Flotation Separation of Chalcopyrite and Molybdenite by Eco-Friendly Microorganism Depressant Bacillus tropicus
Abstract
1. Introduction
2. Materials and Methods
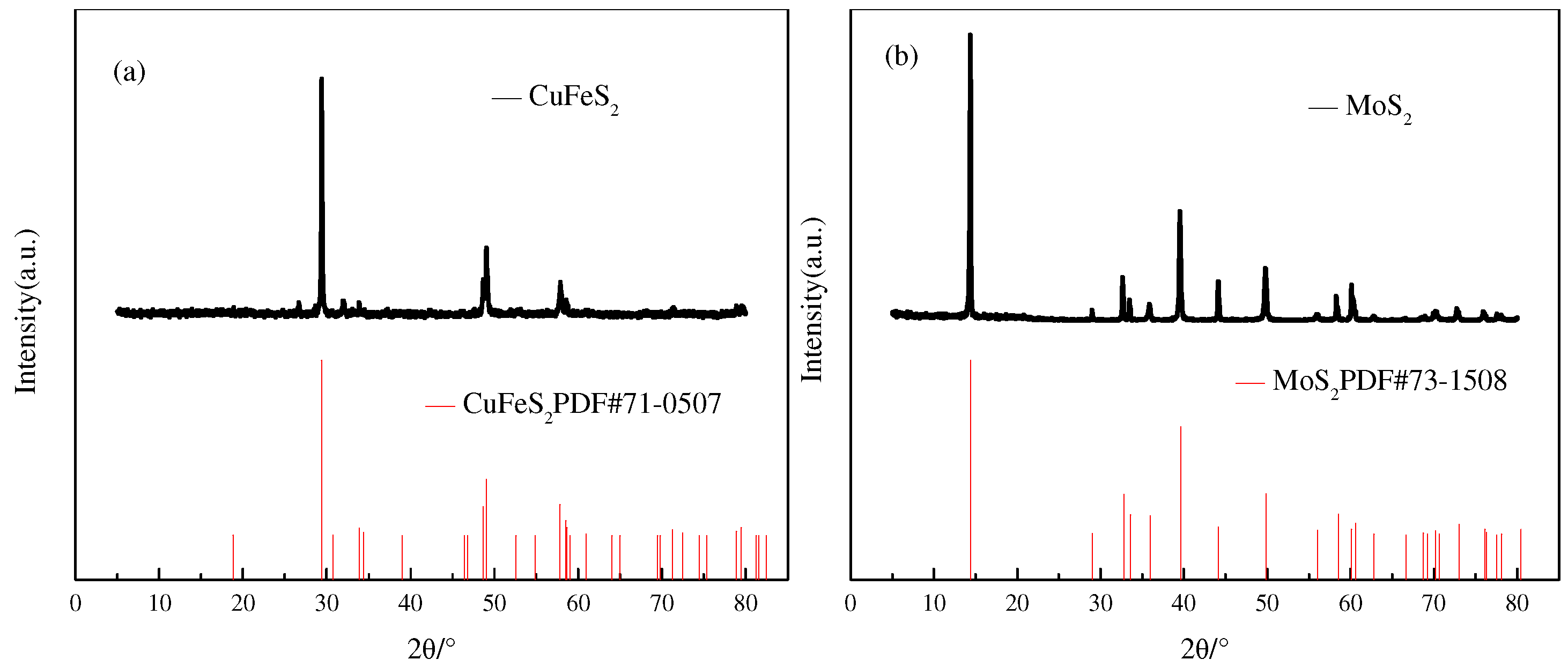
2.1. Samples and Reagents
2.2. Preparation of Bacterial Cultures
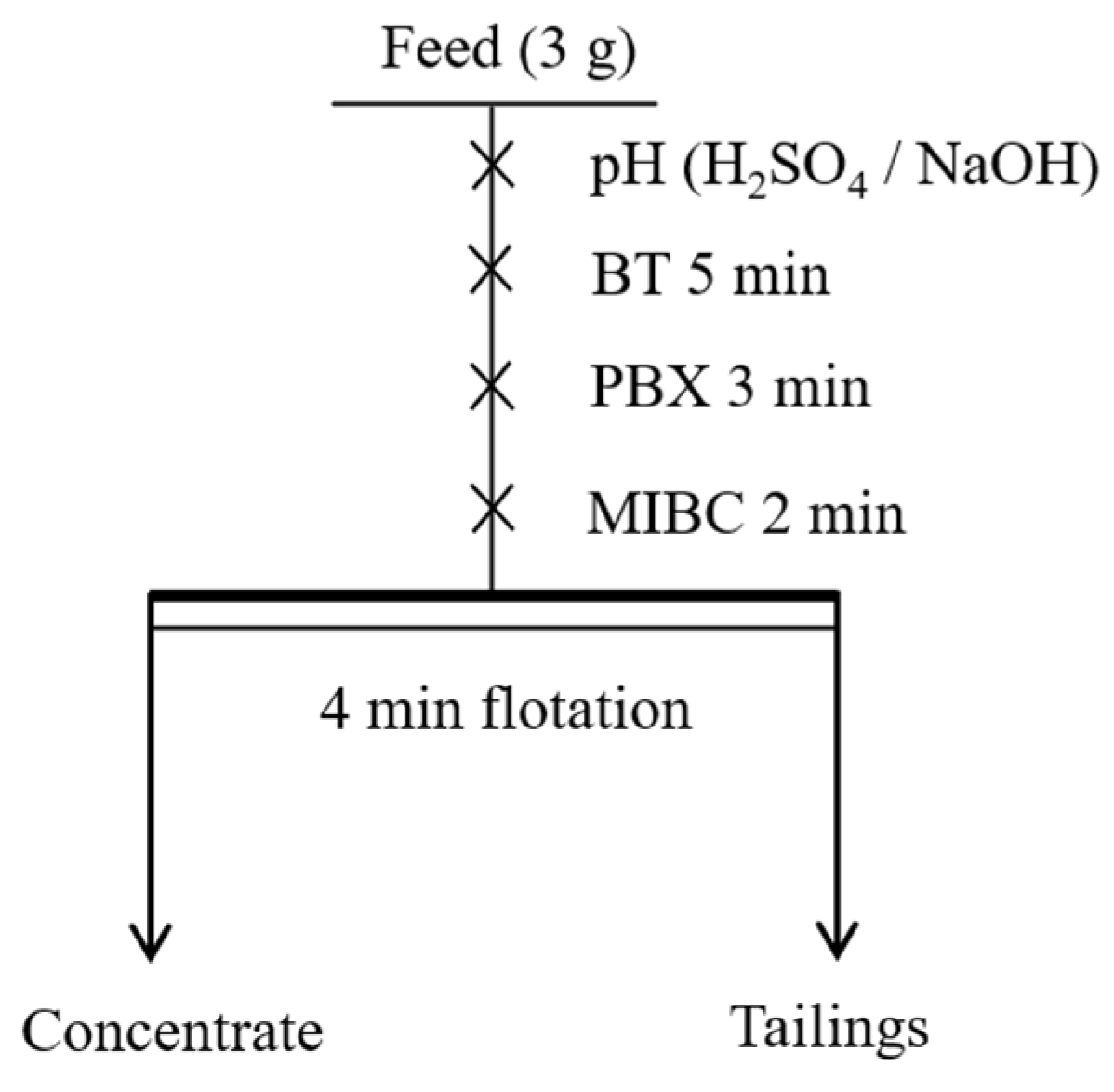
2.3. Flotation Tests
2.4. Physicochemical Analyses
2.4.1. Contact Angle Test
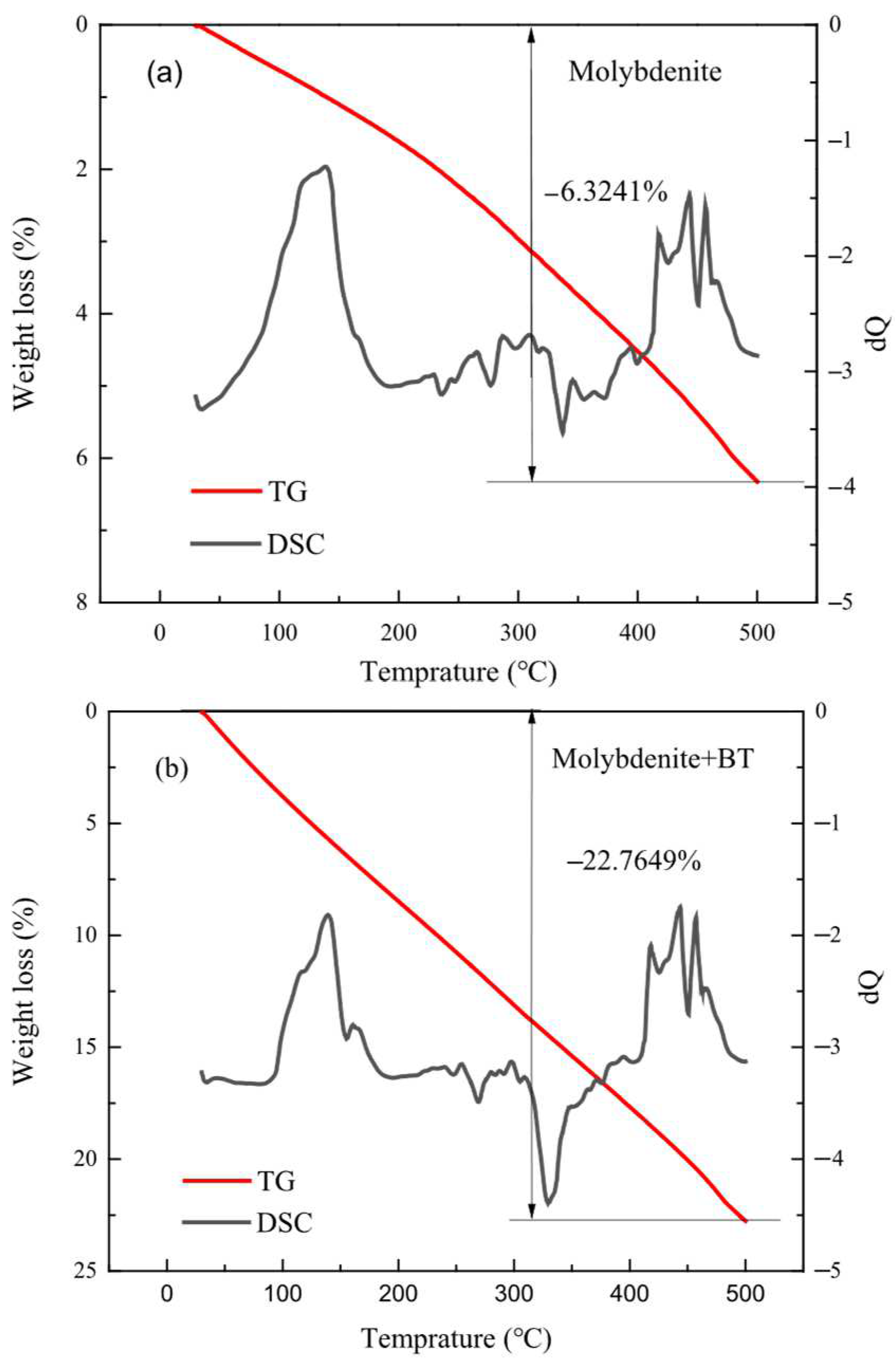
2.4.2. TG-DSC Comprehensive Thermal Analysis
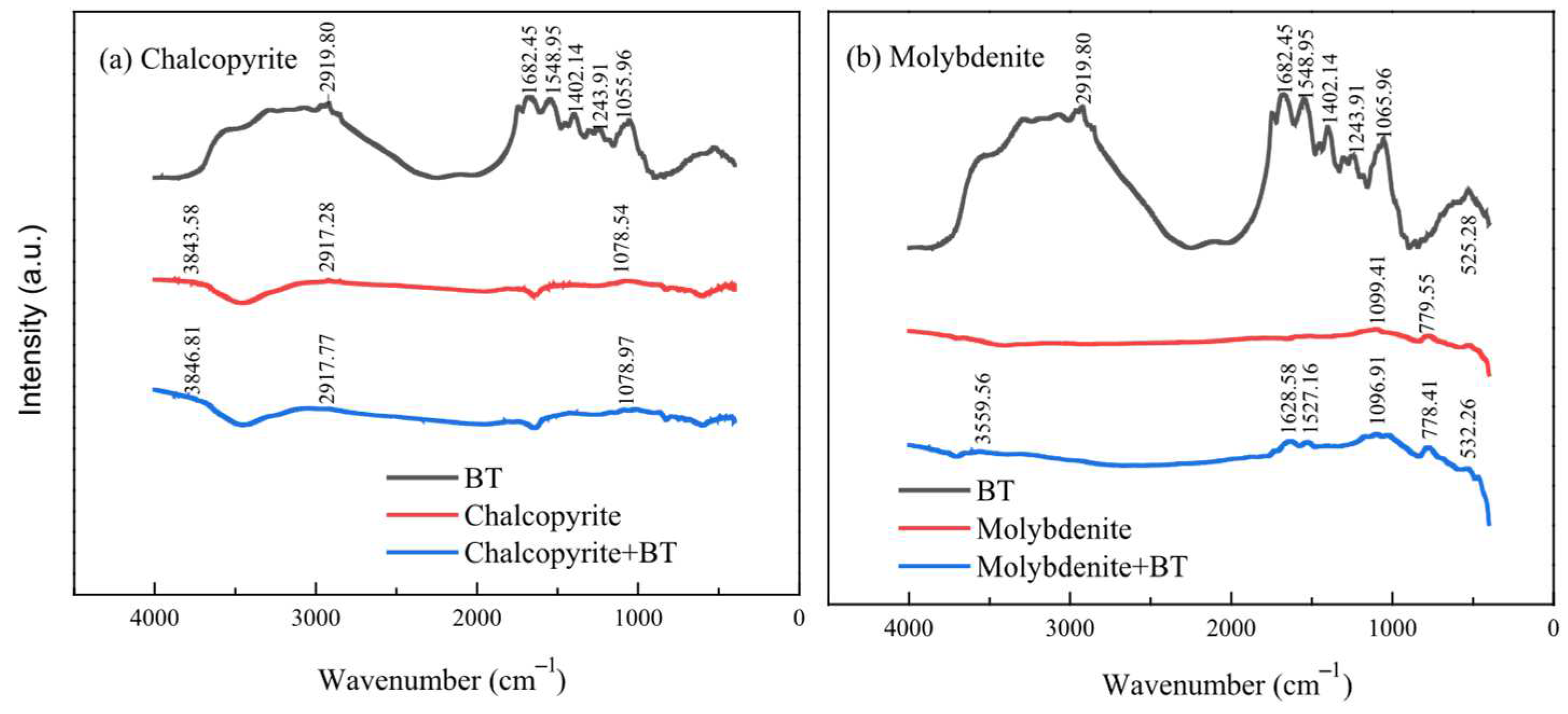
2.4.3. Fourier Infrared Spectroscopy Test
3. Results and Discussion
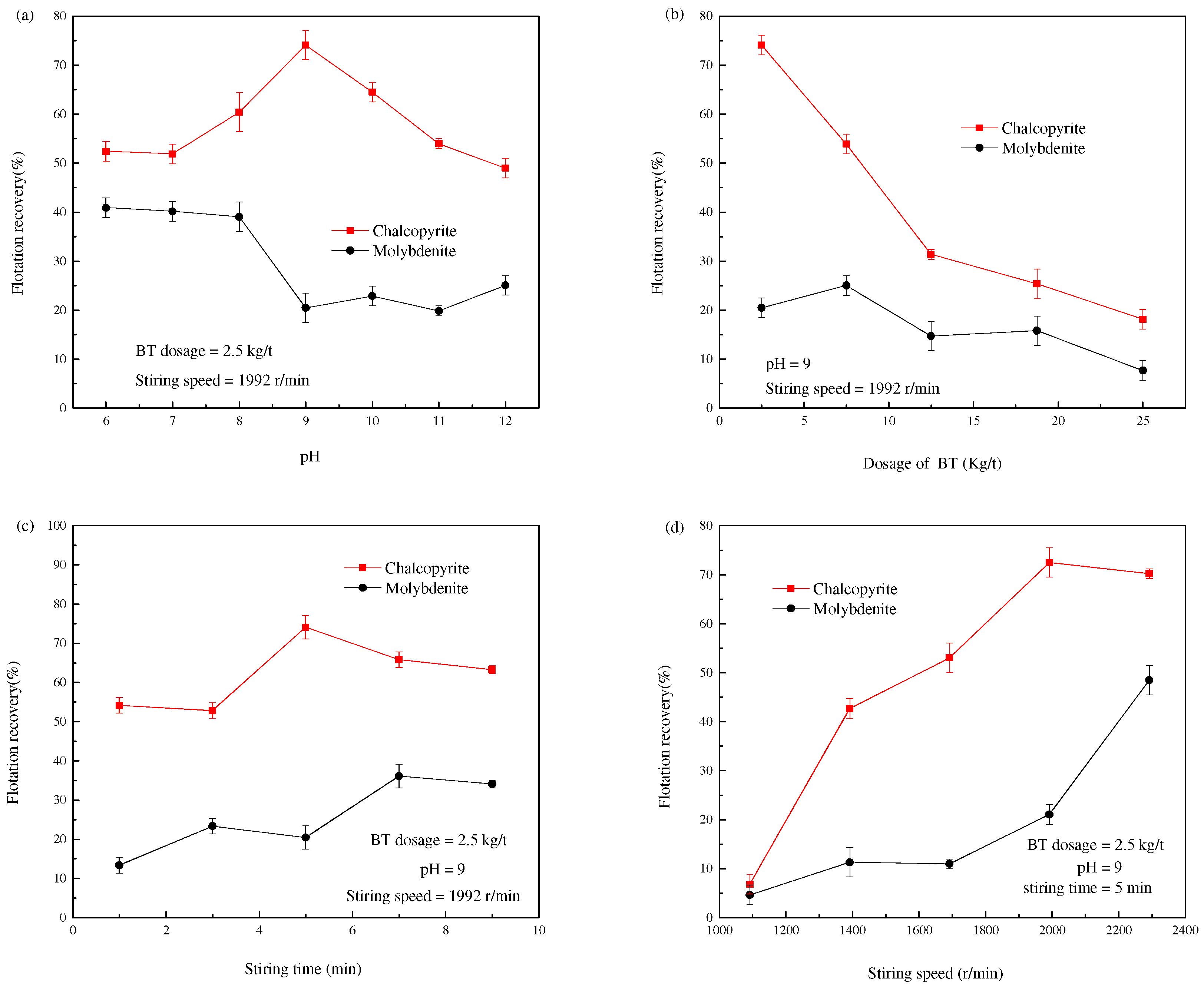
3.1. Flotation Experiment
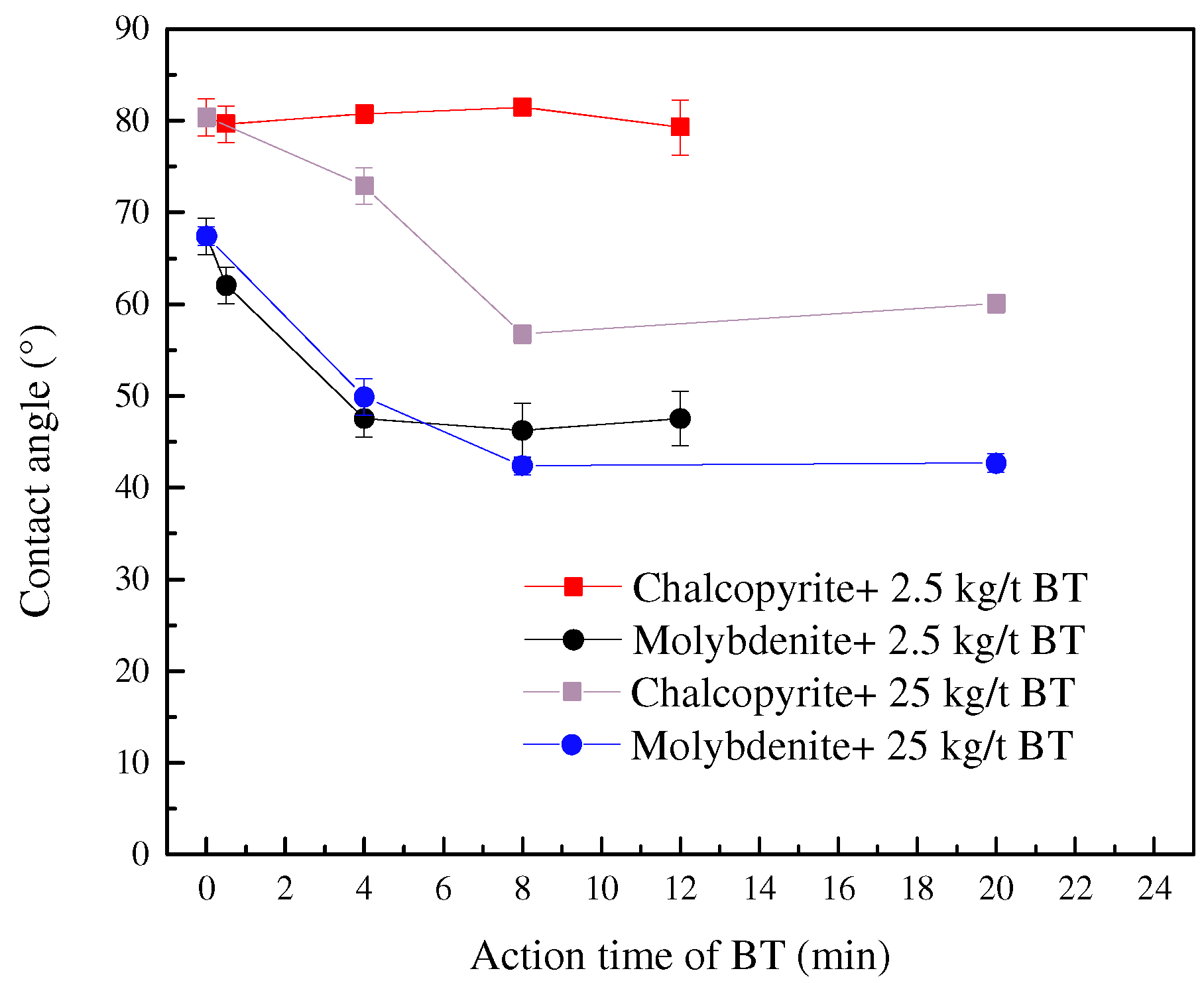
3.2. Contact Angle Analysis
3.3. TG-DSC Thermal Analysis
3.4. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dutta, S.K.; Lodhari, D.R. Molybdenum. In Extraction of Nuclear and Non-Ferrous Metals; Springer: Berlin/Heidelberg, Germany, 2018; pp. 205–209. [Google Scholar]
- Huang, P.; Wang, L.; Liu, Q. Depressant function of high molecular weight polyacrylamide in the xanthate flotation of chalcopyrite and galena. Int. J. Miner. Process. 2014, 128, 6–15. [Google Scholar] [CrossRef]
- Hirajima, T.; Mori, M.; Ichikawa, O.; Sasaki, K.; Miki, H.; Farahat, M.; Sawada, M. Selective flotation of chalcopyrite and molybdenite with plasma pre-treatment. Miner. Eng. 2014, 66–68, 102–111. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, S.; Xu, Z.; Zhang, C.; He, J.; Zou, J.; Chen, D.; Sun, W. Flotation separation of molybdenite from chalcopyrite using an environmentally-efficient depressant L-cysteine and its adsoption mechanism. Miner. Eng. 2020, 156, 106438. [Google Scholar] [CrossRef]
- Zanin, M.; Ametov, I.; Grano, S.; Zhou, L.; Skinner, W. A study of mechanisms affecting molybdenite recovery in a bulk copper/molybdenum flotation circuit. Int. J. Miner. Process. 2009, 93, 256–266. [Google Scholar] [CrossRef]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Adsorption characteristics and mechanisms of O-Carboxymethyl chitosan on chalcopyrite and molybdenite. J. Colloid Interface Sci. 2019, 552, 659–670. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Wang, H.; Chen, Y.; Guo, M. The depression behavior and mechanism of tragacanth gum on chalcopyrite during Cu-Mo flotation separation. Adv. Powder Technol. 2021, 32, 2712–2719. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Q.; Wen, S.; Xian, Y.; Liu, J.; Liang, G. Flotation separation of chalcopyrite from molybdenite with sodium thioglycolate: Mechanistic insights from experiments and MD simulations. Sep. Purif. Technol. 2024, 342, 126958. [Google Scholar] [CrossRef]
- Yang, B.; Yan, H.; Zeng, M.; Huang, P.; Jia, F.; Teng, A. A novel copper depressant for selective flotation of chalcopyrite and molybdenite. Miner. Eng. 2020, 151, 106309. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Zhang, W.; Zhang, L.; Guo, Y.; Wang, T.; Wang, H. The role of sodium alginate in the flotation separation of apatite and dolomite. Powder Technol. 2020, 373, 620–626. [Google Scholar] [CrossRef]
- Feng, B.; Zhong, C.; Zhang, L.; Guo, Y.; Wang, T.; Huang, Z. Effect of surface oxidation on the depression of sphalerite by locust bean gum. Miner. Eng. 2020, 146, 106142. [Google Scholar] [CrossRef]
- Feng, B.; Guo, Y.; Zhang, W.; Peng, J.; Wang, H.; Huang, Z.; Zhou, X. Flotation separation behavior of chalcopyrite and sphalerite in the presence of locust bean gum. Miner. Eng. 2019, 143, 105940. [Google Scholar] [CrossRef]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, Q.; Liu, W.; Zheng, J.; Sun, H. Flotation separation of copper–molybdenum sulfides using chitosan as a selective depressant. Miner. Eng. 2015, 83, 217–222. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhai, J.; Qingjun, G. Evaluation of the replacement of NaCN with depressant mixtures in the separation of copper–molybdenum sulphide ore by flotation. Sep. Purif. Technol. 2017, 173, 9–16. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Wu, M.; Xu, Z.; Tian, M.; Yin, Z.; Sun, W.; Zhang, C. Flotation separation of molybdenite from chalcopyrite using rhodanine-3-acetic acid as a novel and effective depressant. Miner. Eng. 2021, 162, 106747. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Liu, R.; Jiang, W.; Zhang, C.; Guan, Q.; Zhang, C. Synthesis of acetic acid-[(hydrazinylthioxomethyl)thio]-sodium and its application on the flotation separation of molybdenite from galena. J. Ind. Eng. Chem. 2017, 52, 82–88. [Google Scholar] [CrossRef]
- Zhang, X.R.; Lu, L.; Cao, Y.J.; Yang, J.B.; Che, W.F.; Liu, J.T. Relapsed/refractory early T-cell precursor acute lymphoblastic leukemia was salvaged by venetoclax plus HAG regimen. Chem. Eng. J. 2020, 99, 395–397. [Google Scholar] [CrossRef]
- Chen, J.H.; Lan, L.H.; Liao, X.J. Depression effect of pseudo glycolythiourea acid in flotation separation of copper-molybdenum. Trans. Nonferrous Met. Soc. China 2013, 23, 824–831. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, L.; He, J.; Wu, H.; Fang, S.; Khoso, S.A.; Hu, Y.; Sun, W. Evaluation of L-cysteine as an eco-friendly depressant for the selective separation of MoS 2 from PbS by flotation. J. Mol. Liq. 2019, 282, 177–186. [Google Scholar] [CrossRef]
- Li, M.Y.; Wei, D.Z.; Shen, Y.B.; Liu, W.G.; Gao, S.L.; Liang, G.Q. Selective depression effect in flotation separation of copper-molybdenum sulfides using 2,3-disulfanylbutanedioic acid. Trans. Nonferrous Met. Soc. China 2015, 25, 3126–3132. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.; Zheng, M.; Xu, S.; Zheng, R.; Cao, J.; Jin, X.; Gao, Z.; Feng, Z. Prediction of collector flotation performance based on machine learning and quantum chemistry: A case of sulfide minerals. Sep. Purif. Technol. 2024, 342, 126954. [Google Scholar] [CrossRef]
- de Mesquita, L.M.S.; Lins, F.F.; Torem, M.L. Interaction of a hydrophobic bacterium strain in a hematite-quartz flotation system. Int. J. Miner. Process. 2003, 71, 31–44. [Google Scholar] [CrossRef]
- Szymanska, A.; Sadowski, Z. Effects of biosurfactants on surface properties of hematite. Adsorption 2010, 16, 233–239. [Google Scholar] [CrossRef]
- Lin, H.; Dong, Y.; Fu, K. Influencing Factors and Mechanisms of Microbial Leaching of Copper Sulfide; Metallurgical Industry Press: Beijing, China, 2019. [Google Scholar]
- Li, R. Spectrum Analysis of Organic Structure; Tianjin University Press: Tianjin, China, 2002. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, G.; Xiao, G.; Feng, B. Flotation Separation of Chalcopyrite and Molybdenite by Eco-Friendly Microorganism Depressant Bacillus tropicus. Minerals 2025, 15, 762. https://doi.org/10.3390/min15070762
Ai G, Xiao G, Feng B. Flotation Separation of Chalcopyrite and Molybdenite by Eco-Friendly Microorganism Depressant Bacillus tropicus. Minerals. 2025; 15(7):762. https://doi.org/10.3390/min15070762
Chicago/Turabian StyleAi, Guanghua, Guosheng Xiao, and Bo Feng. 2025. "Flotation Separation of Chalcopyrite and Molybdenite by Eco-Friendly Microorganism Depressant Bacillus tropicus" Minerals 15, no. 7: 762. https://doi.org/10.3390/min15070762
APA StyleAi, G., Xiao, G., & Feng, B. (2025). Flotation Separation of Chalcopyrite and Molybdenite by Eco-Friendly Microorganism Depressant Bacillus tropicus. Minerals, 15(7), 762. https://doi.org/10.3390/min15070762




