Mineral Characterization of Gold Ore Occurrences in the Khaptasynnakh Ore Zone, Anabar Shield, Far East Region, Russia
Abstract
1. Introduction
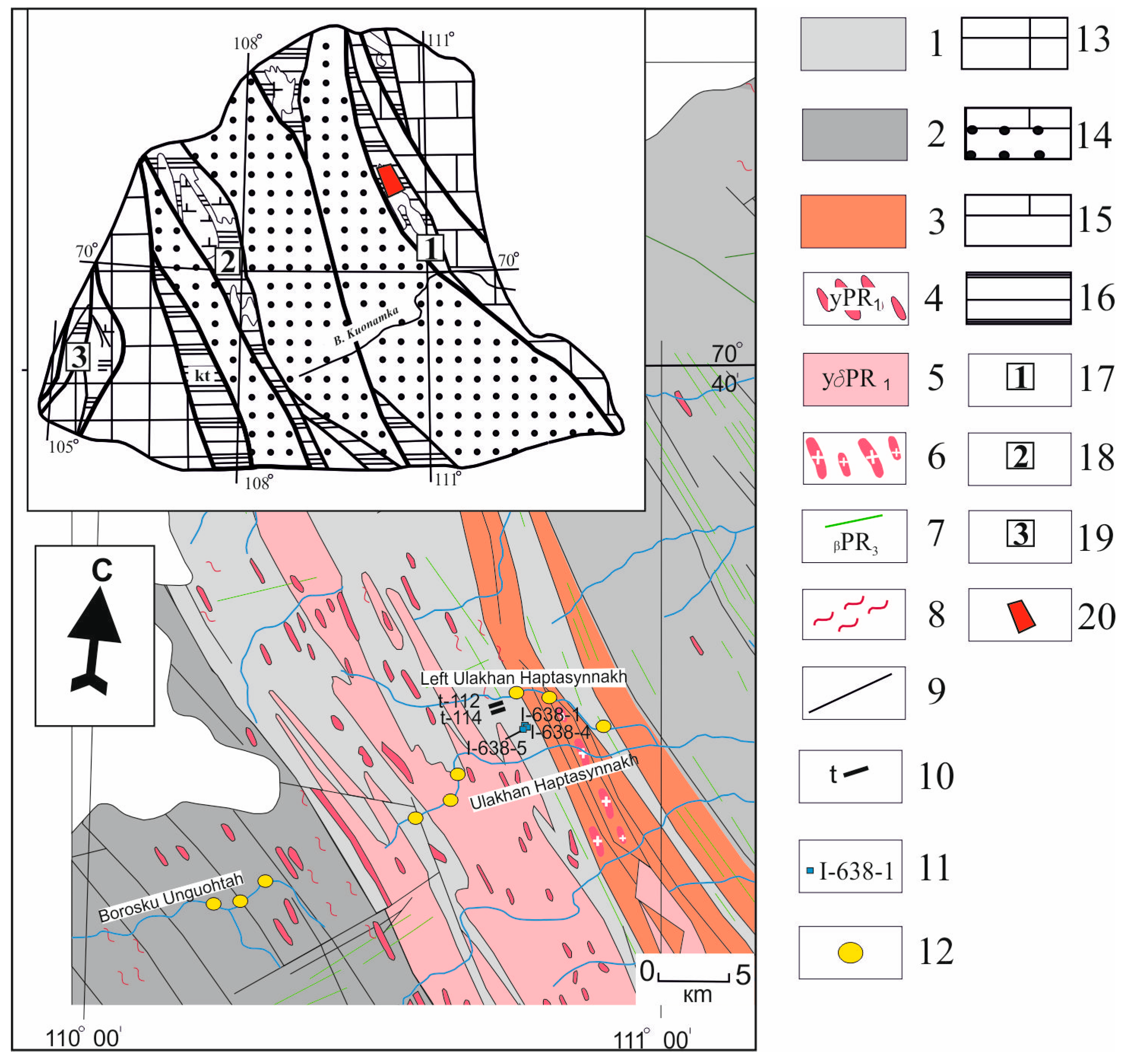
2. Materials and Methods
3. Results
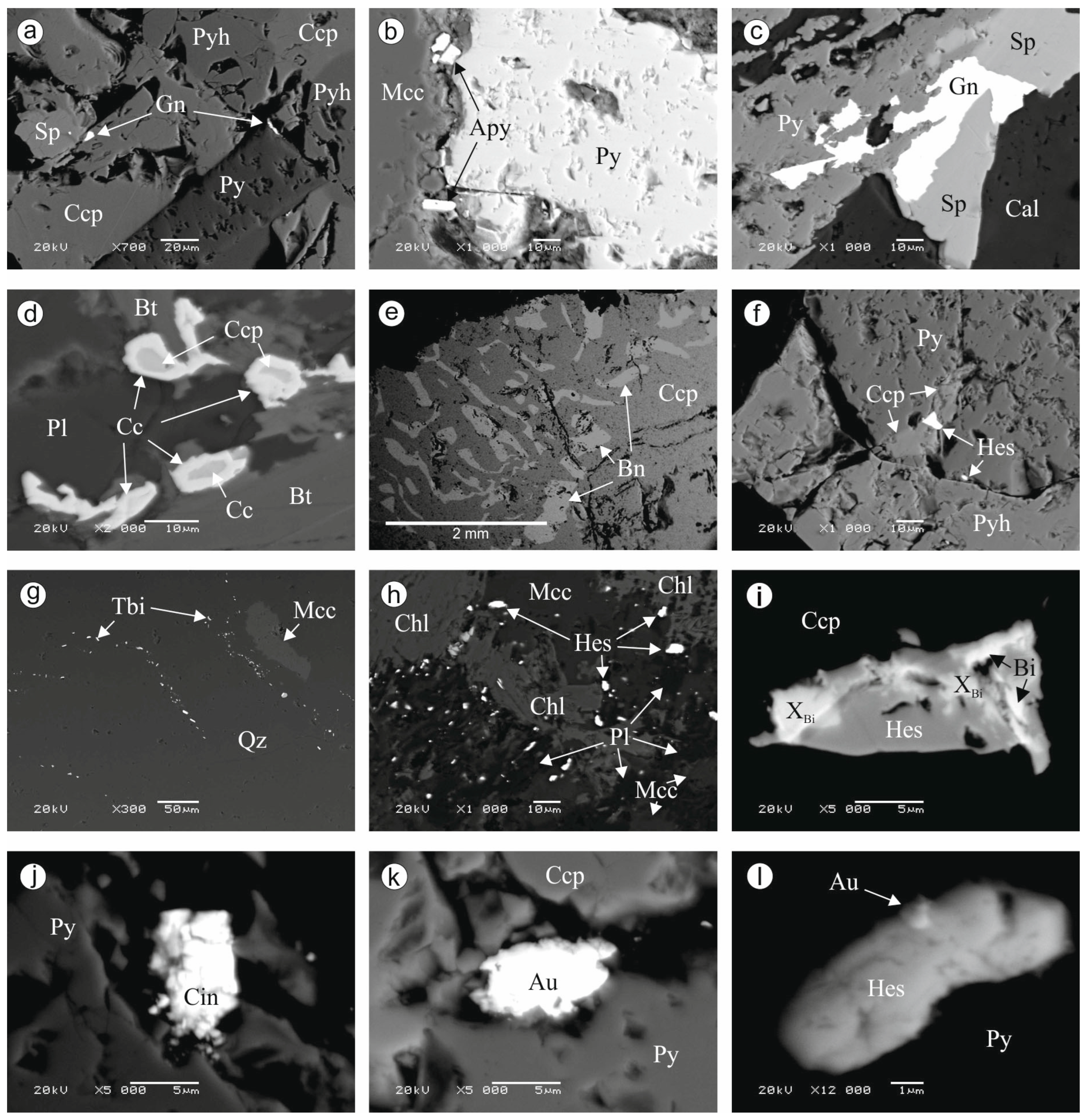
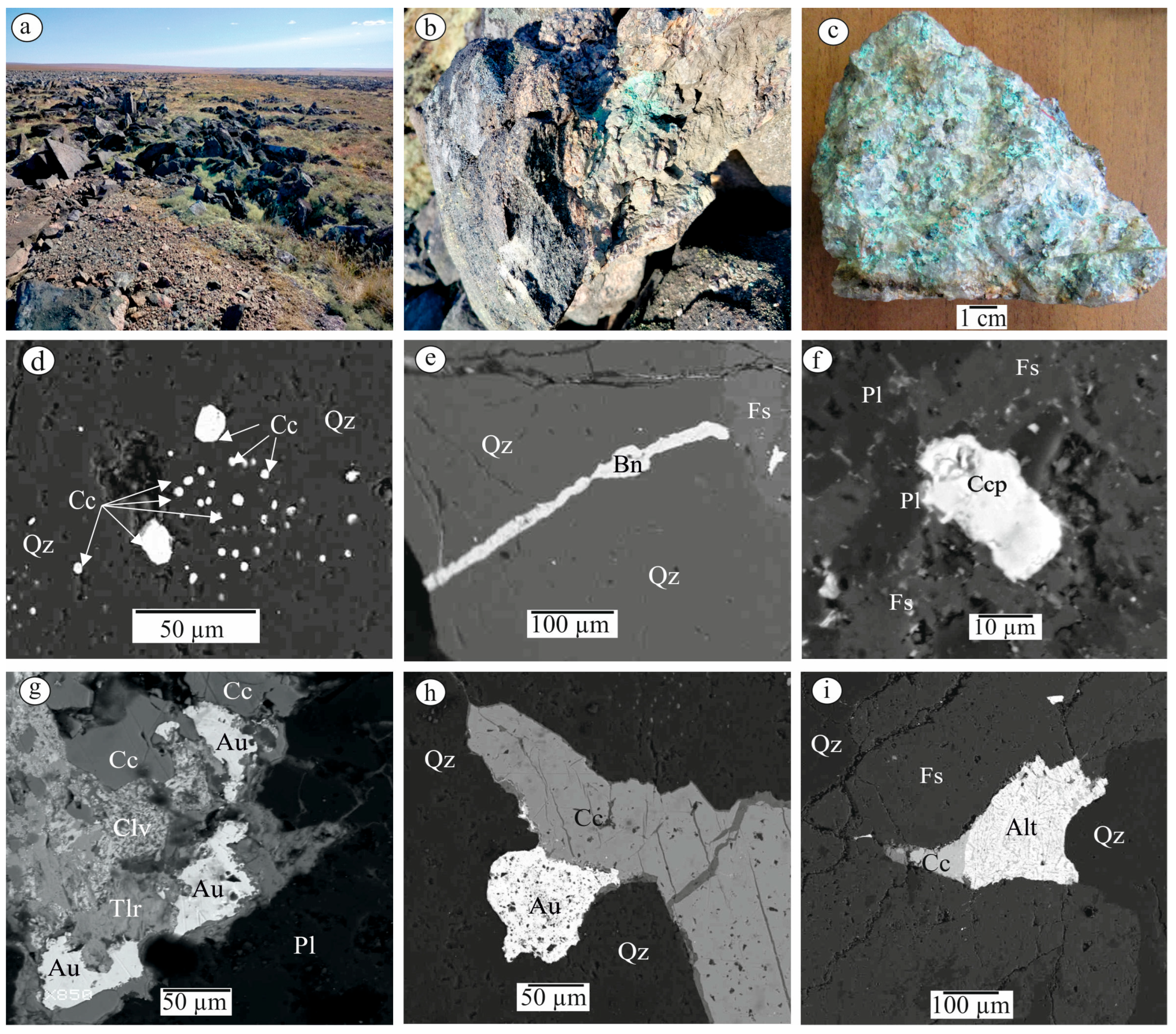
3.1. Mineral Paragenesis of the Khaptasynnakh Ore Zone
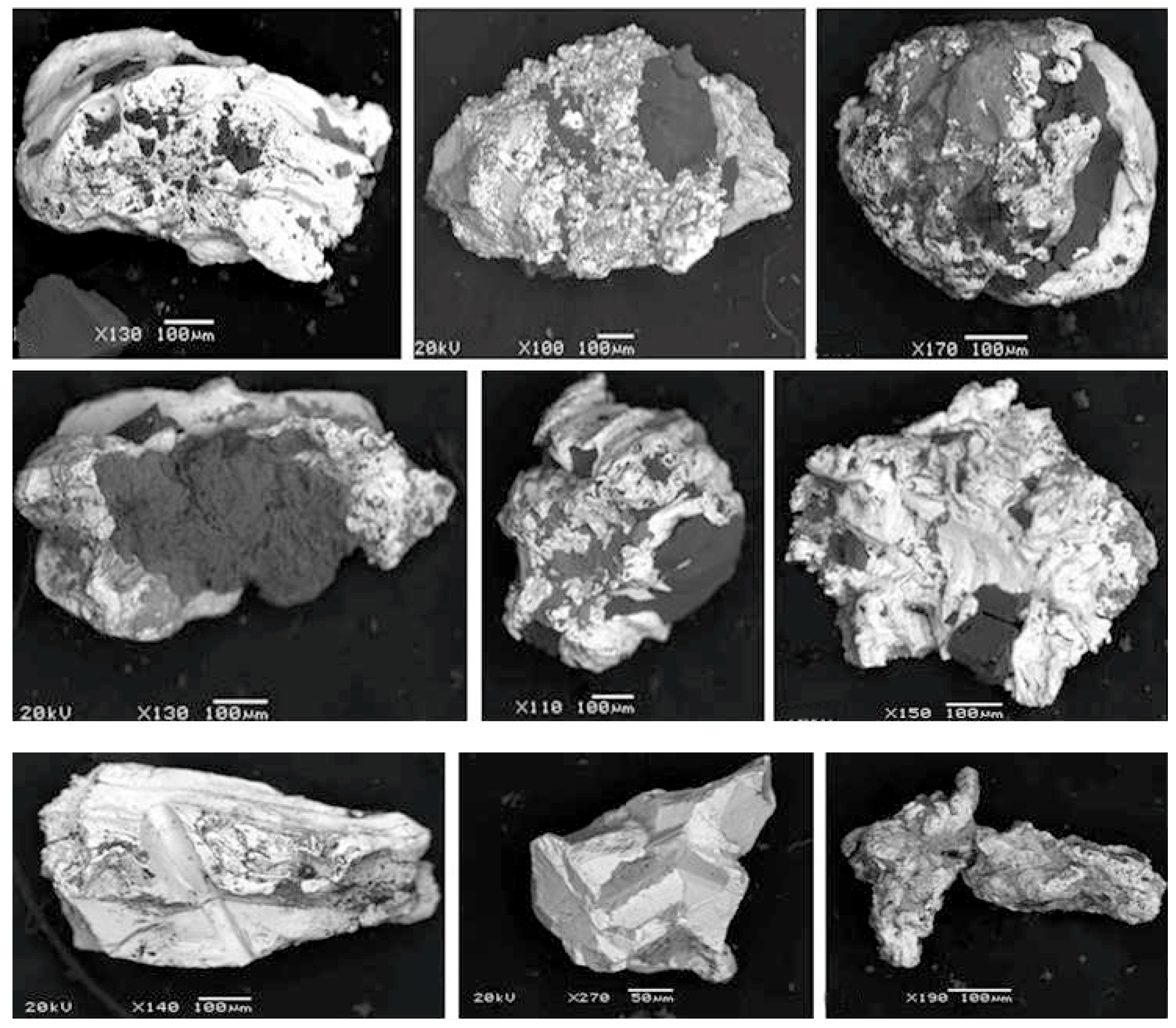
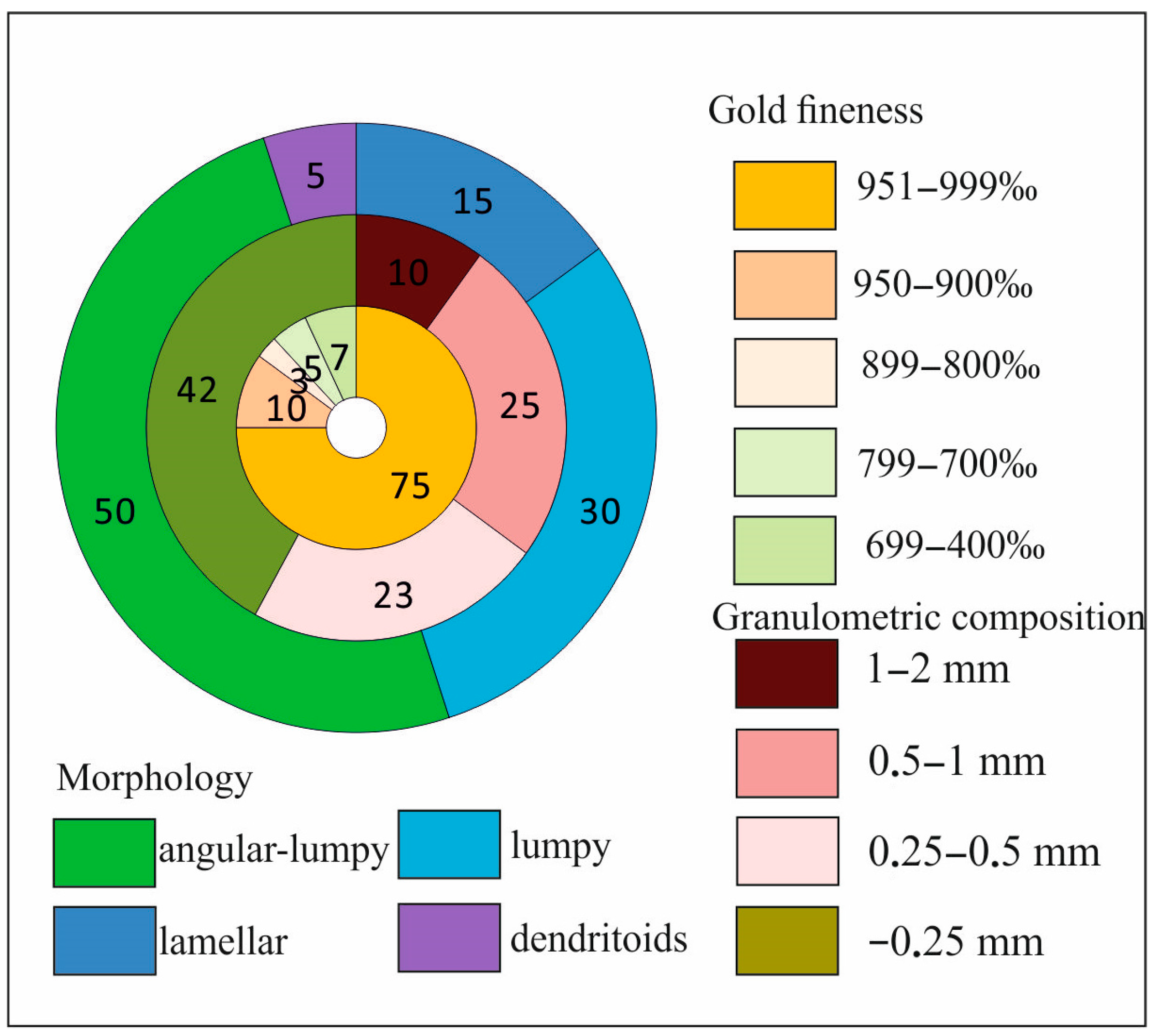
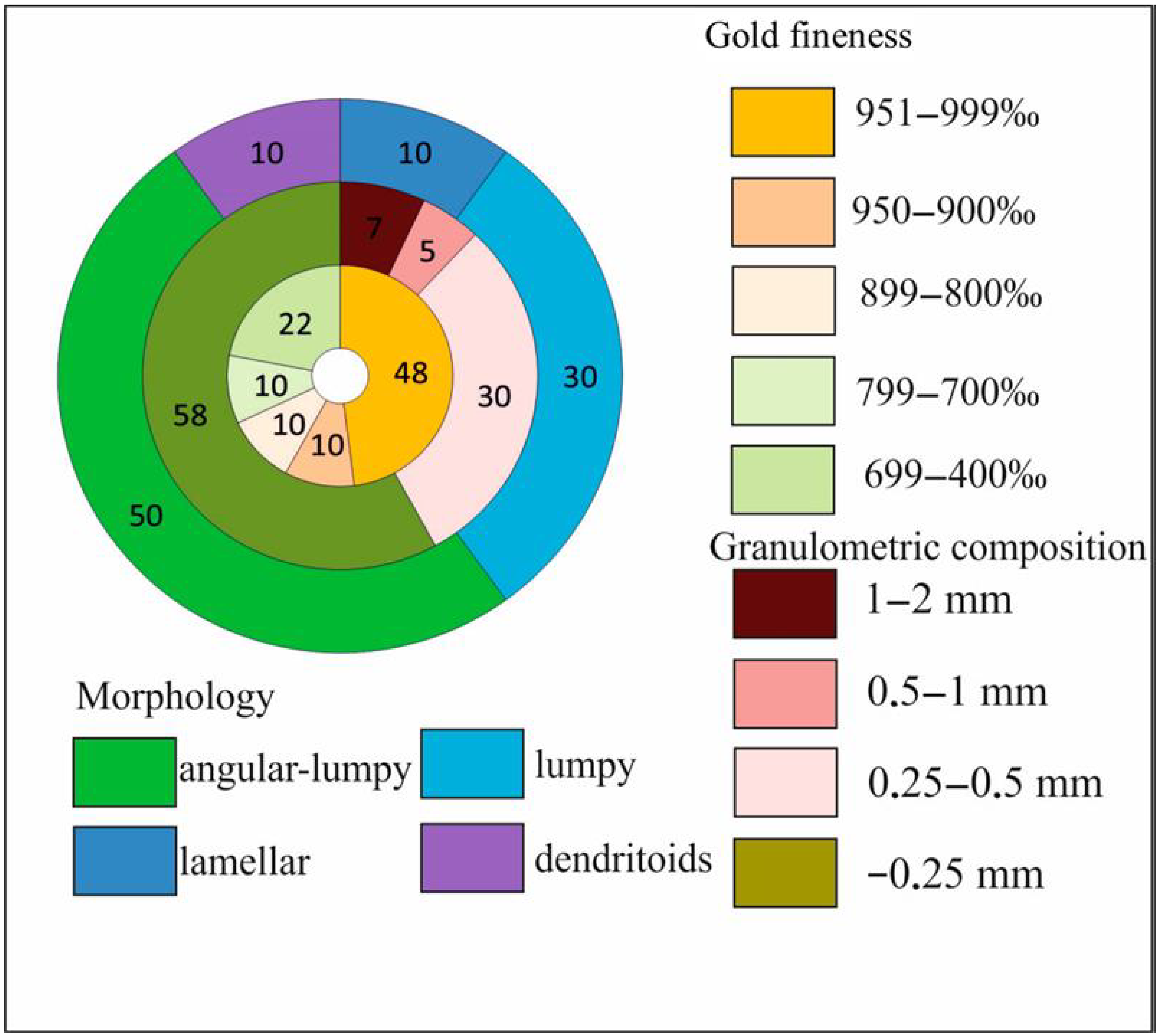
3.2. Characterization of Native Gold from the Khaptasynnakh Zone Using Crushed Samples
4. Discussion
4.1. Possible Genetic Types of Ore Bodies
4.2. Stages of Native Gold Formation in Ore Occurrences and Genesis of Multiphase Particles with Composition Ag—Au—Cu
4.3. The Ore Body–Placer System
5. Conclusions
- For the first time, the results of our study on the mineralogical and geochemical features of ore gold and mineral parageneses at the Khaptasynnakh ore occurrences, located in the central part of the Billyakh zone of tectonic melange in the Anabar Shield, provide grounds for identifying an epithermal gold–telluride type of mineralization on the Anabar Shield. The temperature of the formation of gold–telluride minerals is preliminarily estimated to be in the range of 150–280° C. The general trend during the deposition of tellurides and native gold in the studied ores was a gradual decrease in fTe2 values after initial saturation with tellurium.
- The sequential formation of early sulfides—pyrite, pyrrhotite, and arsenopyrite—and late sulfides—chalcopyrite, sphalerite, galena, bornite, and chalcocite—and the formation at the final stage of gold–telluride mineralization was determined. Gold occurs in association with chalcocite and tellurides, and it is also a component of calaverite and sylvanite.
- The chemical and petrographic sequence of ore gold suggests the two-stage formation of the studied gold mineralization. It is suggested that part of the gold (low grade) entered the system during early potassic metasomatism. Higher-grade gold related to siliceous metasomatism is the result of its additional mobilization by fluids during the late stages of ore formation. At the same time, processes of low-grade gold remobilization took place, which led to the removal of Ag.
- The detected particles consisting of three phases, Au3Cu, AuCu, and Au-Ag solid solutions, were presumably formed by the decomposition of a homogeneous Au-Ag-Cu solid solution at temperatures below 220° C.
- Using mineral inclusion analysis in placer gold, we prove that there is a genetic relation between the gold placers of recent sources identified in the study area and the examined ores. Occurrence of up to 48% of poorly rounded particles of the >0.5 mm fraction in placers suggests the formation of larger native gold in the ores than that found in the course of this study.
- Overall, considering the potential to discover new placers along with recently discovered ones, as well as the presence of gold-bearing ore occurrences, we can report a new Khaptasynnakh ore placer cluster in the North–East Siberian Platform.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gerasimov, B.; Beryozkin, V.; Kravchenko, A. Typomorphic Features of Placer Gold from the Billyakh Tectonic Melange Zone of the Anabar Shield and Its Potential Ore Sources (Northeastern Siberian Platform). Minerals 2020, 10, 281. [Google Scholar] [CrossRef]
- Smelov, A.P.; Kotov, A.B.; Sal’nikova, E.B.; Kovach, V.P.; Beryozkin, V.I.; Kravchenko, A.A.; Dobretsov, V.N.; Velikoslavinskii, S.D.; Yakovleva, S.Z. Age and duration of the formation of the Billyakh tectonic melange zone, Anabar Shield. Petrology 2012, 20, 286–300. (In Russian) [Google Scholar] [CrossRef]
- Smelov, A.P.; Amuzinsky, V.A.; Zedgenizov, A.N.; Berezkin, V.I.; Koval, S.G. Prospects for the Primary Gold Content of Metamorphic and Igneous Complexes of the Anabar Shield. Gold of Siberia and the Far East; Publishing House of the BSC SB RAS: Ulan-Ude, Russia, 2004; pp. 279–281. (In Russian) [Google Scholar]
- Petrovskaya, N.V.; Novgorodova, M.I.; Frolova, K.E. The nature of structures and substructures of endogenous native gold particles. In Mineralogy of Native Elements; Petrovskaya, N., Fatyanov, I., Eds.; FESC AC USSR: Vladivostok, Russia, 1980; pp. 10–20. (In Russian) [Google Scholar]
- Chudnenko, K.V.; Pal’yanova, G.A. Thermodynamic properties of solid solutions in the Ag–Au–Cu system. Russ. Geol. Geophys. 2014, 55, 349–360. (In Russian) [Google Scholar] [CrossRef]
- Petrovskaya, N.V. Native Gold; Nauka: Moscow, Russia, 1973; 347p. (In Russian) [Google Scholar]
- Nikolaeva, L.A.; Gavrilov, A.M.; Nekrasova, A.N.; Yablokova, S.V.; Shatilova, L.V. Atlas of Native Gold of Ore and Placer Deposits in Russia; Tsnigri: Moscow, Russia, 2003; 184p. (In Russian) [Google Scholar]
- Hart, C.J.R. Reduced Intrusion-Related Gold Systems. Mineral Deposits of Canada: A Synthesis of Major Deposit Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Geological Association of Canada, Mineral Deposits Division: St. John’s, NL, Canada, 2007; Volume 5, pp. 95–112. [Google Scholar]
- Robb, L. Introduction to Ore-Forming Processes; Blackwell Publishing: Oxford, UK, 2004; 373p. [Google Scholar]
- Ishihara, S. The magnetite-series and ilmenite-series granitic rocks. Min. Geol. 1977, 26, 293–305. [Google Scholar]
- Gaskov, I.V. The main impurity elements of native gold and their relationship with the conditions of its formation using the example of deposits in the folded belts of Asia. Geol. Geophys. 2017, 58, 1359–1376. (In Russian) [Google Scholar]
- Morrison, G.W.; Rose, W.J.; Jaireth, S. Geological and geochemical controls on the silver content (fineness) of gold in gold-silver deposits. Ore Geol. Rev. 1991, 6, 333–364. [Google Scholar] [CrossRef]
- Townley, B.; Hérail, G.; Maksaev, V.; Palacios, C.; de Parseval, P.; Sepulveda, F.; Orellana, R.; Rivas, P.; Ulloa, C. Gold grain morphology and composition as an exploration tool: Application to gold exploration in covered areas. Geochem. Explor. Environ. Anal. 2003, 3, 29–38. [Google Scholar] [CrossRef]
- Kravchenko, A.A.; Smelov, A.P.; Berezkin, V.I.; Zedgenizov, A.N.; Dobretsov, V.N. Nature of gold mineralization of the early Proterozoic Billyakh granitoid complex according to geochemical data (Anabar shield). Russ. Geol. 2010, 5, 23–29. (In Russian) [Google Scholar]
- Krivtsov, A.I.; Zvezdov, V.S.; Migachev, I.F. Copper-Porphyry Deposits of the World; TsNIGRI: Moscow, Russia, 2001; 236p. (In Russian) [Google Scholar]
- Pavlova, I.G. Geological and genetic models of molybdenum-copper porphyry deposits. In Genetic Models of Endogenous Ore Formations; Nauka: Moscow, Russia, 1983; pp. 127–134. (In Russian) [Google Scholar]
- Popov, V.S. Geology and Genesis of Copper-Molybdenum-Porphyry Deposits; Nauka: Moscow, Russia, 1977; 203p. (In Russian) [Google Scholar]
- Zhang, X.; Spry, P.G. Calculated stability of aqueous tellurium species, calaverite, and hessite at elevated temperatures. Econ. Geol. 1994, 89, 1152–1166. [Google Scholar] [CrossRef]
- Cabri, L.J. Phase relations in the Au-Ag-Te system and theirmineralogical significance. Econ. Geol. 1965, 60, 1569–1606. [Google Scholar] [CrossRef]
- Bortnikov, N.S.; Cramer, H.; Genkin, A.D.; Krapiva, L.Y.; Santa-Cruz, M. Parageneses of gold and silver tellurides at the Florencia gold deposit, Cuba. Geol. Ore Depos. 1988, 2, 49–61. [Google Scholar]
- Afifi, A.M.; Kelly, W.C.; Essene, E.J. Phase relations among tellurides, sulfides, and oxides: I. Thermochemical data and calculated equilibria. Econ. Geol. 1988, 83, 377–394. [Google Scholar] [CrossRef]
- Voudouris, P. A comparative mineralogical study ofTe-rich magmatic-hydrothermal systems in northeastern Greece. Mineral. Petrol. 2006, 87, 241–275. [Google Scholar] [CrossRef]
- Afifi, A.M.; Kelly, W.C.; Essene, E.J. Phase Relations among Tellurides, Sulfides, and Oxides: II. Applications to Telluride-Bearing Ore Deposits. Econ. Geol. 1988, 83, 395–404. [Google Scholar] [CrossRef]
- Gaskov, I.V.; Akimtsev, V.A.; Kovalev, K.R.; Sotnikov, V.I. Gold-bearing mineral associations of copper-ore deposits of the Altai-Sayan folded region. Geol. Geophys. 2006, 47, 996–1004. (In Russian) [Google Scholar]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Chapman, R.J.; Allan, M.M.; Mortensen, J.K.; Wrighton, T.M.; Grimshaw, M.R. A new indicator mineral methodology based on a generic Bi-Pb-Te-S mineral inclusion signature in detrital gold from porphyry and low/intermediate sulfidation epithermal environments in Yukon Territory, Canada. Min. Depos. 2018, 53, 815–834. [Google Scholar] [CrossRef]
- Bukhanova, D.S. Mineralogical and Geochemical Features of the Malmyzh Gold-Copper-Porphyry Deposit, Khabarovsk Region. Ph.D. Thesis, Federal State Budgetary Scientific Institution Institute of Volcanology and Seismology of the Far Eastern Branch of the Russian Academy of Sciences, Petropavlovsk-Kamchatsky, Russia, 2020; 25p. (In Russian). [Google Scholar]
- Migachev, I.F.; Zvezdov, V.S.; Minina, O.V. Formational types of porphyry copper deposits and their ore-magmatic systems. Russ. Geol. 2022, 1, 26–48. (In Russian) [Google Scholar] [CrossRef]
- Plotinskaya, O.Y. Porphyry-Epithermal Systems of the Urals: Sources of Matter, Evolution, Zonality. Ph.D. Thesis, Federal State Budgetary Scientific Institution Institute of Geology of Ore Deposits, Petrography, Mineralogy and Geochemistry of the Russian Academy of Sciences, Moscow, Russia, 2023; p. 39. (In Russian). [Google Scholar]
- Kuznetsov, A.A. Geological-forecast-mineragenic model and prospects for commercial ore-bearing content of the Anabar Shield. Russ. Geol. 2008, 6, 22–34. (In Russian) [Google Scholar]
- Richards, J.P.; Mumin, A.H. Magmatic-hydrothermal processes within an evolving Earth: Iron oxide-copper-gold and porphyry Cu ± Mo ± Au deposits. Geology 2013, 41, 767–770. [Google Scholar] [CrossRef]
- Skirrow, R.G. Iron oxide copper-gold (IOCG) deposits—A review (part 1): Settings, mineralogy, ore geochemistry and classification. Ore Geol. Rev. 2022, 140, 104569. [Google Scholar] [CrossRef]
- Koděra, P.; Lexa, J.; Biroň, A.; Žitňan, J. Gold mineralization and associated alteration zones of the Biely vrch Au-porphyry deposit, Slovakia. Miner. Slovaca 2010, 42, 33–56. [Google Scholar]
- Spiridonov, E.M.; Pletnev, P.A. The Zolotaya Gora Copper Gold Deposit (About the “Gold-Rodingite” Formation); Scientific World: Moscow, Russia, 2002; 220p. (In Russian) [Google Scholar]
- Palyanova, G.A. Gold and silver minerals in sulfide ore. Geol. Ore Depos. 2020, 62, 383–406. [Google Scholar] [CrossRef]
- Onishchenko, S.A.; Kuznetsov, S.K. Exsolution in the Au–Ag–Cu system in a gold-rich area. Geochemistry 2022, 67, 639–654. (In Russian) [Google Scholar] [CrossRef]
- Palyanova, G.A.; Beliaeva, T.V.; Savelyev, D.P.; Seryotkin, Y.V. Minerals of the Au-Cu-Ag System in Grains from the Placers of the Olkhovaya-1 River (Eastern Kamchatka, Russia). Minerals 2024, 14, 448. [Google Scholar] [CrossRef]
- Murzin, V.V.; Varlamov, D.A. Diffusion Processes at Contacts of Native Gold and Copper in the Oxidation Zone of the Anninskoe Deposit in the Middle Urals. Zapiski RMO 2022, 6, 18–28. (In Russian) [Google Scholar] [CrossRef]


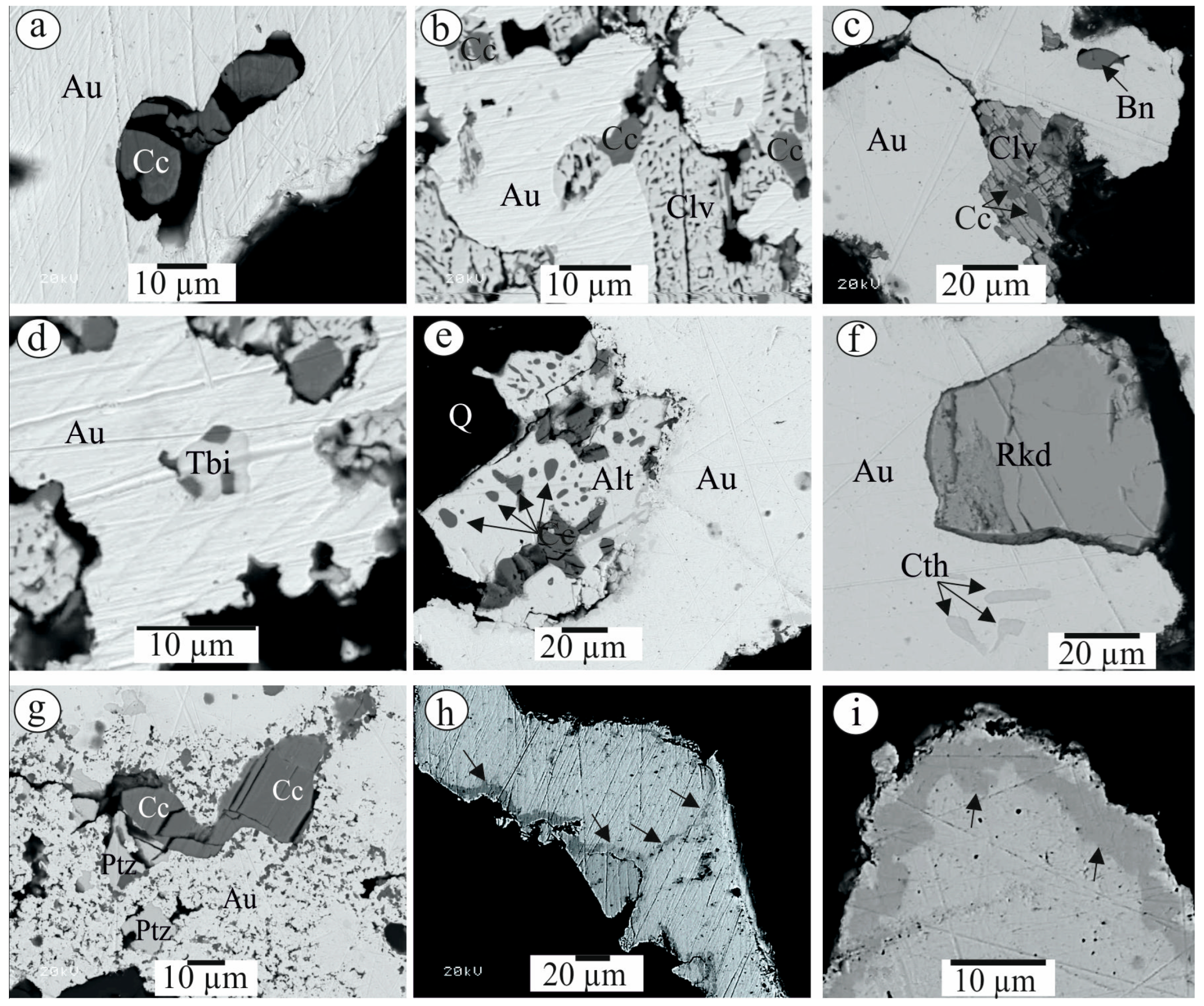
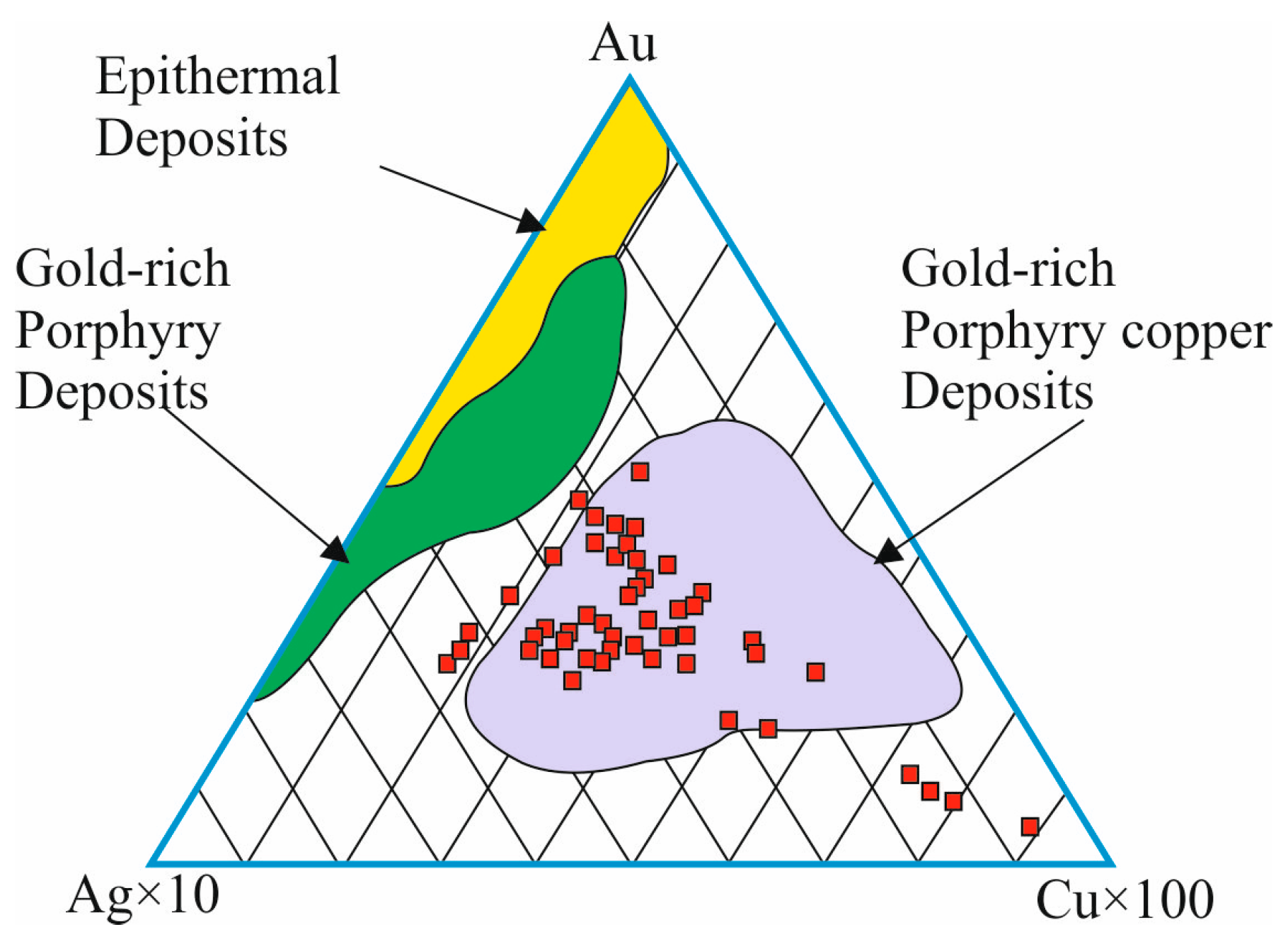


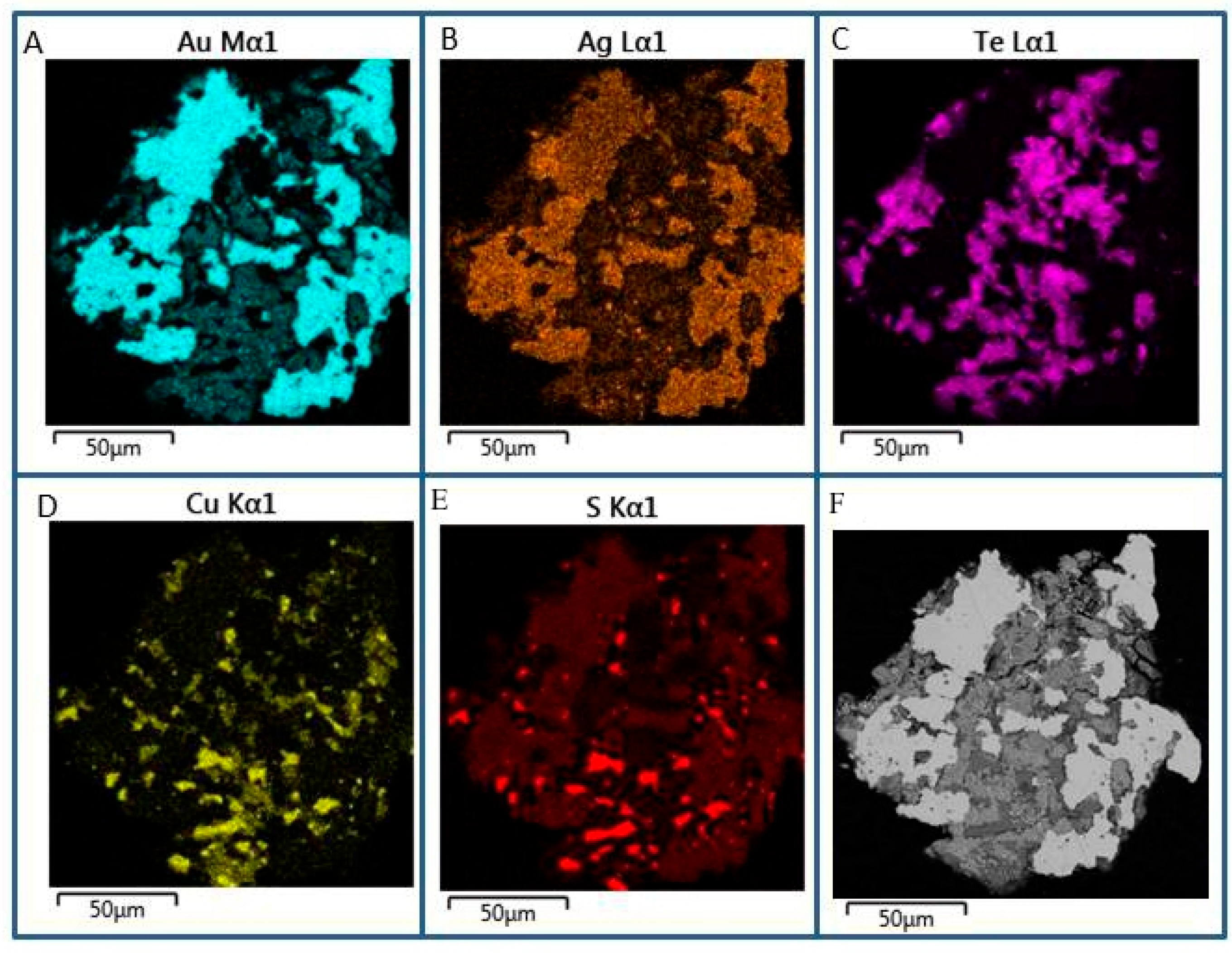
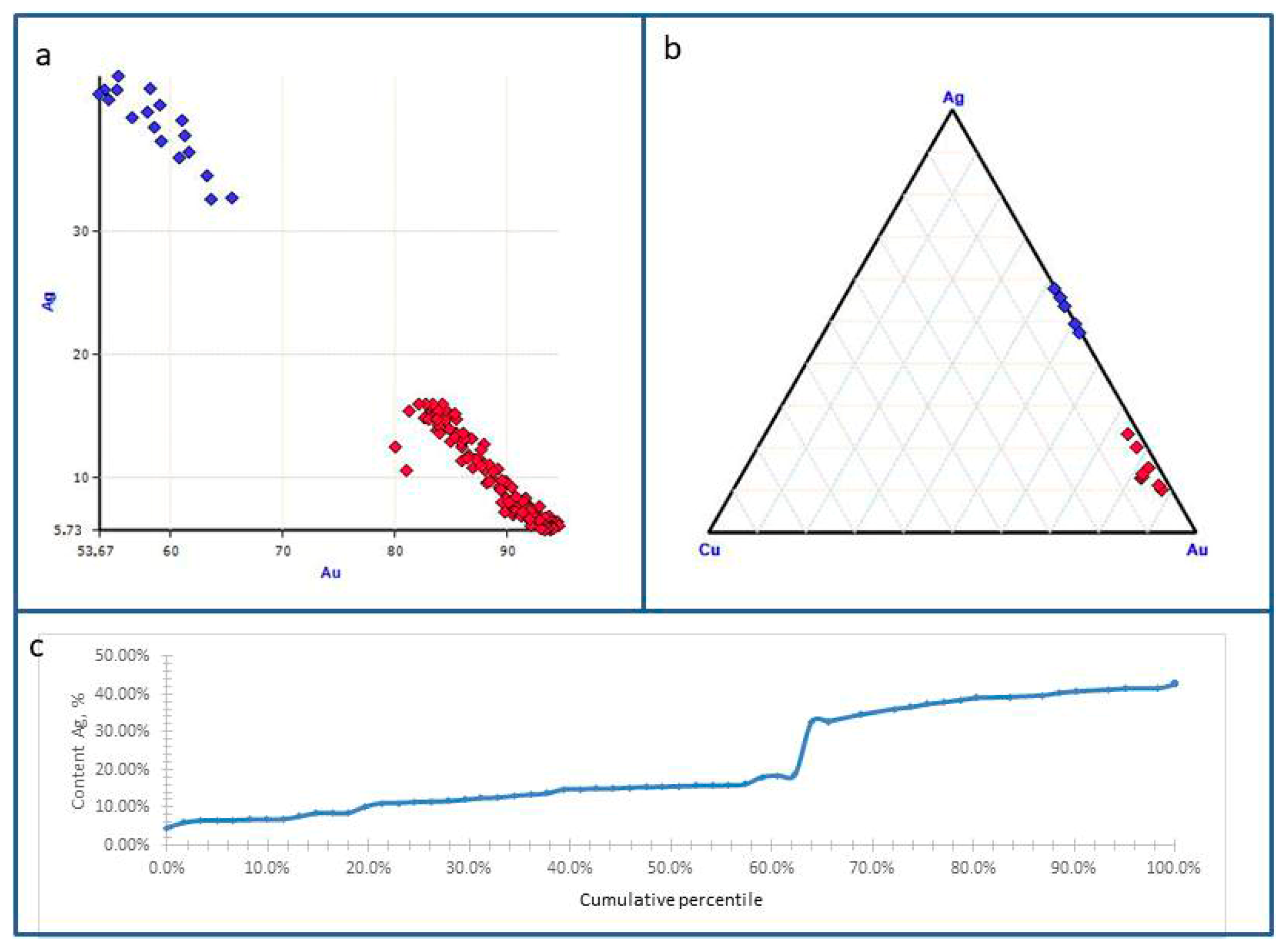
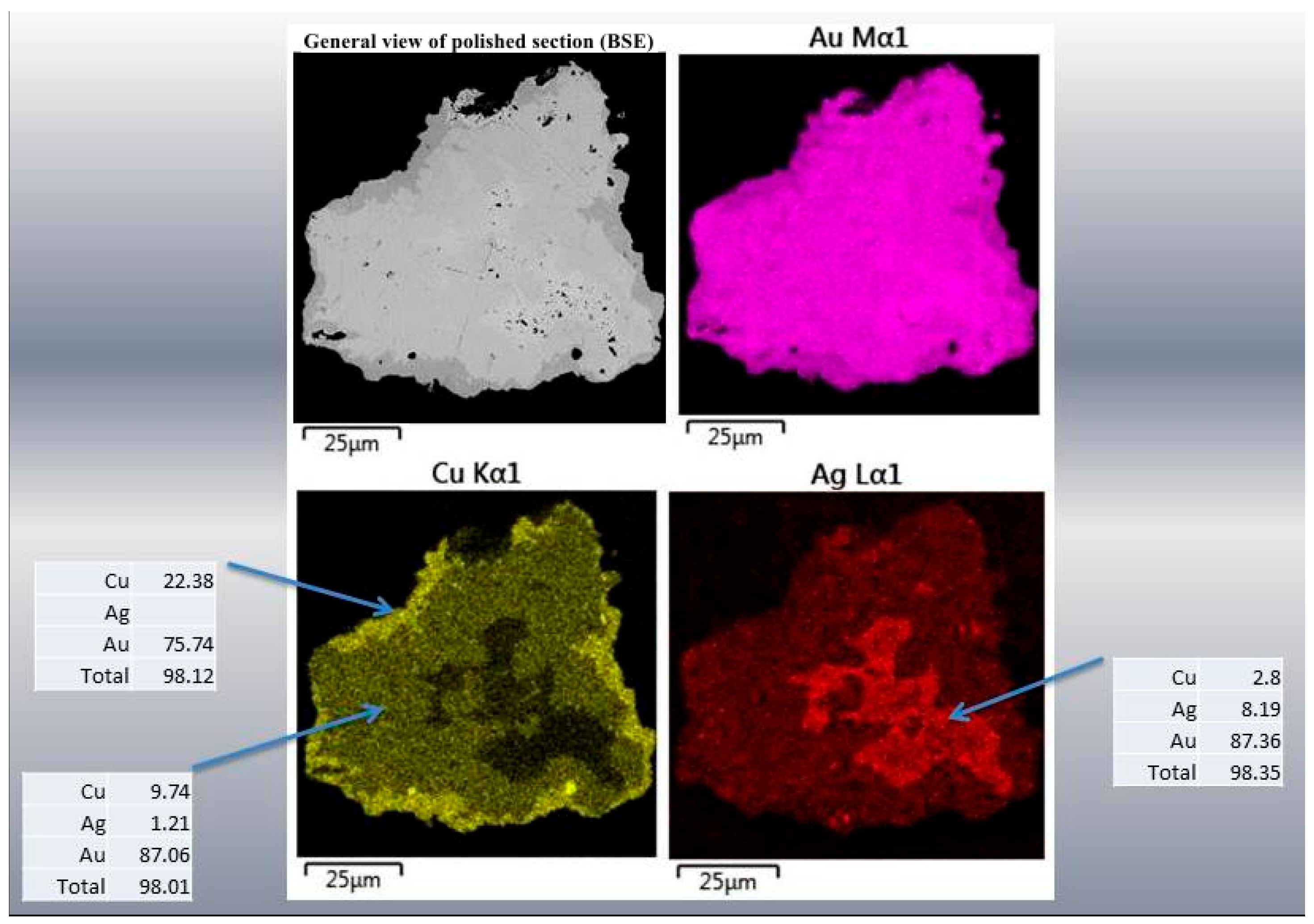
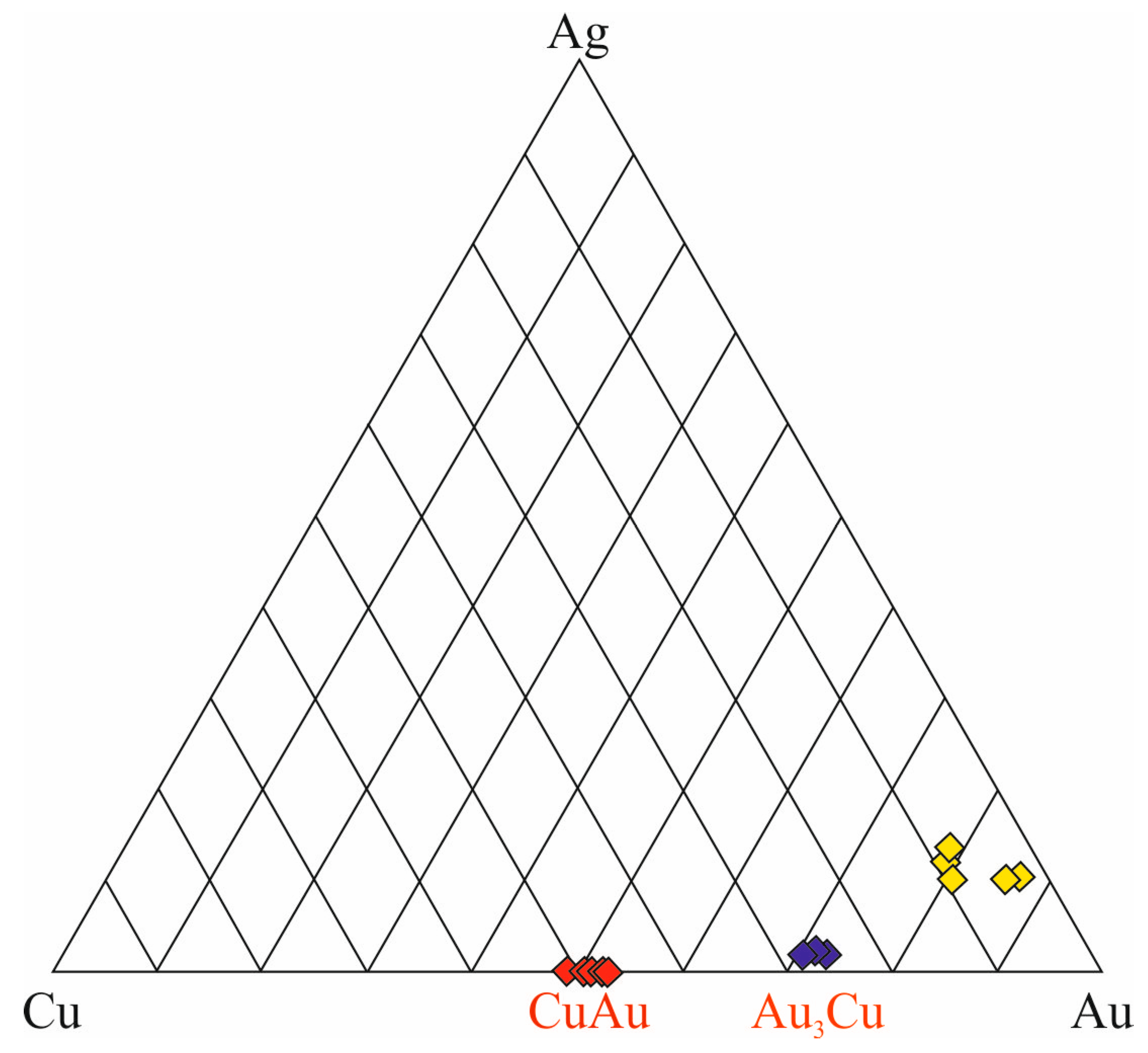
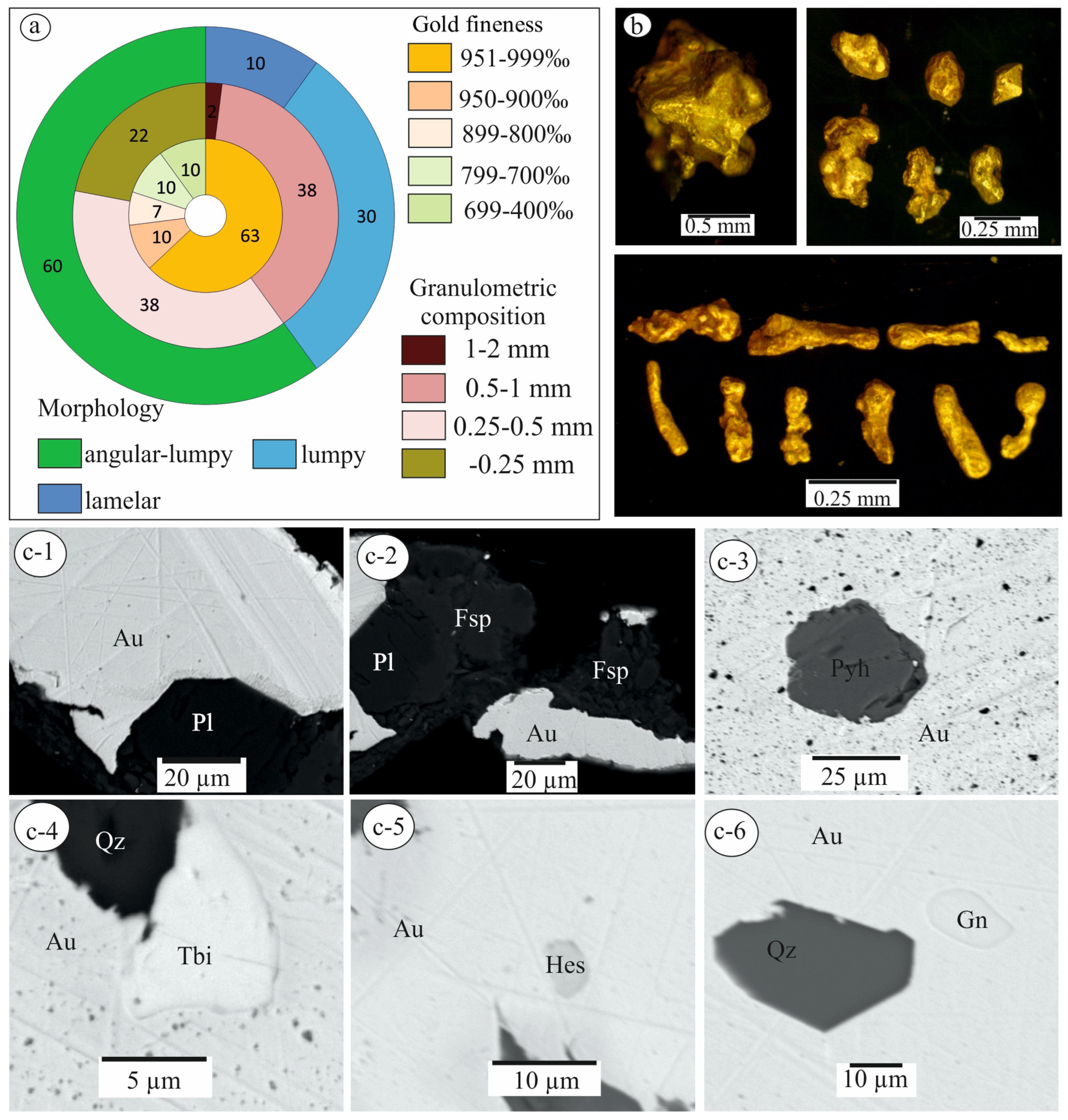
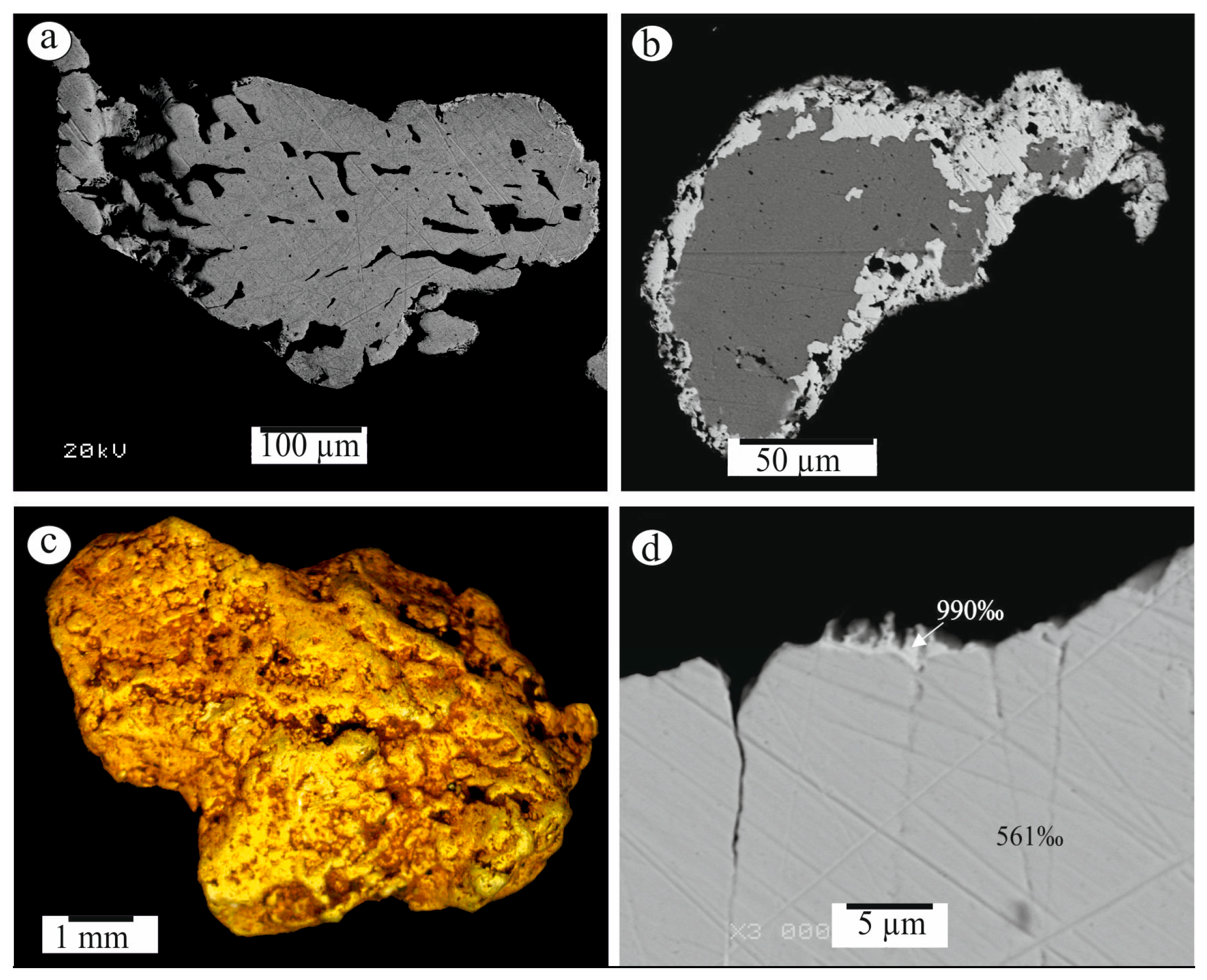
| Sample No. | S | Fe | Se | Ag | Te | Au | Pb | Bi | Total | Mineral |
|---|---|---|---|---|---|---|---|---|---|---|
| 114-20-8-1 | - | - | - | 55.02 | 34.22 | 9.36 | - | - | 98.61 | hessite |
| 114-20-8-2 | - | - | - | 56.33 | 36.48 | 8.18 | - | - | 100.99 | |
| 114-13A12-5 | - | - | - | 55.6 | 36.01 | 7.92 | - | - | 99.54 | |
| 114-20-13-4 | - | - | - | 62.84 | 37.47 | - | - | - | 100.31 | |
| 114-20-21-2 | - | 1.79 | - | 60.91 | 38.04 | - | - | - | 100.75 | |
| 114-20-21-5 | - | 1.33 | - | 61.77 | 35.84 | - | - | - | 98.95 | |
| 114-33-32-1 | 0.99 | - | - | 61.35 | 37.94 | - | - | - | 100.27 | |
| 114-16-1-7-4 | - | - | - | 64.23 | 35.95 | - | - | - | 100.18 | |
| 114-162-5 | 3.12 | 4.64 | - | 56.65 | 35.84 | - | - | - | 100.25 | |
| 114-13A13-6 | - | 2.12 | - | 61.51 | 37.15 | - | - | - | 100.77 | |
| 114-18a-22-2 | - | 1.14 | - | 62.63 | 35.71 | - | - | - | 99.48 | |
| 114-18a-22-8 | - | 0.54 | - | 61.09 | 36.47 | - | - | - | 98.1 | |
| 144-27 | - | 1.96 | - | 16.67 | 42.28 | - | - | 37.01 | 97.92 | volynskite |
| 15-31-7.2-2-1 | - | 3.03 | - | - | 36.72 | - | 59.07 | - | 98.82 | altaite |
| 15-31-7.2-2-3 | - | 3.44 | - | - | 35.09 | - | 60.9 | - | 99.43 | |
| 15-31-7.2-4-2 | - | 2.47 | - | - | 34.58 | - | 62 | - | 99.05 | |
| 114-18a-24-1 | - | - | - | - | 19.24 | - | - | 81.62 | 100.86 | hedleyite |
| 114-20-17-4 | - | 2.16 | 4.2 | - | 19.51 | - | - | 74.43 | 100.29 |
| Sample No. | Au | Ag | Fe | Total | Fineness, ‰ | |
|---|---|---|---|---|---|---|
| 1 | 114-33-9-2 | 56.6 | 39.18 | 2.88 | 98.67 | 574 |
| 2 | 114-33-9-7 | 55.37 | 42.57 | 2.82 | 100.76 | 550 |
| 3 | 114-33-10-4 | 55.29 | 41.44 | 3.16 | 99.89 | 554 |
| 4 | 114-33-10-5 | 57.99 | 39.61 | 2.85 | 100.45 | 577 |
| 5 | 114-33-18-4 | 58.64 | 38.39 | 2.41 | 99.44 | 590 |
| 6 | 114-33-20-3 | 61.35 | 37.74 | 2.41 | 101.5 | 604 |
| 7 | 114-18a-16-2 | 61.64 | 36.44 | 2.44 | 100.52 | 613 |
| 8 | 114-18a-20-1 | 60.82 | 35.99 | 2.63 | 99.45 | 612 |
| 9 | 114-18a-20-2 | 59.27 | 37.3 | 2.45 | 99.01 | 599 |
| Minerals | S | Fe | Cu | Zn | As | Ag | Sb | Te | Hg | Pb | Bi | Total | Formula |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyrite | 53.56 | 46.95 | - | - | - | - | - | - | - | - | - | 100.51 | Fe1.19S1.81 |
| Pyrrhotite | 39.46 | 61.39 | - | - | - | - | - | - | - | - | - | 100.85 | Fe0.94S1.06 |
| Arsenopyrite | 22.58 | 33.3 | - | - | 44.72 | - | - | - | - | - | - | 100.6 | Fe0.94As0.94S1.11 |
| Chalcopyrite | 35.26 | 30.04 | 34.73 | - | - | - | - | - | - | - | - | 100.03 | Cu1.00Fe0.98S2.02 |
| Sphalerite | 33.09 | 5.47 | - | 62.49 | - | - | - | - | - | - | - | 101.05 | Zn0.92Fe0.09S0.99 |
| Galena | 13.8 | - | - | - | - | - | - | - | 85.91 | - | 99.71 | Pb0.98S1.02 | |
| Chalcocite | 20.68 | 78.03 | - | - | - | - | - | - | - | - | 98.71 | Cu1.97S1.03 | |
| Bornite | 24.38 | 10.09 | 64.72 | - | - | - | - | - | - | - | - | 99.19 | Cu5.20Fe0.92S3.88 |
| Hessite | - | - | - | - | - | 62.84 | - | 37.47 | - | - | - | 100.31 | Ag1.99Te1.01 |
| Cinnabar | 14.6 | - | - | - | - | - | - | - | 86.52 | - | - | 101.12 | Hg0.97S1.03 |
| Stibnite | 27.24 | - | - | - | - | - | 72.63 | - | - | - | - | 99.87 | Sb2.06S2.94 |
| Tellurobismuthite | - | - | - | - | - | - | - | 47.95 | - | - | 53.53 | 101.48 | Bi2.03Te2.97 |
| Native Bi | - | - | - | - | - | - | - | 3.64 | - | - | 95.82 | 99.46 | Bi0.94Te0.06 |
| Sample No. | Cu | Ag | Au | Total | Fineness, ‰ |
|---|---|---|---|---|---|
| 1 | 0.85 | 12.74 | 87.97 | 101.56 | 864 |
| 2 | 1.7 | 7.99 | 91.17 | 100.86 | 902 |
| 3 | 0.80 | 6.59 | 93.41 | 100.8 | 926 |
| 4 | 1.09 | 6.7 | 93.02 | 100.81 | 922 |
| 5 | 0.95 | 14.73 | 84.58 | 100.25 | 843 |
| 6 | 1.22 | 9.55 | 88.23 | 99.1 | 890 |
| 7 | 1.47 | 10.74 | 87.03 | 99.24 | 875 |
| 8 | 1.27 | 7.81 | 91.22 | 100.29 | 908 |
| 9 | 2.42 | 14.26 | 83.97 | 100.7 | 833 |
| 10 | 7.87 | 12.53 | 79.04 | 99.44 | 794 |
| Sample No. | Mineral | S | Fe | Cu | Se | As | Te | Pb | Bi | Ag | Au | Total | Formula |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Calaverite | - | - | - | - | - | 58.52 | - | - | - | 39.75 | 98.27 | Au0.92Te2.08 |
| 2 | - | - | - | - | - | 59.35 | - | - | - | 39.9 | 99.25 | Au0.91Te2.09 | |
| 5 | Sylvanite | - | - | 1.91 | - | - | 62.89 | - | - | 12.9 | 23.63 | 101.33 | (Au0.94Ag0.94 Cu0.24)2.12Te3.88 |
| 6 | - | - | - | - | - | 62.74 | - | - | 13.05 | 24.00 | 99.79 | (Au1.00Ag0.98)1.98Te4.02 | |
| 7 | Chalcosite | 21.55 | - | 78.67 | - | - | - | - | - | - | - | 100.22 | Cu1.94S1.06 |
| 8 | 20.08 | - | 80.72 | - | - | - | - | - | - | - | 100.8 | Cu2.01S0.99 | |
| 9 | Bornite | 25.92 | 10.47 | 62.29 | - | - | - | - | - | - | - | 98.68 | Cu4.96Fe0.94S4.1 |
| 10 | 25.05 | 10.38 | 63.57 | - | - | - | - | - | - | - | 99 | Cu5.08Fe0.94S3.98 | |
| 11 | 25.17 | 11.12 | 62.46 | - | - | - | - | - | - | - | 98.76 | Cu4.99Fe1.01S4 | |
| 12 | Altaite | - | - | - | - | - | 35.36 | 63.05 | - | - | - | 98.41 | Pb0.95Te1.05 |
| 13 | - | - | - | - | - | 37.43 | 63.16 | - | - | - | 100.59 | Pb0.98Te1.02 | |
| 14 | - | - | - | 2.8 | - | 35.03 | 63.56 | - | - | - | 101.38 | Pb0.99(Te0.90Se0.11)1.01 | |
| 15 | - | - | - | 3.42 | - | 36.54 | 61.28 | - | - | - | 101.23 | Pb0.95(Te0.91Se0.14)1.05 | |
| 16 | Tellurobismuthite | - | - | - | 8.9 | - | 38.27 | - | 54.07 | - | - | 101.24 | Bi1.93(Te2.23Se0.84)3.07 |
| 17 | - | - | - | - | - | 46.72 | - | 53.08 | - | - | 99.8 | Bi2.05Te2.95 | |
| 18 | Rickardite | - | - | 40.88 | - | - | 58.96 | - | - | - | - | 99.84 | Cu6.98Te5.02 |
| 19 | - | - | 41.84 | - | - | 60.06 | - | - | - | - | 101.9 | Cu6.99Te5.01 | |
| 20 | - | - | 39.86 | - | - | 59.24 | - | - | - | - | 99.11 | Cu6.9Te5.1 | |
| 21 | Petzite | - | - | - | - | - | 35.98 | - | - | 37.41 | 26.15 | 99.54 | Au1.05Ag2.73Te2.22 |
| 22 | Clausthalite | - | - | - | 21.42 | - | - | 77.21 | - | - | - | 98.63 | Pb1.16Se0.84 |
| 23 | - | - | - | 20.65 | 1.15 | 3.77 | 76 | - | - | - | 101.57 | (Pb1.08As0.05)1.13(Te0.09Se0.78)0.87 |
| Sample No. | Cu | Au | Total | Calculating Formula |
|---|---|---|---|---|
| 1 | 25.22 | 73.76 | 98.98 | Au1.02Cu0.97 |
| 2 | 23.27 | 75.78 | 99.05 | Au1Cu0.99 |
| 3 | 24.74 | 76.47 | 101.22 | Au1Cu0.99 |
| 4 | 24.49 | 75.32 | 99.81 | Au1Cu0.99 |
| 5 | 24.62 | 77.17 | 101.79 | Au1Cu0.99 |
| 6 | 23.26 | 77.34 | 100.6 | Au1.03Cu0.96 |
| 7 | 24.38 | 75.98 | 100.36 | Au1Cu0.99 |
| 8 | 25.29 | 76.1 | 101.39 | Au1.01Cu0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimov, B.; Kondratieva, L. Mineral Characterization of Gold Ore Occurrences in the Khaptasynnakh Ore Zone, Anabar Shield, Far East Region, Russia. Minerals 2025, 15, 774. https://doi.org/10.3390/min15080774
Gerasimov B, Kondratieva L. Mineral Characterization of Gold Ore Occurrences in the Khaptasynnakh Ore Zone, Anabar Shield, Far East Region, Russia. Minerals. 2025; 15(8):774. https://doi.org/10.3390/min15080774
Chicago/Turabian StyleGerasimov, Boris, and Larisa Kondratieva. 2025. "Mineral Characterization of Gold Ore Occurrences in the Khaptasynnakh Ore Zone, Anabar Shield, Far East Region, Russia" Minerals 15, no. 8: 774. https://doi.org/10.3390/min15080774
APA StyleGerasimov, B., & Kondratieva, L. (2025). Mineral Characterization of Gold Ore Occurrences in the Khaptasynnakh Ore Zone, Anabar Shield, Far East Region, Russia. Minerals, 15(8), 774. https://doi.org/10.3390/min15080774






