Advances in the Development of Hydrometallurgical Processes in Acidic and Alkaline Environments for the Extraction of Copper from Tailings Deposit
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Selection and Characterization
2.1.1. Selected Sample
2.1.2. Leaching Agents
2.1.3. Leaching Promoter—Activated Carbon
2.1.4. Leaching Tests
3. Results and Discussion
3.1. Characteristics of the Sample
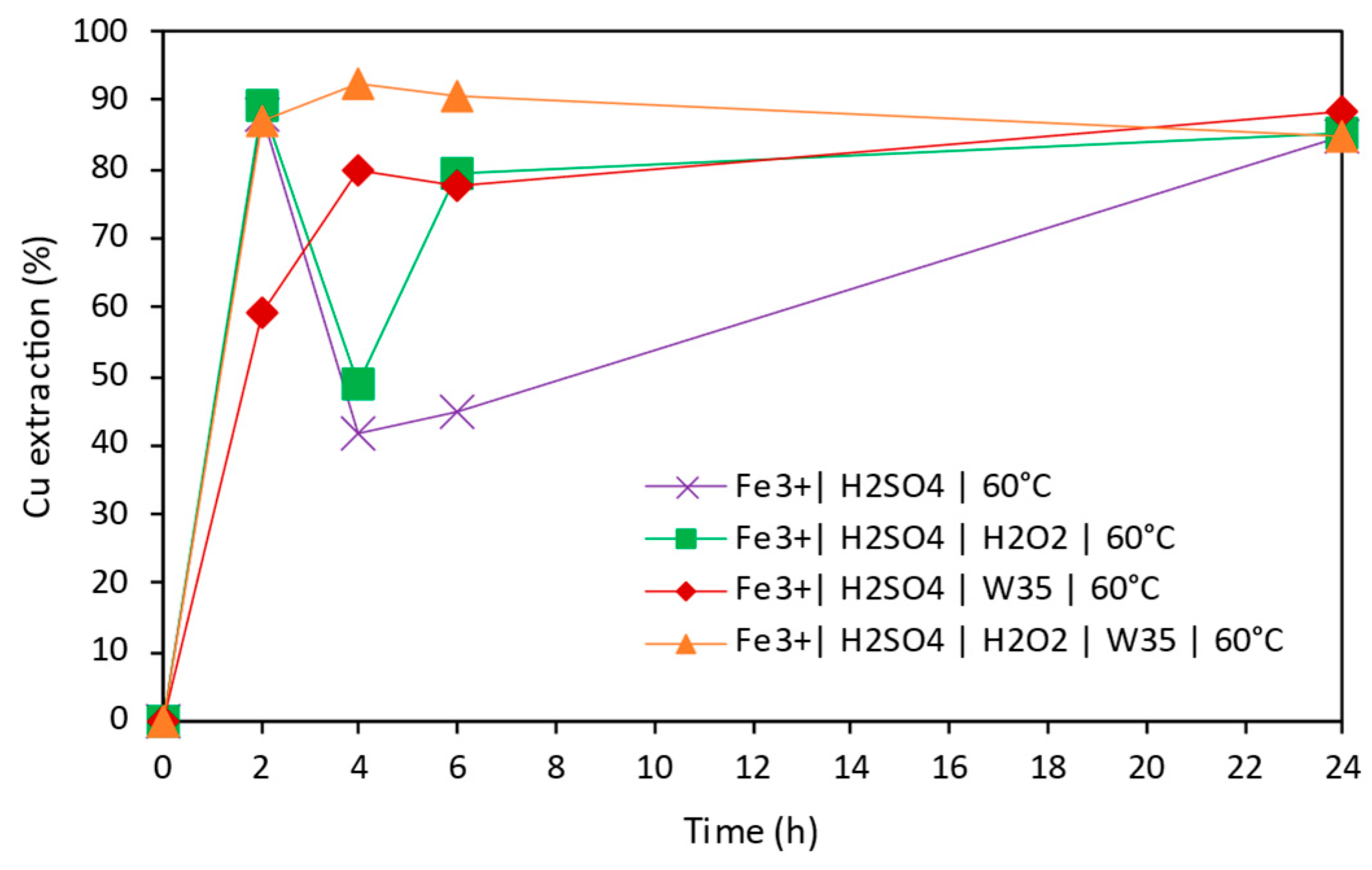
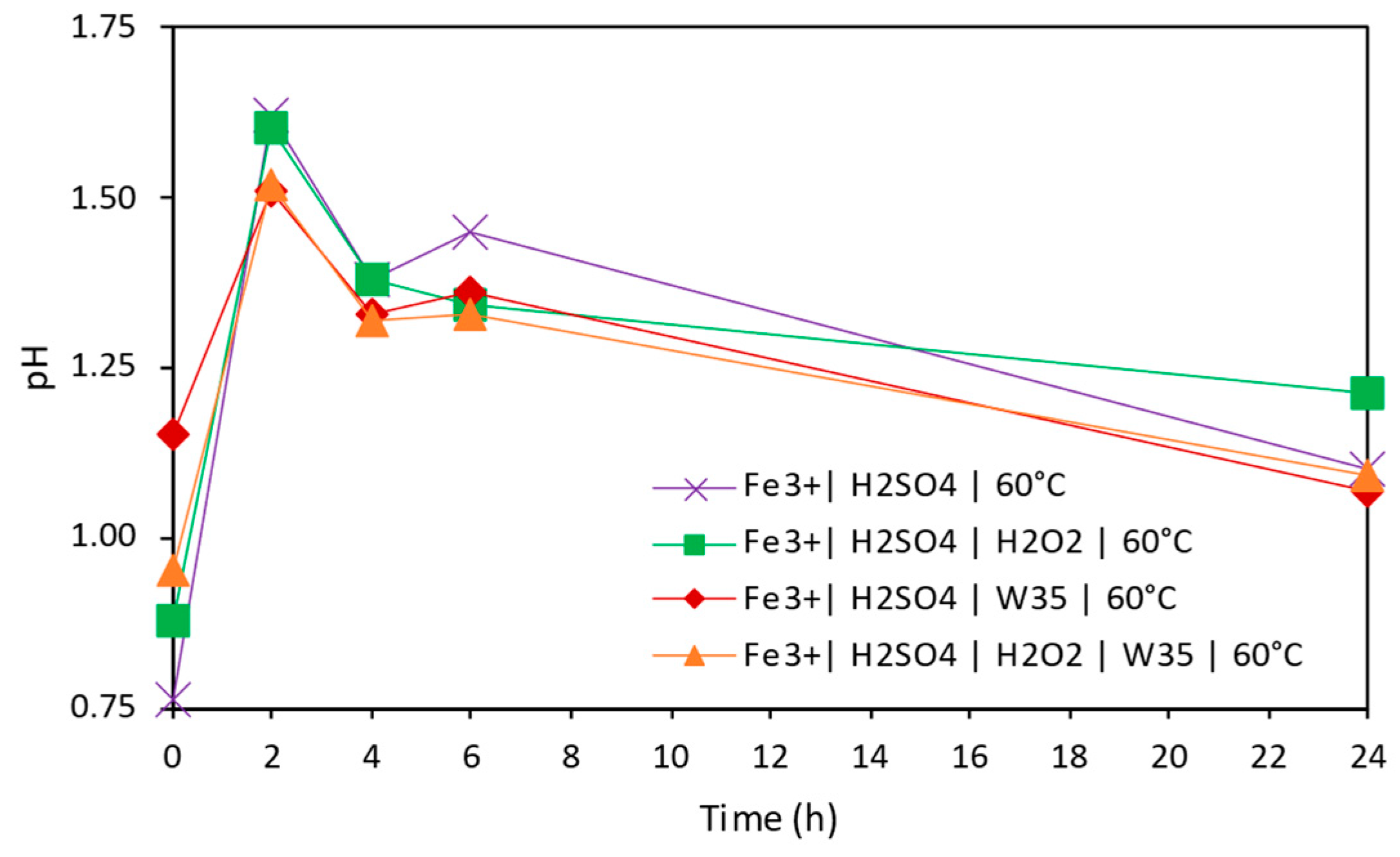
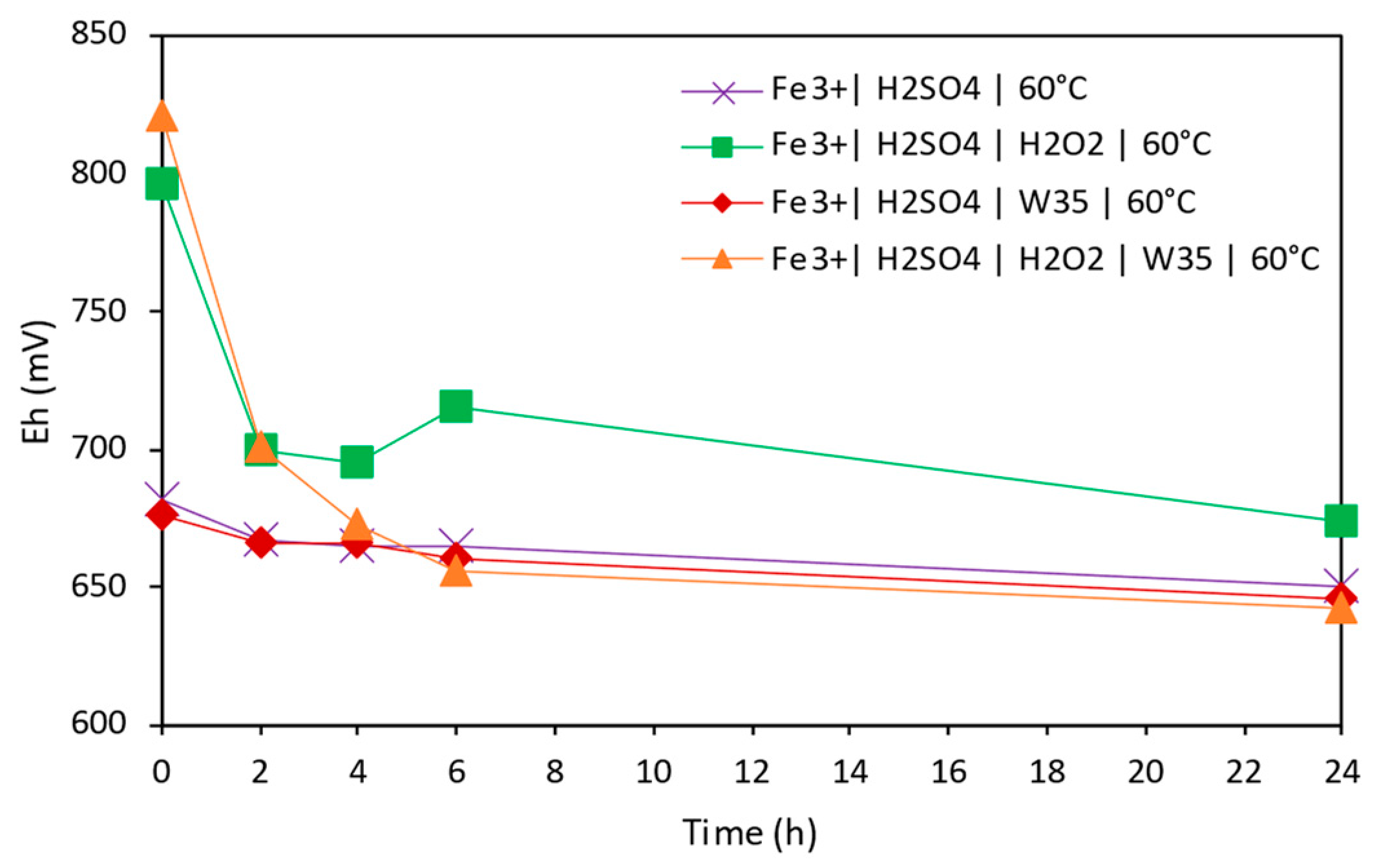
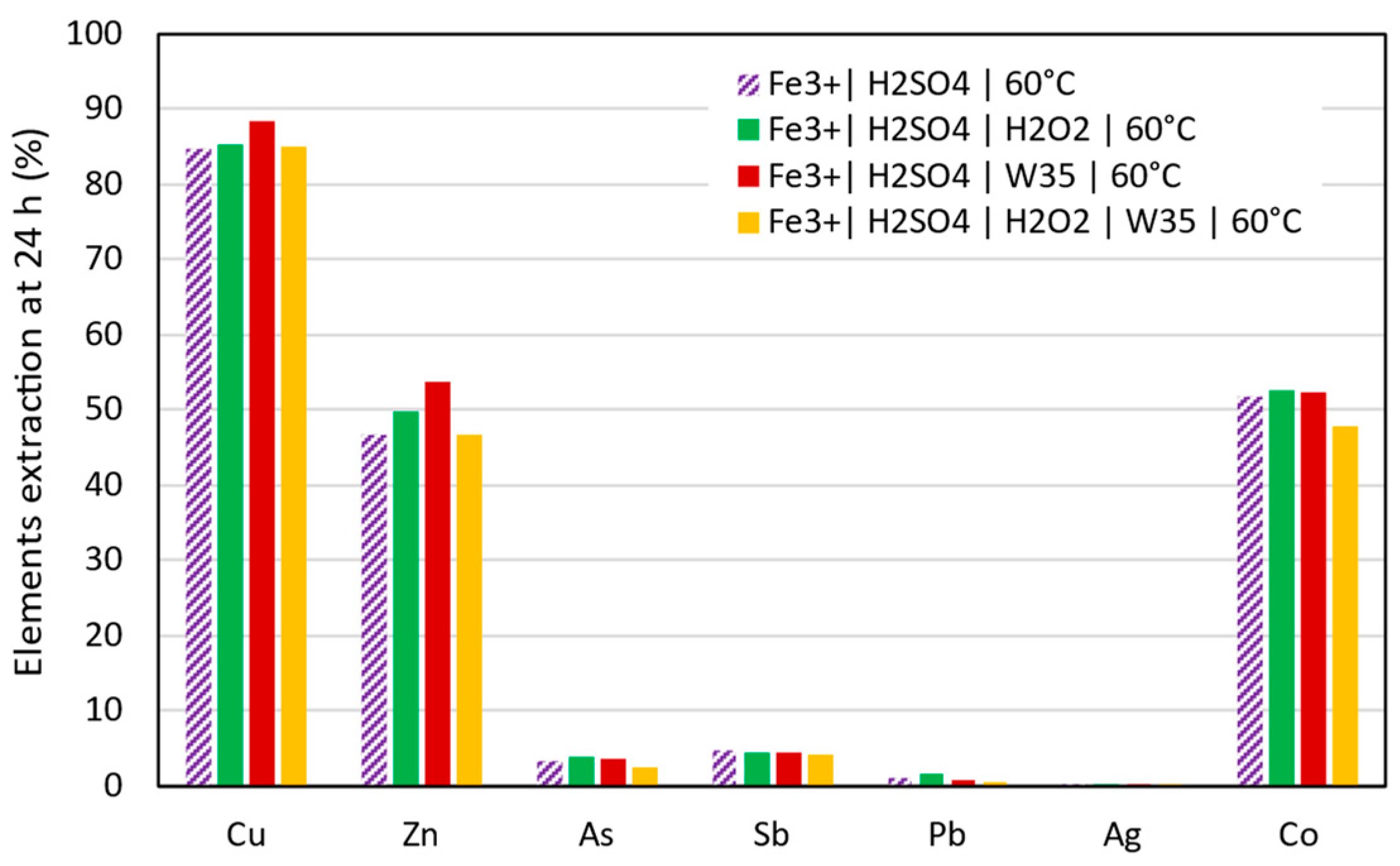
3.2. Copper Extraction in Acidic Conditions
3.3. Copper Extraction in Alkaline Conditions
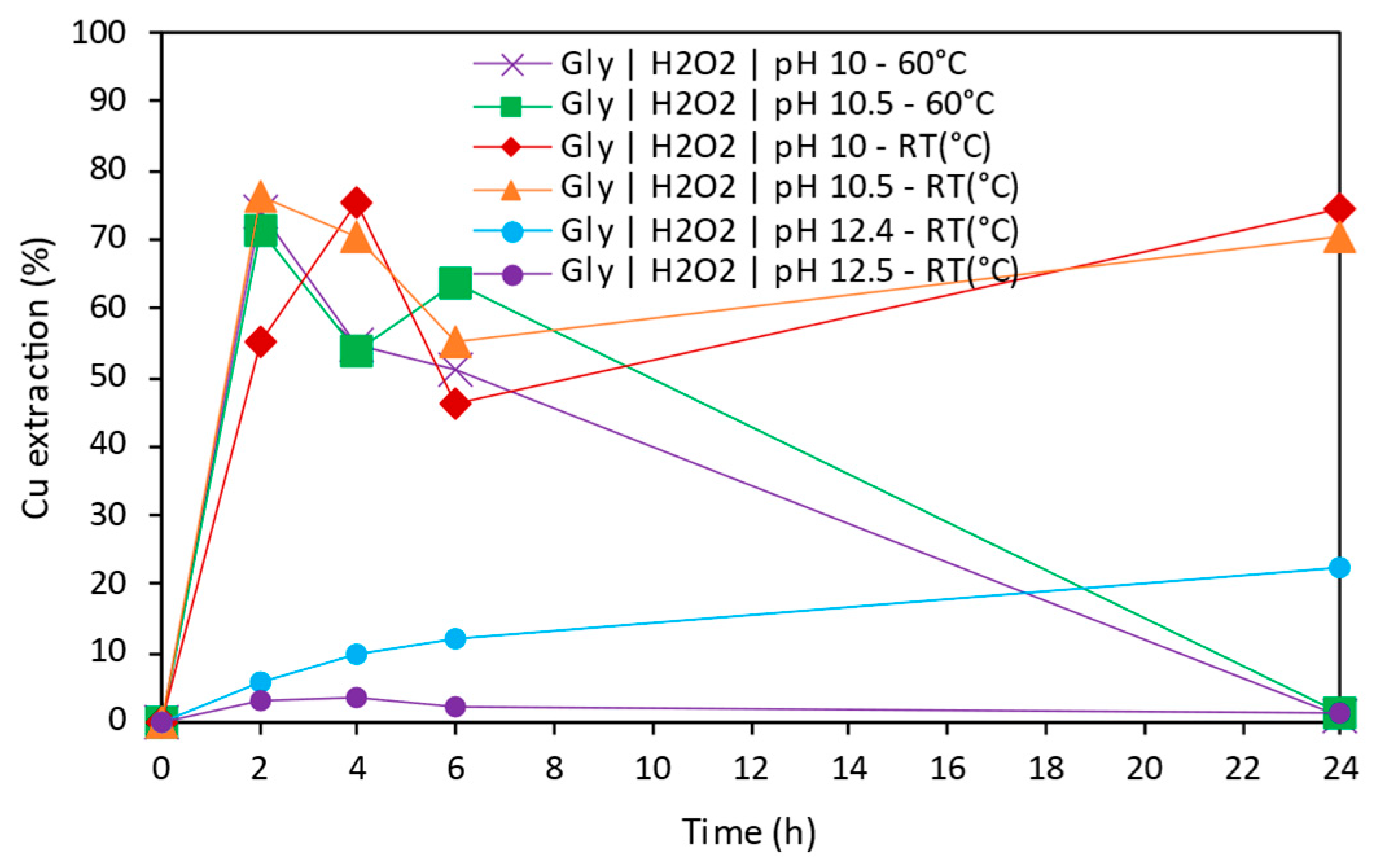
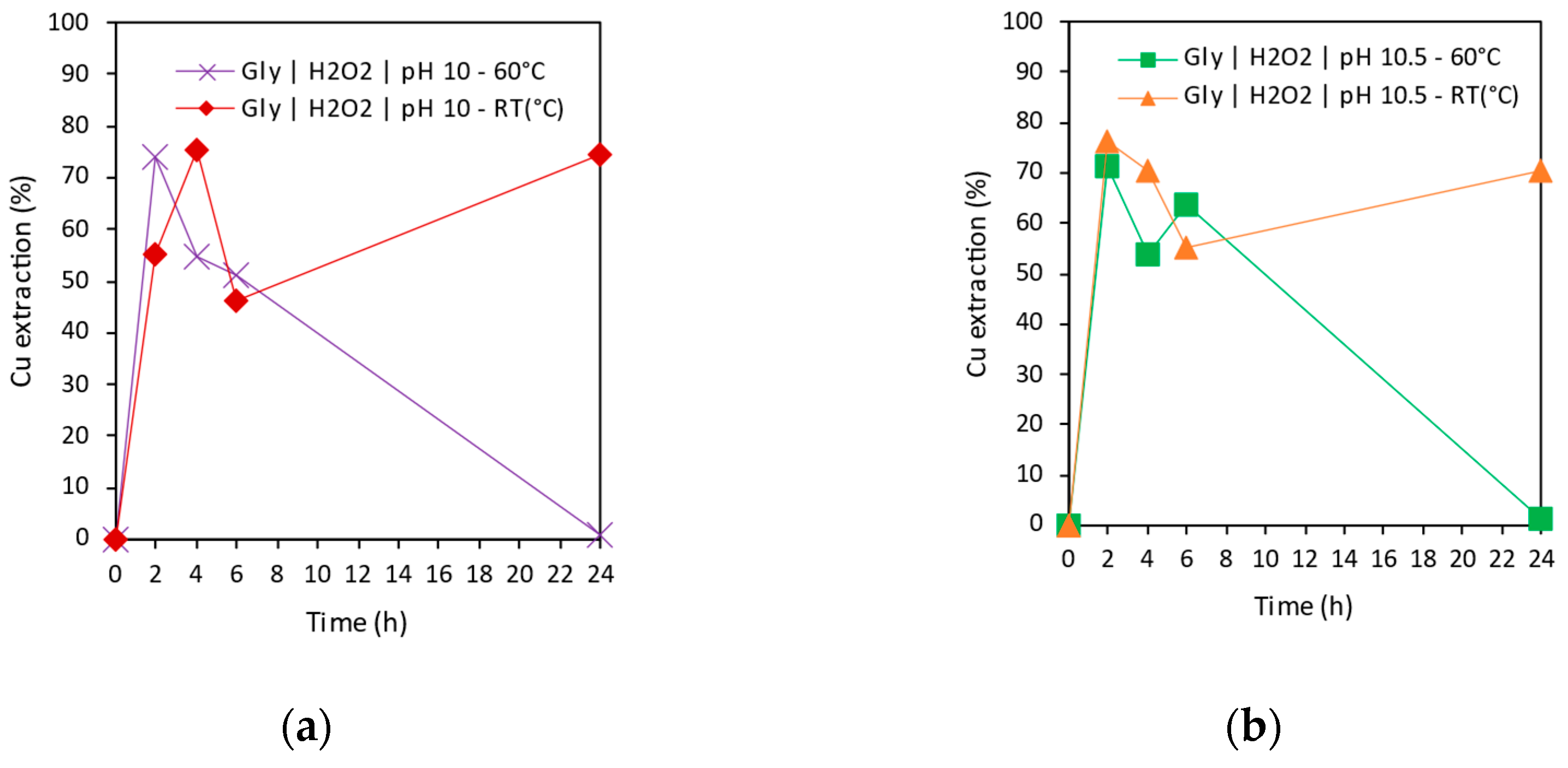
3.3.1. Copper Extraction as a Function of Time
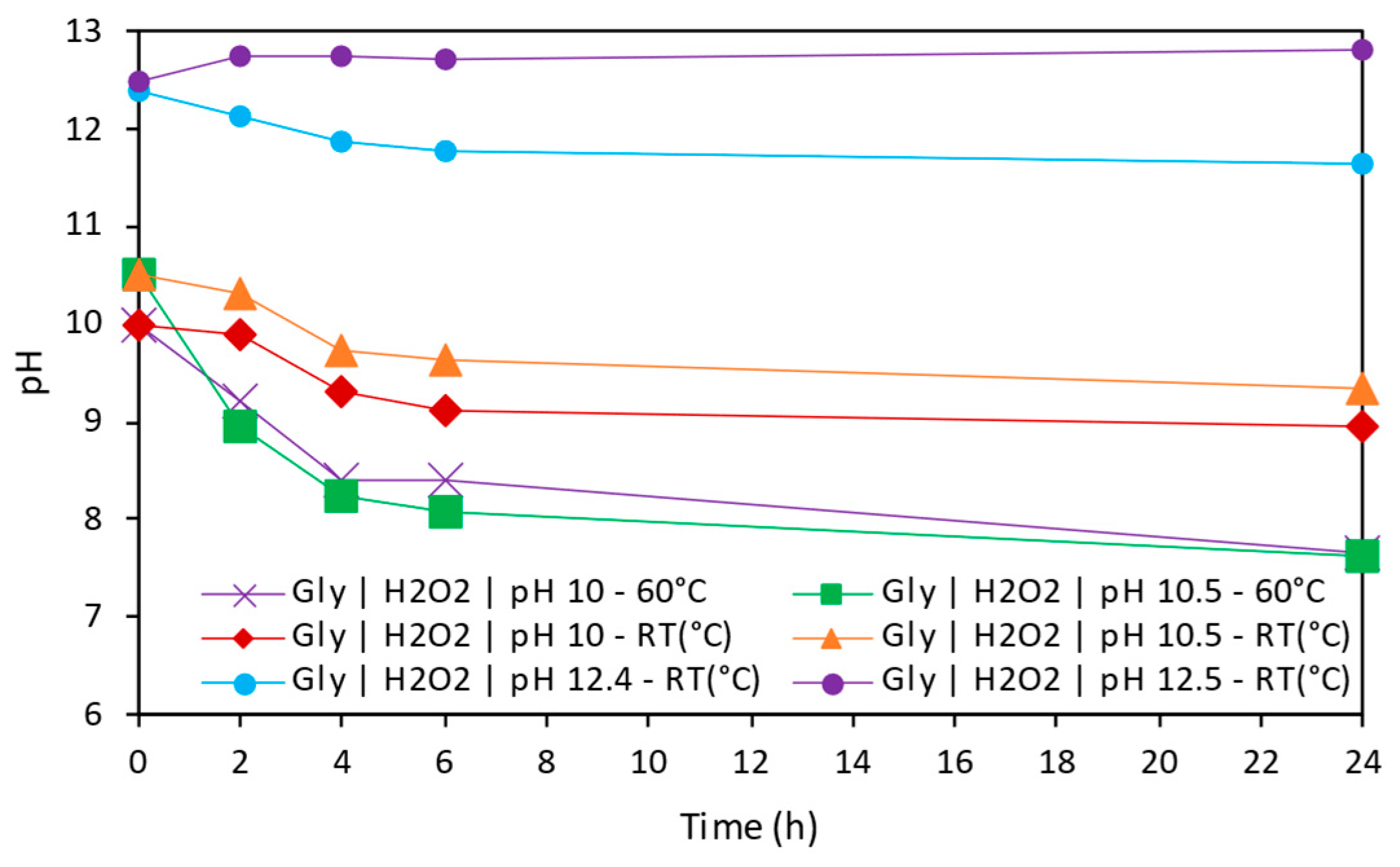
3.3.2. Effect of Temperature in Copper Extraction
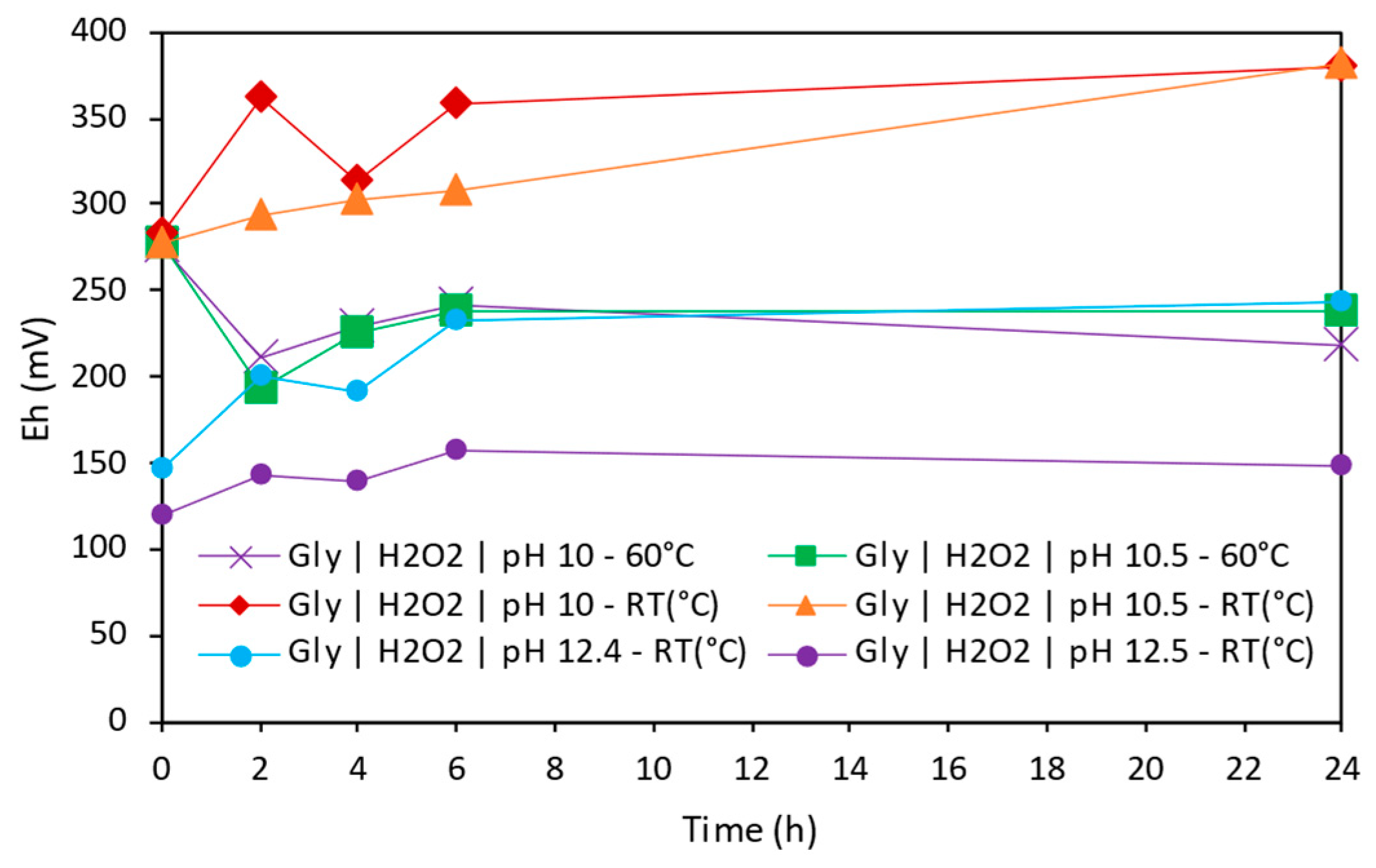
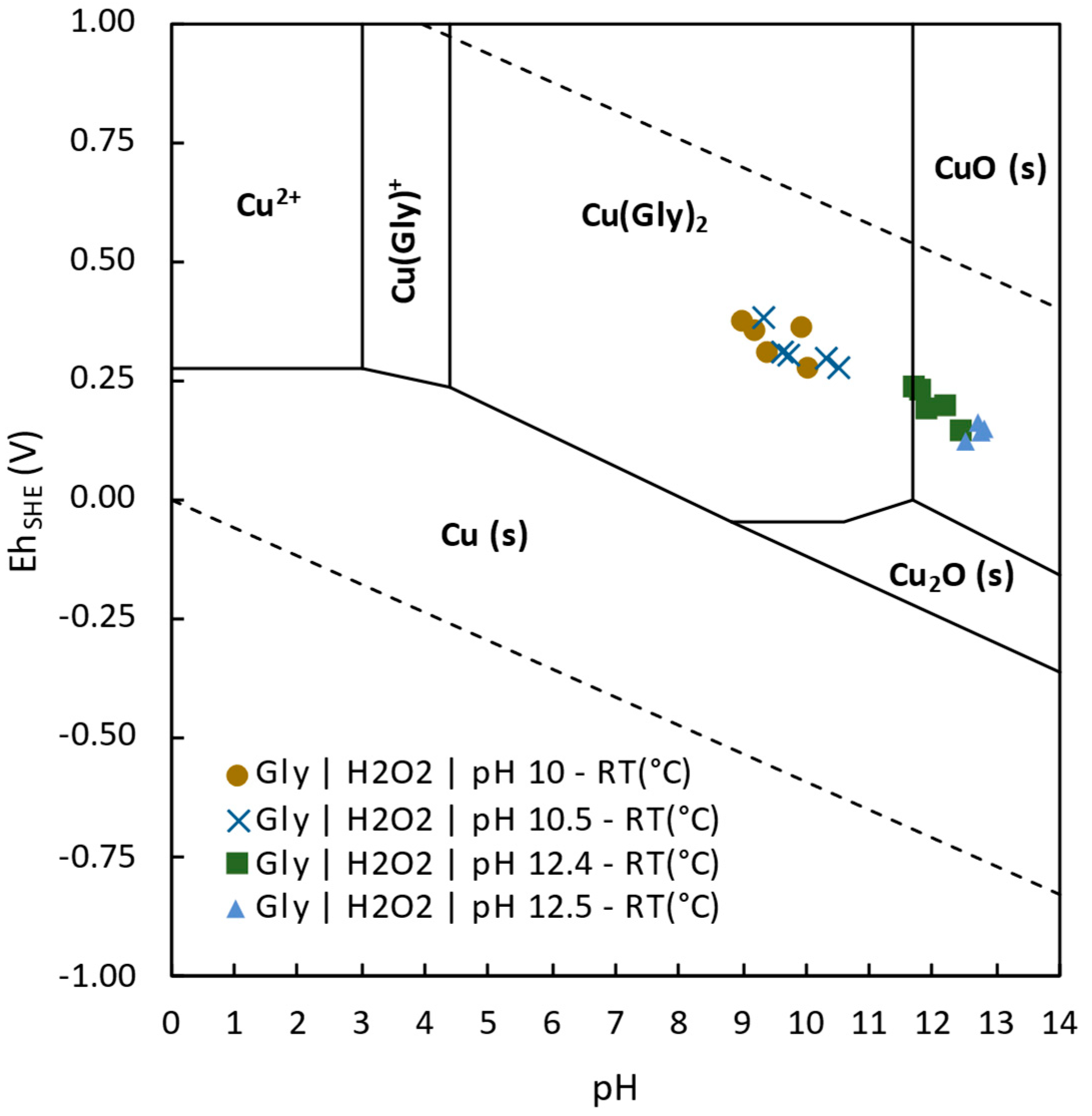
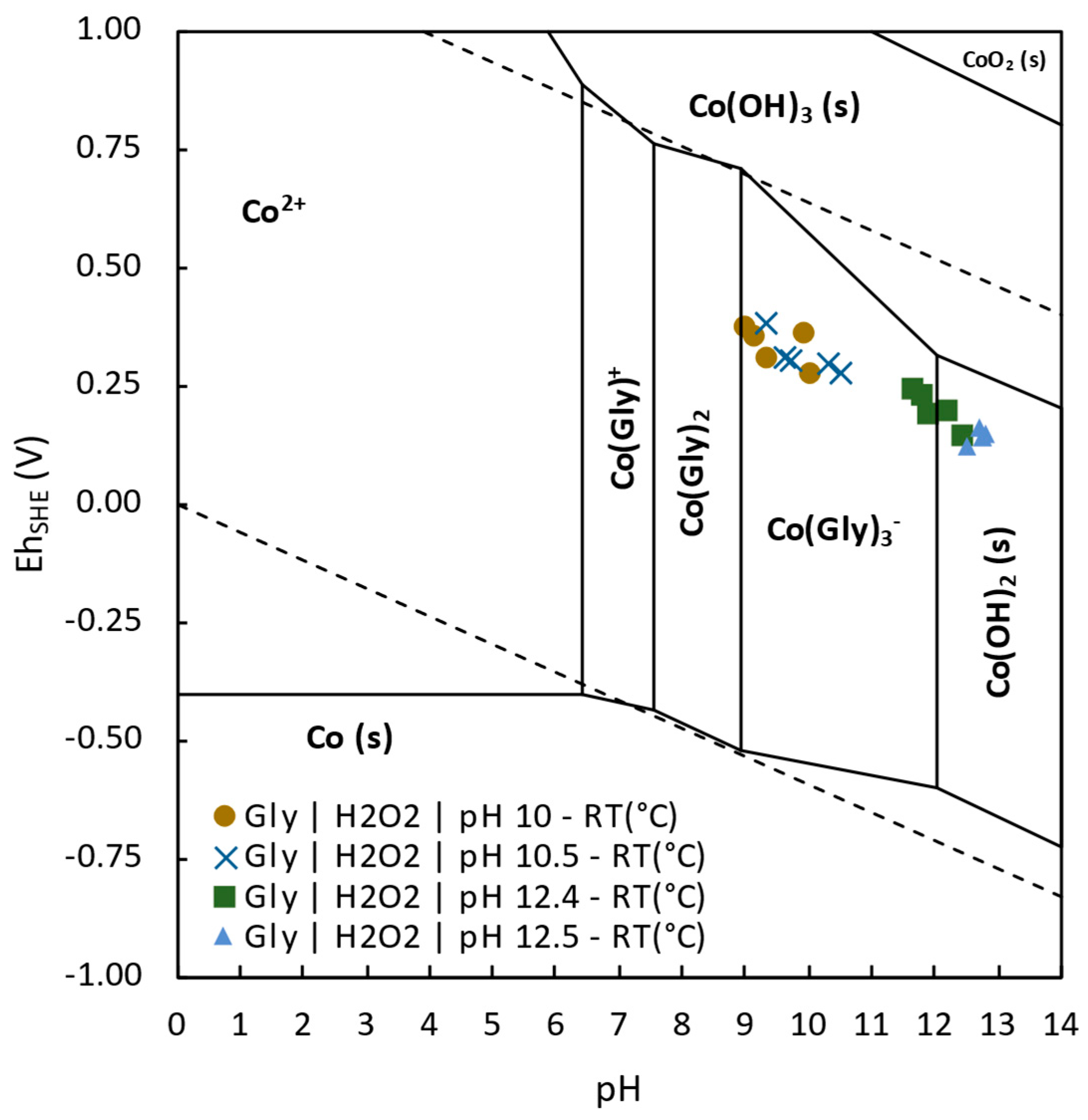
3.3.3. Effect of Electrochemistry in Copper Extraction
4. Conclusions
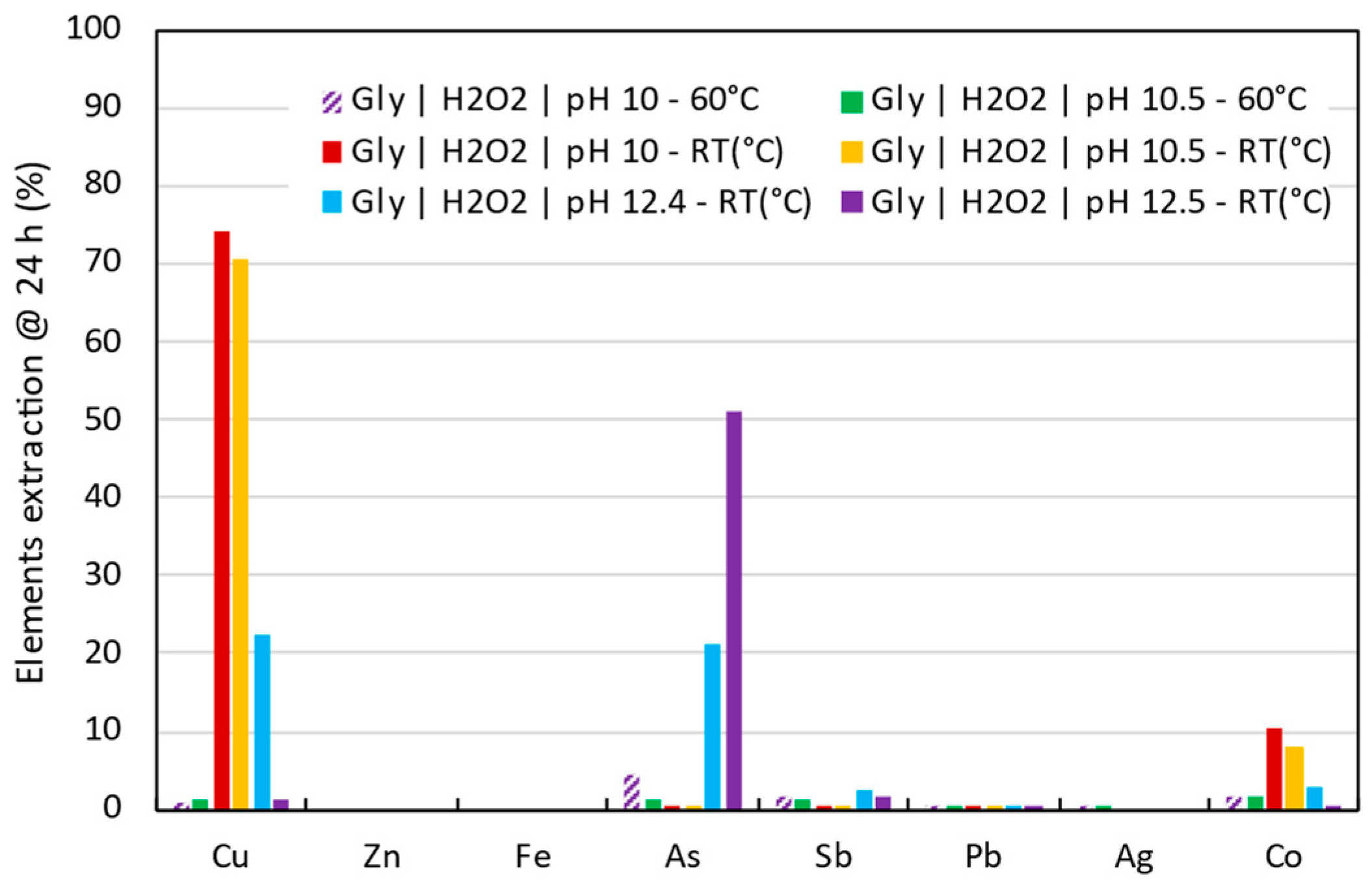
- Three critical raw materials (Cu, Co, and As) and one base metal (Zn) were identified as extractable under the conditions studied. In an acidic medium, Cu, Co, and Zn gave extractions of 92.5%, 52.6%, and 53.7%, respectively. In contrast, under alkaline conditions, extractions of 76.2%, 10.4%, and 50.9% were achieved for Cu, Co, and As.
- The highest extraction of Cu, 92.5%, was achieved in acidic leaching conditions after 2 h when activated carbon and hydrogen peroxide were employed.
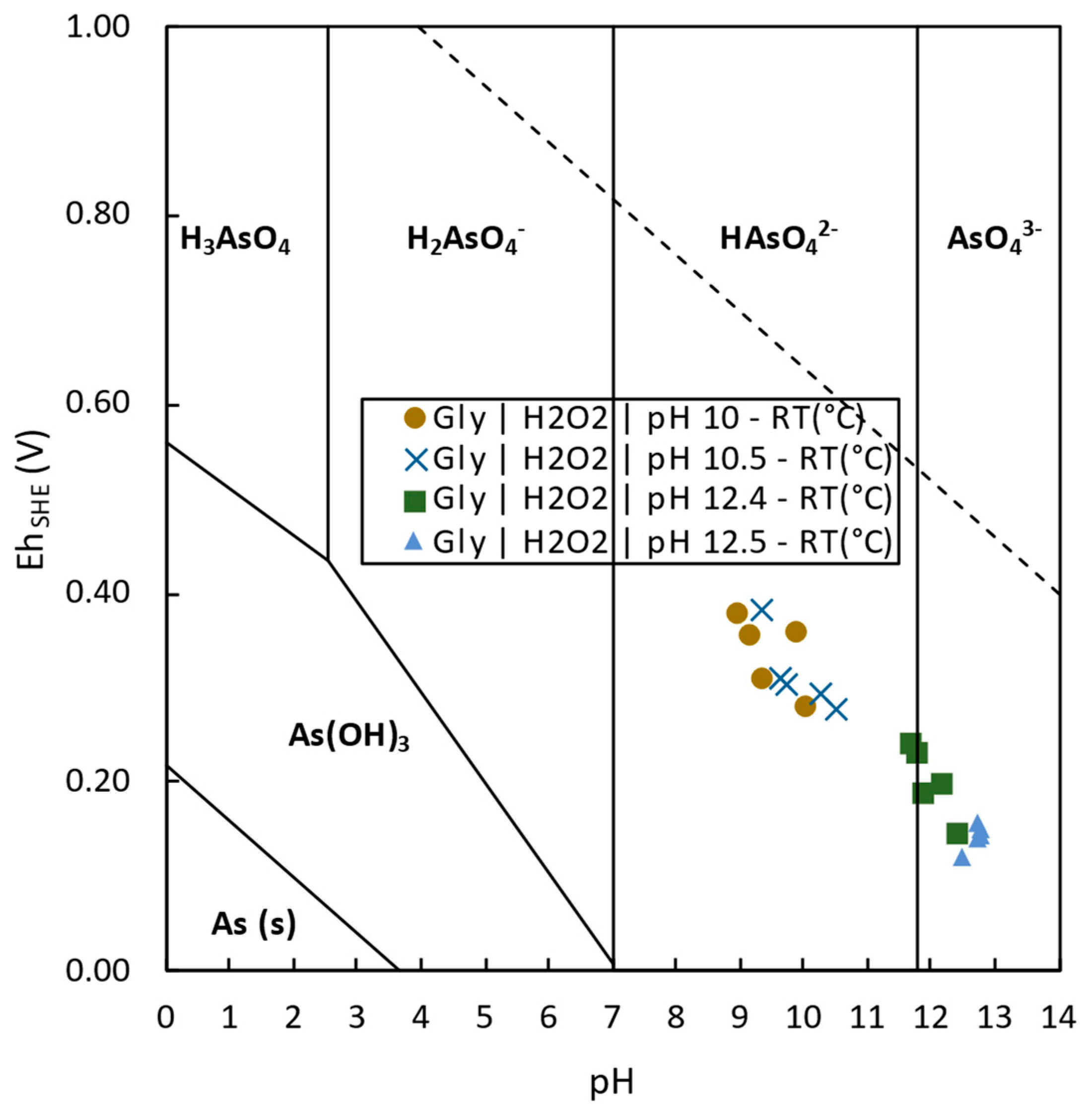
- Glycine was demonstrated to be a promising alternative for tailings reprocessing as its use allowed the selective extraction of Cu, Co, and As over impurities such as Fe, Zn, Pb, or Sb. The highest Cu extraction 76.2% was achieved at pH 10.5, room temperature, after 2 h leaching. Cobalt was able to be extracted up to 10.4% at pH 10 and room temperature after 24 h leaching. Finally, 50.9% of As extraction was achieved at pH 12.5, room temperature, after 24 h leaching time.
- Temperature and pH were identified as key factors for copper extraction in alkaline conditions. Temperature could enhance solubility at early stages; however, it could be the main reason of Cu species precipitation observed in some conditions.
- Further optimization of conditions, such as shorter reaction time, solid/liquid ratios, pH, and temperature should be explored in future investigations in order to optimize alkaline leaching of these wastes with glycine.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IPB | Iberian Pyrite Belt |
| EU | European Union |
| CRM | Critical raw material |
| Gly | Glycine |
| PLS | Pregnant leach solution |
References
- Commission of the European Communities. Communication from the Commission to the European Parliament and the Council. The Materials Initiative—Meeting Our Critical Needs for Growth and Jobs in Europe. 2008, Volume 13. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2008:0699:FIN:en:PDF (accessed on 18 May 2025).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Tackling the Challenges in Commodity Markets and or Raw Materials. 2011, Volume 22. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52011DC0025&from=EN (accessed on 20 December 2024).
- European Commission. Annexes to the Proposal for a Regulation of the European Parliament and of the Council. Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2017, (EU) 2018/858, 2018/1724 and (EU) 2019/1020. 2023, Volume 15. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:903d35cc-c4a2-11ed-a05c-01aa75ed71a1.0001.02/DOC_2&format=PDF (accessed on 20 December 2024).
- Grohol, M.; Veeh, C.; Grow, D.G.; European Commission. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023; Volume 160. [Google Scholar] [CrossRef]
- SCREEN2. Factsheet Updates Based on the EU Factsheets. Copper. 2020, Volume 46. Available online: https://scrreen.eu/wp-content/uploads/2023/03/SCRREEN2_factsheets_COPPER-1.pdf (accessed on 20 December 2024).
- Davoise, D.; Méndez, A. Research of an Abandoned Tailings Deposit in the Iberian Pyritic Belt: Characterization and Gross Reserves Estimation. Processes 2023, 11, 1642. [Google Scholar] [CrossRef]
- Arranz-González, J.C.; Rodríguez-Gómez, V.; Fernández-Naranjo, F.J.; Vadillo-Fernández, L. Assessment of the pollution potential of a special case of abandoned sulfide tailings impoundment in Riotinto mining district (SW Spain). Environ. Sci. Pollut. Res. 2021, 28, 14054–14067. [Google Scholar] [CrossRef] [PubMed]
- Arranz González, J.C.; Cala Rivero, V.; Iribarren Campaña, I. Geochemistry and mineralogy of surface pyritic tailings impoundments at two mining sites of the Iberian pyrite belt (SW Spain). Environ. Earth Sci. 2012, 65, 669–680. [Google Scholar] [CrossRef]
- López-Arce, P.; Garrido, F.; García-Guinea, J.; Voegelin, A.; Göttlicher, J.; Nieto, J.M. Historical roasting of thallium- and arsenic-bearing pyrite: Current Tl pollution in the Riotinto mine area. Sci. Total Environ. 2019, 648, 1263–1274. [Google Scholar] [CrossRef]
- Pérez-López, R.; Sáez, R.; Álvarez-Valero, A.M.; Nieto, J.M.; Pace, G. Combination of sequential chemical extraction and modelling of dam-break wave propagation to aid assessment of risk related to the possible collapse of a roasted sulphide tailings dam. Sci. Total Environ. 2009, 407, 5761–5771. [Google Scholar] [CrossRef]
- Cánovas, C.R.; González, R.M.; Vieira, B.J.C.; Waerenborgh, J.C.; Marques, R.; Macías, F.; Basallote, M.D.; Olias, M.; Prudencio, M.I. Metal mobility and bioaccessibility from cyanide leaching heaps in a historical mine site. J. Hazard. Mater. 2023, 448, 130948. [Google Scholar] [CrossRef]
- Araya, N.; Kraslawski, A.; Cisternas, L.A. Towards mine tailings valorization: Recovery of critical materials from Chilean mine tailings. J. Clean. Prod. 2020, 263, 121555. [Google Scholar] [CrossRef]
- Fleming, C.A.; Brown, J.A.; Botha, M. An economic and environmental case for re-processing gold tailings in South Africa. In Proceedings of the 42nd Annual Meeting of the Canadian Mineral Processors, Ottawa, ON, Canada, 19–21 January 2010; pp. 365–388. [Google Scholar]
- Muravyov, M.I.; Bulaev, A.G.; Kondrat’eva, T.F. Complex treatment of mining and metallurgical wastes for recovery of base metals. Miner. Eng. 2014, 64, 63–66. [Google Scholar] [CrossRef]
- Kang, J.; Wang, Y.; Yu, C.; Wang, X.; Liu, Z. Selective extraction performance of Ni, Cu, Co, and Mn adopted by a coupled leaching of low-grade Ni-sulfide ore and pollymetallic Mn-oxide ore in sulphuric acid solutions. J. Phys. Chem. Solids 2022, 168, 110814. [Google Scholar] [CrossRef]
- Asadi, T.; Azizi, A.; Lee, J.C.; Jahani, M. Leaching of zinc from a lead-zinc flotation tailing sample using ferric sulphate and sulfuric acid media. J. Environ. Chem. Eng. 2017, 5, 4769–4775. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate-chloride and sulfate-nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Guezennec, A.; Bru, K.; Jacob, J.; d’Hugues, P. Co-processing of sulfidic mining wastes and metal-rich post-consumer wastes by biohydrometallurgy. Miner. Eng. 2015, 75, 45–53. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Eksteen, J.J.; Oraby, E.A.; Nguyen, V. Leaching and ion exchange based recovery of nickel and cobalt from a low grade, serpentine-rich sulfide ore using an alkaline glycine lixiviant system. Miner. Eng. 2020, 145, 106073. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. The leaching of gold, silver and their alloys in alkaline glycine-peroxide solutions and their adsorption on carbon. Hydrometallurgy 2015, 152, 199–203. [Google Scholar] [CrossRef]
- Méndez, A.; Álvarez, M.L.; Fidalgo, J.M.; Di Stasi, C.; Manyà, J.J.; Gascó, G. Biomass-derived activated carbon as catalyst in the leaching of metals from a copper sulfide concentrate. Miner. Eng. 2022, 183, 107594. [Google Scholar] [CrossRef]
- Álvarez, M.L.; Fidalgo, J.M.; Gascó, G.; Méndez, A. Hydrometalurgical Recovery of Cu and Zn from a Complex Sulfide Mineral by Fe3+/H2SO4 Leaching in the Presence of Carbon-Based Materials. Metals 2021, 11, 286. [Google Scholar] [CrossRef]
- Collins, M.J.; Kofluk, D.K. Hydrometallurgical Process for the Extraction of Copper from Sulphidic Concentrates. U.S. Patent US5730776A, 24 March 1998. [Google Scholar]
- Nakazawa, H.; Nakamura, S.; Odashima, S.; Hareyama, W. Effect of carbon black to facilitate galvanic leaching of copper from chalcopyrite in the presence of manganese (IV) oxide. Hydrometallurgy 2016, 163, 69–76. [Google Scholar] [CrossRef]
- Nakazawa, H.; Koshiya, S.; Kobayashi, H.; Matsuhashi, T. The effect of carbon black on the oxidative leaching of enargite by manganese (IV) dioxide in sulfuric acid media. Hydrometallurgy 2017, 171, 165–171. [Google Scholar] [CrossRef]
- Jahromi, F.G.; Alvial-Hein, G.; Cowan, D.H.; Ghahreman, A. The kinetics of enargite dissolution in chloride media in the presence of activated carbon and AF 5 catalysts. Miner. Eng. 2019, 143, 106013. [Google Scholar] [CrossRef]
- Nakazawa, H. Effect of carbon black on chalcopyrite leaching in sulfuric acid media at 50 °C. Hydrometallurgy 2018, 177, 100–108. [Google Scholar] [CrossRef]
- Olvera, O.G.; Dixon, D.G.; Asselin, E. Electrochemical study of the dissolution of enargite (Cu3AsS4) in contact with activated carbon. Electrochim. Acta 2013, 107, 525–536. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J.; Mali, T. Extraction of Gold and Copper from Flotation Tailings Using Glycine-Ammonia Solutions in the Presence of Permanganate. Minerals 2022, 12, 612. [Google Scholar] [CrossRef]
- Miličević, A.; Branica, G.; Raos, N. Irving-Williams Order in the Framework of Connectivity Index 3χv Enables Simultaneous Prediction of Stability Constants of Bivalent Transition Metal Complexes. Molecules 2011, 16, 1103–1112. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Oraby, E.; Eksteen, J. Amino acids as lixiviants for metals extraction from natural and secondary resources with emphasis on glycine: A literature review. Hydrometallurgy 2023, 216, 106008. [Google Scholar] [CrossRef]
- Burbano, A.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. Catalytic activity of carbon materials in the oxidation of minerals. Catalysis 2022, 12, 918. [Google Scholar] [CrossRef]
- O’Connor, G.M.; Lepkova, K.; Eksteen, J.J.; Oraby, E.A. Electrochemical behaviour of copper in alkaline glycine solutions. Hydrometallurgy 2018, 181, 221–229. [Google Scholar] [CrossRef]
- Khezri, M.; Rezai, B.; Abdollahzadeh, A.A.; Molaeinasab, M.; Wilson, B.P.; Lundström, M. Glycine leaching of Sarcheshmeh chalcopyrite concentrate at high pulp densities in a stirred tank reactor. Miner. Eng. 2020, 157, 106555. [Google Scholar] [CrossRef]
- Barragán-Mantilla, S.P.; Gascó, G.; Almendros, P.; Méndez, A. Insights into the use of green leaching systems based on glycine for the selective recovery of copper. Miner. Eng. 2024, 206, 108534. [Google Scholar] [CrossRef]
- Oraby, E.; Deng, Z.; Li, H.; Eksteen, J. Selective extraction of nickel and cobalt from disseminated sulfide flotation cleaner tailings using alkaline glycine-ammonia leaching solutions. Miner. Eng. 2023, 204, 108418. [Google Scholar] [CrossRef]
- Yesares, L.; González-Jiménez, J.M.; Jiménez-Cantizano, F.A.; González-Pérez, I.; Caro-Moreno, D.; Sánchez, I.M. Unveiling High-Tech Metals in Roasted Pyrite Wastes from the Iberian Pyrite Belt, SW Spain. Sustainability 2023, 15, 12081. [Google Scholar] [CrossRef]
- Gersztyn, L.; Karczewska, A.; Gałka, B. Influence of pH on the solubility of arsenic in heavily contaminated soils/Wpływ pH na rozpuszczalność arsenu w glebach silnie zanieczyszczonych. Environ. Prot. Nat. Resour. 2013, 24, 7–11. [Google Scholar] [CrossRef]
- Pigna, M.; Caporale, A.G.; Cavalca, L.; Sommella, A.; Violante, A. Arsenic in the Soil Environment: Mobility and Phytoavailability. Environ. Eng. Sci. 2015, 32, 551–563. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Yan, J.; Ye, S. Selective extraction of arsenic and antimony from gold-bearing sludge using two-stage alkaline leaching. Resour. Conserv. Recicling 2021, 167, 105388. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. An investigation into the leaching behaviour of copper oxide minerlas in aqueous alkaline glycine solutions. Hydrometallurgy 2017, 167, 153–162. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. Kinetics of chalcocite leaching in oxygenated alkaline glycine solutions. Hydrometallurgy 2018, 178, 264–273. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Z.; Demopoulos, G.P. Process for Glycine production by antisolvent crystallization using its phase equilibria in the ethylene glycol-NH4Cl-water system. Ind. Eng. Chem. Res. 2016, 55, 2426–2437. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, R.; Qiao, H.; Liu, C. Study on the formation of glycine by Hydantoin and its kinetics. ACS Omega 2020, 5, 13463–13472. [Google Scholar] [CrossRef]
- Grammenou, A.; Petropoulos, S.P.; Thalassinos, G.; Rinklebe, J.; Shaheen, S.M.; Antoniadis, V. Biostimulants in the Soil-Plant Interface: Agro-environmental Implications—A review. Earth Syst. Environ. 2023, 7, 583–600. [Google Scholar] [CrossRef]
- Shooshtari, F.Z.; Souri, M.K.; Hasandokht, M.R.; Jari, S.K. Glycine mitigates fertilizer requirements of agricultural crops: Case study with cucumber as a high fertilizer demanding crop. Chem. Biol. Technol. Agric. 2020, 7, 19. [Google Scholar] [CrossRef]
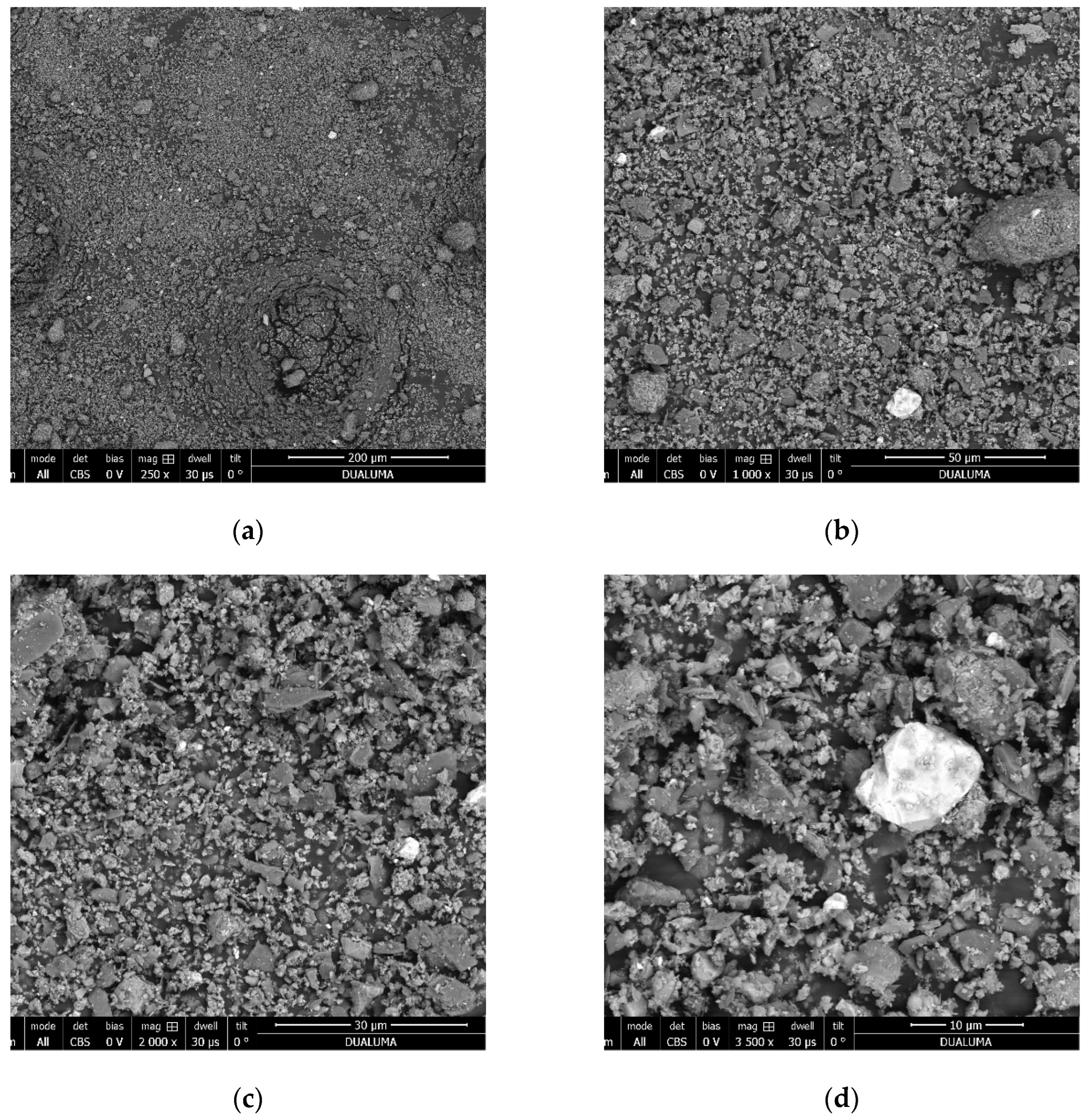
| Concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe (wt%) | As (wt%) | Pb (wt%) | Cu (wt%) | Zn (wt%) | Sb (wt%) | Ag (ppm) | Co (ppm) | O (wt%) | S (wt%) |
| 25.3 | 2.97 | 2.59 | 0.29 | 0.20 | 0.15 | 199 | 35 | 28.48 | 5.00 |
| pH * | Eh (mV) * | Mineralogy-XRD | D80 (µm) | S.G. ** | |||||
| 2.0 | 665 | Py | Hem | Qtz | Ms | Gp | 23.53 | 2.97 | |
| Property | Value |
|---|---|
| pH | 10.3 |
| Eh (mV) | 253 |
| SBET (m2/g) | 985.3 |
| Ash (wt%) | 10.5 |
| C (%) | 82.4 |
| H (%) | 0.38 |
| N (%) | 0.26 |
| O (%) | 6.30 |
| H/C | 0.06 |
| O/C | 0.06 |
| ID | Leaching Agent | Oxidant Agent | Activated Carbon | Temperature (°C) | pH |
|---|---|---|---|---|---|
| E-I | Fe3+/H2SO4 | - | - | 60 | 0.76 |
| E-II | Fe3+/H2SO4 | H2O2 | - | 60 | 0.88 |
| E-III | Fe3+/H2SO4 | - | W35 | 60 | 1.15 |
| E-IV | Fe3+/H2SO4 | H2O2 | W35 | 60 | 0.95 |
| E-V | Glycine | H2O2 | - | 60 | 10.0 |
| E-VI | Glycine | H2O2 | - | 60 | 10.5 |
| E-VII | Glycine | H2O2 | - | Room temperature | 10.0 |
| E-VIII | Glycine | H2O2 | - | Room temperature | 10.5 |
| E-IX | Glycine | H2O2 | - | Room temperature | 12.14 |
| E-X | Glycine | H2O2 | - | Room temperature | 12.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davoise, D.; Méndez, A. Advances in the Development of Hydrometallurgical Processes in Acidic and Alkaline Environments for the Extraction of Copper from Tailings Deposit. Minerals 2025, 15, 550. https://doi.org/10.3390/min15060550
Davoise D, Méndez A. Advances in the Development of Hydrometallurgical Processes in Acidic and Alkaline Environments for the Extraction of Copper from Tailings Deposit. Minerals. 2025; 15(6):550. https://doi.org/10.3390/min15060550
Chicago/Turabian StyleDavoise, Diego, and Ana Méndez. 2025. "Advances in the Development of Hydrometallurgical Processes in Acidic and Alkaline Environments for the Extraction of Copper from Tailings Deposit" Minerals 15, no. 6: 550. https://doi.org/10.3390/min15060550
APA StyleDavoise, D., & Méndez, A. (2025). Advances in the Development of Hydrometallurgical Processes in Acidic and Alkaline Environments for the Extraction of Copper from Tailings Deposit. Minerals, 15(6), 550. https://doi.org/10.3390/min15060550










