Forces During the Film Drainage and Detachment of NMC and Spherical Graphite in Particle–Bubble Interactions Quantified by CP-AFM and Modeling to Understand the Salt Flotation of Battery Black Mass
Abstract
1. Introduction
1.1. Salt Flotation
1.2. Scope
2. Materials and Methods
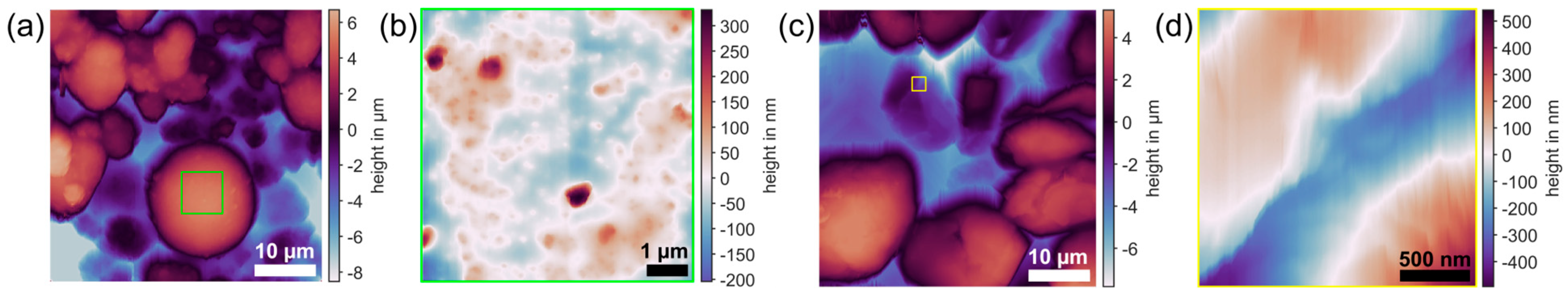
2.1. Particles for CP-AFM
2.1.1. NMC
2.1.2. Spherical Graphite
2.2. Salt Solutions and Surface Tension
2.3. Zeta Potential
2.4. Atomic Force Microscope
2.5. Modeling of Particle–Bubble Interactions
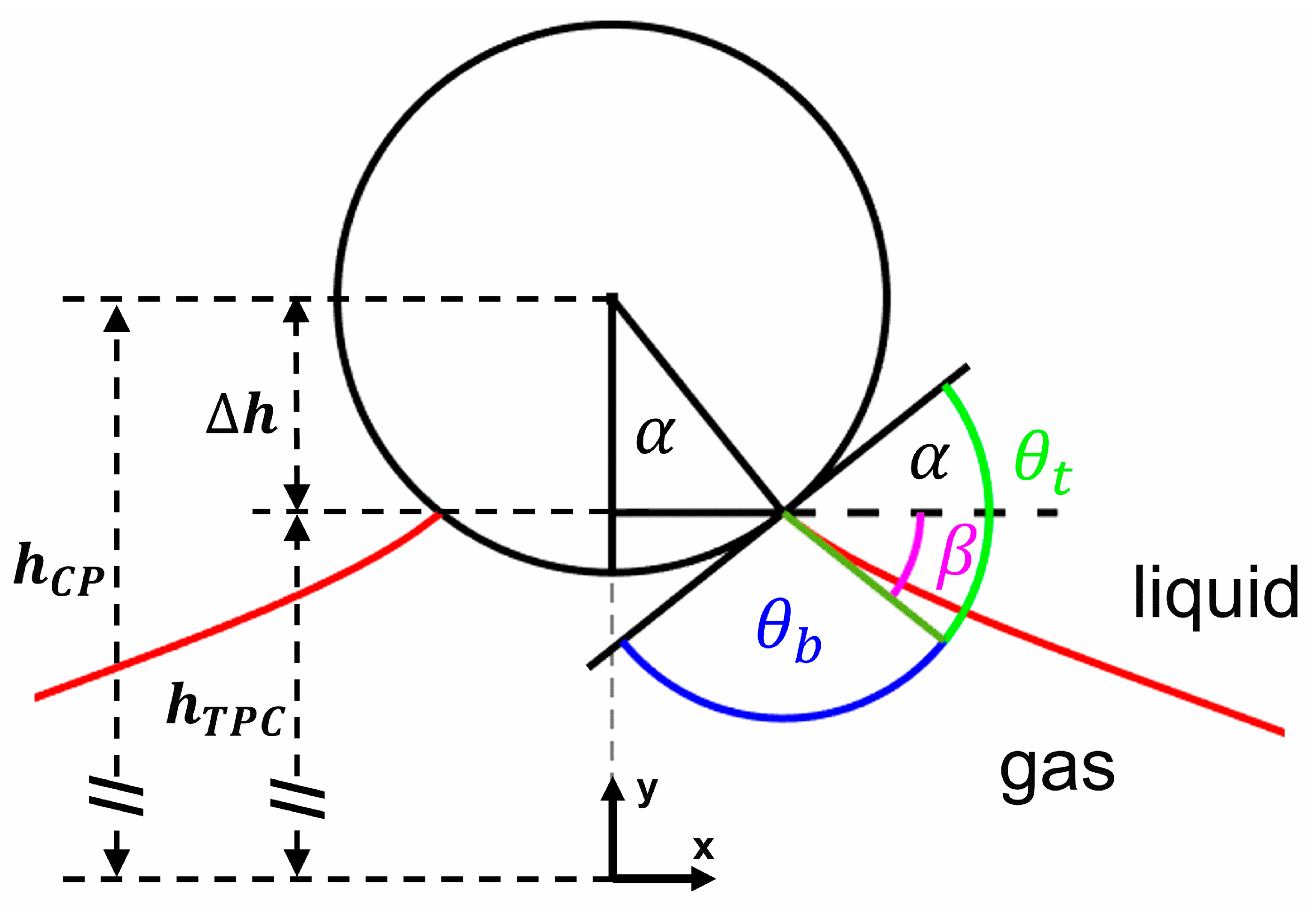
2.5.1. Stokes–Reynolds–Young–Laplace Model
2.5.2. Disjoining Pressure
2.5.3. Electric Double Layer
2.5.4. Van der Waals Interactions
2.5.5. Capillary Interactions
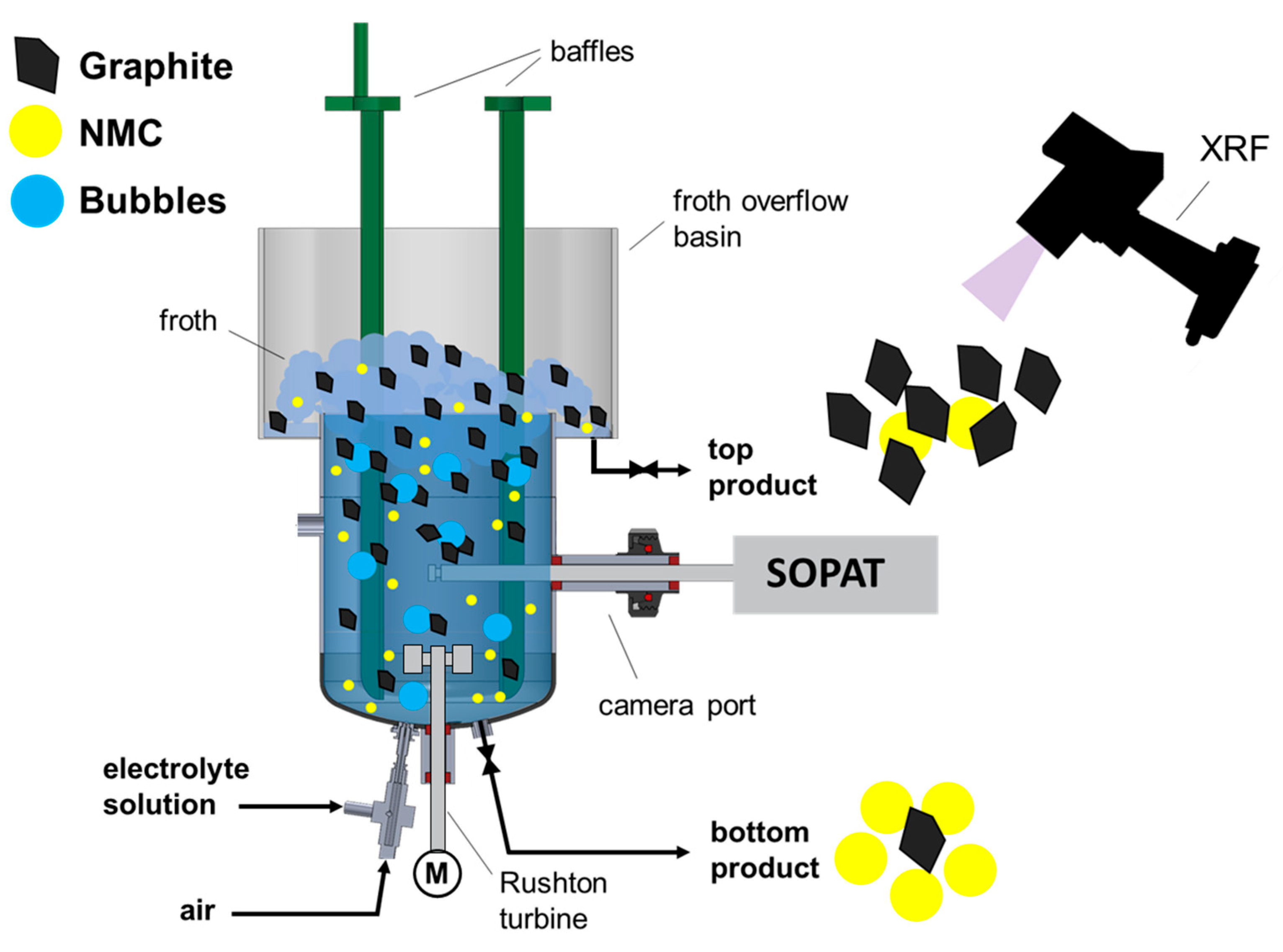
2.6. Flotation Tests
Product Analysis
3. Results and Discussion
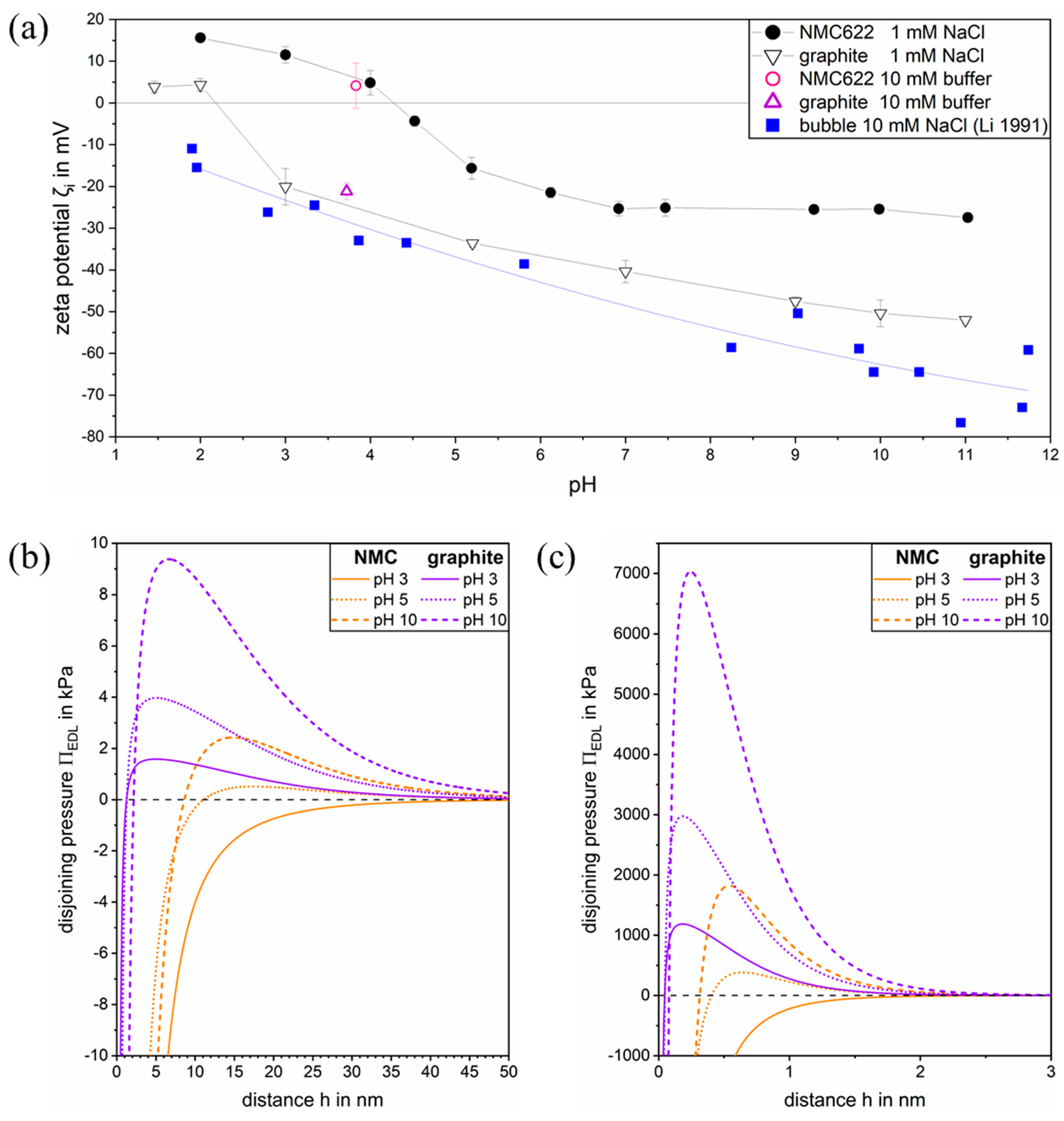
3.1. Zeta Potential of NMC and Graphite
3.2. Electric Double Layer Disjoining Pressure in 1:1 Electrolyte Solution
3.3. Film Drainage and Attachment to Gas Bubbles
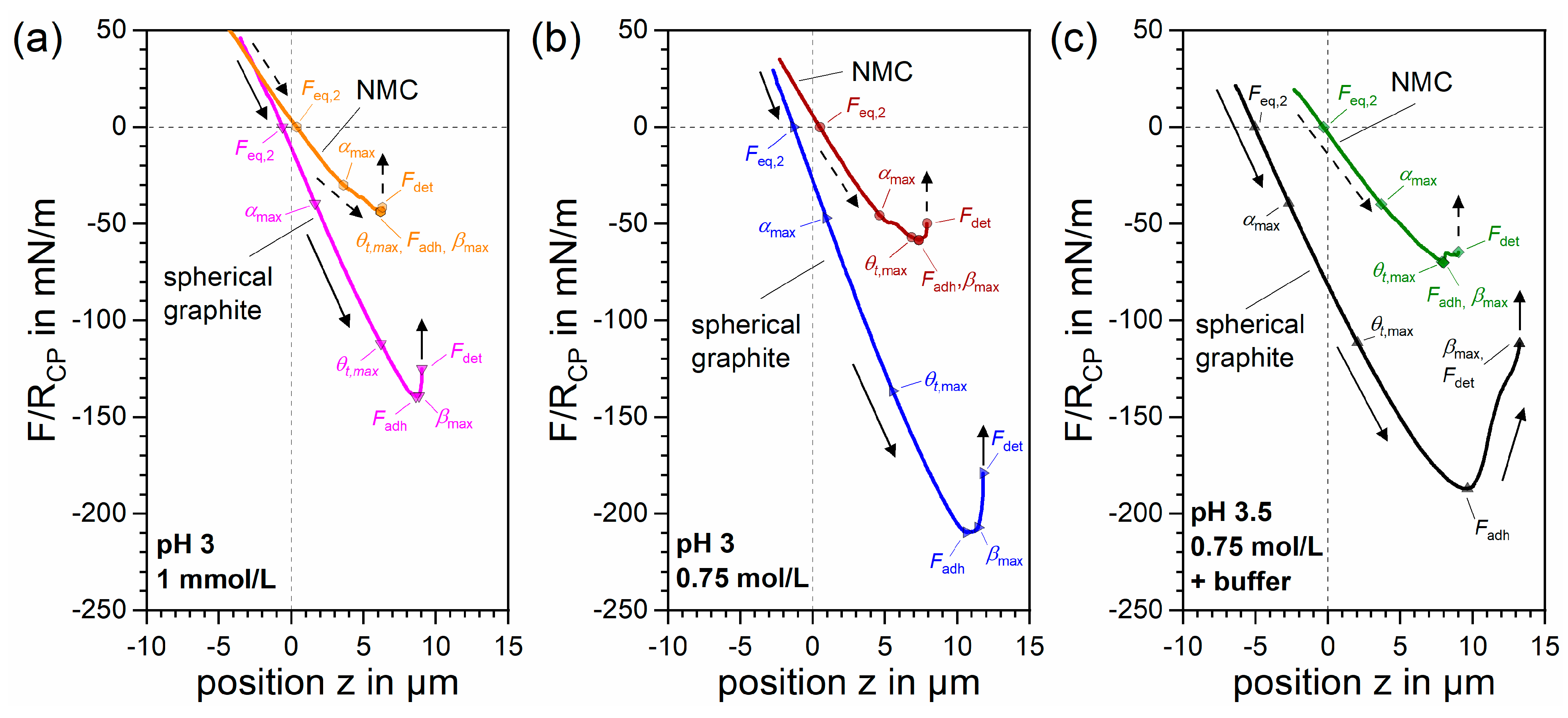
3.3.1. NMC Attachment
3.3.2. Spherical Graphite Attachment
3.3.3. Interactions in 0.75 mol/L NaCl + 0.01 mol/L NaOAc Buffer Solution
3.4. Snap-In Force and Forced Dewetting
3.5. Particle Detachment from Gas Bubbles
3.6. Flotation Test Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscope |
| CP | colloidal probe |
| DLVO | Derjaguin-Landau-Verwey-Overbeek–(theory) |
| EDL | electric double layer |
| HHF | Hogg–Healy–Fuerstenau |
| HOPG | Highly Oriented Pyrolytic Graphite |
| IEP | isoelectric point |
| LIB | lithium-ion battery |
| NMC | nickel manganese cobalt oxide |
| SG | spherical graphite |
| SMD | Sauter mean diameter |
| SRYL | Stokes–Reynolds–Young–Laplace |
| TPCL | three-phase-contact-line |
| TOC | total organic carbon |
| XRF | X-ray fluorescence spectrometer |
References
- Wilke, C.; Werner, D.M.; Kaas, A.; Peuker, U.A. Influence of the Crusher Settings and a Thermal Pre-Treatment on the Properties of the Fine Fraction (Black Mass) from Mechanical Lithium-Ion Battery Recycling. Batteries 2023, 9, 514. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Hayagan, N.; Bachmann, K.; Ferreira, A.; Werner, D.; Horn, D.; Peuker, U.; Serna-Guerrero, R.; Rudolph, M. Lithium-Ion Battery Recycling—Influence of Recycling Processes on Component Liberation and Flotation Separation Efficiency. ACS EST Eng. 2022, 2, 2130–2141. [Google Scholar] [CrossRef]
- Nazari, S.; Vakylabad, A.B.; Asgari, K.; Li, J.; Khoshdast, H.; He, Y.; Hassanzadeh, A. Bubbles to batteries: A review of froth flotation for sustainably recycling spent lithium-ion batteries. J. Energy Storage 2024, 84, 110702. [Google Scholar] [CrossRef]
- Gillies, G.; Kappl, M.; Butt, H.J. Direct measurements of particle-bubble interactions. Adv. Colloid Interface Sci. 2005, 114–115, 165–172. [Google Scholar] [CrossRef]
- Park, S.; Huang, K.; Yoon, R.-H. Predicting bubble coarsening in flotation froth: Effect of contact angle and particle size. Miner. Eng. 2018, 127, 256–264. [Google Scholar] [CrossRef]
- Chan, D.Y.; Klaseboer, E.; Manica, R. Theory of non-equilibrium force measurements involving deformable drops and bubbles. Adv. Colloid Interface Sci. 2011, 165, 70–90. [Google Scholar] [CrossRef]
- Nicklas, J.; Ditscherlein, L.; Peuker, U.A. Particle-Bubble Interactions: An Investigation of the Three-Phase Contact Line by Atomic Force Microscopy. Langmuir 2023, 39, 13630–13640. [Google Scholar] [CrossRef]
- Nicklas, J.; Peuker, U.A. Stability of bubble-particle hetero-coagulates accessed by atomic force microscopy to understand the froth flotation of agglomerating hydrophobic fines. Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134660. [Google Scholar] [CrossRef]
- Ducker, W.A.; Xu, Z.; Israelachvili, J.N. Measurements of Hydrophobic and DLVO Forces in Bubble-Surface Interactions in Aqueous Solutions. Langmuir 1994, 10, 3279–3289. [Google Scholar] [CrossRef]
- Webber, G.B.; Manica, R.; Edwards, S.A.; Carnie, S.L.; Stevens, G.W.; Grieser, F.; Dagastine, R.R.; Chan, D.Y.C. Dynamic Forces between a Moving Particle and a Deformable Drop. J. Phys. Chem. C 2008, 112, 567–574. [Google Scholar] [CrossRef]
- Englert, A.H.; Ren, S.; Masliyah, J.H.; Xu, Z. Interaction forces between a deformable air bubble and a spherical particle of tuneable hydrophobicity and surface charge in aqueous solutions. J. Colloid Interface Sci. 2012, 379, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Anachkov, S.E.; Lesov, I.; Zanini, M.; Kralchevsky, P.A.; Denkov, N.D.; Isa, L. Particle detachment from fluid interfaces: Theory vs. experiments. Soft Matter 2016, 12, 7632–7643. [Google Scholar] [CrossRef]
- Verrelli, D.I.; Koh, P.T.L.; Nguyen, A.V. Particle–bubble interaction and attachment in flotation. Chem. Eng. Sci. 2011, 66, 5910–5921. [Google Scholar] [CrossRef]
- Nguyen, A.V. Hydrodynamics of liquid flows around air bubbles in flotation: A review. Int. J. Miner. Process. 1999, 56, 165–205. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Dukhin, S.S. Theory of flotation of small and medium-size particles. Prog. Surf. Sci. 1993, 43, 241–266. [Google Scholar] [CrossRef]
- Schulze, H.J. New theoretical and experimental investigations on stability of bubble/particle aggregates in flotation: A theory on the upper particles size of floatability. Int. J. Miner. Process. 1977, 4, 241–259. [Google Scholar] [CrossRef]
- De, F.; Gontijo, C.; Fornasiero, D.; Ralston, J. The Limits of Fine and Coarse Particle Flotation. Can. J. Chem. Eng. 2008, 85, 739–747. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.-J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Moradi, B.; Botte, G.G. Recycling of graphite anodes for the next generation of lithium ion batteries. J. Appl. Electrochem. 2015, 46, 123–148. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Sygusch, J.; Rudolph, M.; Serna-Guerrero, R. A contribution to understanding the flotation behavior of lithium metal oxides and spheroidized graphite for lithium-ion battery recycling. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127111. [Google Scholar] [CrossRef]
- Zhan, R.; Yang, Z.; Bloom, I.; Pan, L. Significance of a Solid Electrolyte Interphase on Separation of Anode and Cathode Materials from Spent Li-Ion Batteries by Froth Flotation. ACS Sustain. Chem. Eng. 2020, 9, 531–540. [Google Scholar] [CrossRef]
- Schubert, H. On the turbulence-controlled microprocesses in flotation machines. Int. J. Miner. Process. 1999, 56, 257–276. [Google Scholar] [CrossRef]
- Tabor, R.F.; Grieser, F.; Dagastine, R.R.; Chan, D.Y. Measurement and analysis of forces in bubble and droplet systems using AFM. J. Colloid Interface Sci. 2012, 371, 1–14. [Google Scholar] [CrossRef]
- Neethling, S.J.; Cilliers, J.J. Modelling flotation froths. Int. J. Miner. Process. 2003, 72, 267–287. [Google Scholar] [CrossRef]
- Folayan, T.-O.; Lipson, A.L.; Durham, J.L.; Pinegar, H.; Liu, D.; Pan, L. Direct Recycling of Blended Cathode Materials by Froth Flotation. Energy Technol. 2021, 9, 2100468. [Google Scholar] [CrossRef]
- Li, W.; MCKinnan, W.R.; Dahn, J.R. Lithium Intercalation from Aqueous Solutions. J. Electrochem. Soc. 1994, 141, 2310–2316. [Google Scholar] [CrossRef]
- Verdugo, L.; Zhang, L.; Saito, K.; Bruckard, W.; Menacho, J.; Hoadley, A. Flotation behavior of the most common electrode materials in lithium ion batteries. Sep. Purif. Technol. 2022, 301, 121885. [Google Scholar] [CrossRef]
- Bauer, W.; Çetinel, F.A.; Müller, M.; Kaufmann, U. Effects of pH control by acid addition at the aqueous processing of cathodes for lithium ion batteries. Electrochim. Acta 2019, 317, 112–119. [Google Scholar] [CrossRef]
- Yoon, R.H.; Sabey, J.B. Coal Flotation in Inorganic Salt Solutions; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA; Virginia Center for Coal and Energy Research: Blacksburg, VA, USA, 1982. [Google Scholar]
- Pegram, L.M.; Record, M.T. Hofmeister Salt Effects on Surface Tension Arise from Partitioning of Anions and Cations between Bulk Water and the Air—Water Interface. J. Phys. Chem. B 2007, 111, 5411–5417. [Google Scholar] [CrossRef] [PubMed]
- Fuerstenau, D.W.; Pradip. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114–115, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Battino, R.; Rettich, T.R.; Tominaga, T. The Solubility of Nitrogen and Air in Liquids. J. Phys. Chem. Ref. Data 1984, 13, 563–600. [Google Scholar] [CrossRef]
- Morrison, T.J.; Billett, F. The salting-out of non-electrolytes. Part II. The effect of variation in non-electrolyte. J. Chem. Soc. 1952, 3819–3822. [Google Scholar] [CrossRef]
- Paulson, O.; Pugh, R.J. Flotation of Inherently Hydrophobic Particles in Aqueous Solutions of Inorganic Electrolytes. Langmuir 1996, 12, 4808–4813. [Google Scholar] [CrossRef]
- Ditscherlein, L.; Knüpfer, P.; Peuker, U.A. The influence of nanobubbles on the interaction forces between alumina particles and ceramic foam filters. Powder Technol. 2019, 357, 408–416. [Google Scholar] [CrossRef]
- Knüpfer, P.; Ditscherlein, L.; Peuker, U.A. Nanobubble enhanced agglomeration of hydrophobic powders. Colloids Surf. A Physicochem. Eng. Asp. 2017, 530, 117–123. [Google Scholar] [CrossRef]
- Aziz, H.A.; Ghazali, M.F.; Yusoff, M.S.; Hung, Y.-T. Waste Treatment and Management in Chlor-Alkali Industries. In Handbook of Advanced Industrial and Hazardous Wastes Management; Wang, L.K., Wang, M.-H.S., Hung, Y.-T., Shammas, N.K., Chen, J.P., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 611–655. [Google Scholar]
- Warren, J.K. Solution Mining and Salt Cavern Usage. In Evaporites: A Geological Compendium; Warren, J.K., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1303–1374. [Google Scholar]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Panagopoulos, A. Study and evaluation of the characteristics of saline wastewater (brine) produced by desalination and industrial plants. Environ. Sci. Pollut. Res. Int. 2022, 29, 23736–23749. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xie, Y.; Sun, W.; Xu, Y.; Sun, Y. Research Progress of High-Salinity Wastewater Treatment Technology. Water 2023, 15, 684. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y. The effect of saline water on mineral flotation—A critical review. Miner. Eng. 2014, 66–68, 13–24. [Google Scholar] [CrossRef]
- Cao, L.; Peng, Y.; Liu, Q. Interactions of NaCl with frother surfactants in producing microbubbles through modifying air/water interfacial energy and interfacial charge. J. Mol. Liq. 2023, 389, 122889. [Google Scholar] [CrossRef]
- Li, C.; Somasundaran, P. Reversal of bubble charge in multivalent inorganic salt solutions—Effect of magnesium. J. Colloid Interface Sci. 1991, 146, 215–218. [Google Scholar] [CrossRef]
- Liu, B.; Manica, R.; Zhang, X.; Bussonniere, A.; Xu, Z.; Xie, G.; Liu, Q. Dynamic Interaction between a Millimeter-Sized Bubble and Surface Microbubbles in Water. Langmuir 2018, 34, 11667–11675. [Google Scholar] [CrossRef] [PubMed]
- Vakarelski, I.U.; Manica, R.; Tang, X.; O’Shea, S.J.; Stevens, G.W.; Grieser, F.; Dagastine, R.R.; Chan, D.Y. Dynamic interactions between microbubbles in water. Proc. Natl. Acad. Sci. USA 2010, 107, 11177–11182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Manica, R.; Tang, Y.; Liu, Q.; Xu, Z. Bubbles with tunable mobility of surfaces in ethanol-NaCl aqueous solutions. J. Colloid Interface Sci. 2019, 556, 345–351. [Google Scholar] [CrossRef]
- Carnie, S.L.C.; Derek, Y.C.; Lewis, C.; Manica, R.; Dagastine, R.R. Carnie_Dagastine_2005_Measurement of Dynamical Forces between Deformable Drops Using the Atomic Force Microscope. I. Theory. Langmuir 2005, 21, 2912–2922. [Google Scholar] [CrossRef]
- Dagastine, R.R.; Webber, G.B.; Manica, R.; Stevens, G.W.; Grieser, F.; Chan, D.Y.C. Viscosity Effects on Hydrodynamic Drainage Force Measurements Involving Deformable Bodies. Langmuir 2010, 26, 11921–11927. [Google Scholar] [CrossRef]
- Lin, C.Y.; Slattery, J.C. Thinning of a liquid film as a small drop or bubble approaches a solid plane. AIChE J. 1982, 28, 147–156. [Google Scholar] [CrossRef]
- Vinogradova, O.I. Drainage of a thin liquid film confined between hydrophobic surfaces. Langmuir 1995, 11, 2213–2220. [Google Scholar] [CrossRef]
- Zhang, X.; Manica, R.; Tang, Y.; Tchoukov, P.; Liu, Q.; Xu, Z. Probing Boundary Conditions at Hydrophobic Solid-Water Interfaces by Dynamic Film Drainage Measurement. Langmuir 2018, 34, 12025–12035. [Google Scholar] [CrossRef]
- Chan, D.Y.; Dagastine, R.R.; White, L.R. Forces between a Rigid Probe Particle and a Liquid Interface. J. Colloid Interface Sci. 2001, 236, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Aston, D.E.; Berg, J.C. Thin-Film Hydrodynamics in Fluid Interface-Atomic Force Microscopy. Ind. Eng. Chem. Res. 2001, 41, 389–396. [Google Scholar] [CrossRef]
- Shi, C.; Chan, D.Y.C.; Liu, Q.; Zeng, H. Probing the Hydrophobic Interaction between Air Bubbles and Partially Hydrophobic Surfaces Using Atomic Force Microscopy. J. Phys. Chem. C 2014, 118, 25000–25008. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y. Effect of bubble size on bubble-particle attachment and film drainage kinetics—A theoretical study. Powder Technol. 2017, 322, 140–146. [Google Scholar] [CrossRef]
- Hogg, R.; Healy, T.W.; Fuerstenau, D.W. Mutual Coagulation of Colloidal Dispersions. Trans. Faraday Soc. 1966, 62, 1638–1651. [Google Scholar] [CrossRef]
- Pushkarova, R.A.; Horn, R.G. Bubble-Solid Interactions in Water and Electrolyte Solutions. Langmuir 2008, 24, 8726–8734. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: New York, NY, USA; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Parsons, D.F.; Bostrom, M.; Lo Nostro, P.; Ninham, B.W. Hofmeister effects: Interplay of hydration, nonelectrostatic potentials, and ion size. Phys. Chem. Chem. Phys. 2011, 13, 12352–12367. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, M.; Liu, Q. The effect of non-polar oil on fine hematite flocculation and flotation using sodium oleate or hydroxamic acids as a collector. Miner. Eng. 2018, 119, 105–115. [Google Scholar] [CrossRef]
- Huang, K.; Yoon, R.-H. Effect of ζ-Potentials on Bubble-Particle Interactions. Min. Metall. Explor. 2019, 36, 21–34. [Google Scholar] [CrossRef]
- Sunkel, J.M.; Berg, J.C. The Stability Behavior of Sol-Emulsion Systems. J. Colloid Interface Sci. 1996, 179, 618–624. [Google Scholar] [CrossRef]
- Duan, J.; Wang, J.; Guo, T.; Gregory, J. Zeta potentials and sizes of aluminum salt precipitates—Effect of anions and organics and implications for coagulation mechanisms. J. Water Process Eng. 2014, 4, 224–232. [Google Scholar] [CrossRef]
- Krasowska, M.; Carnie, S.L.; Fornasiero, D.; Ralston, J. Ultrathin Wetting Films on Hydrophilic Titania Surfaces: Equilibrium and Dynamic Behavior. J. Phys. Chem. C 2011, 115, 11065–11076. [Google Scholar] [CrossRef]
- Bowen, W.R.; Jenner, F. The calculation of dispersion forces for engineering applications. Adv. Colloid Interface Sci. 1995, 56, 201–243. [Google Scholar] [CrossRef]
- Tabor, D.; Winterton, R.H.S. The Direct Measurement of Normal and Retarded van der Waals Forces. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1969, 312, 435–450. [Google Scholar] [CrossRef]
- Hough, D.B.; White, L.R. The calculation of hamaker constants from liftshitz theory with applications to wetting phenomena. Adv. Colloid Interface Sci. 1980, 14, 3–41. [Google Scholar] [CrossRef]
- Owens, N.F.; Richmond, P. Hamaker Constants and Combining Rules. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1978, 74, 691–695. [Google Scholar] [CrossRef]
- Parsegian, A.V. Van der Waals Forces: A Handbook for Biologists, Chemists, Engineers, and Physicists; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Bergström, L. Hamaker constants of inorganic materials. Adv. Colloid Interface Sci. 1997, 70, 125–169. [Google Scholar] [CrossRef]
- Dagastine, R.R.; Prieve, D.C.; White, L.R. Calculations of van der Waals forces in 2-dimensionally anisotropic materials and its application to carbon black. J. Colloid Interface Sci. 2002, 249, 78–83. [Google Scholar] [CrossRef]
- Rubin, M.; von Rottkay, K.; Wen, S.-J.; Özer, N.; Slack, J. Optical indices of lithiated electrochromic oxides. Sol. Energy Mater. Sol. Cells 1998, 54, 49–57. [Google Scholar] [CrossRef]
- Draine, B.T. Graphite Revisited. Astrophys. J. 2016, 831, 109. [Google Scholar] [CrossRef]
- Tolias, P. Lifshitz calculations of Hamaker constants for fusion relevant materials. Fusion Eng. Des. 2018, 133, 110–116. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Evans, G.M.; Schulze, H.J. Prediction of van der Waals interaction in bubble–particle attachment in flotation. Int. J. Miner. Process. 2001, 61, 155–169. [Google Scholar] [CrossRef]
- Sherman, H.; Nguyen, A.V.; Bruckard, W. An Analysis of Bubble Deformation by a Sphere Relevant to the Measurements of Bubble-Particle Contact Interaction and Detachment Forces. Langmuir 2016, 32, 12022–12030. [Google Scholar] [CrossRef]
- Nguyen, A.; Schulze, H.J. Colloidal Science of Flotation; CRC Press: Boca Raton, FL, USA, 2003; Volume 118. [Google Scholar]
- Shi, C.; Cui, X.; Xie, L.; Liu, Q.; Chan, D.Y.; Israelachvili, J.N.; Zeng, H. Measuring Forces and Spatiotemporal Evolution of Thin Water Films between an Air Bubble and Solid Surfaces of Different Hydrophobicity. ACS Nano 2015, 9, 95–104. [Google Scholar] [CrossRef]
- Liu, B.; Manica, R.; Liu, Q.; Klaseboer, E.; Xu, Z.; Xie, G. Coalescence of Bubbles with Mobile Interfaces in Water. Phys. Rev. Lett. 2019, 122, 194501. [Google Scholar] [CrossRef]
- Zhang, X.; Manica, R.; Tchoukov, P.; Liu, Q.; Xu, Z. Effect of Approach Velocity on Thin Liquid Film Drainage between an Air Bubble and a Flat Solid Surface. J. Phys. Chem. C 2017, 121, 5573–5584. [Google Scholar] [CrossRef]
- Lee, T.; Charrault, E.; Neto, C. Interfacial slip on rough, patterned and soft surfaces: A review of experiments and simulations. Adv. Colloid Interface Sci. 2014, 210, 21–38. [Google Scholar] [CrossRef]
- Guriyanova, S.; Semin, B.; Rodrigues, T.S.; Butt, H.J.; Bonaccurso, E. Hydrodynamic drainage force in a highly confined geometry: Role of surface roughness on different length scales. Microfluid. Nanofluidics 2009, 8, 653–663. [Google Scholar] [CrossRef]
- Miksis, M.J.; Davis, S.H. Slip over rough and coated surfaces. J. Fluid Mech. 1994, 273, 125–139. [Google Scholar] [CrossRef]
- Parkinson, L.; Ralston, J. The Interaction between a Very Small Rising Bubble and a Hydrophilic Titania Surface. J. Phys. Chem. C 2010, 114, 2273–2281. [Google Scholar] [CrossRef]
- Schulze, H.J. Hydrodynamics of Bubble-Mineral Particle Collisions. Miner. Process. Extr. Metall. Rev. 1989, 5, 43–76. [Google Scholar] [CrossRef]









| Liquid Phase | Surface Tension γ in mN/m |
|---|---|
| 1 mmol/L NaCl | |
| 0.75 mol/L NaCl | |
| 0.75 mol/L NaCl + 0.01 mol/L buffer |
| Parameter | SRYL 1 | SRYL 2 | SRYL 3 | SRYL 4 | Unit |
|---|---|---|---|---|---|
| pH value | 3 | 10 | 3 | 10 | - |
| salt concentration cNaCl | 0.001 | 0.001 | 0.750 | 0.750 | mol/L |
| bubble surface potential ψ1 | −25 | −60 | −25 | −60 | mV |
| NMC surface potential ψ2 | 11 | −25 | 11 | −25 | mV |
| Debye length κ−1 | 9.6 | 9.6 | 0.35 | 0.35 | nm |
| radius of bubble Rb | 81 | 108 | 56 | 106 | µm |
| radius of Colloidal Probe RCP | 6 | 11 | 7.5 | 6 | µm |
| effective Hamaker constant Aeff | −5 × 10−20 | −5 × 10−20 | −5 × 10−20 | −5 × 10−20 | J |
| slip length b | 300 | 300 | 300 | 300 | nm |
| overlap ΔXmax | - | 10 | 10 | 10 | nm |
| interfacial tension γ | 71.4 | 71.4 | 73.4 | 73.4 | mN/m |
| dynamic viscosity η | 0.79 | 0.79 | 0.79 | 0.79 | mPas |
| substrate contact angle θ0 | 90 | 90 | 90 | 90 | ° |
| velocity v | ∓30 | ∓30 | ∓30 | ∓30 | µm/s |
| cantilever spring constant kc | 0.055 | 0.067 | 0.054 | 0.055 | N/m |
| Liquid-Phase | NMC | Spherical Graphite | |||
|---|---|---|---|---|---|
| in | |||||
| pH 3-1 mmol/L NaCl | 5.31 × 10−8 | 21.3 | 0.47 | 64.6 | 8.35 |
| pH 10-1 mmol/L NaCl | 4.91 × 10−8 | - | 0.55 | 25.9 | 12.68 |
| pH 3-0.75 mol/L NaCl | 9.94 × 10−9 | 29.3 | 2.55 | 113.3 | 15.56 |
| pH 10-0.75 mol/L NaCl | 1.17 × 10−7 | 28.1 | 4.22 | 46.3 | 34.48 |
| pH 3.5-0.75 mol/L NaCl + 0.01 mol/L NaOAc buffer | 1.17 × 10−8 | 26.5 | 2.73 | 76.3 | 27.02 |
| Liquid-Phase | Particle | in ° | in ° | in ° | in µm | in mN/m | in J/m2 |
|---|---|---|---|---|---|---|---|
| pH 3 1 mmol/L NaCl | NMC | 102.5 | 15.9 | 87.0 | 5.922 | 43.8 | 0.011 |
| graphite | 103.7 | 23.1 | 89.3 | 9.638 | 139.3 | 0.108 | |
| pH 3 0.75 mol/L NaCl | NMC | 102.2 | 15.1 | 88.0 | 7.405 | 58.5 | 0.020 |
| graphite | 106.2 | 36.0 | 89.9 | 13.075 | 209.7 | 0.228 | |
| pH 3.5-0.75 mol/L NaCl + 0.01 mol/L NaOAc buffer | NMC | 103.7 | 17.0 | 87.8 | 9.324 | 70.0 | 0.028 |
| graphite | 106.3 | 60.6 | 90.0 | 14.548 | 187.0 | 0.296 | |
| pH 10 1 mmol/L NaCl | NMC | 100.5 | 13.0 | 88.2 | 7.089 | 48.4 | 0.014 |
| graphite | 104.2 | 26.5 | 90.8 | 13.519 | 158.1 | 0.180 | |
| pH 10 0.75 mol/L NaCl | NMC | 103.9 | 17.3 | 87.4 | 7.842 | 60.6 | 0.022 |
| graphite | 108.8 | 65.7 | 90.5 | 2.334 | 234.7 | 0.294 |
| Parameter | pH 3 | pH 10 |
|---|---|---|
| kgraphite in s−1 | 8.1 × 10−3 | 5.2 × 10−3 |
| kNMC in s−1 | 2.3 × 10−4 | 2.5 × 10−4 |
| R∞,graphite | 0.98 | 0.98 |
| R∞,NMC | 0.22 | 0.18 |
| SMD in µm | 379 | 390 |
| in L/min | 1.1 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicklas, J.; Heilmann, C.; Ditscherlein, L.; Peuker, U.A. Forces During the Film Drainage and Detachment of NMC and Spherical Graphite in Particle–Bubble Interactions Quantified by CP-AFM and Modeling to Understand the Salt Flotation of Battery Black Mass. Minerals 2025, 15, 809. https://doi.org/10.3390/min15080809
Nicklas J, Heilmann C, Ditscherlein L, Peuker UA. Forces During the Film Drainage and Detachment of NMC and Spherical Graphite in Particle–Bubble Interactions Quantified by CP-AFM and Modeling to Understand the Salt Flotation of Battery Black Mass. Minerals. 2025; 15(8):809. https://doi.org/10.3390/min15080809
Chicago/Turabian StyleNicklas, Jan, Claudia Heilmann, Lisa Ditscherlein, and Urs A. Peuker. 2025. "Forces During the Film Drainage and Detachment of NMC and Spherical Graphite in Particle–Bubble Interactions Quantified by CP-AFM and Modeling to Understand the Salt Flotation of Battery Black Mass" Minerals 15, no. 8: 809. https://doi.org/10.3390/min15080809
APA StyleNicklas, J., Heilmann, C., Ditscherlein, L., & Peuker, U. A. (2025). Forces During the Film Drainage and Detachment of NMC and Spherical Graphite in Particle–Bubble Interactions Quantified by CP-AFM and Modeling to Understand the Salt Flotation of Battery Black Mass. Minerals, 15(8), 809. https://doi.org/10.3390/min15080809








