1. Introduction
Copper is a critical metal in modern society and plays an indispensable role in the global economy. Its unique properties, including high electrical and thermal conductivity, corrosion resistance, and malleability, make it an essential material in various industries. Copper is extensively utilized in the construction of infrastructure, electrical power generation and transmission systems, telecommunications, transportation networks, and advanced manufacturing sectors. Additionally, copper’s significance is increasing with the growth of renewable energy technologies, electric vehicles, and energy-efficient systems, further setting its importance in driving sustainable economic development worldwide [
1]. Global copper production has surged significantly, reaching 28.50 million tons in 2019. Currently, more than 80% of the world’s copper products are produced through the beneficiation of copper sulfide concentrates, followed by the pyrometallurgical process. This method has been known for its excellent adaptability to raw materials, low energy costs, and high production efficiency [
2], although hydrometallurgy is now gaining popularity in mineral processing industries for its sustainability and low carbon footprint towards meeting the goal of NetZero. During the smelting and converting stages of copper production by pyrometallurgy, copper slag is generated as a byproduct, which is typically discarded as waste. A literature search revealed that for every ton of copper produced, approximately 2.0–3.0 tons of copper slag are generated throughout the process [
3,
4,
5,
6]. Copper slag generally contains significant amounts of valuable metals, including copper and iron, making it a promising secondary resource for recycling and further utilization [
7]. The global production of copper slag is estimated at 30–38 million tons per year [
8,
9], and during 2000 to 2024, approximately 966 million tons of copper slag waste have been produced globally [
10]. The copper content in the copper slag lies in the range of 0.5%–2% [
11], which accounts for about 1 million tons of copper dumped into the environment with copper slag wastes every year.
With the increased demand for copper in emerging clean technologies, such as solar cells, electric vehicles (EVs), and battery materials, it is important to recover copper from copper slag as a value-added critical mineral. Many researchers used different methods to optimize the metal recovery from copper slags. Some adopted the concept of a new, more environmentally friendly, and cost-effective technology for utilizing water as a lixiviant after roasting to recover valuable metals, such as lithium, copper, nickel, cobalt, and zinc from secondary sources [
12,
13]. This study proposes an innovative method of pyrometallurgy using chlorinating roasting, where different chlorine donors such as NaCl, AlCl
3, and CaCl
2 were evaluated using HSC Chemistry 941 software and in experiments. The authors claimed that chlorinating roasting is the best strategy for metal recovery from copper slag. However, this process has several disadvantages, as chlorine is a corrosive chemical, and it requires corrosion-resistant materials for constructing the process plant, resulting in higher capital expenses (CAPEX). Also, gaseous chlorine produced in roasting is poisonous, which is classified as a pulmonary irritant.
In several other studies, a direct reduction–magnetic separation method using Na
2CO
3 and CaO was studied to recover iron and copper from copper slag containing 40.33% Fe and 0.65% Cu. The optimal conditions at 0.5 basicity with 8% Na
2CO
3 achieved a recovery of 94.3% Fe, but only 86.5% Cu. The authors mentioned that the magnetic concentrate (90.5% Fe, 1.2% Cu) produced in this process is a suitable feedstock for the steel-making industry, while nonmagnetic tailings can be used in cement production. Additionally, in their process, 99.16% Zn- and 91.89% Pb-rich dust containing 50.65% Zn and 8.56% Pb, respectively, were recovered for further extraction of valuable metals [
14,
15]. The recovery of copper from copper slag by physical beneficiation using froth flotation has also been studied by several researchers [
16,
17], and Bruckard et al. found that only 80–87% Cu can be recovered from the copper slag using a two-stage flotation process [
17].
A novel process for joint waste processing to extract valuable metals (cobalt, nickel, and copper) from smelter slag using high-temperature exhaust gas (SO
2) has been proposed and tested. In that process, nickel and copper were extracted from smelter slag using a high-temperature effluent gas, sulfur dioxide. The sulfatous oxidation followed by water leaching was conducted under specially developed thermodynamic conditions. This enabled the selective formation of sulfates of cobalt, nickel, and copper, while simultaneously separating iron in the form of oxide. The parameters of the investigated process included the temperature, time, the addition of Na
2SO
4, and the selection of the leaching agent, and the results demonstrated that 95.8% of nickel, 91.8% of cobalt, and 81.6% of copper were extracted. Moreover, the process of sulfatous smelting and aqueous leaching was validated as both effective and feasible at the laboratory scale. The mechanism of the process was defined and verified through the examination of microstructural alterations. Based on preliminary assessments of the environmental benefits, economic advantages, and heat recovery potential, the authors proposed that this is a prospective environmentally benign industrial process, although the copper recovery in their process is below 82% [
18]. This low recovery of copper is due to the heterogeneous and crystalline behaviors of copper smelter slag which happen during the molten oxidation process, where Fe
2SiO
4 and FeS undergo stepwise oxidation, provided that sufficient oxygen partial pressure is available. The transformation follows the sequence: Fe
2SiO
4 → Fe
3O
4 → Fe
2O
3, and FeS → FeO → Fe
3O
4 → Fe
2O
3. In this process, Fe
2+ ions, released from Fe
2SiO
4 and FeS, are progressively oxidized as oxygen diffuses through the system, leading to an increased Fe
3+ content. The amount of sulfur (S) in the slag is very low (about 1%), and it mostly escapes as gas during the process. Silicon (Si) changes to sodium metasilicate (Na
2SiO
3) during treatment, and later, sodium metasilicate dissolves in water and precipitates out as pure silica (SiO
2). The formation of Fe
3+ facilitates the generation of magnetite, which occurs through interactions between Fe
3+, Fe
2+, and O
2− [
19,
20].
Copper slag contains significant amounts of valuable metals, such as copper (Cu), zinc (Zn), and iron (Fe) that are often trapped within complex mineral structures, including fayalite (Fe
2SiO
4), which is formed when pyrite is oxidized to iron oxide in the presence of silica [
21,
22]. The recovery of these valuable metals is essential not only for economic reasons but also for environmental sustainability, as copper slag presents a significant waste management challenge. Fayalite, a dominant phase in copper slag, forms a highly stable matrix that binds metals, making their extraction difficult through conventional methods. Therefore, developing innovative techniques to break down this structure is crucial for improving metal recovery rates. In the present research, sodium sulfate (Na
2SO
4) has been identified as a promising agent for enhancing the recovery of valuable metals from complex slags. The addition of Na
2SO
4 at elevated temperatures can disrupt the fayalite structure, promoting the release of trapped metals and facilitating their subsequent extraction. This study aims to investigate the effect of Na
2SO
4 on the decomposition of fayalite and other minerals present in copper slag under varying conditions of temperature and time. By optimizing these parameters, it is possible to increase the efficiency of metal recovery and reduce the environmental impact of slag disposal. The primary objective of this research is to explore the transformation of mineral structures within copper slag through the addition of Na
2SO
4 and to identify the optimal conditions for the selective recovery of valuable metals. This study contributes to the development of more sustainable processing methods for copper slag, potentially offering a viable solution to both the environmental and economic challenges faced by the metallurgical industry.
2. Materials and Methods
In this study, copper slag, primarily composed of complex mineral phases such as fayalite (Fe
2SiO
4) and spinel (Fe
3O
4, MgAl
2O
4), was selected as the primary material for investigation. Its detailed chemical composition is presented in
Table 1. To optimize subsequent processing stages and maximize the exposure of reactive surface areas, the slag was meticulously ground to a fine particle size of 0.074 mm, enhancing its reactivity during treatment. Sodium sulfate (Na
2SO
4) was employed as a key reagent to disrupt the stable and complex mineral structures of the slag, particularly targeting fayalite and spinel, thereby facilitating the liberation of valuable metals, including copper and iron. The synergy between finely ground slag and Na
2SO
4 is anticipated to significantly enhance the efficiency of metal recovery by promoting the decomposition of these refractory phases, ultimately laying the groundwork for a more effective and sustainable extraction process.
During the initial phase of the experiment, five samples were prepared to determine the optimal concentration of sodium sulfate (Na2SO4) for decomposing fayalite in copper slag. Each sample consisted of 100 g of slag in addition to varying amounts (wt%) of 100% pure Na2SO4 added as follows: 10% for Sample 1, 15% for Sample 2, 20% for Sample 3, 25% for Sample 4, and 30% for Sample 5. The prepared samples were subjected to thermal treatment in a muffle furnace (SNOL 7.2/1300 LSC01, manufactured in Tver, Russia) to evaluate the effect of Na2SO4 concentration on fayalite decomposition. After thermal treatment, the samples were analyzed using an X-ray Diffractometer (XRD-6100, manufactured by Shimadzu, Japan) to precisely identify the mineral phases present. This analytical technique provided detailed insights into the structural changes induced by Na2SO4 at different concentrations.
To thoroughly investigate the influence of temperature on the treatment process, a series of controlled experiments were performed at distinct temperature levels: 600 °C, 650 °C, 700 °C, 750 °C, 800 °C, 850 °C, 900 °C, 950 °C, and 1000 °C. Each temperature was selected to systematically evaluate its effect on the decomposition of complex mineral phases within copper slag during thermal treatment. These experiments aimed to determine the temperature at which the mineral structures, particularly fayalite and spinel, undergo the most efficient breakdown, thereby liberating valuable metals for subsequent recovery.
The effect of reaction time on the decomposition of fayalite and spinel was systematically examined to assess its role in enhancing the breakdown efficiency of the mineral and facilitating the release of valuable metals. Thermal treatment experiments were meticulously designed and conducted over a series of controlled time intervals: 30, 50, 70, 90, 110, and 130 min. These specific durations were selected to evaluate the progressive impact of prolonged exposure on the structural integrity of fayalite and its transformation into more stable or decomposed phases.
For the calculation of the decomposition values, almost all SiO2 in the slag was combined with FeO. After thermal treatment, the formed Na2SiO3 is dissolved in water, and then pure SiO2 is separated by precipitation. The degree of decomposition was determined based on the amount of SiO2 that was separated. Following the breakdown of the refractory nature of fayalite and spinel structure with sodium sulfate, both treated and untreated copper slags were leached for 2 h at 90 °C at varying H2SO4 concentration of solutions to examine the leaching efficiency. In the leaching test, 500 mL of sulfuric acid was added to 100 g of slag.
3. Results and Discussions
This study highlights the critical role of sodium sulfate (Na
2SO
4) concentration, temperature, and reaction time in breaking down fayalite and spinel, a key mineral in copper slag. The results, illustrated in
Figure 1, reveal the relationship between Na
2SO
4 concentration and the degree of fayalite and spinel decomposition. The findings underscore the effectiveness of higher Na
2SO
4 concentrations in promoting the breakdown of fayalite and spinel, thereby enhancing the recovery of valuable metals from the slag. As illustrated in
Figure 1, the decomposition rate reached 89% for fayalite and 85% for spinel when the sodium sulfate concentration was 20%. This result demonstrates the high effectiveness of Na
2SO
4 in disrupting fayalite’s structure, allowing valuable metals trapped within to be released for recovery. Interestingly, increasing the Na
2SO
4 concentration beyond 20% did not lead to any significant improvement in the decomposition rate. This finding indicates that 20% is the optimal concentration, ensuring maximum efficiency without the need for additional reagents. These results provide important insights for designing an efficient and cost-effective process for extracting metals from copper slag.
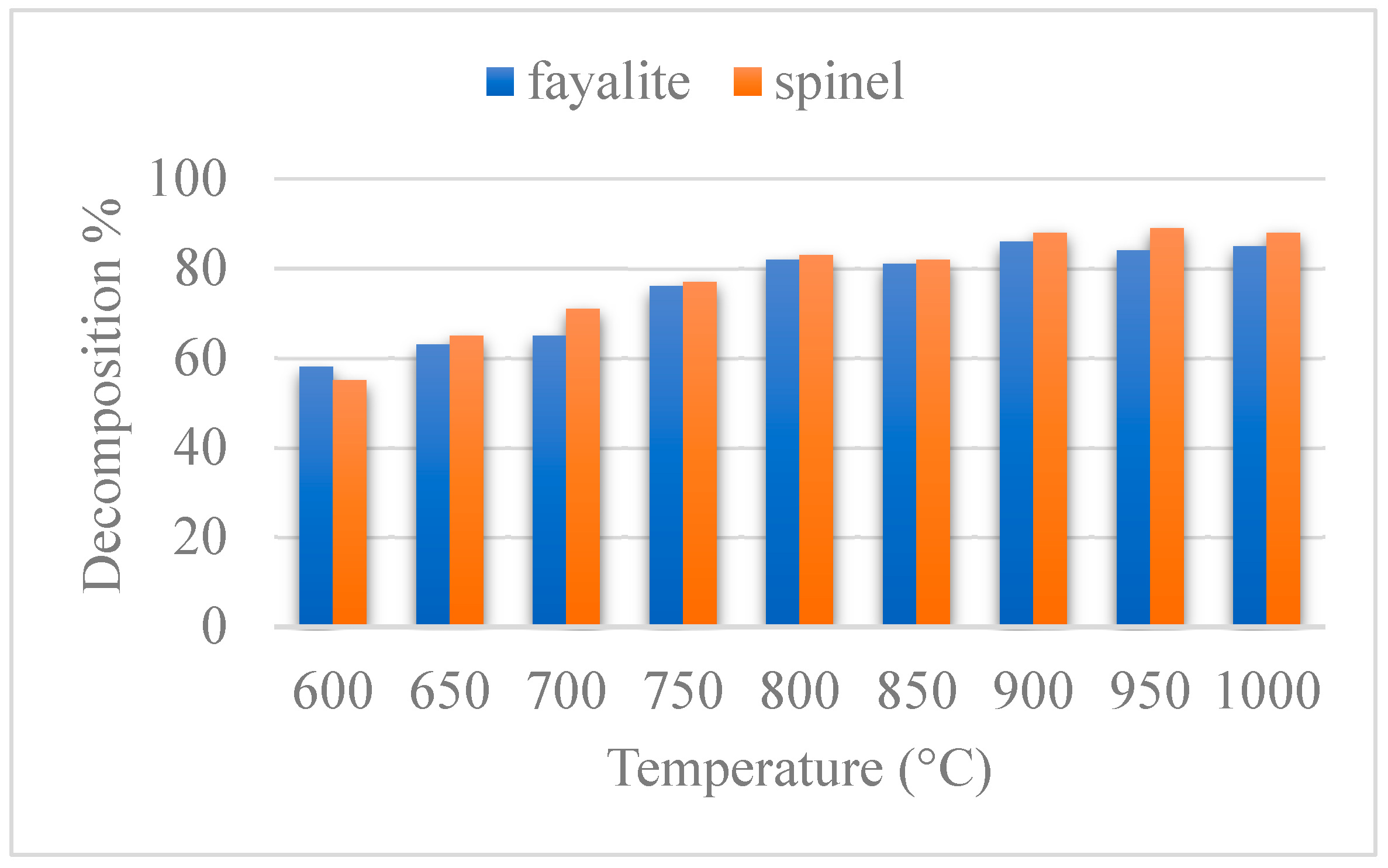
Temperature plays a crucial role in breaking down fayalite in copper slag. The results, illustrated in
Figure 2, demonstrate the relationship between temperature and the extent of mineral decomposition, showcasing a clear trend in the thermal reactivity of the slag. These findings provide valuable insights into the thermal behavior of the material and pinpoint the optimal temperature conditions required to maximize metal recovery while ensuring process efficiency and sustainability. As shown in
Figure 2, the optimal temperature for fayalite and spinel decomposition is 900 °C. At this temperature, the decomposition rate reaches its highest efficiency, as the heat provides sufficient energy to drive the reactions and break apart the mineral structure effectively. Importantly, increasing the temperature beyond 900 °C does not improve the decomposition rate. This indicates that 900 °C is the most efficient temperature for the process, balancing energy input and reaction effectiveness. Operating at this optimal temperature ensures maximum fayalite breakdown while avoiding unnecessary energy use, making it an efficient and practical choice for metal recovery processes.
The effect of reaction time on fayalite and spinel decomposition was also carefully studied. The findings, illustrated in
Figure 3, demonstrate a clear and consistent trend. As shown with increasing reaction time, the decomposition of fayalite and spinel becomes increasingly pronounced, significantly improving the liberation and recovery rates of encapsulated metals. Notably, after the critical reaction time, the rate of decomposition reaches a plateau, suggesting a limit to the effectiveness of extended treatment. This critical insight emphasizes the necessity of identifying the optimal reaction time that balances maximum mineral breakdown with process efficiency. Establishing this parameter is essential for optimizing the overall extraction process, minimizing energy consumption, and maximizing the yield of valuable metals from copper slag. These results offer valuable insights into the kinetics of fayalite and spinel decomposition and provide a foundation for refining industrial metal recovery practices. As shown in
Figure 3, the results revealed that a reaction time of 90 min yielded the best outcome, achieving the highest breakdown efficiency and metal recovery. This indicates that allowing sufficient time for sodium sulfate to fully interact with fayalite is essential for effective mineral decomposition. Interestingly, extending the reaction time beyond 90 min did not improve the results further. This suggests that 90 min is the optimal duration, ensuring maximum fayalite and spinel breakdown without wasting additional time or resources. These findings highlight the importance of selecting the right reaction time to balance efficiency and practicality in metal recovery processes from copper slag.
During the thermal treatment of copper slag with sodium sulfate (Na2SO4), several key chemical reactions occur, facilitating the decomposition of fayalite (Fe2SiO4) and spinel (MgAl2O4) while promoting the liberation of valuable metals. The primary reactions governing these transformations are as follows:
Decomposition of copper-containing fayalite: In this reaction, copper ions (Cu2+) within the fayalite matrix are released as copper (II) oxide (CuO), enhancing their subsequent recovery.
Breakdown of fayalite into iron and silicate phases: Sodium sulfate actively reacts with fayalite, leading to the formation of sodium silicate (Na2SiO3), iron (II) oxide (FeO), and sulfur trioxide (SO3), which further influence the overall slag chemistry.
Oxidative transformation of fayalite: Under specific thermal conditions, fayalite undergoes oxidation, forming iron (III) oxide (Fe2O3) while sodium silicate (Na2SiO3) and sulfur dioxide (SO2) are generated as reaction products.
Reaction of spinel (MgAl2O4) with sodium sulfate: The interaction of sodium sulfate with spinel leads to the formation of sodium aluminate (NaAlO2), magnesium oxide (MgO), and sulfur trioxide (SO3), which play a significant role in altering the mineralogical composition of the treated slag. The calculated thermodynamic data of reaction (4) are ΔH (298 K) = −558.86 kJ/mol, ΔS (298 K) = 183.73 J/mol·K, and ΔG (298 K) = −613.62 kJ/mol, which shows that the reaction is likely to occur.
These reactions collectively contribute to the destabilization of the refractory mineral phases present in copper slag, thereby improving the efficiency of metal extraction. By facilitating the conversion of complex silicate and spinel structures into more soluble or easily separable compounds, this approach enhances the overall recovery process while ensuring greater selectivity in metal separation.
Figure 4 illustrates how varying concentrations of sodium sulfate (Na
2SO
4) affect the treated samples, providing a clear visual representation of its influence. The figure showcases the structural and chemical changes that occur when different amounts of Na
2SO
4 are added during the treatment process. This comparison offers valuable insights into how Na
2SO
4 interacts with fayalite and other minerals in the slag, impacting the overall reaction dynamics. By analyzing these variations, the figure helps identify the most effective concentration of Na
2SO
4 for breaking down the mineral structure and enhancing the release of valuable metals, which was found to be 20 wt%. This information is crucial for optimizing the treatment process and improving metal recovery efficiency.
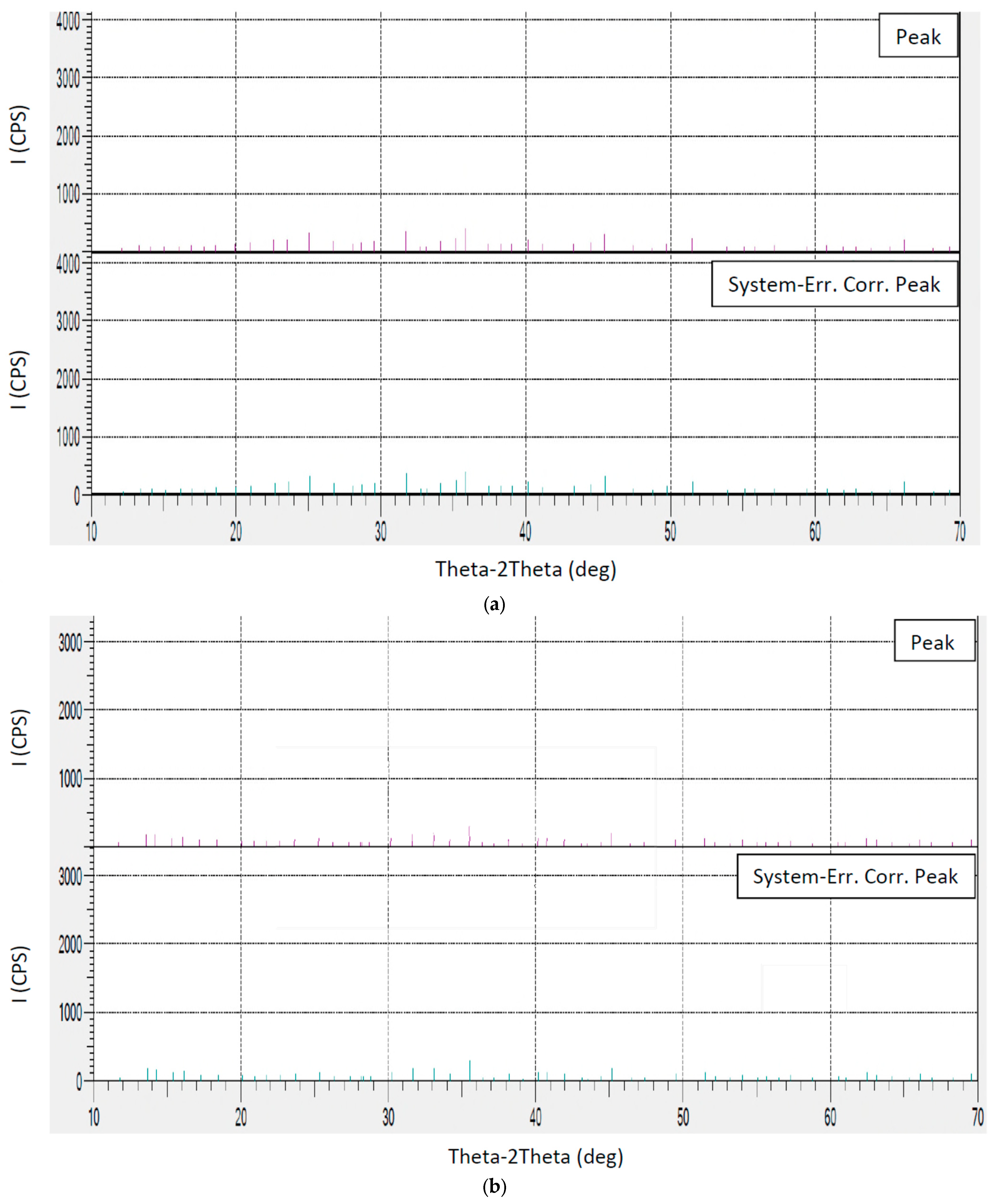
Figure 5a,b show the XRD diagrams of untreated and treated copper slags, respectively, and
Table 2 shows the peak details.
As shown in
Figure 5a and
Table 2, for the untreated sample, sharp and intense peaks are observed, which correspond to well crystallized fayalite (Fe
2SiO
4) and spinel phases such as magnesium aluminate (MgAl
2O
4). These peaks indicate that the original slag material contains highly stable and crystalline mineral structures. However, after the thermal treatment with Na
2SO
4 (
Figure 5b and
Table 2), the intensity of peaks at 2Thetas at around 36 and 32 degrees decreases significantly. For the untreated sample, the 2Theta at around 25 deg is 177 counts, whereas it was found to be 62 counts for the treated sample. Also, at 2Theta of around 45 deg, the intensity of peaks for untreated and treated samples was found to be 171 and 98 counts, respectively. The drops in peak intensity make it clear that the crystalline structure of fayalite and spinel has been largely destroyed during treatment, where the sodium sulfate reacts with these phases during heating, disrupting their structure and converting them into more reactive or amorphous forms.
Examination of the Effect of the Leaching Solution and Filtration on the Decomposition of Fayalite:
To gain a more comprehensive understanding of the research findings, a comparative analysis was conducted between samples treated with sodium sulfate (Na
2SO
4) and those that underwent no treatment in terms of their solubility in sulfuric acid (H
2SO
4). The experiments were carried out under controlled conditions with a sulfuric acid concentration as specified in
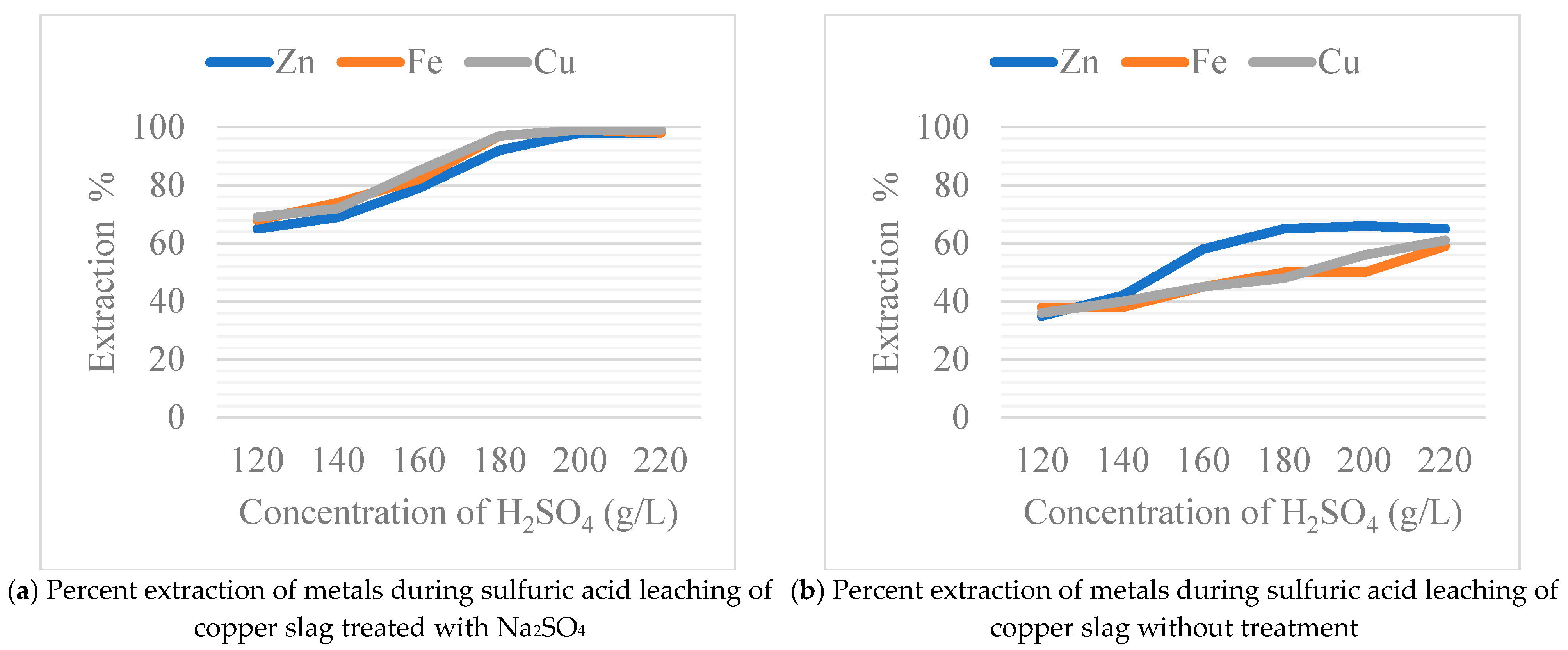
Figure 6, a temperature of 90 °C, and a reaction time of 120 min with a solid–liquid ratio of 1:5 [
23]. The results of this comparison, presented in
Figure 6, reveal significant differences in the dissolution rates of the two sets of samples. Specifically, the sodium sulfate-treated samples exhibited markedly higher dissolution efficiencies, resulting in impressive metal recovery rates of 97% for copper (Cu), 96% for iron (Fe), and 93% for zinc (Zn). In contrast, the untreated samples demonstrated significantly lower recovery rates, with copper at 61%, iron at 59%, and zinc at 65%. This comparative analysis underscores the substantial impact of sodium sulfate treatment on enhancing the dissolution behavior of the samples in sulfuric acid, thereby improving the recovery of valuable metals. The elevated dissolution rates observed in the Na
2SO
4-treated samples emphasize the potential of this method for optimizing metal extraction processes, thereby suggesting its effectiveness in promoting the efficient recovery of copper, iron, and zinc from copper slag.
After dissolving in sulfuric acid, significant differences were observed between the filtration of sodium sulfate (Na2SO4)-treated slag and untreated slag. The filtration process of the Na2SO4-treated slag showed excellent efficiency, with the liquid passing through the filter smoothly and the filter remaining clean. This can be attributed to changes in the mineral composition of the slag and the role of Na2SO4 in facilitating the breakdown of the slag structure, which resulted in an easier filtration process. In contrast, after leaching the untreated slag in sulfuric acid, the filtration process stopped, indicating that some components of the slag hindered smooth filtration and the passage of liquid. This is because the slag contains large amounts of fayalite (Fe2SiO4), and during acid leaching it reacts and forms silica gel (SiO2·nH2O), which blocks the filter and stops the filtration process. These results demonstrate that Na2SO4 treatment effectively improves the slag structure and enhances its filtration properties. Video clips for the filtration process of leaching of both untreated and treated slags are given in the links below: