Coupled Eu Anomalies and Fe Isotopes Reveal a Hydrothermal Iron Source for Superior-Type Iron Formations: A Case Study from the Wilgena Hill Iron Formation, South Australia
Abstract
1. Introduction
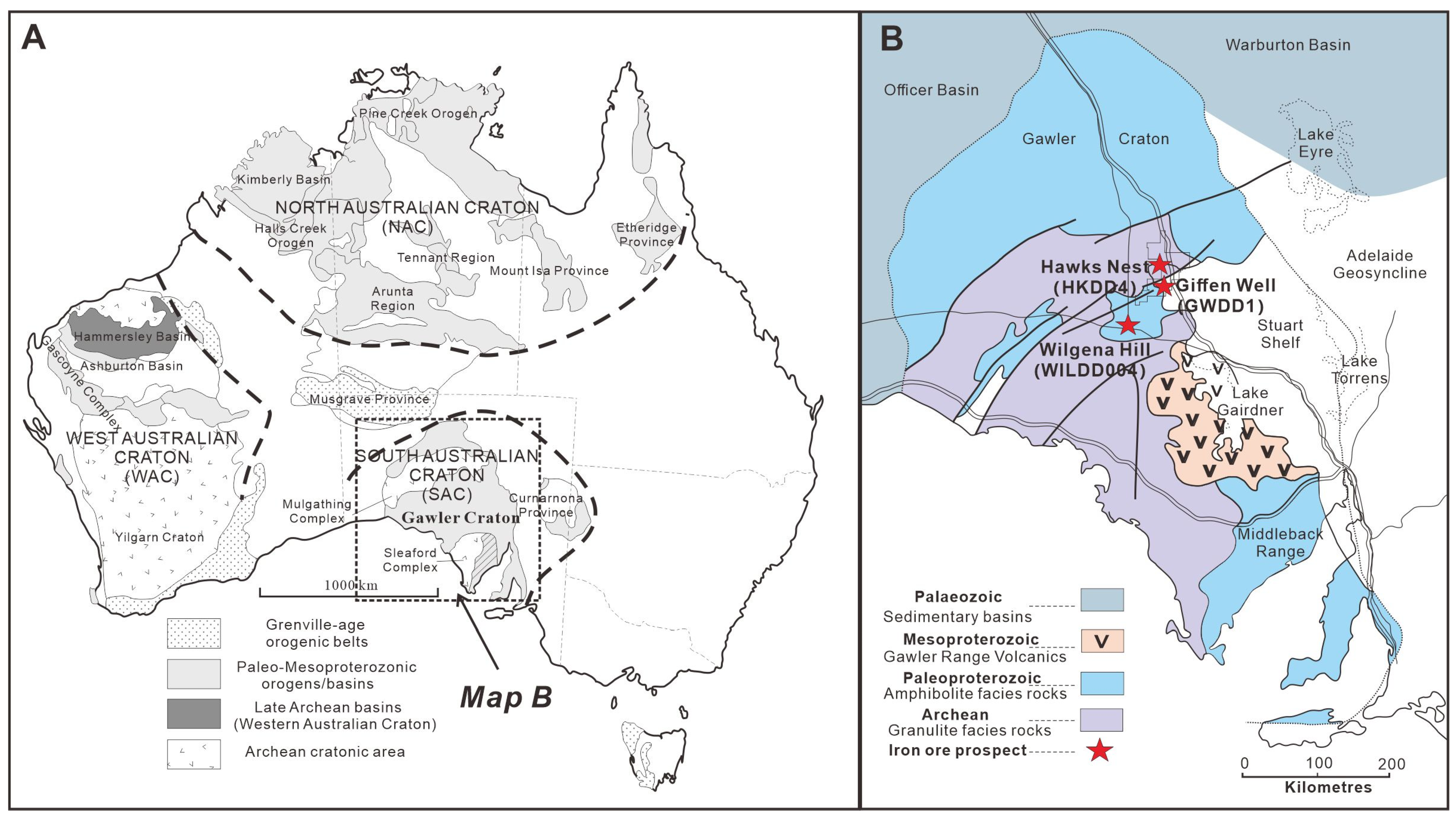
2. Geological Background
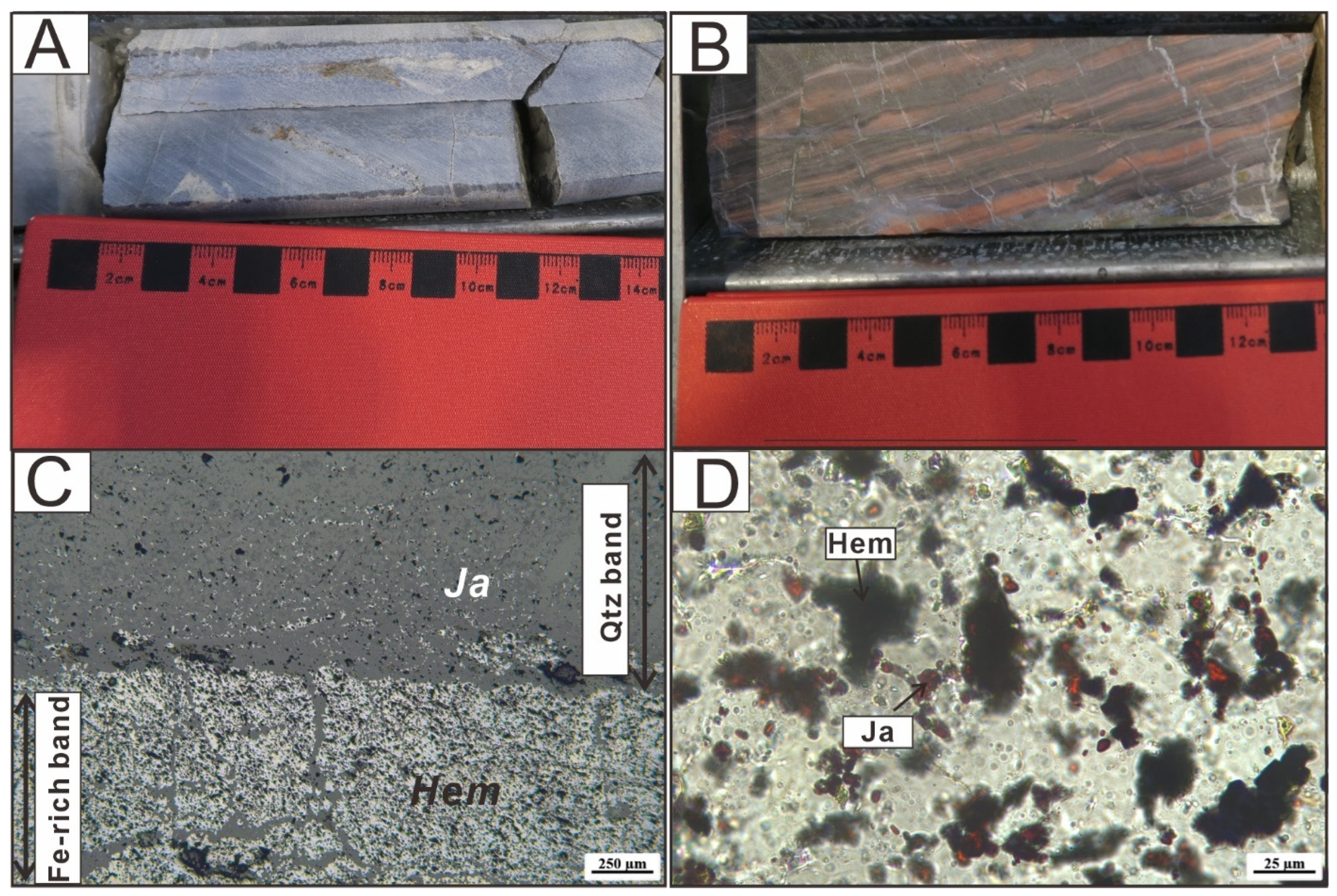
3. Petrography
4. Methods
4.1. Sample Collection and Preparation
4.2. Major and Trace Element Analyses
4.3. Iron Isotope Analyses
5. Results
5.1. Major Element Compositions
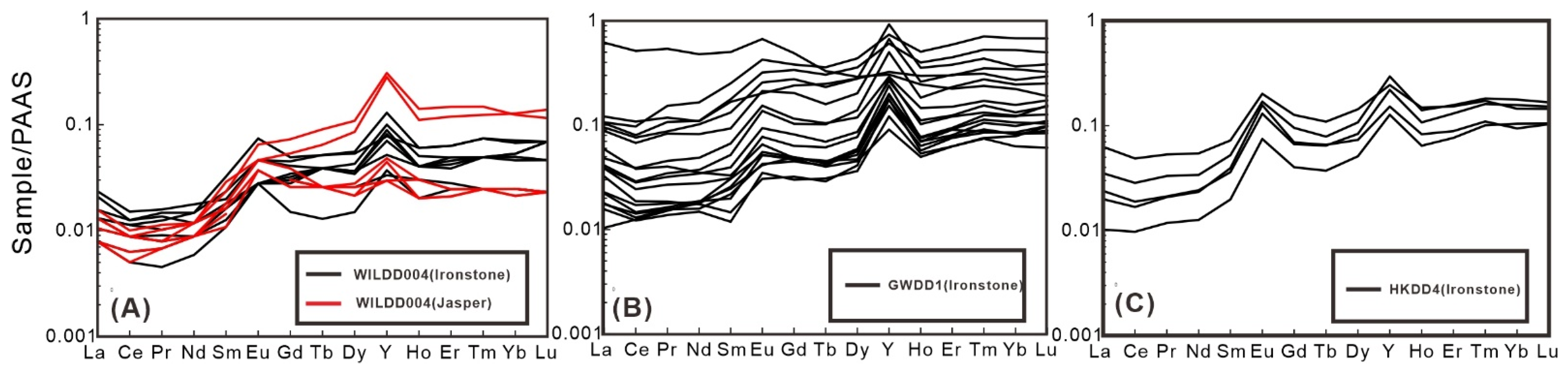
5.2. Trace Element Compositions
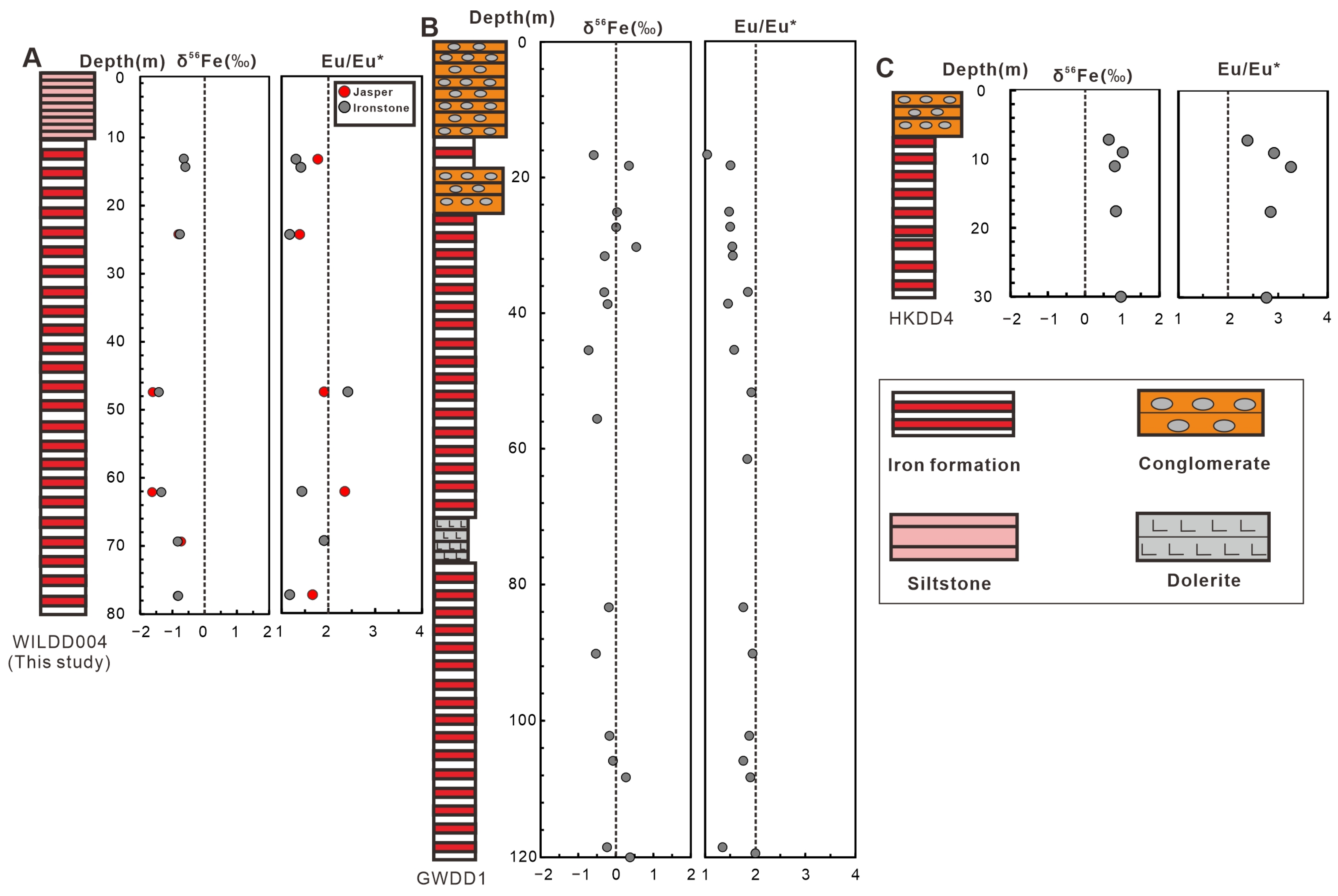
5.3. Fe Isotope Compositions
6. Discussion
6.1. Iron Source of the Wilgena Hill Iron Formation
6.2. Coupled δ56Fe–Eu/Eu* and Hydrothermal Transport
6.3. Implications for Genetic Models of Superior-Type IFs
7. Conclusions
- (1)
- All three drill cores exhibit positive Eu anomalies, indicative of hydrothermal contributions across the basin.
- (2)
- A clear spatial co-variation exists between δ56Fe and Eu/Eu*. This provides direct geochemical evidence that both iron and REEs were sourced from the same hydrothermal fluids and fractionated during lateral transport and oxidation in the basin.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, H.L. Sedimentary facies of iron-formation. Econ. Geol. 1954, 49, 235–293. [Google Scholar] [CrossRef]
- Gross, G.A. A classification of iron formations based on depositional environments. Can. Mineral. 1980, 18, 215–222. [Google Scholar]
- Isley, A.E.; Abbott, D.H. Plume-related mafic volcanism and the deposition of banded iron formation. J. Geophys. Res. Solid Earth 1999, 104, 15461–15477. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhu, X.K.; Tang, S.H. Characters of Fe isotopes and rare earth elements of banded iron formations from Anshan-Benxi area: Implications for Fe source. Acta Petrol. Et Mineral. 2008, 27, 285–290. [Google Scholar]
- Bekker, A.; Slack, J.F.; Planavsky, N.; Krapez, B.; Hofmann, A.; Konhauser, K.O.; Rouxel, O.J. Iron Formation: The Sedimentary Product of a Complex Interplay among Mantle, Tectonic, Oceanic, and Biospheric Processes. Econ. Geol. 2010, 105, 467–508. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Planavsky, N.J.; Hardisty, D.S.; Robbins, L.J.; Warchola, T.J.; Haugaard, R.; Lalonde, S.V.; Partin, C.A.; Oonk, P.B.H.; Tsikos, H.; et al. Iron formations: A global record of Neoarchaean to Palaeoproterozoic environmental history. Earth-Sci. Rev. 2017, 172, 140–177. [Google Scholar] [CrossRef]
- Cloud, P.E. Atmospheric and Hydrospheric Evolution on the Primitive Earth: Both secular accretion and biological and geochemical processes have affected earth’s volatile envelope. Science 1968, 160, 729–736. [Google Scholar] [CrossRef]
- Alexander, B.W.; Bau, M.; Andersson, P.; Dulski, P. Continentally-derived solutes in shallow Archean seawater: Rare earth element and Nd isotope evidence in iron formation from the 2.9Ga Pongola Supergroup, South Africa. Geochim. Cosmochim. Acta 2008, 72, 378–394. [Google Scholar] [CrossRef]
- Haugaard, R.; Frei, R.; Stendal, H.; Konhauser, K. Petrology and geochemistry of the ∼2.9Ga Itilliarsuk banded iron formation and associated supracrustal rocks, West Greenland: Source characteristics and depositional environment. Precambrian Res. 2013, 229, 150–176. [Google Scholar] [CrossRef]
- Li, W.; Beard, B.L.; Johnson, C.M. Biologically recycled continental iron is a major component in banded iron formations. Proc. Natl. Acad. Sci. USA 2015, 112, 8193–8198. [Google Scholar] [CrossRef] [PubMed]
- Michard, A.; Michard, G.; Stüben, D.; Stoffers, P.; Cheminée, J.L.; Binard, N. Submarine thermal springs associated with young volcanoes: The Teahitia vents, Society Islands, Pacific Ocean. Geochim. Cosmochim. Acta. 1993, 57, 4977–4986. [Google Scholar] [CrossRef]
- Schnetzler, C.C.; Philpotts, J.A. Partition coefficients of rare-earth elements between igneous matrix material and rock-forming mineral phenocrysts—II. Geochim. Cosmochim. Acta 1970, 34, 331–340. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Europium redox equilibria in aqueous solution. Earth Plant. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Bau, M.; Möller, P. Rare earth element systematics of the chemically precipitated component in early precambrian iron formations and the evolution of the terrestrial atmosphere-hydrosphere-lithosphere system. Geochim. Cosmochim. Acta 1993, 57, 2239–2249. [Google Scholar] [CrossRef]
- Viehmann, S.; Bau, M.; Hoffmann, J.E.; Münker, C. Geochemistry of the Krivoy Rog Banded Iron Formation, Ukraine, and the impact of peak episodes of increased global magmatic activity on the trace element composition of Precambrian seawater. Precambrian Res. 2015, 270, 165–180. [Google Scholar] [CrossRef]
- Li, F.; Zhu, X.; Ding, H.; Zhang, K. Local hydrothermal sources for Superior-type iron formations: Insights from the Animikie Basin. Precambrian Res. 2022, 377, 106736. [Google Scholar] [CrossRef]
- Anbar, A.D.; Jarzecki, A.A.; Spiro, T.G. Theoretical investigation of iron isotope fractionation between Fe (H2O)63+ and Fe (H2O)62+: Implications for iron stable isotope geochemistry. Geochim. Cosmochim. Acta 2005, 69, 825–837. [Google Scholar] [CrossRef]
- Myers, J.S.; Shaw, R.D.; Tyler, I.M. Tectonic evolution of Proterozoic Australia. Tectonics 1996, 15, 1431–1446. [Google Scholar] [CrossRef]
- Cawood, P.A.; Korsch, R.J. Assembling Australia: Proterozoic building of a continent. Precambrian Res. 2008, 166, 1–35. [Google Scholar] [CrossRef]
- Szpunar, M.; Hand, M.; Barovich, K.; Jagodzinski, E.; Belousova, E. Isotopic and geochemical constraints on the Paleoproterozoic Hutchison Group, southern Australia: Implications for Paleoproterozoic continental reconstructions. Precambrian Res. 2011, 187, 99–126. [Google Scholar] [CrossRef]
- Davies, M.B.; Morris, B.J.; Crettenden, P.P. South Australian Steel and Energy Project. Coober Pedy Iron Ore Investigation; Hawks Nest prospect, Report Book 97/00011; Mines and Energy South Australia: Adelaide, SA, Australia, 1997. [Google Scholar]
- Fanning, C.; Reid, A.; Teale, G. A geochronological framework for the Gawler Craton, South Australia. Bull. Geol. Surv. S. Aust. 2007, 55. [Google Scholar]
- Cowley, W.M.; Martin, A.R. KINGOONYA, South Australia. 1:250000 Geological Series–Explanatory Notes; Primary Industries and Resources South Australia: Adelaide, SA, Australia, 1991; p. 64. [Google Scholar]
- Daly, S.J.; Fanning, C.M.; Fairclough, M.C. Tectonic evolution and exploration potential of the Gawler Craton South Australia. AGSO J. Aust. Geol. Geophys. 1998, 17, 145–168. [Google Scholar]
- Budd, A. The Tarcoola Goldfield of the Central Gawler Gold Province, and the Hiltaba Association Granites, Gawler Craton, South Australia. Ph.D. Thesis, Australian National University, Canberra, ACT, Australia, 2006. [Google Scholar]
- Reid, A.; Flint, R.; Maas, R.; Howard, K.; Belousova, E. Geochronological and isotopic constraints on Palaeoproterozoic skarn base metal mineralisation in the central Gawler Craton, South Australia. Ore Geol. Rev. 2009, 36, 350–362. [Google Scholar] [CrossRef]
- Davies, M.; Twining, M. Magnetite: South Australia’s resource potential. MSEA J. 2018, 86, 30–44. [Google Scholar]
- Wilkins, L. Fe Isotope Analysis of South Australian Banded-Iron Formations. Bachelor’s Thesis, University of Adelaide, Adelaide, SA, Australia, 2009. [Google Scholar]
- Davies, M.B.; Morris, B.J.; Crettenden, P.P. South Australian Steel and Energy Project. Coober Pedy Iron Ore Investigation; Giffen Well prospect, Report Book 97/00010; Mines and Energy South Australia: Adelaide, SA, Australia, 1997. [Google Scholar]
- Zhu, X.K.; Li, Z.H.; Zhao, X.M.; Tang, S.H.; He, X.X.; Belshaw, N.S. High-precision measurements of Fe isotopes using MC-ICP-MS and Fe isotope compositions of geological reference materials. Acta Petrol. Miner. 2008, 27, 263–272. (In Chinese) [Google Scholar]
- Belshaw, N.S.; Zhu, X.K.; Guo, Y.; O’Nions, R.K. High precision measurement of iron isotopes by plasma source mass spectrometry. Int. J. Mass Spectrom. 2000, 197, 191–195. [Google Scholar] [CrossRef]
- Craddock, P.R.; Dauphas, N. Iron Isotopic Compositions of Geological Reference Materials and Chondrites. Geostand. Geoanalytical Res. 2011, 35, 101–123. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; p. 312. [Google Scholar]
- Trendall, A.F.; Blockley, J.G. The Iron Formations of the Precambrian Hamersley Group Western Australia With Special Reference to the Associated Crocidolite; Geological Survey of Western Australia: East Perth, WA, Australia, 1970. [Google Scholar]
- Zhou, Z.; Zhu, X.; Sun, J.; Li, Z. Crucial roles of local hydrothermal activities in the generation of large-scale iron formations during Mesoproterozoic tectonic quiescence. Chem. Geol. 2025, 688, 122858. [Google Scholar] [CrossRef]
- McLennan, S.M.; Taylor, S.R. Geochemical standards for sedimentary rocks: Trace-element data for U.S.G.S. standards SCo-1, MAG-1 and SGR-1. Chem. Geol. 1980, 29, 333–343. [Google Scholar] [CrossRef]
- Dauphas, N.; Van Zuilen, M.; Busigny, V.; Lepland, A.; Wadhwa, M.; Janney, P.E. Iron isotope, major and trace element characterization of early Archean supracrustal rocks from SW Greenland: Protolith identification and metamorphic overprint. Geochim. Cosmochim. Acta 2007, 71, 4745–4770. [Google Scholar] [CrossRef]
- Planavsky, N.; Rouxel, O.J.; Bekker, A.; Hofmann, A.; Little, C.T.S.; Lyons, T.W. Iron isotope composition of some Archean and Proterozoic iron formations. Geochim. Cosmochim. Acta 2012, 80, 158–169. [Google Scholar] [CrossRef]



| Sample | Lithology | SiO2 | Fe2O3 | Al2O3 | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | Eu/Eu* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | wt% | wt% | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | |||
| WIL4-1 | Siltstone | 75.32 | 3.43 | 10.01 | 24.3 | 49.1 | 5.52 | 19.6 | 3.8 | 0.91 | 3.38 | 0.53 | 3.05 | 19 | 0.66 | 1.89 | 0.28 | 1.85 | 0.29 | 1.23 |
| WIL4-4-1 | Ironstone | 28.94 | 71.82 | <0.01 | 0.5 | 0.7 | 0.08 | 0.3 | 0.07 | 0.03 | 0.14 | 0.03 | 0.16 | 1.9 | 0.04 | 0.11 | 0.02 | 0.14 | 0.02 | 1.31 |
| WIL4-4-2 | Jasper | 73.50 | 24.99 | <0.01 | 0.9 | 1.2 | 0.14 | 0.6 | 0.11 | 0.05 | 0.19 | 0.03 | 0.2 | 2.7 | 0.05 | 0.14 | 0.02 | 0.15 | 0.03 | 1.78 |
| WIL4-5 | Ironstone | 0.8 | 1 | 0.13 | 0.5 | 0.13 | 0.05 | 0.21 | 0.04 | 0.26 | 3.5 | 0.06 | 0.18 | 0.03 | 0.2 | 0.03 | 1.41 | |||
| WIL4-10-1 | Ironstone | 0.6 | 1 | 0.12 | 0.4 | 0.09 | 0.03 | 0.15 | 0.03 | 0.17 | 2.4 | 0.04 | 0.14 | 0.02 | 0.15 | 0.03 | 1.17 | |||
| WIL4-10-2 | Jasper | 0.3 | 0.4 | 0.06 | 0.3 | 0.1 | 0.05 | 0.25 | 0.05 | 0.4 | 7.6 | 0.11 | 0.34 | 0.05 | 0.36 | 0.06 | 1.39 | |||
| WIL4-16-1 | Ironstone | 31.45 | 69.52 | 0.03 | 0.3 | 0.4 | 0.04 | 0.2 | 0.06 | 0.03 | 0.07 | 0.01 | 0.07 | 1 | 0.02 | 0.07 | 0.01 | 0.07 | 0.01 | 2.41 |
| WIL4-16-2 | Jasper | 79.34 | 19.46 | <0.01 | 0.5 | 0.7 | 0.07 | 0.4 | 0.09 | 0.04 | 0.12 | 0.02 | 0.1 | 1.2 | 0.02 | 0.06 | 0.01 | 0.07 | 0.01 | 1.91 |
| WIL4-22-1 | Ironstone | 0.4 | 0.7 | 0.07 | 0.3 | 0.09 | 0.03 | 0.14 | 0.02 | 0.12 | 0.9 | 0.03 | 0.08 | 0.01 | 0.07 | 0.01 | 1.43 | |||
| WIL4-22-2 | Jasper | 0.3 | 0.5 | 0.06 | 0.3 | 0.06 | 0.04 | 0.12 | 0.02 | 0.1 | 0.8 | 0.02 | 0.06 | 0.01 | 0.06 | 0.01 | 2.35 | |||
| WIL4-28-1 | Ironstone | 0.5 | 0.9 | 0.11 | 0.5 | 0.18 | 0.08 | 0.23 | 0.04 | 0.25 | 2.1 | 0.06 | 0.18 | 0.03 | 0.19 | 0.03 | 1.91 | |||
| WIL4-28-2 | Jasper | 0.4 | 0.7 | 0.07 | 0.3 | 0.08 | <0.03 | 0.14 | 0.02 | 0.12 | 0.8 | 0.03 | 0.07 | 0.01 | 0.06 | 0.01 | ||||
| WIL4-32-1 | Ironstone | 17.98 | 82.83 | 0.07 | 0.3 | 0.5 | 0.06 | 0.3 | 0.09 | 0.03 | 0.13 | 0.03 | 0.17 | 1.4 | 0.04 | 0.12 | 0.02 | 0.13 | 0.02 | 1.17 |
| WIL4-32-2 | Jasper | 0.6 | 0.8 | 0.1 | 0.4 | 0.16 | 0.05 | 0.18 | 0.02 | 0.13 | 1.3 | 0.03 | 0.07 | 0.01 | 0.07 | 0.01 | 1.66 | |||
| WIL4-36-1 | Ironstone | 21.56 | 79.52 | 0.01 | 0.5 | 0.9 | 0.09 | 0.4 | 0.1 | 0.03 | 0.16 | 0.03 | 0.17 | 2.2 | 0.04 | 0.13 | 0.02 | 0.14 | 0.02 | 1.12 |
| WIL4-36-2 | Jasper | 77.01 | 22.38 | <0.01 | 0.5 | 0.7 | 0.09 | 0.4 | 0.13 | 0.07 | 0.34 | 0.07 | 0.51 | 8.3 | 0.14 | 0.42 | 0.06 | 0.35 | 0.05 | 1.42 |
| Sample | Lithology | n | δ56Fe (‰) | 2SD | δ57Fe (‰) | 2SD |
|---|---|---|---|---|---|---|
| WIL4-1 | Siltstone | 2 | 0.06 | 0.00 | 0.21 | 0.06 |
| WIL4-4-1 | Ironstone | 3 | −0.65 | 0.12 | −0.95 | 0.15 |
| WIL4-5 | Ironstone | 2 | −0.60 | 0.14 | −0.93 | 0.17 |
| WIL4-10-1 | Ironstone | 2 | −0.77 | 0.20 | −1.06 | 0.11 |
| WIL4-10-2 | Jasper | 2 | −0.82 | 0.03 | −1.17 | 0.19 |
| WIL4-16-1 | Ironstone | 2 | −1.42 | 0.02 | −2.10 | 0.04 |
| WIL4-16-2 | Jasper | 3 | −1.61 | 0.03 | −2.30 | 0.15 |
| WIL4-22-1 | Ironstone | 2 | −1.34 | 0.20 | −1.93 | 0.17 |
| WIL4-22-2 | Jasper | 2 | −1.63 | 0.14 | −2.36 | 0.22 |
| WIL4-28-1 | Ironstone | 2 | −0.84 | 0.06 | −1.22 | 0.12 |
| WIL4-28-2 | Jasper | 2 | −0.73 | 0.06 | −1.12 | 0.21 |
| WIL4-32-1 | Ironstone | 2 | −0.82 | 0.13 | −1.21 | 0.24 |
| WIL4-32-2 | Jasper | 2 | −0.83 | 0.09 | −1.28 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Sun, J.; Zhu, X.; Chen, Y. Coupled Eu Anomalies and Fe Isotopes Reveal a Hydrothermal Iron Source for Superior-Type Iron Formations: A Case Study from the Wilgena Hill Iron Formation, South Australia. Minerals 2025, 15, 824. https://doi.org/10.3390/min15080824
Chen S, Sun J, Zhu X, Chen Y. Coupled Eu Anomalies and Fe Isotopes Reveal a Hydrothermal Iron Source for Superior-Type Iron Formations: A Case Study from the Wilgena Hill Iron Formation, South Australia. Minerals. 2025; 15(8):824. https://doi.org/10.3390/min15080824
Chicago/Turabian StyleChen, Shuo, Jian Sun, Xiangkun Zhu, and Yuelong Chen. 2025. "Coupled Eu Anomalies and Fe Isotopes Reveal a Hydrothermal Iron Source for Superior-Type Iron Formations: A Case Study from the Wilgena Hill Iron Formation, South Australia" Minerals 15, no. 8: 824. https://doi.org/10.3390/min15080824
APA StyleChen, S., Sun, J., Zhu, X., & Chen, Y. (2025). Coupled Eu Anomalies and Fe Isotopes Reveal a Hydrothermal Iron Source for Superior-Type Iron Formations: A Case Study from the Wilgena Hill Iron Formation, South Australia. Minerals, 15(8), 824. https://doi.org/10.3390/min15080824






