Mineral–Soil–Plant–Nutrient Synergism: Carbonate Rock Leachate Irrigation Enhances Soil Nutrient Availability, Improving Crop Yield and Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Source and Characterization Methods of Samples
2.2. Preparation and Analysis of Carbonate Rock Leachates
2.3. Lettuce Pot Experiment
2.3.1. Design and Management of the Lettuce Pot Experiment
2.3.2. Measurement of Growth Data, Root Activity, and Nutritional Quality in the Lettuce Pot Experiment
2.4. Peanut Field Experiment
2.5. Soil Sampling and Physicochemical Property Analysis
2.6. Determination of Soil Microbial Communities
2.7. Statistical Analysis
3. Results
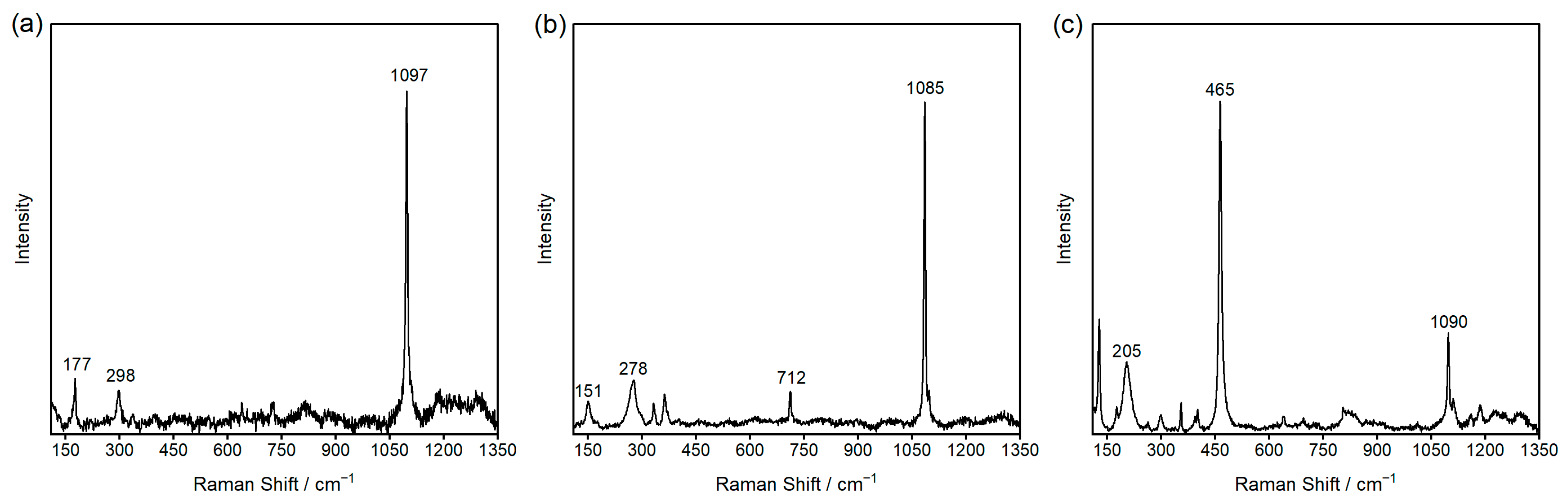
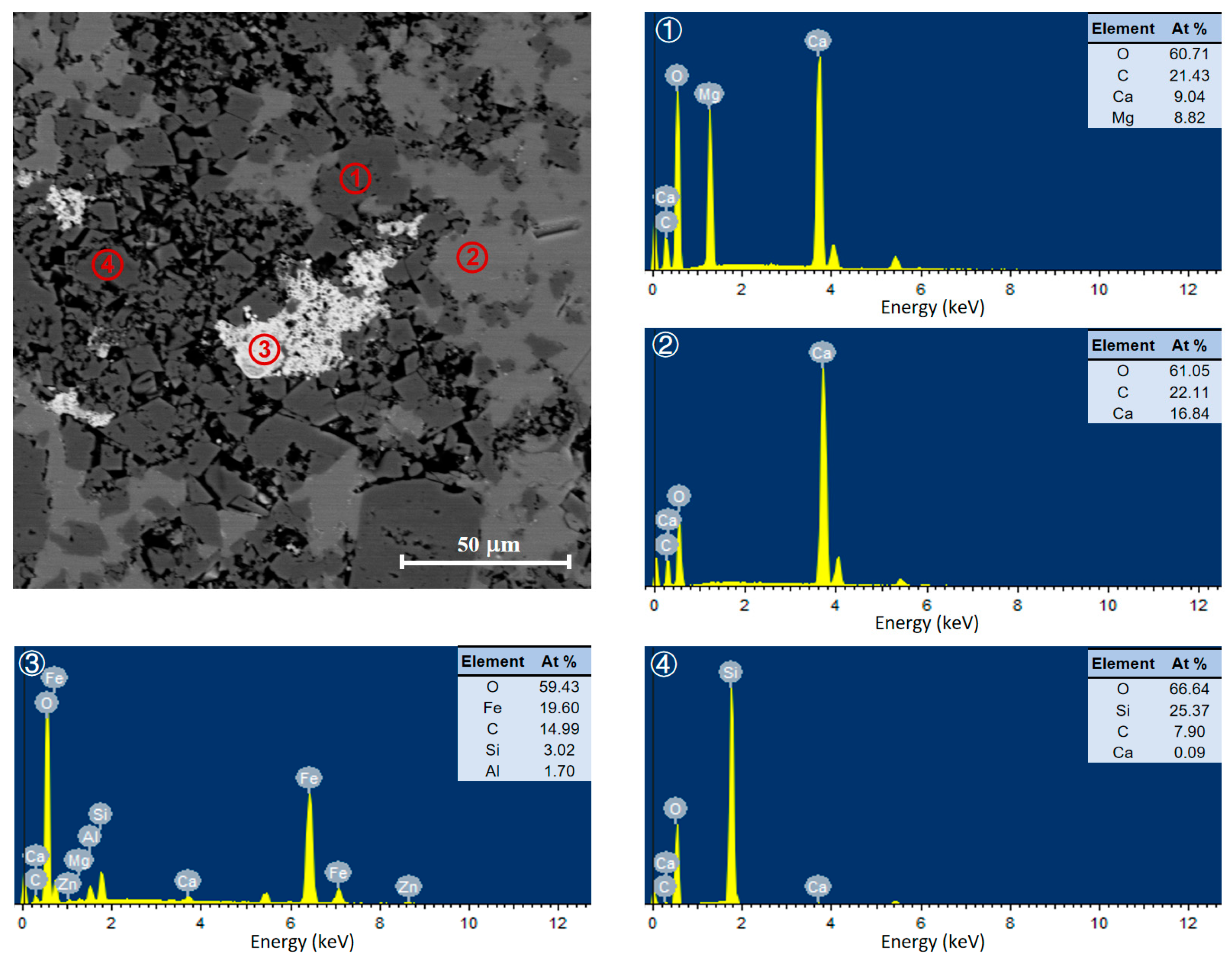
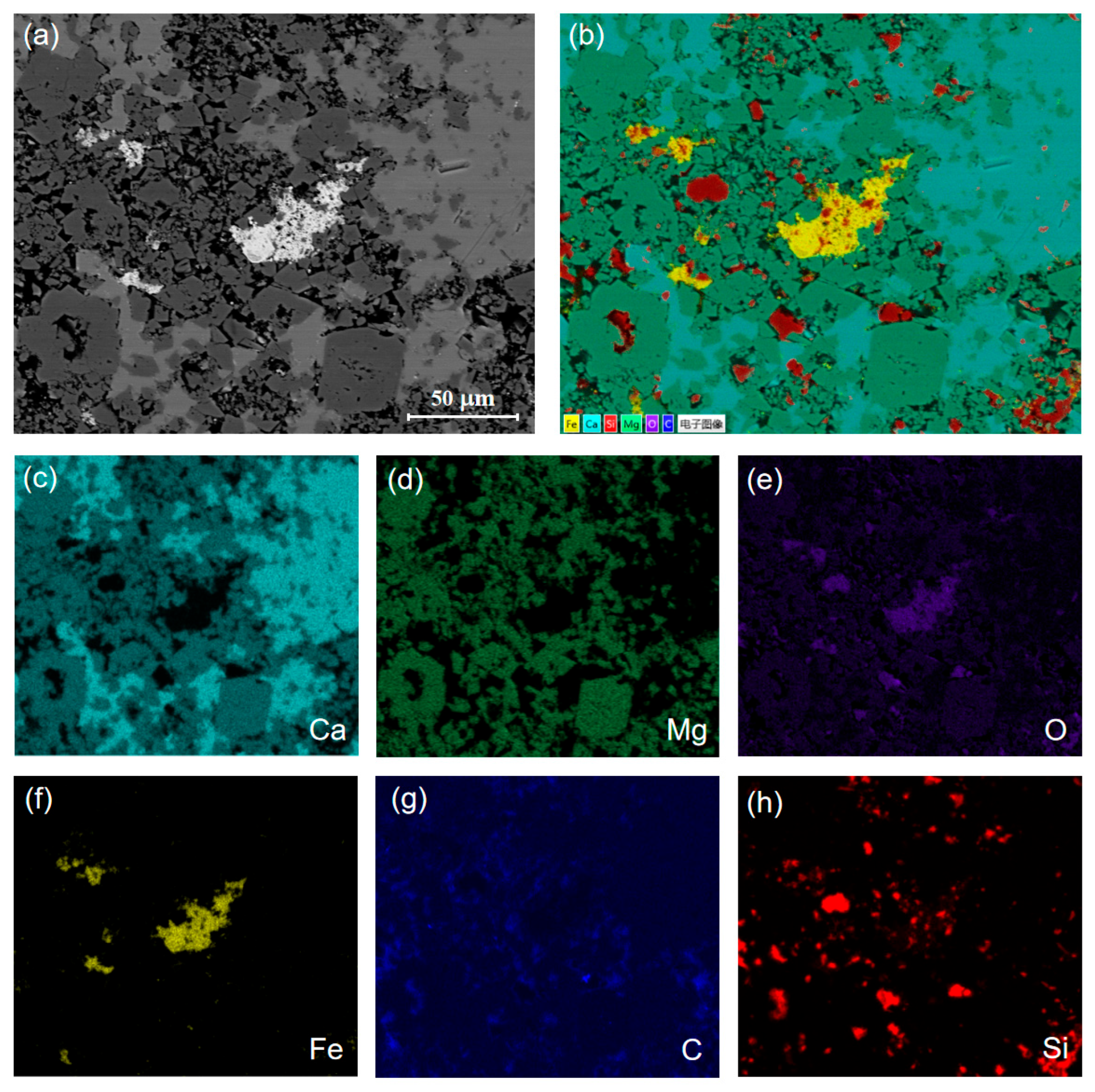
3.1. Characterization of Carbonate Rocks
3.2. Determination and Analysis of Carbonate Rock Leachates
3.3. Effect of Carbonate Rock Leachates on Lettuce Growth and Nutritional Quality in the Pot Experiment
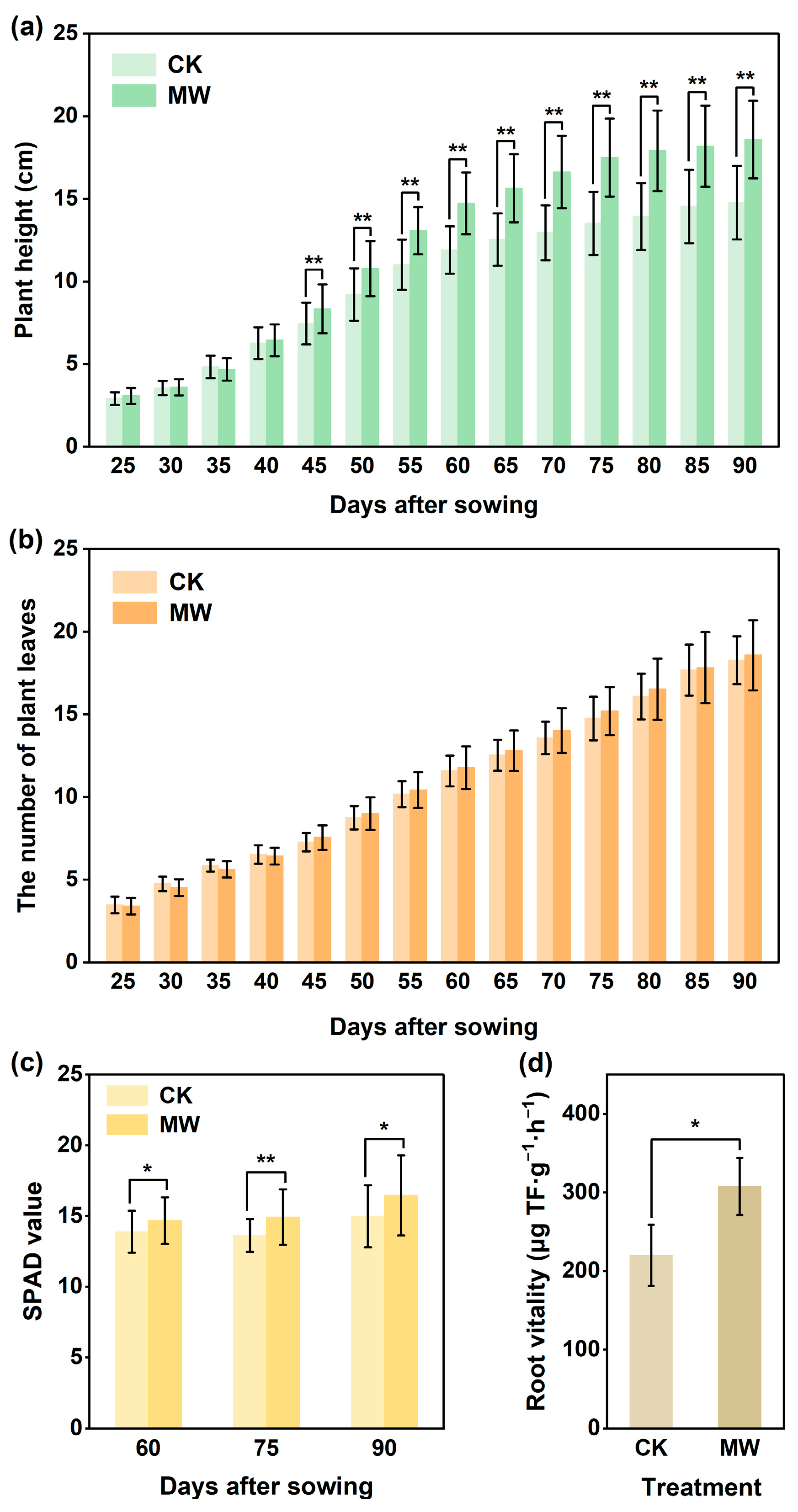
3.3.1. Effect of Carbonate Rock Leachates on Lettuce Biomass, Leaf SPAD Value, and Root Activity
3.3.2. Effect of Carbonate Rock Leachates on Lettuce Nutritional Quality
3.4. Effects of Carbonate Rock Leachates on Soil Physicochemical Properties and Microbial Community Structure in the Pot Experiment
3.4.1. Effects of Carbonate Rock Leachates on Soil Physicochemical Properties
3.4.2. Effects of Carbonate Rock Leachates on Soil Microbial Communities
3.5. Effects on Soil Physicochemical Properties in the Field Experiment
4. Discussion
4.1. Mechanism of Carbonate Rock Leachates on Soil Nutrient Availability Enhancement
4.2. Changes in Soil Microbial Community Structure and Their Functional Implications
4.3. Crop Yield and Quality Responses to Carbonate Rock Leachate Irrigation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mills, G.; Sharps, K.; Simpson, D.; Pleijel, H.; Frei, M.; Burkey, K.; Emberson, L.; Uddling, J.; Broberg, M.; Feng, Z.; et al. Closing the global ozone yield gap: Quantification and cobenefits for multistress tolerance. Glob. Change Biol. 2018, 24, 4869–4893. [Google Scholar] [CrossRef]
- Ortiz-Bobea, A.; Ault, T.R.; Carrillo, C.M.; Chambers, R.G.; Lobell, D.B. Anthropogenic climate change has slowed global agricultural productivity growth. Nat. Clim. Change 2021, 11, 306–312. [Google Scholar] [CrossRef]
- Evenson, R.E.; Gollin, D. Assessing the Impact of the Green Revolution, 1960 to 2000. Science 2003, 300, 758–762. [Google Scholar] [CrossRef]
- Khush, G.S. Green revolution: The way forward. Nat. Rev. Genet. 2001, 2, 815–822. [Google Scholar] [CrossRef]
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Steadman, C.E.; Stevenson, D.; Coyle, M.; Rees, R.M.; Skiba, U.M.; Sutton, M.A.; Cape, J.N.; Dore, A.J.; Vieno, M.; et al. Effects of global change during the 21st century on the nitrogen cycle. Atmos. Chem. Phys. 2015, 15, 13849–13893. [Google Scholar] [CrossRef]
- Yuan, Z.; Jiang, S.; Sheng, H.; Liu, X.; Hua, H.; Liu, X.; Zhang, Y. Human Perturbation of the Global Phosphorus Cycle: Changes and Consequences. Environ. Sci. Technol. 2018, 52, 2438–2450. [Google Scholar] [CrossRef] [PubMed]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural Intensification and Ecosystem Properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef]
- Bonner, M.R.; Alavanja, M.C.R. Pesticides, human health, and food security. Food Energy Secur. 2017, 6, 89–93. [Google Scholar] [CrossRef]
- Bodirsky, B.L.; Popp, A.; Lotze-Campen, H.; Dietrich, J.P.; Rolinski, S.; Weindl, I.; Schmitz, C.; Müller, C.; Bonsch, M.; Humpenöder, F.; et al. Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution. Nat. Commun. 2014, 5, 3858. [Google Scholar] [CrossRef]
- Srivastav, A.L. Chemical fertilizers and pesticides: Role in groundwater contamination. In Agrochemicals Detection, Treatment and Remediation; Chapter 6; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 143–159. [Google Scholar]
- Iqbal, S.; Riaz, U.; Murtaza, G.; Jamil, M.; Ahmed, M.; Hussain, A.; Abbas, Z. Chemical Fertilizers, Formulation, and Their Influence on Soil Health. In Microbiota and Biofertilizers: A Sustainable Continuum for Plant and Soil Health; Hakeem, K.R., Dar, G.H., Mehmood, M.A., Bhat, R.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–15. [Google Scholar]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y.; Huang, H.; He, X.; Yang, M.; Zhu, S. Soil Acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Naveed, M.; Moldrup, P.; Vogel, H.-J.; Lamandé, M.; Wildenschild, D.; Tuller, M.; de Jonge, L.W. Impact of long-term fertilization practice on soil structure evolution. Geoderma 2014, 217–218, 181–189. [Google Scholar] [CrossRef]
- Bisht, N.; Chauhan, P.S. Excessive and Disproportionate Use of Chemicals Cause Soil Contamination and Nutritional Stress. In Soil Contamination—Threats and Sustainable Solutions; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Bai, Y.-C.; Chang, Y.-Y.; Hussain, M.; Lu, B.; Zhang, J.-P.; Song, X.-B.; Lei, X.-S.; Pei, D. Soil Chemical and Microbiological Properties Are Changed by Long-Term Chemical Fertilizers That Limit Ecosystem Functioning. Microorganisms 2020, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In Agrochemicals Detection, Treatment and Remediation; Chapter 2; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54. [Google Scholar]
- Haque, F.; Santos, R.M.; Dutta, A.; Thimmanagari, M.; Chiang, Y.W. Co-Benefits of Wollastonite Weathering in Agriculture: CO2 Sequestration and Promoted Plant Growth. ACS Omega 2019, 4, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, H.; Haque, F.; Vanderburgt, S.; Santos, R.M.; Chiang, Y.W. Mineral–Soil–Plant–Nutrient Synergisms of Enhanced Weathering for Agriculture: Short-Term Investigations Using Fast-Weathering Wollastonite Skarn. Front. Plant Sci. 2022, 13, 929457. [Google Scholar] [CrossRef]
- Levy, C.R.; Almaraz, M.; Beerling, D.J.; Raymond, P.; Reinhard, C.T.; Suhrhoff, T.J.; Taylor, L. Enhanced Rock Weathering for Carbon Removal–Monitoring and Mitigating Potential Environmental Impacts on Agricultural Land. Environ. Sci. Technol. 2024, 58, 17215–17226. [Google Scholar] [CrossRef]
- Andrews, M.G.; Taylor, L.L. Combating Climate Change Through Enhanced Weathering of Agricultural Soils. Elements 2019, 15, 253–258. [Google Scholar] [CrossRef]
- Kelland, M.E.; Wade, P.W.; Lewis, A.L.; Taylor, L.L.; Sarkar, B.; Andrews, M.G.; Lomas, M.R.; Cotton, T.A.; Kemp, S.J.; James, R.H.; et al. Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Global Change Biol. 2020, 26, 3658–3676. [Google Scholar] [CrossRef]
- Beerling, D.J.; Leake, J.R.; Long, S.P.; Scholes, J.D.; Ton, J.; Nelson, P.N.; Bird, M.; Kantzas, E.; Taylor, L.L.; Sarkar, B.; et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 2018, 4, 138–147. [Google Scholar] [CrossRef]
- Edwards, D.P.; Lim, F.; James, R.H.; Pearce, C.R.; Scholes, J.; Freckleton, R.P.; Beerling, D.J. Climate change mitigation: Potential benefits and pitfalls of enhanced rock weathering in tropical agriculture. Biol. Lett. 2017, 13, 20160715. [Google Scholar] [CrossRef]
- Renforth, P.; von Strandmann, P.P.; Henderson, G.M. The dissolution of olivine added to soil: Implications for enhanced weathering. Appl. Geochem. 2015, 61, 109–118. [Google Scholar] [CrossRef]
- Ten Berge, H.F.; Van der Meer, H.G.; Steenhuizen, J.W.; Goedhart, P.W.; Knops, P.; Verhagen, J. Olivine Weathering in Soil, and Its Effects on Growth and Nutrient Uptake in Ryegrass (Lolium perenne L.): A Pot Experiment. PLoS ONE 2012, 7, e42098. [Google Scholar] [CrossRef]
- Hartmann, J.; West, A.J.; Renforth, P.; Köhler, P.; De La Rocha, C.L.; Wolf-Gladrow, D.A.; Dürr, H.H.; Scheffran, J. Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Rev. Geophys. 2013, 51, 113–149. [Google Scholar] [CrossRef]
- Nicola, J.H.; Scott, J.F.; Couto, R.M.; Correa, M.M. Raman spectra of dolomite [CaMg(CO3)2]. Phys. Rev. 1976, 14, 4676–4678. [Google Scholar] [CrossRef]
- Herman, R.G.; Bogdan, C.E.; Sommer, A.J.; Simpson, D.R. Discrimination among Carbonate Minerals by Raman Spectroscopy Using the Laser Microprobe. Appl. Spectrosc. 1987, 41, 437–440. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Lounejeva, E.; Ostroumov, M.; Sánchez-Rubio, G. Micro-Raman and optical identification of coesite in suevite from Chicxulub. In Catastrophic Events and Mass Extinctions: Impacts and Beyond; Koeberl, C., MacLeod, K.G., Eds.; Geological Society of America: Boulder, CO, USA, 2002. [Google Scholar]
- Morse, J.W. The surface chemistry of calcium carbonate minerals in natural waters: An overview. Mar. Chem. 1986, 20, 91–112. [Google Scholar] [CrossRef]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Bacteria as Emerging Indicators of Soil Condition. Appl. Environ. Microbiol. 2016, 83, e02826-16. [Google Scholar] [CrossRef]
- Morris, S.J.; Blackwood, C.B. Chapter 10—The ecology of soil biota and their function. In Soil Microbiology, Ecology and Biochemistry, 5th ed.; Paul, E.A., Frey, S.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 275–302. [Google Scholar]
- Tao, R.; Liang, Y.; Wakelin, S.A.; Chu, G. Supplementing chemical fertilizer with an organic component increases soil biological function and quality. Appl. Soil Ecol. 2015, 96, 42–51. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Shen, J.; Yuan, Q.; Fan, F.; Wei, W.; Li, Y.; Wu, J. Biochar alters soil microbial communities and potential functions 3–4 years after amendment in a double rice cropping system. Agric. Ecosyst. Environ. 2021, 311, 107291. [Google Scholar] [CrossRef]
- Ramírez-Builes, V.H.; Küsters, J.; de Souza, T.R.; Simmes, C. Calcium Nutrition in Coffee and Its Influence on Growth, Stress Tolerance, Cations Uptake, and Productivity. Front. Agron. 2020, 2, 590892. [Google Scholar] [CrossRef]
- Tang, R.-J.; Zhao, F.-G.; Yang, Y.; Wang, C.; Li, K.; Kleist, T.J.; Lemaux, P.G.; Luan, S. A calcium signalling network activates vacuolar K+ remobilization to enable plant adaptation to low-K environments. Nat. Plants 2020, 6, 384–393. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, X.; Xu, G.; Dai, Z.; Chen, P.; Zhang, T.; Zhang, H. The Ca2+-CaM Signaling Pathway Mediates Potassium Uptake by Regulating Reactive Oxygen Species Homeostasis in Tobacco Roots Under Low-K+ Stress. Front. Plant Sci. 2021, 12, 658609. [Google Scholar] [CrossRef]
- Pauget, B.; Gimbert, F.; Scheifler, R.; Coeurdassier, M.; de Vaufleury, A. Soil parameters are key factors to predict metal bioavailability to snails based on chemical extractant data. Sci. Total Environ. 2012, 431, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; Delhaize, E.; Jones, D.L. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhou, Y.; Liang, X.; Li, J.; Huang, Y.; Li, Q. A novel carbon-nitrogen coupled metabolic pathway promotes the recyclability of nitrogen in composting habitats. Bioresour. Technol. 2023, 381, 129134. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Liang, A.; Li, Y.; Song, Q.; Li, X.; Li, D.; Hou, N. Insight into the soil aggregate-mediated restoration mechanism of degraded black soil via biochar addition: Emphasizing the driving role of core microbial communities and nutrient cycling. Environ. Res. 2023, 228, 115895. [Google Scholar] [CrossRef] [PubMed]
- Borggaard, O.K.; Raben-Lange, B.; Gimsing, A.L.; Strobel, B.W. Influence of humic substances on phosphate adsorption by aluminium and iron oxides. Geoderma 2005, 127, 270–279. [Google Scholar] [CrossRef]
- Brear, E.M.; Day, D.A.; Smith, P.M.C. Iron: An essential micronutrient for the legume-rhizobium symbiosis. Front. Plant Sci. 2013, 4, 359. [Google Scholar] [CrossRef]
- Li, R.; Chen, H.; Yang, Z.; Yuan, S.; Zhou, X.A. Research status of soybean symbiosis nitrogen fixation. Oil Crop Sci. 2020, 5, 6–10. [Google Scholar]
- Su, Y.; Zhang, Z.; Su, G.; Liu, J.; Liu, C.; Shi, G. Genotypic Differences in Spectral and Photosynthetic Response of Peanut to Iron Deficiency. J. Plant Nutr. 2015, 38, 145–160. [Google Scholar] [CrossRef]
- Tang, C.; Robson, A.D.; Dilworth, M.J. The role of iron in nodulation and nitrogen fixation in Lupinus angustifolius L. New Phytol. 1990, 114, 173–182. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Yang, S.; Li, X.; Top, E.M.; Wang, R.; Zhang, Y.; Cai, J.; Yao, F.; Han, X.; et al. Responses of Soil Bacterial Communities to Nitrogen Deposition and Precipitation Increment Are Closely Linked with Aboveground Community Variation. Microb. Ecol. 2016, 71, 974–989. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Yang, S.; Wang, Z.; Feng, X.; Liu, H.; Jiang, Y. Variations in soil bacterial taxonomic profiles and putative functions in response to straw incorporation combined with N fertilization during the maize growing season. Agric. Ecosyst. Environ. 2019, 283, 106578. [Google Scholar] [CrossRef]
- Lian, J.; Wang, H.; Deng, Y.; Xu, M.; Liu, S.; Zhou, B.; Jangid, K.; Duan, Y. Impact of long-term application of manure and inorganic fertilizers on common soil bacteria in different soil types. Agric. Ecosyst. Environ. 2022, 337, 108044. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, J.; Yuan, H.; Xie, X.; Gao, Y.; Lu, Y.; Liao, Y.; Nie, J. Long-term application of legume green manure improves rhizosphere soil bacterial stability and reduces bulk soil bacterial stability in rice. Eur. J. Soil. Biol. 2024, 122, 103652. [Google Scholar] [CrossRef]
- Zhu, R.; Azene, B.; Gruba, P.; Pan, K.; Nigussie, Y.; Guadie, A.; Sun, X.; Wu, X.; Zhang, L. Response of carbohydrate-degrading enzymes and microorganisms to land use change in the southeastern Qinghai-Tibetan Plateau, China. Appl. Soil. Ecol. 2024, 200, 105442. [Google Scholar] [CrossRef]
- van der Bom, F.; Nunes, I.; Raymond, N.S.; Hansen, V.; Bonnichsen, L.; Magid, J.; Nybroe, O.; Jensen, L.S. Long-term fertilisation form, level and duration affect the diversity, structure and functioning of soil microbial communities in the field. Soil. Biol. Biochem. 2018, 122, 91–103. [Google Scholar] [CrossRef]
- Ye, G.; Banerjee, S.; He, J.-Z.; Fan, J.; Wang, Z.; Wei, X.; Hu, H.-W.; Zheng, Y.; Duan, C.; Wan, S.; et al. Manure application increases microbiome complexity in soil aggregate fractions: Results of an 18-year field experiment. Agric. Ecosyst. Environ. 2021, 307, 107249. [Google Scholar] [CrossRef]
- Kong, C.; Zhang, S.; Yuan, S.; Wang, W.; Song, X.; Guo, D.; Lawi, A.S. Soil bacterial community characteristics and its effect on organic carbon under different fertilization treatments. Front. Microbiol. 2024, 15, 1356171. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Sivojiene, D.; Kacergius, A.; Baksiene, E.; Maseviciene, A.; Zickiene, L. The Influence of Organic Fertilizers on the Abundance of Soil Microorganism Communities, Agrochemical Indicators, and Yield in East Lithuanian Light Soils. Plants 2021, 10, 2648. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ghosh, T.K.; Kabir, A.H.; Abdelrahman, M.; Rahman Khan, M.A.; Mochida, K.; Tran, L.-S.P. Potassium in plant physiological adaptation to abiotic stresses. Plant Physiol. Biochem. 2022, 186, 279–289. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Szczerba, M.W.; Britto, D.T.; Kronzucker, H.J. K+ transport in plants: Physiology and molecular biology. J. Plant Physiol. 2009, 166, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive compounds in lettuce: Highlighting the benefits to human health and impacts of preharvest and postharvest practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef]

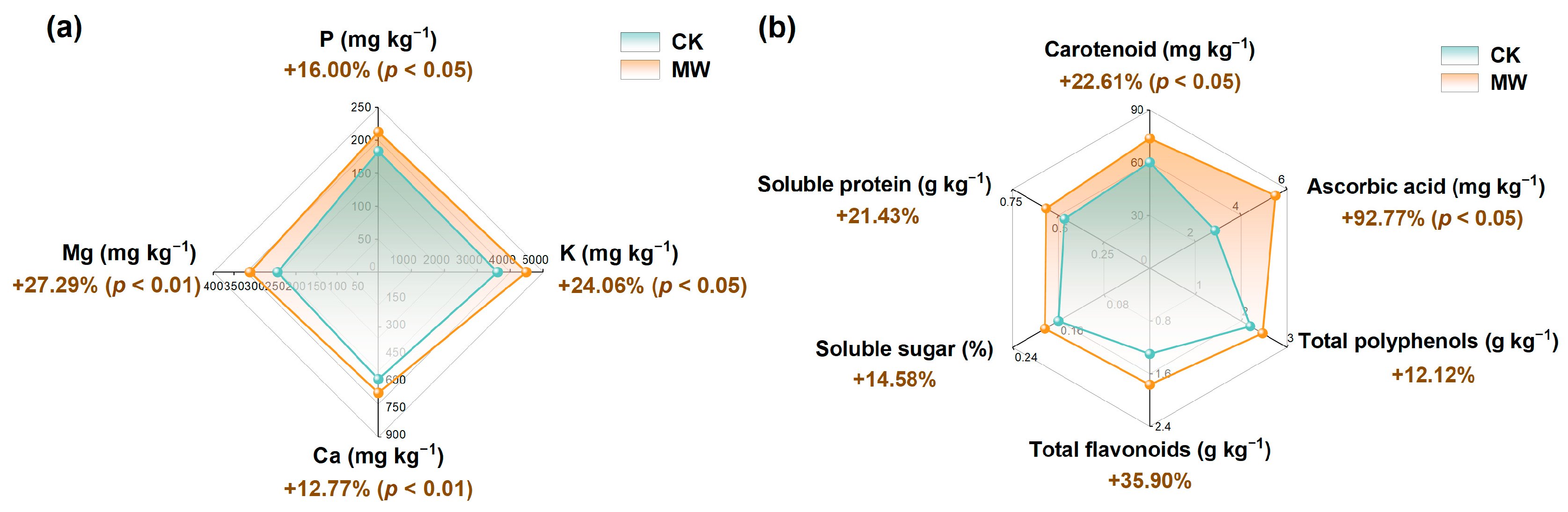
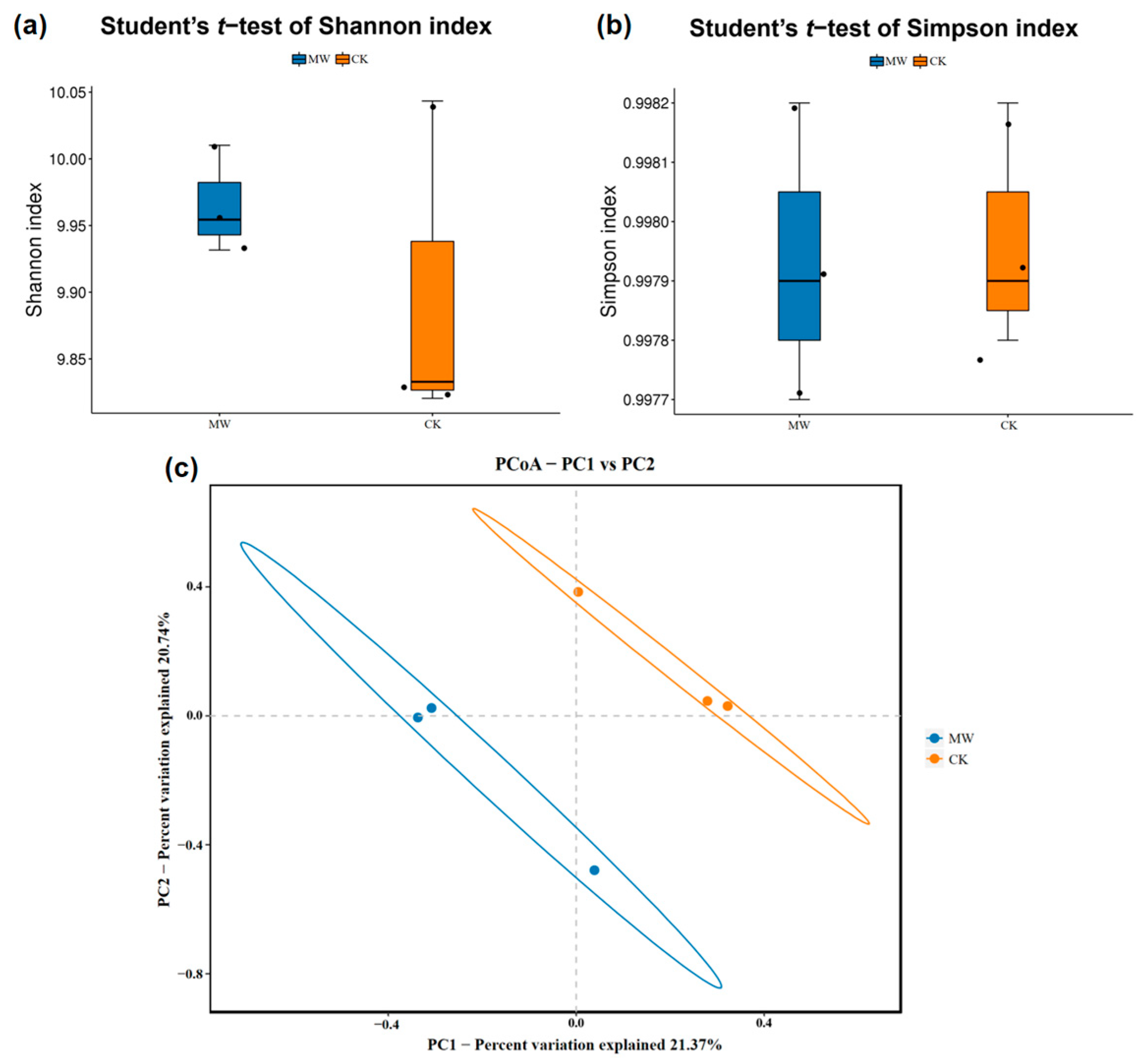

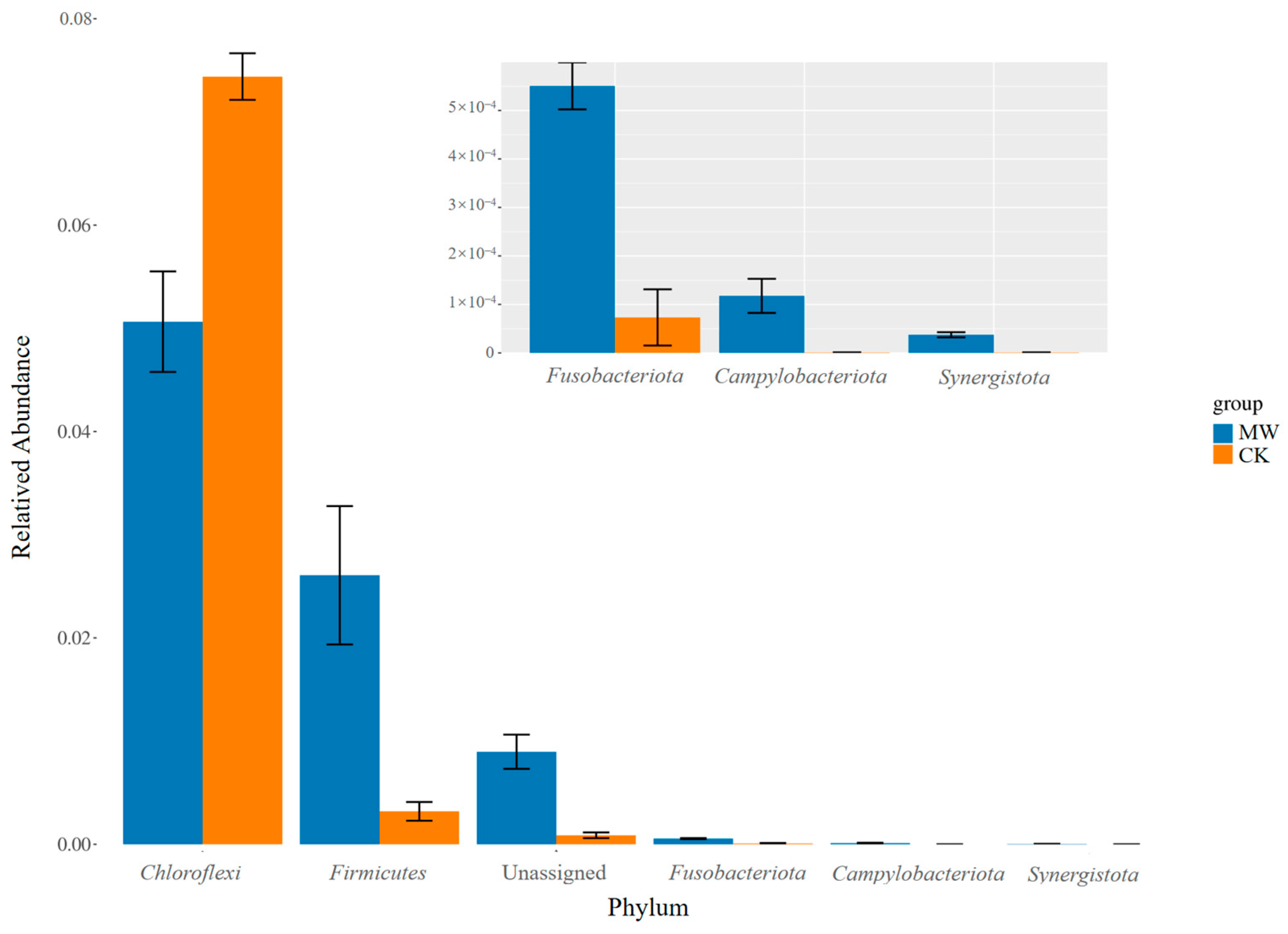
| Pot—CK | Pot—MW | |
|---|---|---|
| pH | 9.02 ± 0.04 | 9.11 ± 0.06 |
| Organic matter (g kg−1) | 65.04 ± 4.52 | 63.27 ± 3.73 |
| Ammonium nitrogen (mg kg−1) | 0.71 ± 0.13 | 0.85 ± 0.18 |
| Nitrate nitrogen (mg kg−1) | 1.41 ± 0.27 | 1.37 ± 0.16 |
| Available potassium (mg kg−1) | 742.34 ± 37.68 | 729.67 ± 45.29 |
| Available phosphorus (mg kg−1) | 9.33 ± 0.39 | 9.63 ± 0.57 |
| Ca (mg g−1) | 30.44 ± 3.52 | 31.68 ± 4.29 |
| Mg (mg g−1) | 10.95 ± 0.63 | 10.07 ± 0.51 |
| Fe (mg g−1) | 23.14 ± 2.78 | 22.56 ± 2.13 |
| Field—CK | Field—MW | |
|---|---|---|
| pH | 6.51 ± 0.06 | 6.45 ± 0.05 |
| Organic matter (g kg−1) | 5.27 ± 0.39 | 5.46 ± 0.53 |
| Ammonium nitrogen (mg kg−1) | 0.45 ± 0.05 | 0.42 ± 0.04 |
| Nitrate nitrogen (mg kg−1) | 4.75 ± 0.09 | 4.81 ± 0.14 |
| Available potassium (mg kg−1) | 40.57 ± 2.49 | 42.35 ± 3.18 |
| Available phosphorus (mg kg−1) | 5.07 ± 0.08 | 5.13 ± 0.11 |
| Ca (mg g−1) | 15.52 ± 3.36 | 13.89 ± 2.14 |
| Mg (mg g−1) | 6.71 ± 1.14 | 7.13 ± 0.69 |
| Fe (mg g−1) | 29.47 ± 5.38 | 29.08 ± 4.13 |
| Dolomite (%) | Calcite (%) | Quartz (%) | |
|---|---|---|---|
| S-1 | 74 | 20 | 6 |
| S-2 | 87 | 9 | 4 |
| S-3 | 71 | 15 | 14 |
| S-4 | 76 | 16 | 8 |
| S-5 | 73 | 19 | 8 |
| S-6 | 84 | 11 | 5 |
| S-7 | 79 | 14 | 7 |
| S-8 | 81 | 11 | 8 |
| S-9 | 72 | 18 | 10 |
| S-10 | 77 | 12 | 11 |
| CaO | ZnO | MgO | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | K2O | L.O.I. | |
|---|---|---|---|---|---|---|---|---|---|---|
| S-1 | 30.56 | 1.39 | 18.31 | 1.86 | 0.10 | 0.45 | 4.70 | 0.12 | 0.63 | 41.84 |
| S-2 | 29.22 | 1.37 | 20.81 | 1.46 | 0.12 | 0.37 | 4.37 | 0.12 | 0.54 | 41.53 |
| S-3 | 29.74 | 1.38 | 18.34 | 1.34 | 0.10 | 0.34 | 4.39 | 0.12 | 0.39 | 43.79 |
| S-4 | 29.19 | 1.49 | 21.19 | 2.19 | 0.04 | 0.23 | 3.81 | 0.13 | 0.21 | 41.43 |
| S-5 | 30.83 | 1.37 | 22.28 | 1.65 | 0.16 | 0.48 | 3.44 | 0.12 | 0.48 | 39.08 |
| AVE | 29.91 | 1.40 | 20.19 | 1.70 | 0.10 | 0.37 | 4.14 | 0.12 | 0.45 | 41.53 |
| SS-1 | SS-2 | SS-3 | SS-4 | SS-5 | |
|---|---|---|---|---|---|
| Li | 9.07 | 9.02 | 4.99 | 4.14 | 9.22 |
| Be | 0.18 | 0.21 | 0.17 | 0.11 | 0.24 |
| Sc | 1.63 | 2.37 | 1.83 | 1.34 | 2.09 |
| Ti | 155.94 | 562.77 | 234.47 | 110.3 | 309.84 |
| V | 10.88 | 16.92 | 12.88 | 8.49 | 26.86 |
| Cr | 3.12 | 7.46 | 3.84 | 2.39 | 5.19 |
| Mn | 525.52 | 496.17 | 505.61 | 409.84 | 459.74 |
| Co | 1.82 | 2.87 | 2.38 | 2.31 | 3.17 |
| Ni | 3.77 | 4.68 | 4 | 4.65 | 7.96 |
| Cu | 2.49 | 4.75 | 2.83 | 2.69 | 7.84 |
| Zn | 99.19 | 82.35 | 47.37 | 27.81 | 34.2 |
| Ga | 0.67 | 1.4 | 0.78 | 0.41 | 1.33 |
| Ge | 0.2 | 0.19 | 0.14 | 0.1 | 0.17 |
| Pb | 9.32 | 8.67 | 5.39 | 8.53 | 7.61 |
| As | 2.23 | 3.35 | 2.03 | 1.86 | 5.94 |
| Rb | 4.19 | 10.78 | 6.06 | 2.11 | 8.65 |
| Sr | 69.04 | 64 | 37.91 | 42.59 | 26.49 |
| Y | 3.14 | 3.19 | 2.12 | 2.02 | 3.3 |
| Zr | 15.19 | 19.41 | 9.3 | 6.31 | 15.02 |
| Nb | 0.69 | 2.38 | 1.05 | 0.45 | 1.59 |
| Mo | 0.11 | 0.1 | 0.09 | 0.1 | 0.24 |
| Cd | 0.07 | 0.11 | 0.03 | 0.02 | 0.02 |
| Ca (mg L−1) | Mg (mg L−1) | Na (mg L−1) | K (mg L−1) | pH | |
|---|---|---|---|---|---|
| Pot—CK | 0.843 ± 0.010 | / | 2.327 ± 0.089 | 2.867 ± 0.032 | 7.06 ± 0.04 |
| Pot—MW | 5.355 ± 0.087 | 0.049 ± 0.013 | 2.313 ± 0.024 | 2.860 ± 0.035 | 7.39 ± 0.03 |
| t-value | −88.850 | −6.384 | 0.276 | 0.230 | 0.031 |
| p-value | 0.000 | 0.003 | 0.796 | 0.829 | 0.000 |
| Field—CK | 38.305 ± 1.507 | 6.688 ± 0.748 | 13.033 ± 0.210 | 3.527 ± 0.083 | 7.89 ± 0.03 |
| Field—MW | 19.098 ± 0.498 | 3.825 ± 0.133 | 11.165 ± 0.111 | 3.303 ± 0.021 | 7.59 ± 0.05 |
| t-value | 20.961 | 6.526 | 13.613 | 4.530 | 9.701 |
| p-value | 0.000 | 0.003 | 0.000 | 0.011 | 0.001 |
| Growth Index | Leaf Fresh Weight | Leaf Dry Weight | Root Fresh Weight | Root Dry Weight |
|---|---|---|---|---|
| CK | 20.14 ± 3.71 | 1.53 ± 0.21 | 2.33 ± 0.57 | 0.34 ± 0.09 |
| MW | 24.93 ± 3.29 ** | 1.98 ± 0.17 ** | 2.56 ± 0.52 | 0.38 ± 0.11 |
| CK | MW | |
|---|---|---|
| pH | 8.95 ± 0.03 | 8.62 ± 0.08 ** |
| Organic matter (g kg−1) | 70.66 ± 4.04 | 75.99 ± 4.91 |
| Ammonium nitrogen (mg kg−1) | 1.66 ± 0.10 | 1.84 ± 0.57 |
| Nitrate nitrogen (mg kg−1) | 7.31 ± 1.25 | 14.78 ± 1.37 ** |
| Available potassium (mg kg−1) | 1005.33 ± 29.48 | 1170.67 ± 50.96 ** |
| Available phosphorus (mg kg−1) | 24.76 ± 2.13 | 29.20 ± 1.62 * |
| Ca (mg g−1) | 36.71 ± 0.44 | 38.93 ± 0.40 ** |
| Mg (mg g−1) | 11.55 ± 0.17 | 12.90 ± 0.07 ** |
| Fe (mg g−1) | 26.68 ± 0.94 | 29.77 ± 0.65 ** |
| CK | MW | |
|---|---|---|
| pH | 6.84 ± 0.19 | 7.12 ± 0.32 |
| Organic matter (g kg−1) | 6.32 ± 0.18 | 7.28 ± 1.60 |
| Ammonium nitrogen (mg kg−1) | 0.49 ± 0.08 | 1.56 ± 0.41 * |
| Nitrate nitrogen (mg kg−1) | 6.95 ± 0.41 | 13.08 ± 2.73 * |
| Available potassium (mg kg−1) | 55.67 ± 8.62 | 56.00 ± 6.24 |
| Available phosphorus (mg kg−1) | 6.09 ± 0.23 | 9.81 ± 3.55 |
| Ca (mg g−1) | 18.72 ± 0.75 | 19.58 ± 0.56 |
| Mg (mg g−1) | 8.61 ± 0.48 | 8.91 ± 0.64 |
| Fe (mg g−1) | 31.70 ± 0.57 | 35.01 ± 1.56 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Ge, X.; Du, Y.; Ding, H.; Lu, A. Mineral–Soil–Plant–Nutrient Synergism: Carbonate Rock Leachate Irrigation Enhances Soil Nutrient Availability, Improving Crop Yield and Quality. Minerals 2025, 15, 825. https://doi.org/10.3390/min15080825
Du Y, Ge X, Du Y, Ding H, Lu A. Mineral–Soil–Plant–Nutrient Synergism: Carbonate Rock Leachate Irrigation Enhances Soil Nutrient Availability, Improving Crop Yield and Quality. Minerals. 2025; 15(8):825. https://doi.org/10.3390/min15080825
Chicago/Turabian StyleDu, Yifei, Xiao Ge, Yimei Du, Hongrui Ding, and Anhuai Lu. 2025. "Mineral–Soil–Plant–Nutrient Synergism: Carbonate Rock Leachate Irrigation Enhances Soil Nutrient Availability, Improving Crop Yield and Quality" Minerals 15, no. 8: 825. https://doi.org/10.3390/min15080825
APA StyleDu, Y., Ge, X., Du, Y., Ding, H., & Lu, A. (2025). Mineral–Soil–Plant–Nutrient Synergism: Carbonate Rock Leachate Irrigation Enhances Soil Nutrient Availability, Improving Crop Yield and Quality. Minerals, 15(8), 825. https://doi.org/10.3390/min15080825






