Chemical Peculiarities of Quartz from Peralkaline Granitoids
Abstract
1. Introduction
2. Geology
3. Analytical Methods
3.1. Cathodoluminescence (CL)
3.2. Trace Elements in Quartz
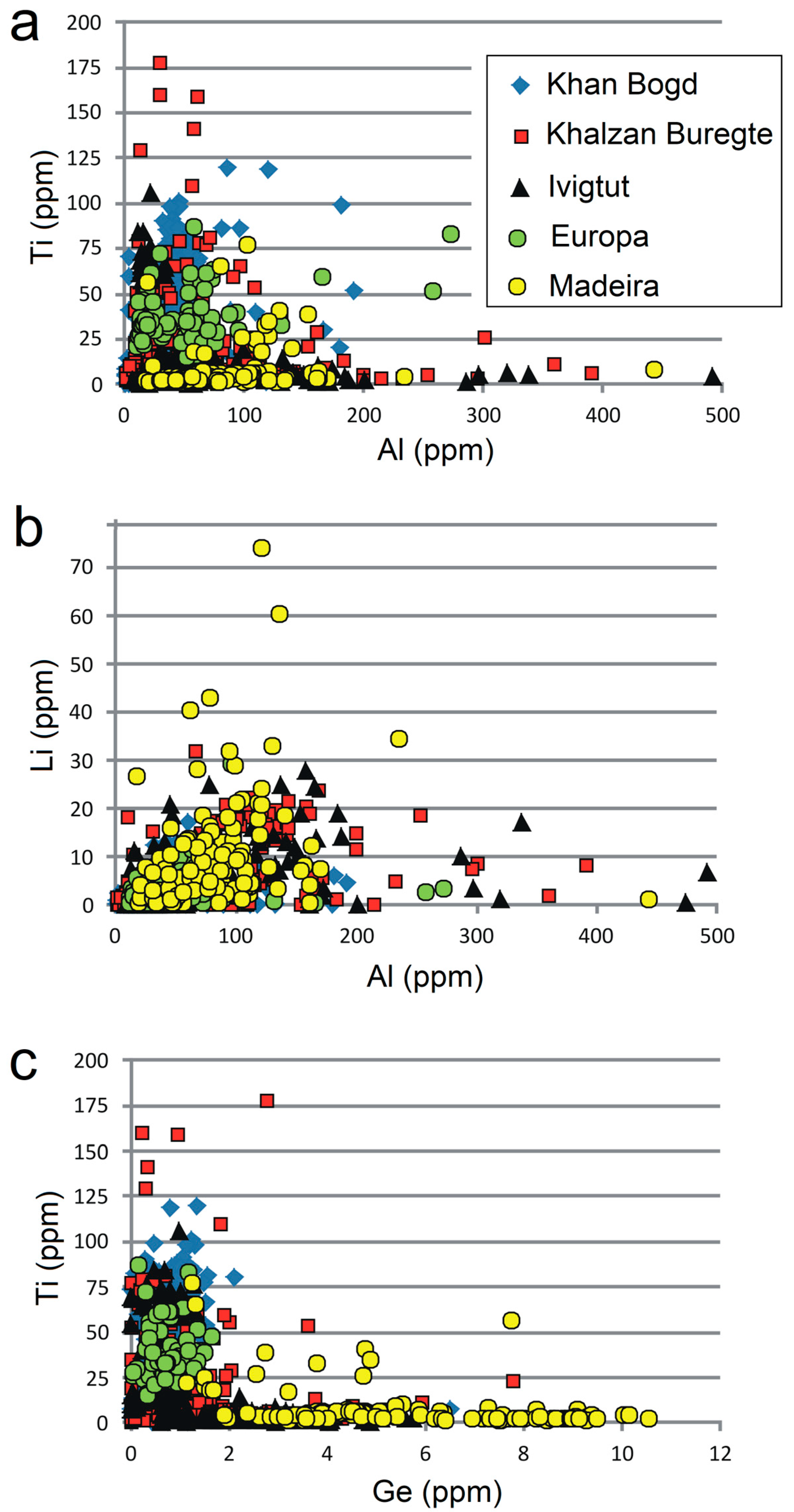
4. Results
4.1. Zoning of Quartz Crystals in Cathodoluminescence (CL)
4.2. Chemical Composition of Quartz
4.3. Chemical Zoning of Quartz Grains
5. Discussion
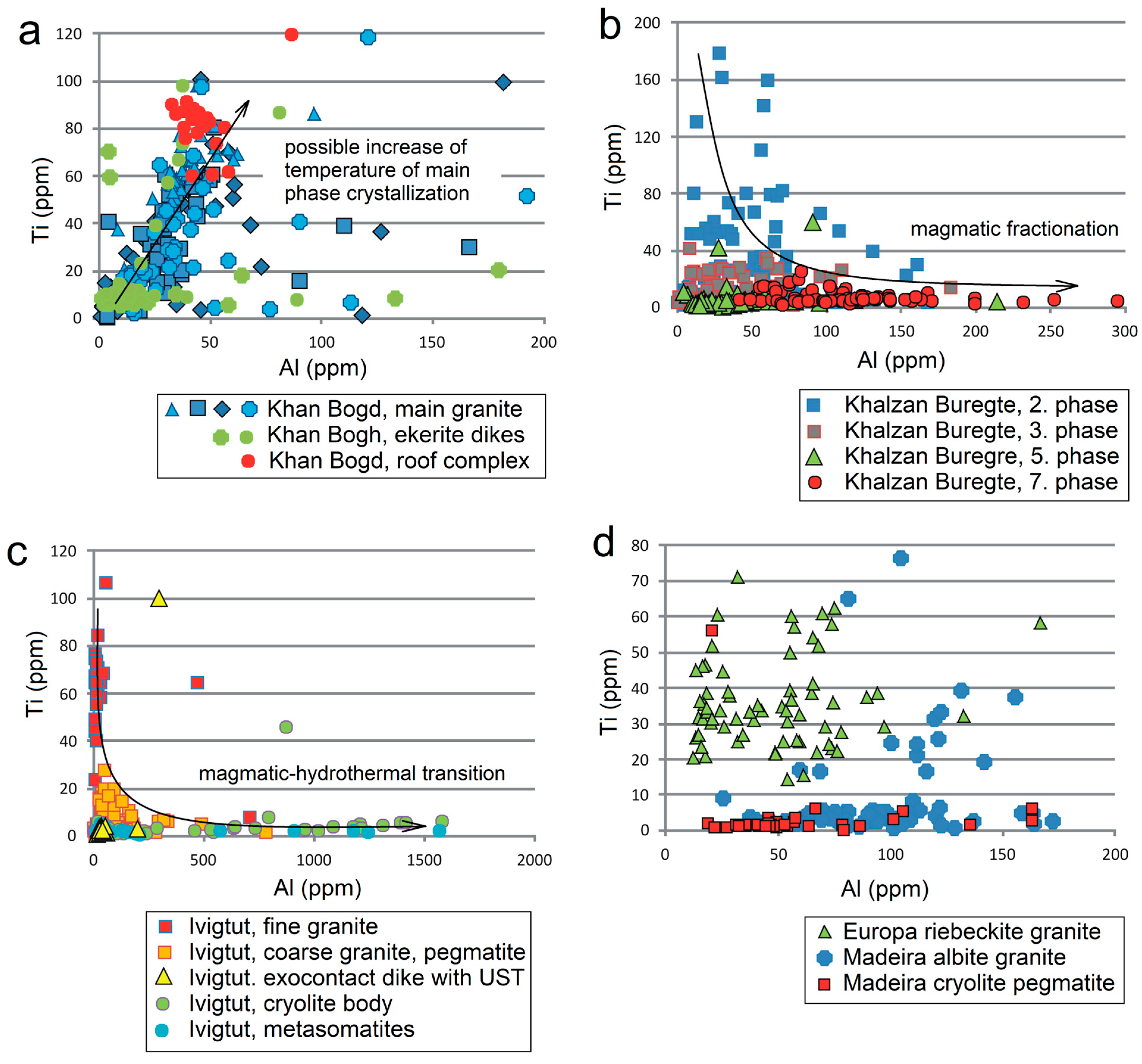
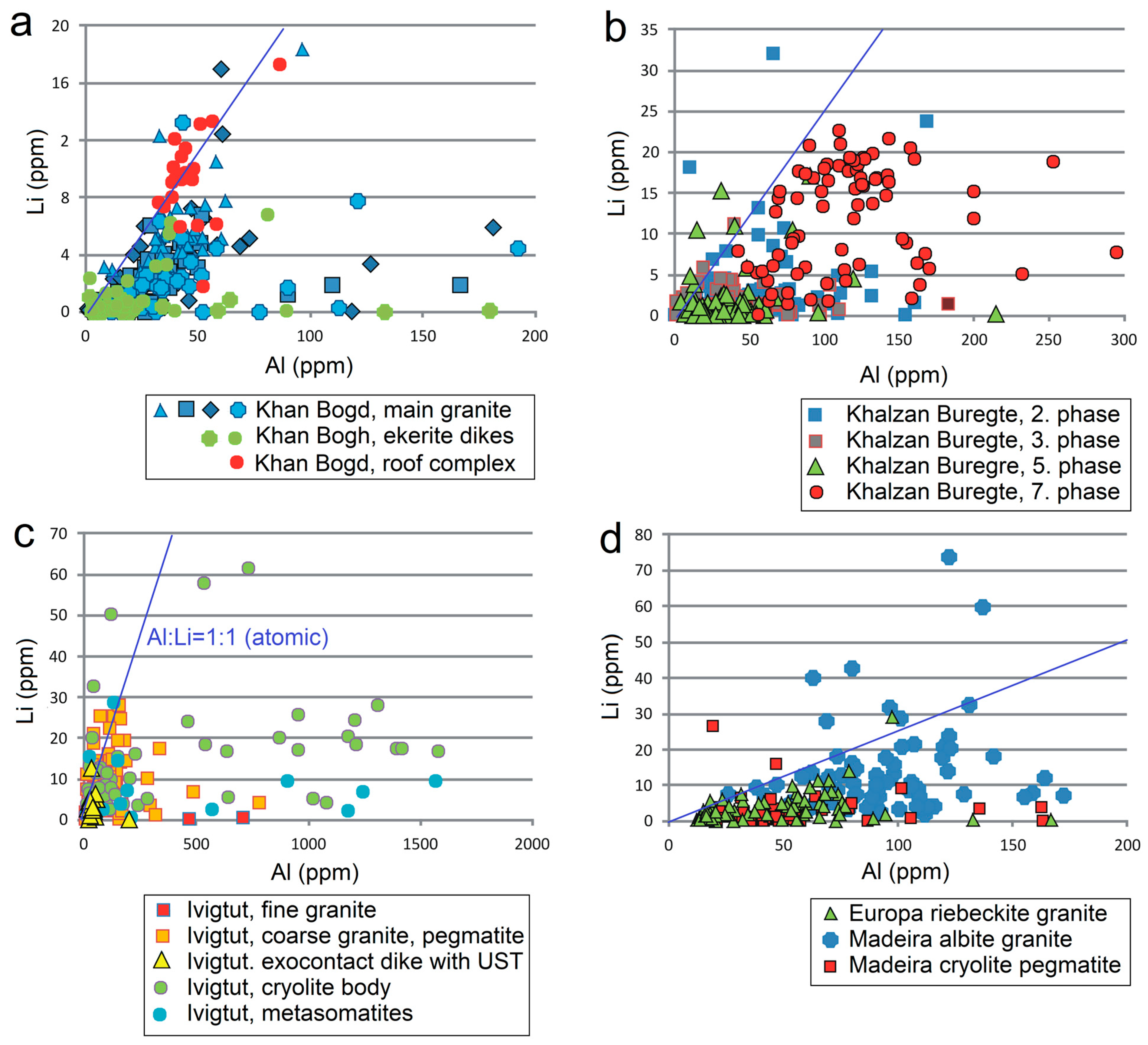
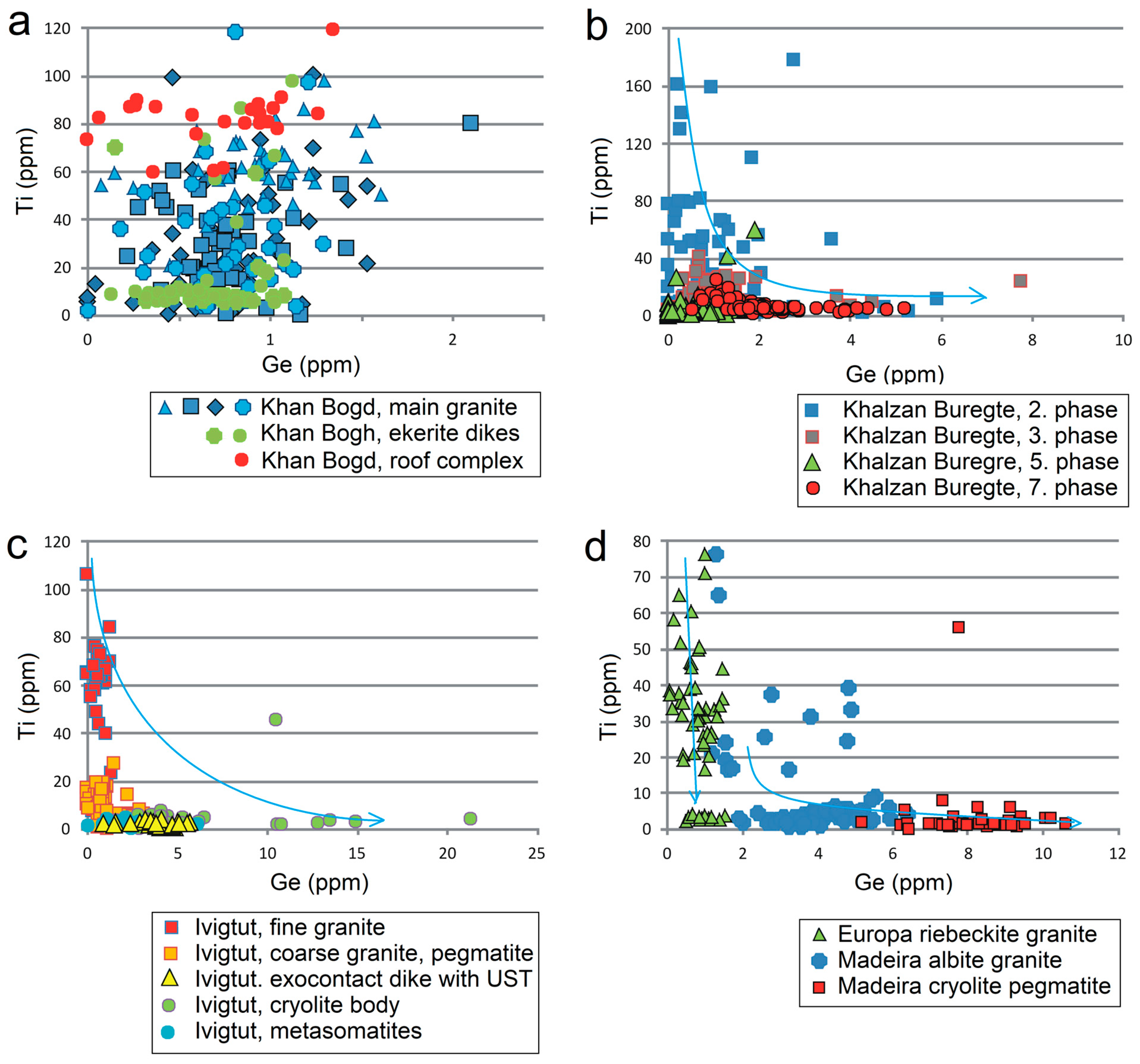
5.1. Evolution of Individual Magmatic Systems
5.2. Comparison of Quartz from Peralkaline Granitoids with Quartz of Other Granite Types
6. Conclusions
- Quartz from peralkaline granitoids is statistically poorer in Al and Li (medians 42 and 2.4 ppm, respectively) in comparison with quartz from peraluminous S-type granites (medians 448 and 37 ppm, respectively) and subaluminous A-type granites (medians 160 and 15 ppm, respectively). Content of Ti and Ge is similar in all three groups of granitoids (medians in peralkaline quartz 8.2 and 0.98 ppm, respectively).
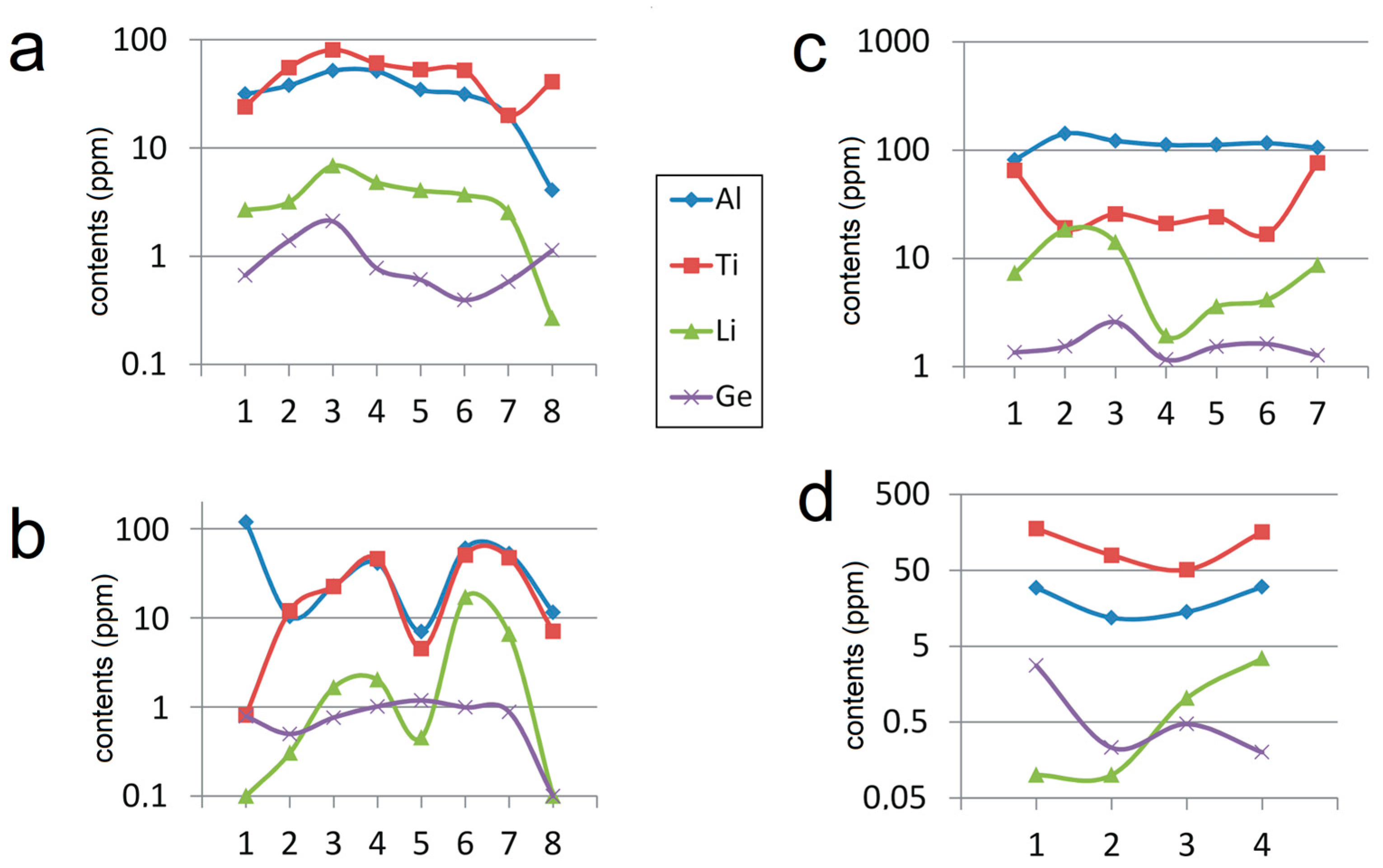
- Quartz from large homogeneous bodies of riebeckite-bearing granitoids (Khan Bogd, older suite of Khalzan Buregte, Europa) shows no Ti/Ge fractionation and displays either a positive Ti–Al correlation (Khan Bogd) or no Ti–Al correlation.
- Quartz from more evolved late intrusive phases (5–7th phase of Khalzan Buregte, late granite/pegmatite Ivigtut) and the strongly fractionated Madeira pluton is characterized by a moderate enrichment in Al and strong increase in the Ge/Ti ratio (Figure 3 and Figure 5), i.e., features already well-known from rare-metal granites of both A- and S-type affiliation [3,11,16,17,18,49]. This can be used as an auxiliary indicator in exploration work for granite-related mineral deposits.
- A statistically significant dataset confirmed that quartz from the three basic geochemical types of granitoids differs significantly in Al and Li content, while the Ti and Ge content are similar. However, even in the case of Al and Li, there is a wide interval of overlap in the content across all rock types. This limits the practical applicability for analysis of quartz’s provenance [29,54].
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and mineral chemistry of quartz: A review. Mineral. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]
- Jacamon, F.; Larsen, R.B. Trace element evolution of quartz in the charnockitic Kleivan granite, SW Norway: The Ge/Ti ratio of quartz as an index of igneous differentiation. Lithos 2009, 107, 281–291. [Google Scholar] [CrossRef]
- Breiter, K.; Müller, A. Evolution of rare-metal granitic magmas documented by quartz chemistry. Eur. J. Mineral. 2009, 21, 335–346. [Google Scholar] [CrossRef]
- Müller, A.; Kerkhof, A.M.; Behr, H.-J.; Kronz, A.; Koch-Müller, M. The evolution of late-Hercynian granites and rhyolites documented by quartz—A review. Trans. R. Soc. Edinb. 2010, 100, 185–204. [Google Scholar]
- Müller, A.; Herklotz, G.; Giegling, H. Chemistry of quartz related to the Zinnwald/Cínovec Sn-W-Li greisen-type deposit, Eastern Erzgebirge, Germany. J. Geochem. Explor. 2018, 190, 357–373. [Google Scholar] [CrossRef]
- Drivenes, K.; Larsen, R.B.; Müller, A.; Sorensen, B.E. Crystallization and uplift path of late Variscan granites evidenced by quartz chemistry and fluid inclusions: Example from the Land’s End granite, SW England. Lithos 2016, 252–253, 57–75. [Google Scholar] [CrossRef]
- Breiter, K.; Ackerman, L.; Svojtka, M.; Müller, A. Behavior of trace elements in quartz from plutons of different geochemical signature: A case study from the Bohemian Massif, Czech Republic. Lithos 2013, 175–176, 54–67. [Google Scholar] [CrossRef]
- Breiter, K.; Ďurišová, J.; Dosbaba, M. Quartz chemistry—A step to understanding magmatic-hydrothermal processes in ore-bearing granites: Cínovec/Zinnwald Sn-W-Li deposit Central Europe. Ore Geol. Rev. 2017, 90, 25–35. [Google Scholar] [CrossRef]
- Breiter, K.; Ďurišová, J.; Dosbaba, M. Chemical signature of quartz from S- and A-type rare-metal granites—A summary. Ore Geol. Rev. 2020, 125, 103674. [Google Scholar] [CrossRef]
- Monnier, L.; Lach, P.; Salvi, S.; Melleton, J.; Bailly, L.; Béziat, D.; Monnier, Y.; Gouy, S. Quartz trace-element composition by LA-ICP-MS as proxy for granite differentiation, hydrothermal episodes, and related mineralization: The Beauvoir granite (Echassieres district), France. Lithos 2018, 320–321, 355–377. [Google Scholar] [CrossRef]
- Breiter, K.; Svojtka, M.; Ackerman, L.; Švecová, K. Trace element composition of quartz from the Variscan Teplice caldera (Krušné hory/Erzgebirge Mts., Czech Republic/Germany): Insights into the volcano-plutonic complex evolution. Chem. Geol. 2012, 326–327, 36–50. [Google Scholar] [CrossRef]
- Leeman, W.P.; MacRae, C.M.; Wilson, N.C.; Torpy, A.; Lee, C.-T.A.; Student, J.J.; Thomas, J.B.; Vicenzi, E.P. A study of cathodoluminescence and trace element compositional zoning in natural quartz from volcanic rocks: Mapping titanium content in quartz. Microscop. Microanal. 2012, 18, 1322–1341. [Google Scholar] [CrossRef]
- Aud’etat, A. Origin of Ti-rich rims in quartz phenocrysts from the Upper Bandelier Tuff and the Tunnel Spring Tuff, southwestern USA. Chem. Geol. 2013, 360–361, 99–104. [Google Scholar] [CrossRef]
- Fiedrich, A.M.; Laurent, O.; Heinrich, C.A.; Bachmann, O. Melt and fluid evolution in an upper-crustal magma reservoir, preserved by inclusions in juvenile clasts from the Kos Plateau Tuff, Aegean Arc, Greece. Geochim. Cosmochim. Acta 2020, 280, 237–262. [Google Scholar] [CrossRef]
- Larsen, R.B.; Henderson, I.; Ihlen, P.M. Distribution and petrogenetic behavior of trace elements in granitic pegmatite quartz from South Norway. Contrib. Mineral. Petrol. 2004, 147, 615–628. [Google Scholar] [CrossRef]
- Beurlen, H.; Müller, A.; Silva, D.; Silva, M.R.R.D. Petrogenetic significance of LA-ICP-MS trace-element data on quartz from the Borborema pegmatite province, northeast Brazil. Mineral. Mag. 2011, 75, 2703–2719. [Google Scholar] [CrossRef]
- Breiter, K.; Ackerman, L.; Ďurišová, J.; Svojtka, M.; Novák, M. Trace element composition of quartz from different types of pegmatites: A case study from the Moldanubian Zone of the Bohemian Massif (Czech Republic). Mineral. Mag. 2014, 78, 703–722. [Google Scholar] [CrossRef]
- Garate-Olave, I.; Műller, A.; Roda-Robles, E.; Gil-Grespo, P.P.; Pesquera, A. Extreme fractionation in a granite-pegmatite system documented by quartz chemistry: The case study of Tres Arroyos (Central Iberian Zone, Spain). Lithos 2017, 286–287, 162–174. [Google Scholar] [CrossRef]
- Müller, A.; Keyser, W.; Simmons, W.B.; Webber, K.; Wise, M.; Beurlen, H.; Garate-Olave, I.; Roda-Robles, E.; Galliski, M.A. Quartz chemistry of granitic pegmatites: Implications for classification, genesis and exploration. Chem. Geol. 2021, 584, 120507. [Google Scholar] [CrossRef]
- Rusk, B.G.; Reed, M.H.; Dilles, J.H.; Kent, A.J.R. Intensity of quartz cathodoluminescence and trace-element content in quartz from the porphyry copper deposit at Butte, Montana. Am. Mineral. 2006, 91, 1300–1312. [Google Scholar] [CrossRef]
- Takahashi, R.; Müller, A.; Matsueda, H.; Okrugin, V.M.; Ono, S.; Kerkhof, A.; Kronz, A.; Andreeva, E.D. Cathodoluminescence and trace elements in quartz: Clues to metal precipitation mechanisms at the Asachinskoe gold deposit in Kamchatka. In Proceedings of the International Symposium Origin and Evolution of Natural Diversity, Sapporo, Japan, 1–5 October 2007; pp. 175–184. [Google Scholar]
- Rusk, B. Cathodoluminescent textures and trace elements in hydrothermal quartz. In Quartz: Deposits, Mineralogy and Analytics; Götze, J., Möckel, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 307–329. [Google Scholar]
- Wertich, V.; Leichmann, J.; Dosbaba, M.; Götze, J. Multi-stage evolution of gold-bearing hydrothermal quartz veins at the Mokrsko gold deposit (Czech Republic) based on cathodoluminescence, spectroscopic, and trace elements analyses. Minerals 2018, 8, 335. [Google Scholar] [CrossRef]
- Pacák, K.; Zachariáš, J.; Strnad, L. Trace-element chemistry of barren and orebearing quartz of selected Au, Au-Ag and Sb-Au deposits from the Bohemian Massif. J. Geosci. 2019, 64, 19–35. [Google Scholar] [CrossRef]
- Rottier, B.; Casanova, V. Trace element composition of quartz from porphyry systems: A tracer of the mineralizing fluid evolution. Miner. Depos. 2021, 56, 843–862. [Google Scholar] [CrossRef]
- Maydagán, L.; Franchini, M.; Rusk, B.; Lentz, R.; McFarlane, C.; Impiccini, A.; Ríos, F.J.; Rey, R. Porphyry to epithermal transition in the Altar Cu-(Au-Mo) deposit, Argentina. Studied by cathodoluminescence, LA-ICP-MS, and fluid inclusions analysis. Econ. Geol. 2015, 110, 889–923. [Google Scholar] [CrossRef]
- Monecke, T.; Kempe, U.; Götze, J. Genetic significance of the trace element content in metamorphic and hydrothermal quartz: A reconnaissance study. Earth Planet. Sci. Lett. 2002, 202, 709–724. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, W.T.; Zhang, X.-C.; Tang, Y.-W. The trace element chemistry of quartz in carbonatite-related REE deposits: Implication for REE exploration. Ore Geol. Rev. 2022, 149, 105068. [Google Scholar] [CrossRef]
- Ackerson, R.A.; Tailby, N.D.; Watson, E.B. Trace elements in quartz shed light on sediment provenence. Geochem. Geophys. Geosyst. 2015, 16, 1894–1904. [Google Scholar] [CrossRef]
- Shand, S.J. Eruptive Rocks; John Wiley and Sons: New York, NY, USA, 1949; pp. 1–488. [Google Scholar]
- Breiter, K.; Costi, H.T.; Vašinová Galiová, M.; Hložková, M.; Kynický, J.; Korbelová, Z.; Dosbaba, M. Trace element composition of quartz from alkaline granites—A factor supporting genetic considerations: Case study of the Pitinga Sn-Nb-Ta-Th-cryolite deposit. J. S. Am. Earth Sci. 2022, 119, 104025. [Google Scholar] [CrossRef]
- Kovalenko, V.I.; Yarmolyuk, V.V.; Sal’nikova, E.B.; Kozlovsky, A.M.; Kotov, A.B.; Kovach, V.P.; Savatenkov, V.M.; Vladykin, N.V.; Ponomarchuk, V.A. Geology, geochronology, and geodynamics of the Khan Bogd alkali granite pluton in Southern Mongolia. Geotectonics 2006, 40, 450–466. [Google Scholar] [CrossRef]
- Kynický, J.; Chakhmouradian, A.R.; Xu, C.; Krmíček, L.; Galiová, M. Distribution and evolution of zirconium mineralization in peralkaline granites and associate pegmatites of the Khan Bogd complex, Southern Mongolia. Can. Mineral. 2011, 49, 947–965. [Google Scholar] [CrossRef]
- Kovalenko, V.I.; Yarmolyuk, V.V.; SaL’Nikova, E.B.; Listratova, E.N.; Yakovleva, S.Z. The Khaldzan-Buregtei massif of peralkaline rare-metal igneous rocks: Structure, geochronology, and geodynamic setting in the Caledonides of Western Mongolia. Petrology 2004, 12, 412–436. [Google Scholar]
- Kovalenko, V.I.; Yarmolyuk, V.V.; Kovach, V.P.; Kotov, A.B.; Khanchuk, A.I. Multiple magma sources for the peralkaline granitoids and related rocks of the Khaldzan Buregte group of Massifs, Western Mongolia: Isotopic (neodymium, strontium, and oxygen) and geochemical data. Petrology 2004, 12, 497–518. [Google Scholar]
- Kovalenko, V.I.; Tsaryeva, G.M.; Goreglyad, A.V.; Yarmolyuk, V.V.; Troitsky, V.A. The peralkaline granite-related Khaldzan-Buregtey rare metal (Zr, Nb, REE) deposit, Western Mongolia. Econ. Geol. 1995, 90, 530–547. [Google Scholar] [CrossRef]
- Kempe, U.; Möckel, R.; Graupner, T.; Kynický, J.; Dombon, E. The genesis of Zr-Nb-REE mineralisation at Khalzan Buregte (Western Mongolia) reconsidered. Ore Geol. Rev. 2015, 64, 602–625. [Google Scholar] [CrossRef]
- Sarangua, N.; Watanabe, Y.; Echigo, T.; Hoshino, M. Chemical characteristic of zircon from Khaldzan Burgedei peralkaline complex, Western Mongolia. Minerals 2019, 9, 10. [Google Scholar] [CrossRef]
- Blaxland, A.B. Rb-Sr isotopic evidence for the age and origin of the Ivigtut granite and associated cryolite body, South Greeland. Econ. Geol. 1976, 71, 864–869. [Google Scholar] [CrossRef]
- Pauly, H.; Bailey, J.C. Genesis and evolution of the Ivigtut cryolite deposit, SW Greenland. Meddelelser Om Gronl. Geosci. 1999, 37, 1–60. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Upton, B.G.J.; Ellam, R.M. Geochemical evolution of the Ivigtut granite, South Greenland: A fluorine-rich “A-type” intrusion. Lithos 2000, 51, 205–221. [Google Scholar] [CrossRef]
- Köhler, J.; Schönenberger, J.; Upton, B.; Markl, G. Halogen and trace-element chemistry in the Gardar province, South Greenland: Subduction-related mantle metasomatism and fluid exsolution from alkalic melts. Lithos 2009, 113, 731–747. [Google Scholar] [CrossRef]
- Costi, H.T.; Dall’Agnoll, R.; Moura, C.A.V. Geology and Pb-Pb geochronology of Paleoproterozoic volcanic and granitic rocks of Pitinga province, Amazonian craton, northern Brazil. Int. Geol. Rev. 2000, 42, 832–849. [Google Scholar] [CrossRef]
- Bastos Neto, A.C.; Pereira, V.P.; Ronchi, L.H.; Lima, E.F.; Frantz, J.C. The world-class Sn, Nb, Ta, F (Y, REE, Li) deposit and the massive cryolite associated with the albite-enriched facies of the Madeira A-type granite, Pitinga mining district, Amazonas State, Brazil. Can. Mineral. 2009, 47, 1329–1357. [Google Scholar] [CrossRef]
- Costi, H.T.; Borges, R.M.; Dall’Agnoll, R. Tin deposits at the Pitinga mine, Amazonas state. In Caracterizaçao de Depositos Minerais da Amazonia; Marini, O.J., Queiroz, E.T., Ramos, B.W., Eds.; DNPM-CT/MINERAL-ADIMB: Brasilia, Brazil, 2005; pp. 391–475. (In Portuguese) [Google Scholar]
- Paludo, C.M.; Bastos Neto, A.C.; Pereira, V.P.; Botelho, N.F. Mineralogy and geochemistry of REE, F and alkali metal-rich pegmatites associated with albite granite facies in the Sn-Nb-Ta- (F, REE, U, Th) Madeira deposit (Pitinga mine, AM, Brazil). Pesqui. Em Geocienc. 2018, 45, e0747, (In Portuguese, with English Abstract). [Google Scholar]
- Breiter, K.; Gardenová, N.; Kanický, V.; Vaculovič, T. Galium and germanium geochemistry during magmatic fractionation and post-magmatic alteration in different types of granitoids: A case study from the Bohemian Massif, Czech Republic. Geol. Carpath. 2013, 64, 171–180. [Google Scholar] [CrossRef]
- Mysen, B.O. Magmatic silicate melts: Relation between bulk composition, structure and properties. In Magmatic Processes: Physicochemical Principles; Mysen, B.O., Ed.; The Geochemical Society, Pennsylvania State University: Lancaster, PA, USA; Lancaster Press, Inc.: Lancaster, PA, USA, 1987; Special publication, no. 1.; pp. 375–399. [Google Scholar]
- Müller, A.; Kronz, A.; Breiter, K. Trace elements and growth patterns in quartz: A fingerprint of the evolution of the subvolcanic Podlesí Granite System (Krušné Hory, Czech Republic). Bull. Czech Geol. Surv. 2002, 77, 135–145. [Google Scholar]
- He, D.; Lee, C.-T.A.; Yu, X.; Farner, M. Ge/Si partitioning in igneous systems: Constraints from laser ablation ICP-MS measurements on natural samples. Geochem. Geophys. Geosyst. 2019, 20, 4472–4486. [Google Scholar] [CrossRef]
- Huang, R.; Audétat, A. The titanium-in-quartz (TitaniQ) thermobarometer: A critical examination and re-calibration. Geochim. Cosmochim. Acta 2012, 84, 75–89. [Google Scholar] [CrossRef]
- Breiter, K.; Ďurišová, J.; Korbelová, Z.; Lima, A.; Vašinová Galiová, M.; Hložková, M.; Dosbaba, M. Rock textures and mineral zoning—A clue to understanding rare-metal granite evolution: Argemela stock, Central-Eastern Portugal. Lithos 2022, 410–411, 106562. [Google Scholar] [CrossRef]
- Breiter, K.; Ďurišová, J.; Korbelová, Z.; Vašinová Galiová, M.; Hložková, M. Granite Pluton at the Panasqueira Tungsten Deposit, Portugal: Genetic Implications as Revealed from New Geochemical Data. Minerals 2023, 13, 163. [Google Scholar] [CrossRef]
- Müller, A.; Knies, J. Trace elements and cathodoluminescence of detrital quartz in Arctic marine sediments—A new ice-rafted debris provenance proxy. Clim. Past 2013, 9, 2615–2630. [Google Scholar] [CrossRef]


| N | Li Mean | Li Median | Al Mean | Al Median | Ti Mean | Ti Median | Ge Mean | Ge Median | |
|---|---|---|---|---|---|---|---|---|---|
| Khan Bogd, sample 1 | 42 | 3.15 | 2.21 | 37.39 | 27.80 | 31.34 | 23.63 | 0.78 | 0.80 |
| Khan Bogd, sample 2 | 39 | 2.57 | 2.52 | 36.46 | 29.47 | 33.05 | 30.99 | 0.76 | 0.72 |
| Khan Bogd, sample 3 | 38 | 5.69 | 5.14 | 40.52 | 38.24 | 62.14 | 61.36 | 0.93 | 0.95 |
| Khan Bogd, sample 4 | 38 | 1.99 | 1.38 | 41.06 | 32.25 | 30.53 | 23.27 | 0.75 | 0.78 |
| Khan Bogd ekerite | 45 | 0.89 | 0.25 | 26.29 | 13.97 | 19.55 | 8.48 | 0.82 | 0.70 |
| Khan Bogd roof complex | 23 | 9.31 | 9.34 | 46.45 | 43.10 | 81.78 | 83.10 | 0.71 | 0.75 |
| Khalzan Buregte, phase 2 | 145 | 2.03 | 0.90 | 41.70 | 34.61 | 21.40 | 7.20 | 0.90 | 0.75 |
| Khalzan Buregte, phase 3 | 67 | 1.61 | 1.06 | 44.38 | 31.17 | 12.46 | 9.02 | 1.17 | 0.92 |
| Khalzan Buregte, phase 5 | 63 | 2.15 | 0.83 | 46.72 | 29.97 | 5.52 | 3.11 | 0.53 | 0.39 |
| Khalzan Buregte, phase 7 | 77 | 11.81 | 13.44 | 122.82 | 115.14 | 6.34 | 4.89 | 2.01 | 2.06 |
| Ivigtut hypersolvus granite | 39 | 0.15 | 0.11 | 20.50 | 16.91 | 63.34 | 64.37 | 0.64 | 0.62 |
| Ivigtut subsolvus granite and pegmatite | 102 | 6.81 | 4.58 | 81.30 | 54.90 | 6.06 | 4.15 | 1.72 | 1.78 |
| Ivigtut UST zircon-rich dike | 23 | 3.26 | 2.65 | 35.40 | 34.50 | 2.06 | 2.03 | 3.76 | 3.84 |
| Ivigtut cryolite body | 69 | 14.58 | 6.93 | 329.00 | 97.17 | 2.11 | 1.58 | 4.42 | 3.63 |
| Ivigtut metasomatite | 26 | 4.52 | 2.12 | 269.00 | 45.20 | 1.91 | 1.80 | 1.99 | 1.82 |
| Europa riebeckite granite | 71 | 4.02 | 2.91 | 54.40 | 52.20 | 37.33 | 33.60 | 0.81 | 0.82 |
| Madeira albite granite | 73 | 14.00 | 10.20 | 96.80 | 92.40 | 7.40 | 4.47 | 3.88 | 3.94 |
| Madeira cryolite pegmatite | 41 | 1.87 | 1.06 | 56.30 | 46.62 | 1.98 | 1.41 | 8.23 | 8.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breiter, K.; Kynický, J.; Vašinová Galiová, M.; Hložková, M. Chemical Peculiarities of Quartz from Peralkaline Granitoids. Minerals 2025, 15, 790. https://doi.org/10.3390/min15080790
Breiter K, Kynický J, Vašinová Galiová M, Hložková M. Chemical Peculiarities of Quartz from Peralkaline Granitoids. Minerals. 2025; 15(8):790. https://doi.org/10.3390/min15080790
Chicago/Turabian StyleBreiter, Karel, Jindřich Kynický, Michaela Vašinová Galiová, and Michaela Hložková. 2025. "Chemical Peculiarities of Quartz from Peralkaline Granitoids" Minerals 15, no. 8: 790. https://doi.org/10.3390/min15080790
APA StyleBreiter, K., Kynický, J., Vašinová Galiová, M., & Hložková, M. (2025). Chemical Peculiarities of Quartz from Peralkaline Granitoids. Minerals, 15(8), 790. https://doi.org/10.3390/min15080790









