Mineralogy and Preparation of High-Purity Quartz: A Case Study from Pegmatite in the Eastern Sector of the North Qinling Orogenic Belt
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Quartz Processing
2.3. Calcination and Water Quenching
2.4. Acid Leaching Test
2.5. Mineral Composition Analysis
2.6. Trace Element Analysis
3. Results and Discussion
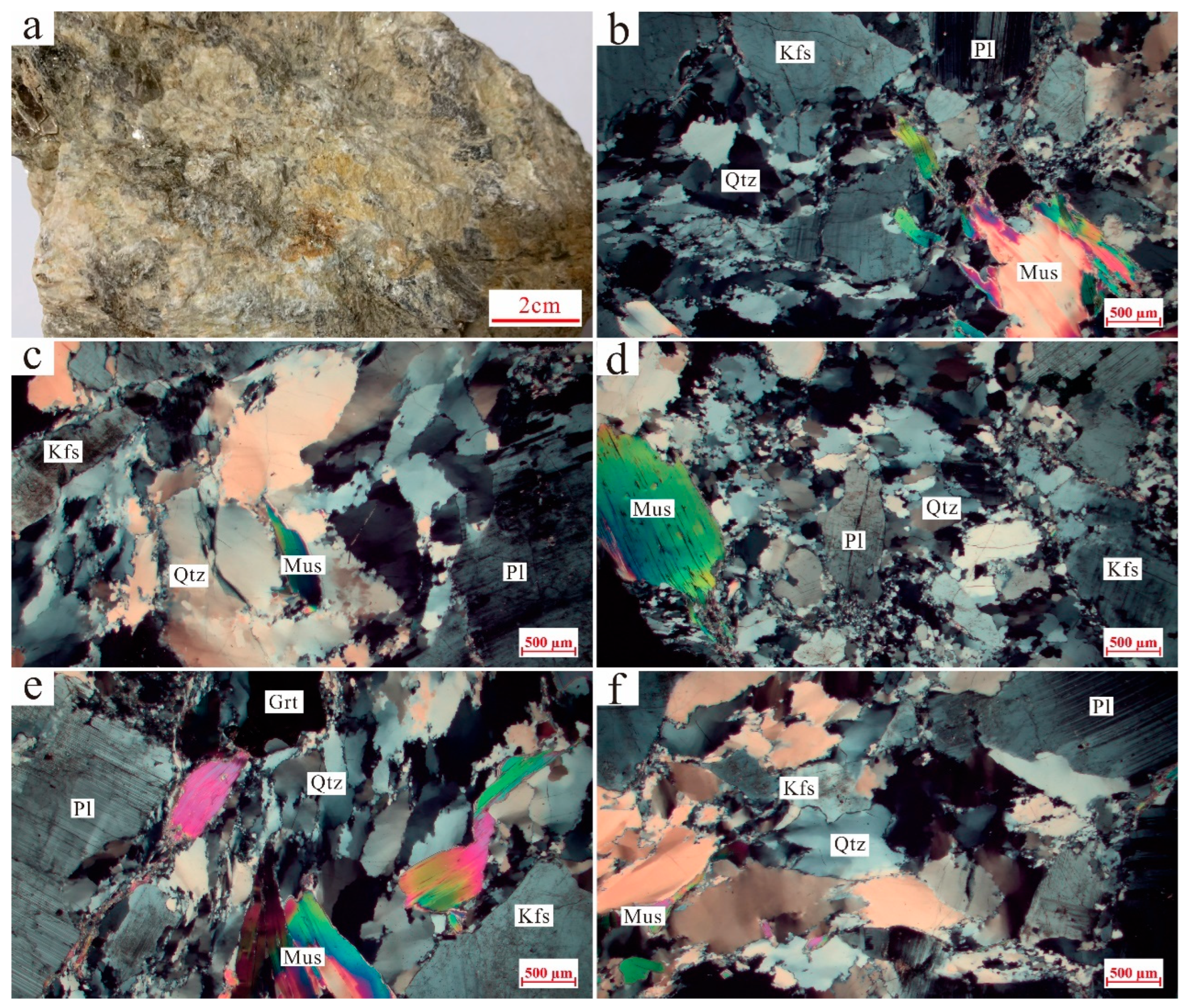
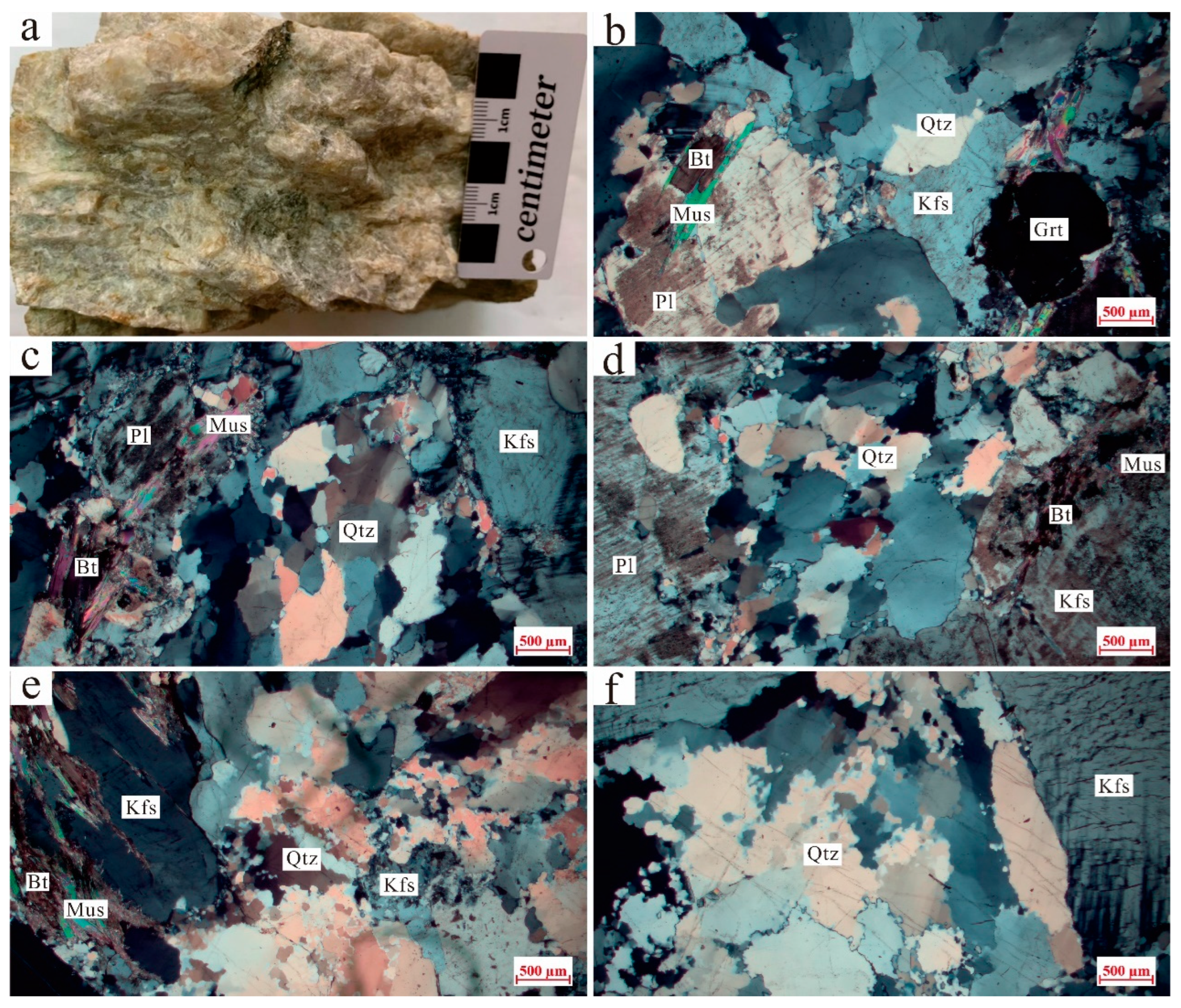
3.1. Petrography
3.2. Mineral Composition in Raw Ore, Raw Sand, and Purified Sand
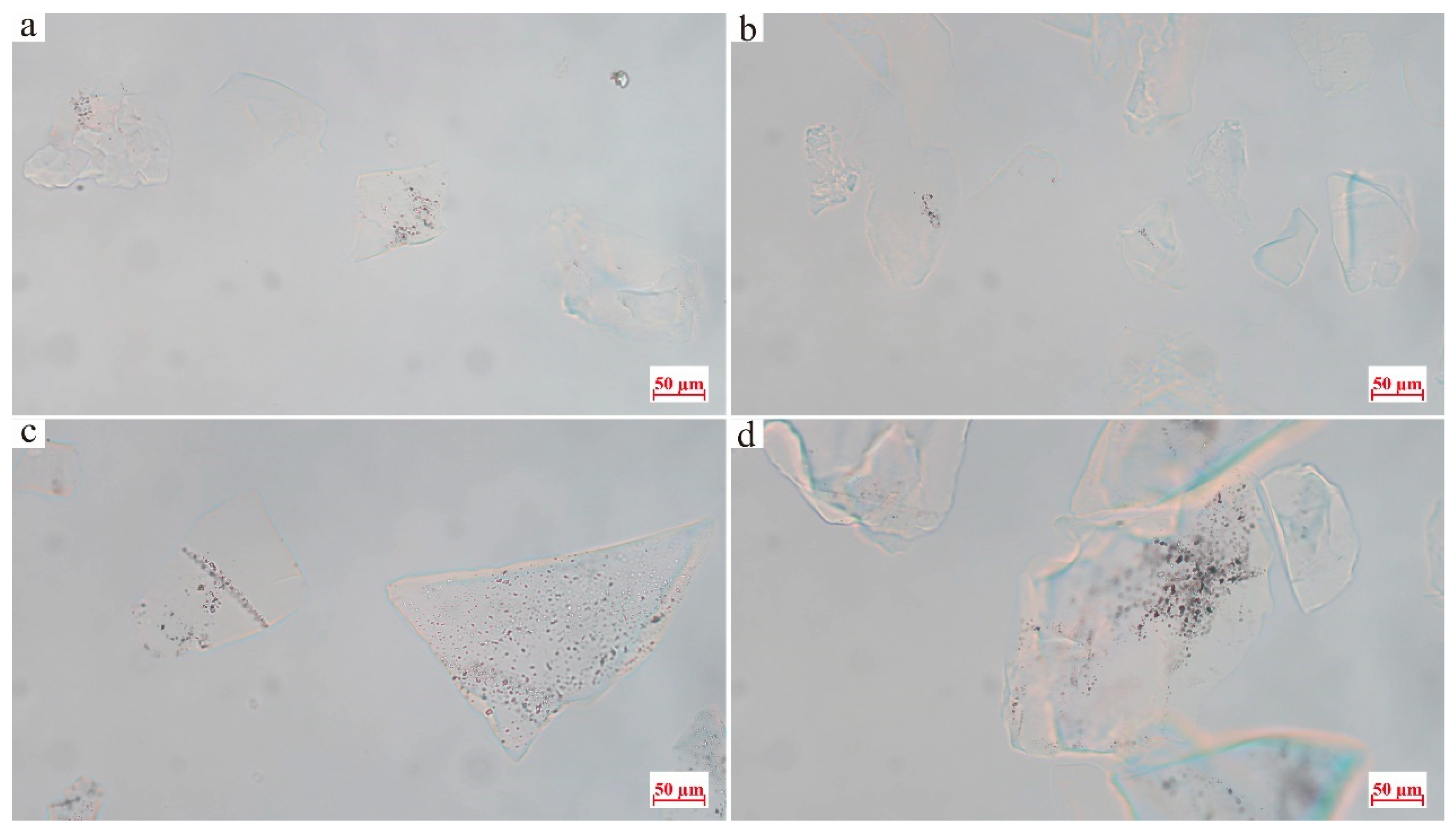
3.3. Fluid Inclusion Characteristics
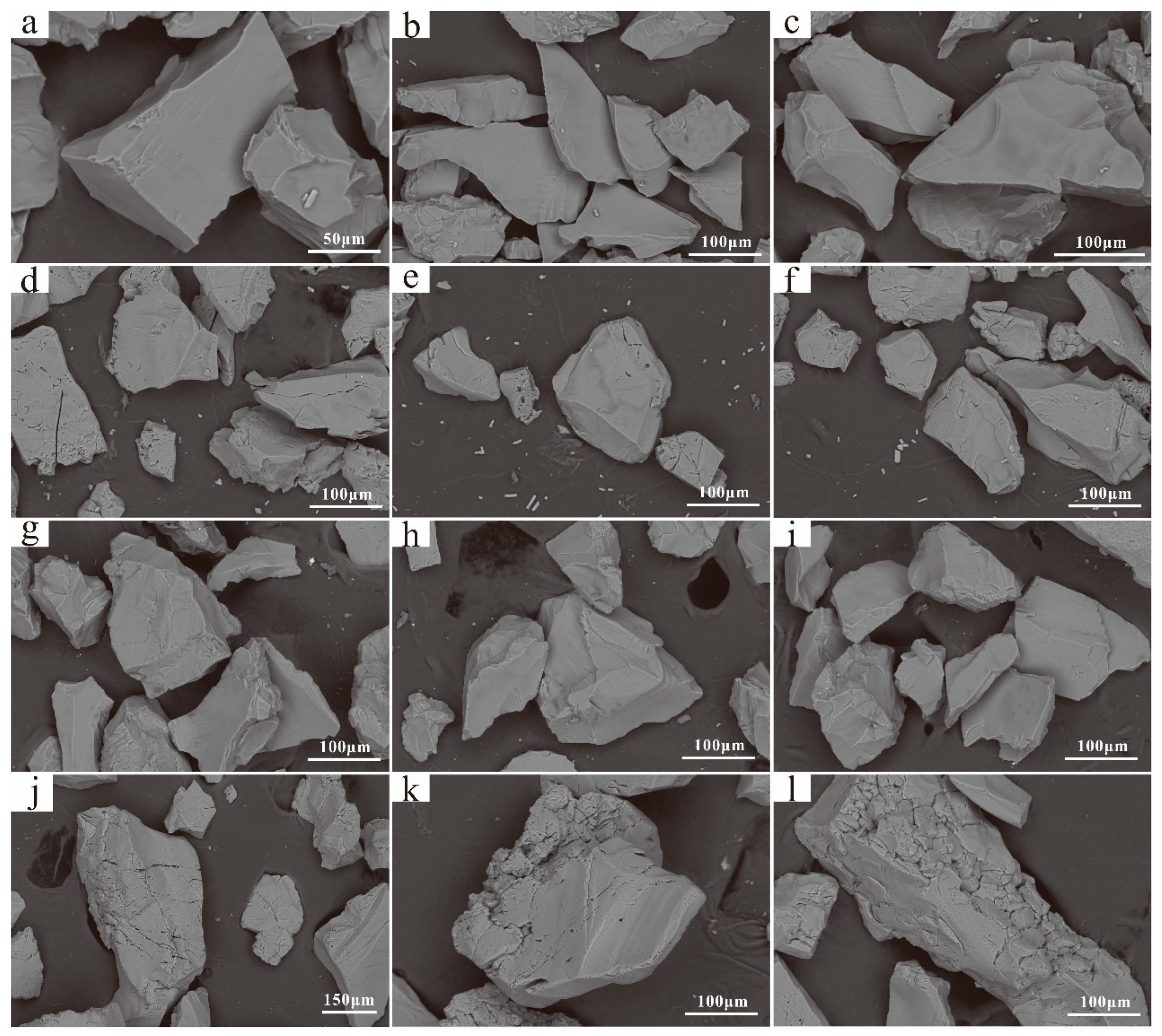
3.4. Surface Morphology of Quartz
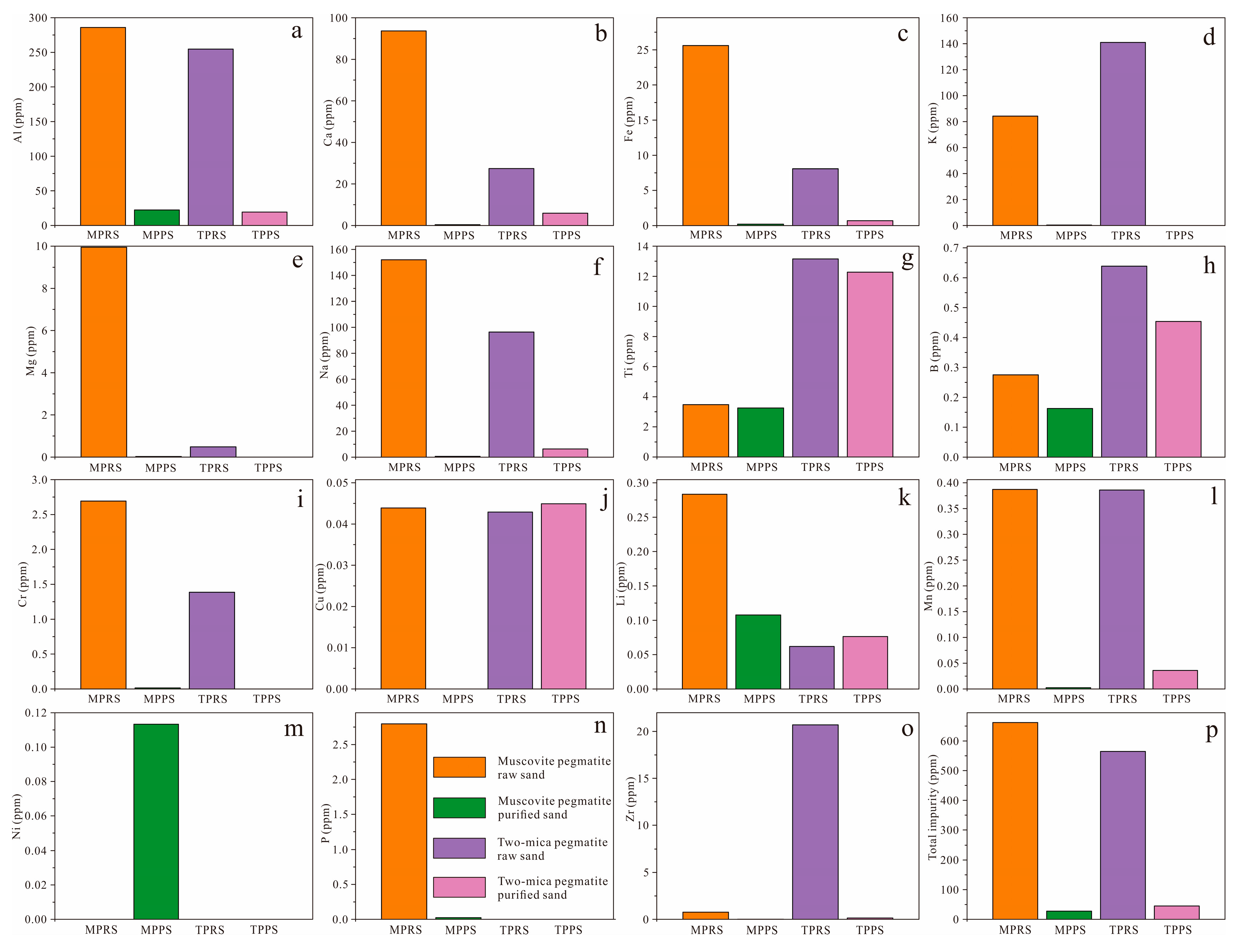
3.5. Chemical Composition
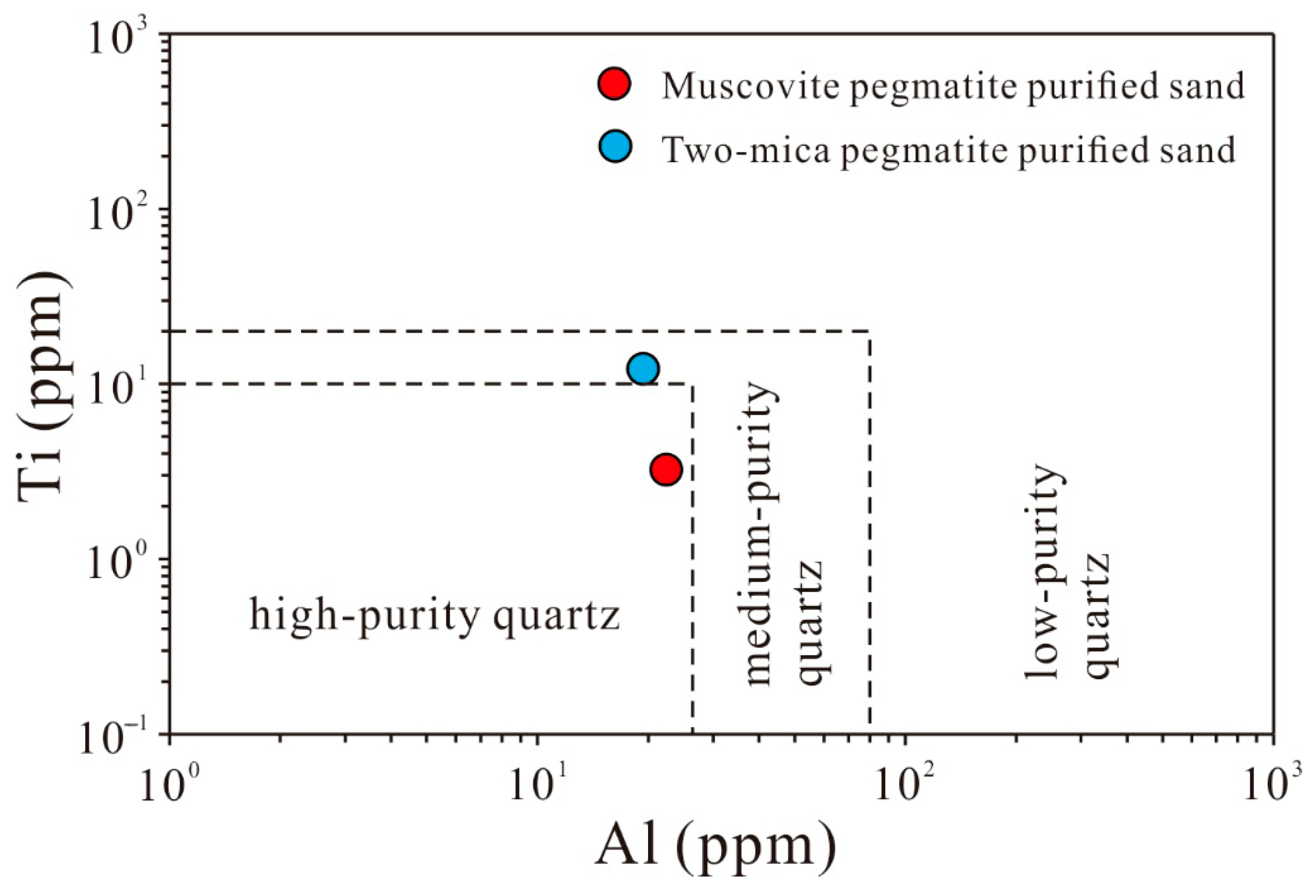
3.6. Discussion
4. Conclusions
- (1)
- Fluid inclusions in both muscovite and two-mica pegmatite quartz exhibit small sizes, yet the former demonstrates significantly lower inclusion density.
- (2)
- Surface discontinuity (i.e., cracks, pits, cavities) development is more pronounced in two-mica pegmatite purified quartz, correlating with its elevated fluid inclusion abundance.
- (3)
- Elevated Ti in purified sand from two-mica pegmatite is likely related to biotite in two-mica pegmatite, whereas persistent Ca and Na suggest contributions from fluid inclusions or undetected microscopic mineral inclusions (e.g., plagioclase).
- (4)
- Purified quartz from muscovite pegmatite achieves reduced trace element concentrations, demonstrating enhanced potential for high-purity quartz.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.Y.; Sun, C.; Cao, J.Y.; Shi, J.B. High purity quartz: Research progress and perspective review. Earth Sci. Front. 2022, 29, 231–244, (In Chinese with English Abstract). [Google Scholar]
- Harben, P.W. The Industrial Minerals Handbook: A Guide to Markets, Specification & Prices; Metal Bulletin Books Ltd.: London, UK, 1994; p. 284. [Google Scholar]
- Ma, Y.M.; Li, J.G.; Wu, Z.C.; Zhang, H.Q.; Tan, X.M.; Yi, Y.J.; Tan, Q.; Liu, L. Characteristics of high-purity quartz raw materials for crucibles and exploration of key purification technologies. Miner. Eng. 2025, 231, 109446. [Google Scholar] [CrossRef]
- Haus, R.; Prinz, S.; Priess, C. Assessment of high purity quartz resources. In Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–51. [Google Scholar]
- Ma, Y.M.; Li, J.G.; Wu, Z.C.; Zhang, H.Q.; Tan, X.M.; Zhang, X.F.; Yi, Y.J. Extraction and purification of a high purity quartz in the Altay orogenic belt and its potential evaluation. Miner. Eng. 2025, 228, 109347. [Google Scholar] [CrossRef]
- Pan, X.D.; Li, S.Q.; Li, Y.K.; Guo, P.H.; Zhao, X.; Cai, Y.S. Resource, characteristic, purification and application of quartz: A review. Miner. Eng. 2022, 183, 107600. [Google Scholar] [CrossRef]
- Long, H.L.; Zhu, D.Q.; Pan, J.; Li, S.W.; Yang, C.C.; Guo, Z.Q. Advanced processing techniques and impurity management for high-purity quartz in diverse industrial applications. Minerals 2024, 14, 571. [Google Scholar] [CrossRef]
- Zhong, T.S.; Yu, W.H.; Shen, C.; Wu, X.W. Research on Preparation and Characterisation of High-purity Silica Sands by Purification of Quartz Vein Ore from Dabie Mountain. Silicon 2022, 14, 4723–4729. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, Q.; Ku, J.G.; Shang, H.L.; Shen, Z.C. Purification technologies for high-purity quartz: From mineralogy to applications. Sep. Purif. Rev. 2025, 1–18. [Google Scholar] [CrossRef]
- Müller, A.; Wanvik, J.E.; Ihlen, P.M. Petrological and chemical characterization of high-purity quartz deposits with examples from Norway. In Quartz: Deposits, Mineralogy and Analytics; Götze, J., Möckel, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 71–118. [Google Scholar]
- Liu, M.; Wang, G.F.; Zhao, F.Y.; Li, W.F.; Zhu, G.; Liang, G.C.; Jian, W.; Liao, L.B.; Lv, G.C. Advances in purification technologies and applications of high-purity quartz resources. Prog. Nat. Sci. Mater. Int. 2025, 35, 51–64. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Z.Y.; Wei, Y.; Liu, B.; Meng, Y.; Qiu, H.; Lei, S.M.; Zhang, X.; Li, Y.B. A critical review on the mineralogy and processing for high-grade quartz. Min. Metall. Explor. 2020, 37, 1627–1639. [Google Scholar] [CrossRef]
- Larsen, R.B.; Polvé, M.; Juve, G. Granite pegmatite quartz from Evje-Iveland: Trace element chemistry and implications for the formation of high-purity quartz. Nor. Geol. Unders. Bull. 2000, 436, 57–65. [Google Scholar]
- Harben, P.W. The Industrial Minerals Handybook: A Guide to Markets, Specifications and Prices, 4th ed.; Industrial Mineral Information: London, UK, 2002; p. 412. [Google Scholar]
- Hao, W.J.; Feng, S.W.; Zhan, J.H.; Zhang, X.; Li, G.H. Current situation, production, consumption and trade pattern of high purity quartz in the world. China Non-Met. Miner. Ind. 2020, 5, 15–19, (In Chinese with English Abstract). [Google Scholar]
- Wang, J.Y. Global high purity quartz deposits: Resources distribution and exploitation status. Acta. Petrol. Miner. 2021, 40, 131–141, (In Chinese with English Abstract). [Google Scholar]
- Wang, L. Concept of high purity quartz and classification of its raw materials. Conserv. Util. Mineral. Resour. 2022, 42, 55–63, (In Chinese with English Abstract). [Google Scholar]
- Wang, L. Principles of Mineral Material Science; Geological Publishing House: Beijing, China, 2021; p. 660. (In Chinese) [Google Scholar]
- Gao, R.B.; Huang, Y.H.; Shang, B.Z.; Yu, B.W.; Hu, Z.T.; Song, X.Y.; Peng, W.J. Research status and prospect of high purity quartz processing and purification technology. China Min. Mag. 2025, 13, 1505, (In Chinese with English Abstract). [Google Scholar]
- Lü, M.D.; Li, H.Z.; Zhao, M.Z.; Ma, Z.W.; Yang, Z.J.; Liang, J. Study on the Order Degree and Geochemical Characteristics of Major Elements of Siliceous Rock in Eastern Qinling Area, China. Spectrosc. Spectr. Anal. 2014, 34, 3005–3010. [Google Scholar]
- Deng, Q.; Ren, Z.J.; Song, Y.H.; He, Y.H.; Li, P.Y.; Yin, H. Purification of different-sized quartz crystals in granitic pegmatite. Miner. Eng. 2024, 216, 108856. [Google Scholar]
- Zhang, Y.; Pan, J.Y.; Xia, F.; Zhao, H.B.; Xu, Z.; Liu, G.Q.; Zhong, F.J.; Zhang, X.T.; Liu, Y.; Du, G.F.; et al. Textures and chemical compositions of muscovite and quartz: Implications for granite-hosted high-purity quartz mineralization and exploration in South China. Ore. Geol. Rev. 2023, 161, 105635. [Google Scholar] [CrossRef]
- Wang, Z.H.; Xi, W.; Wang, L. The mineral characteristics of high-purity quartz raw materials from the granite pegmatite in the Spruce Pine Area. Mineral. Petrol. 2025, 45, 106–116, (In Chinese with English Abstract). [Google Scholar]
- Medjahed, S.; Kheloufi, A.; Bobocioiu, E.; Kefaifi, A.; Kerkar, F.; Lebbou, K. Quartz Ore Beneficiation by Reverse Flotation for Silicon Production. Silicon 2022, 14, 87–97. [Google Scholar] [CrossRef]
- Cunha, F.R.; Sobral, Y.D. Characterization of the physical parameters in a process of magnetic separation and pressure-driven flow of a magnetic fluid. Phys. A 2004, 343, 36–64. [Google Scholar] [CrossRef]
- Podoynitsyn, S.N.; Sorokina, O.N.; Kovarski, A.L. High-gradient magnetic separation using ferromagnetic membrane. J. Magn. Magn. Mater. 2016, 397, 51–56. [Google Scholar] [CrossRef]
- Vieira, A.M.; Peres, A.E.C. The effect of amine type, pH, and size range in the flotation of quartz. Miner. Eng. 2007, 20, 1008–1013. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.H.; Xu, L.H. Adsorption mechanism of mixed anionic/cationic collectors in muscovite-quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.Q.; Hu, Y.H.; Liu, J.P.; Sun, W. Adsorption behavior of mixed cationic/anionic surfactants and their depression mechanism on the flotation of quartz. Powder Technol. 2016, 302, 15–20. [Google Scholar] [CrossRef]
- Yusupov, T.S.; Kirillova, E.A.; Denisov, G.A. Dressing of Quartz-Feldspar ores on the basis of selective grinding and mechanical activation. J. Min. Sci. 2003, 39, 174–177. [Google Scholar] [CrossRef]
- Liu, G.X.; Ma, Y.M.; Liu, L.; Zhang, H.L.; Zhu, L.K.; Guo, L.X.; Cao, F. Study on deep impurity removal technology of a granite pegmatite-type high-purity quartz in Altay region of Xinjiang. Conserv. Util. Miner. Resour. 2022, 5, 8–14, (In Chinese with English Abstract). [Google Scholar]
- Ma, Y.M.; Zhang, H.Q.; Tan, X.M.; Liu, G.X.; Yi, Y.J.; Liu, L. Research on chemical deep purification technology of high-purity quartz in a mining area. Conserv. Util. Miner. Resour. 2022, 5, 22–27, (In Chinese with English Abstract). [Google Scholar]
- Lu, H.Z.; Fan, H.R.; Ni, P.; Ou, G.X.; Shen, K.; Zhang, W.H. Fluid Inclusions; Science Press: Beijing, China, 2004; pp. 1–487, (In Chinese with English abstract). [Google Scholar]
- Chi, G.X.; Haid, T.; Quirt, D.; Fayek, M.; Blamey, N.; Chu, H.X. Petrography, fluid inclusion analysis, and geochronology of the end uranium deposit, Kiggavik, Nunavut, Canada. Mineral. Deposita 2017, 52, 211–232. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions. Rev. Mineral 1984, 12, 644. [Google Scholar]
- Thomas, R.; Davidson, P.; Badanina, E. Water- and boron-rich melt inclusions in quartz from the Malkhan pegmatite. Transbaikalia, Russia. Minerals 2012, 2, 435–458. [Google Scholar] [CrossRef]
- Götze, J.; Pan, Y.; Müller, A.; Kotova, E.; Cerin, D. Trace element compositions and defect Structures of High-Purity Quartz from the Southern Ural Region, Russia. Minerals 2017, 7, 189. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xie, Z.F.; Wang, C.L.; Hu, Y.F. Trace element concentrations and mineralogy of quartz vein deposits from southeastern Hubei Province, China. Minerals 2022, 12, 814. [Google Scholar] [CrossRef]
- Wang, S.J.; Yu, D.S.; Ma, C.; Wei, F.S.; Zhang, H.Q. A new insight into the influence of fluid inclusions in high-purity quartz sand on the bubble defects in quartz glass: A case study from vein quartz in the Dabie Mountain. Minerals 2024, 14, 794. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, C.; Ni, W.; Yuan, J.; Zhou, Y.; Liu, X. Research status and challenges of high-purity quartz processing technology from a mineralogical perspective in China. Minerals 2023, 13, 1505. [Google Scholar] [CrossRef]
- Xia, M.; Yang, X.; Hou, Z. Preparation of high-purity quartz sand by vein quartz purification and characteristics: A case study of Pakistan vein quartz. Minerals 2024, 14, 727. [Google Scholar] [CrossRef]
- Xie, Y.; Xia, M.; Yang, X.; Khan, I.; Hou, Z. Research on 4N8 high-purity quartz purification technology prepared using vein quartz from Pakistan. Minerals 2024, 14, 1049. [Google Scholar] [CrossRef]
- Müller, A.; Ihlen, P.M.; Wanvik, J.E.; Flem, B. High-purity quartz mineralization in kyanite quartzites, Norway. Miner. Depos. 2007, 42, 523–535. [Google Scholar] [CrossRef]
- Xia, M.; Sun, C.; Yang, X.; Chen, J. Assessment of gold-bearing quartz vein as a potential high-purity quartz resource: Evidence from mineralogy, geochemistry, and technological purification. Minerals 2023, 13, 261. [Google Scholar] [CrossRef]
- Magar, J.T.; Yang, X.; Li, K.; Xia, M.; Li, X.; Cai, Z. Mineralogical characteristics and purification experiments of quartz from a pegmatite: A Case study in the Lushi region of the Qinling Orogenic Belt, Central China. Minerals 2024, 14, 1225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Ma, Y.; Wang, S.; Ma, C.; Wei, F. Mineralogy and Preparation of High-Purity Quartz: A Case Study from Pegmatite in the Eastern Sector of the North Qinling Orogenic Belt. Minerals 2025, 15, 788. https://doi.org/10.3390/min15080788
Yu D, Ma Y, Wang S, Ma C, Wei F. Mineralogy and Preparation of High-Purity Quartz: A Case Study from Pegmatite in the Eastern Sector of the North Qinling Orogenic Belt. Minerals. 2025; 15(8):788. https://doi.org/10.3390/min15080788
Chicago/Turabian StyleYu, Deshui, Yameng Ma, Shoujing Wang, Chi Ma, and Fushuai Wei. 2025. "Mineralogy and Preparation of High-Purity Quartz: A Case Study from Pegmatite in the Eastern Sector of the North Qinling Orogenic Belt" Minerals 15, no. 8: 788. https://doi.org/10.3390/min15080788
APA StyleYu, D., Ma, Y., Wang, S., Ma, C., & Wei, F. (2025). Mineralogy and Preparation of High-Purity Quartz: A Case Study from Pegmatite in the Eastern Sector of the North Qinling Orogenic Belt. Minerals, 15(8), 788. https://doi.org/10.3390/min15080788




