sub Eom, Go-Gi Lee, and Richard Diaz Alorro. 2025. "Advances in Graphite Recycling from Spent Lithium-Ion Batteries: Towards Sustainable Resource Utilization" Minerals 15, no. 8: 832. https://doi.org/10.3390/min15080832
APA StyleBondoc, M. J. C., Jorolan, J. H., Eom, H.-S., Lee, G.-G., & Alorro, R. D. (2025). Advances in Graphite Recycling from Spent Lithium-Ion Batteries: Towards Sustainable Resource Utilization. Minerals, 15(8), 832. https://doi.org/10.3390/min15080832












